Abstract
The surface tensions of binary mixtures alkyl levulinate (methyl levulinate and ethyl levulinate) + n-alkanols (methanol, ethanol, 1-propanol, and 1-butanol) at several temperatures (283.15 K, 298.15 K, and 313.15 K) and at atmospheric pressure were reported. For each binary mixture, the surface tension deviations were obtained and correlated with composition by using the Redlich–Kister polynomial expansion. These surface tension deviations vary from positive values for methanol to negative ones for 1-butanol. Regarding the behavior of surface tension deviation with alkyl levulinate, ethyl levulinate presents higher positive values or less negative ones than methyl levulinate. The computation of the surface tension was obtained with the linear square gradient theory plus the Peng–Robinson–Stryjek–Vera (PRSV-EoS). Phase equilibria for all the mixtures were predicted, because \(k_{12} = 0\) was set. Then, the densities of the homogeneous phases were obtained and used in the calculation of the surface tension, which was obtained according to two approaches, i.e., prediction and fitted, and using values constant and correlations for the parameters for both approaches. The predictive approach was not adequate because a high global deviation was obtained (3.97 %), while two adjustable parameters for the mixture in LSGT improved the representation of the variation of experimental surface tension with temperature (deviation = 1.08 %). Therefore, the simplified version of square gradient theory named LSGT guarantees good results of fitting the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to its potential use as substitutes for the traditional solvents in chemical processes and even as biodiesel, levulinic acid and its esters have attracted the interest of the scientific community [1, 2]. Our research group is interested in the thermophysical properties of systems containing an alkyl levulinate and a short-chain alkanol [3, 4]. Herein, we present the surface tension of the binary mixtures containing an alkyl levulinate (methyl levulinate and ethyl levulinate) and n-alkanol (methanol, ethanol, 1-propanol, and 1-butanol) in the temperature range (283.15 K to 313.15 K) and at atmospheric pressure.
The surface tension modeling for the experimentally studied systems in the range of 283.15 K to 313.15 K was obtained by applying the linear square gradient theory (LSGT), i.e., the simplified version of square gradient theory (SGT). This theoretical approach (SGT) [5, 6] requires an expression for the Helmholtz energy density, which can be obtained from an equation of state (EoS). As the computation of surface tension through gradient theory requires phase equilibrium properties, this work will also model vapor-liquid phase equilibrium for binary mixtures. According to a literature search, only Resk et al. [7] show experimental data on the phase equilibrium for the ethanol + ethyl levulinate mixture at 60 °C. Therefore, it is interesting to theoretically know the bubble and dew curves for these systems that have practically not been studied experimentally. For the application of SGT to binary mixtures, it is necessary to obtain a parameter named symmetric parameter from the fit of experimental surface tension data. If this parameter is zero, a system of nonlinear algebraic equations must be solved to obtain the surface tension, and SGT is used as a predictive approach, i.e., it would not be necessary to fit the experimental surface tension. On the other hand, when symmetric parameter is not zero, SGT requires the solution of a system of complex differential equations. The disadvantage of null symmetric parameter is that the calculation becomes complex when mixtures present components with simultaneous adsorption and desorption. For this reason, the linear approach proposed by Zuo and Stenby [8, 9] has been widely used to efficiently calculate the surface tension of complex systems, without the need to know the density profile at the interface, because the authors [8, 9] propose a linear profile.
Therefore, Peng–Robinson–Stryjek–Vera equation of state (PRSV-EoS) + quadratic mixing rule (QMR) will be used to obtain the phase equilibrium, and the surface tension computation will be obtained with PRSV + QMR + LSGT.
We have found a paper [2] reporting the surface tensions of alkyl levulinates with n-alkanols (methyl levulinate + methanol, ethyl levulinate + ethanol, and butyl levulinate + butanol) at four temperatures and at atmospheric pressure.
2 Materials and Methods
The chemicals were used without further purification Their purities were obtained using gas chromatography by the suppliers, while a Karl-Fischer titration was employed in our laboratory to determine their water content using a Crison KF 1S-2B. This information is reported in Table 1.
The mixtures were prepared by mass using a CP225-D Sartorius Semimicro mass balance, being the uncertainty \(1\times 10^{-5}\) g. The corresponding uncertainty in the mole fraction is 0.001.
The surface tensions at the liquid–air interface, \(\sigma,\) of both the pure liquids and their mixtures, were determined using a Lauda TVT-2 drop volume tensiometer. The density of the liquid samples needed to obtain the surface tension was calculated from previous papers [3, 4]. The temperature was maintained constant within ± 0.01 K using a Lauda E-200 thermostat. The surface tension uncertainty is 0.2 mN·m−1.
3 Theoretical Models
3.1 Equation of State
Peng–Robinson–Stryjek–Vera equation of state (PRSV-EoS) [10, 11] has been successfully applied to quantitatively represent thermodynamic properties [12,13,14,15,16,17]. This equation of state proposes that pressure, i.e., P is given by Eq. 1:
where R is the universal ideal gas constant, T is the temperature, \(\rho\) is the molar density, a is the attractive parameter, and b is the repulsive term. These parameters for a pure fluid (\(i=1, 2\)) are given by Eq. 2:
where \(T_{c,i}\) and \(P_{c,i}\) are the critical temperature and critical pressure, respectively. \(c_1\) and \(c_2\) are the fitted parameters, which are directly fitted for vapor pressure data.
For the binary mixtures, the quadratic mixing rule was used. This mixing rule proposes that attractive and repulsive parameters can be obtained by Eq. 3:
where subscripts 1 and 2 are methyl levulinate or ethyl levulinate and alcohol (methanol, ethanol, 1-propanol, and 1-butanol) as pure fluids, respectively. \(w_i\) is the mole fraction of the component i in the mixture, which can be in the liquid phase or vapor phase. On the other hand, \(k_{12}\) is the adjustable parameter.
3.2 Linear Square Gradient Theory (LSGT)
According to approach published by Zuo and Stenby [8, 9], the surface tension for the fluid mixture is given by Eq. 4:
where L, V, subscript ref, and superscript 0 are the condition of liquid and vapor at equilibrium, reference, and the condition in the bulk equilibria phases, respectively, \(f_0\) is the Helmholtz energy density of homogeneous fluid, \(\mu\) is the chemical potential, and \(\chi\) is the influence parameter of the mixture, which contains the information of the intermolecular geometry of the interfacial region, and can be obtained by Eq. 5:
where \(\Delta \rho _ i = \rho _ i ^L - \rho _ i ^V\) for \(i= 1, 2\) and \(\beta _{12}\) is the symmetric parameter, which can be obtained by experimental surface tension adjustment of the binary mixture. It is important to note that the reference density is calculated according to Eq. 6:
On the other hand, the chemical potential of the component i (\(i=1, 2\)) in the mixture is defined by Eq. 7 [18]:
Also, using Eq. 8 [13], the Helmholtz energy density from PRSV-EoS can be obtained by Eq. 8:
Therefore, to obtain the surface tension using Eq. 4, it will be necessary follow main steps (i) to (iv):
-
(i)
Select the reference fluid.
-
(ii)
Calculate the density profiles using Eq. 9, i.e., \(\rho _1(z)\) and \(\rho _2(z)\),
$$\begin{aligned} \rho _i (z) = \rho _i ^{V} + \left( \frac{ \rho _i ^{L} - \rho _i ^{V} }{n-1} \right) z \end{aligned}$$(9)where n is the number of points used in the discretization of z.
-
(iii)
Calculate the influence parameters of pure fluids (\(\chi _{1}\) and \(\chi _{2}\)) considering that is a constant value and on the other hand, considering that it can be obtained by means of a linear correlation dependent on temperature. To calculate the surface tension of the pure fluid, Eq. 4 can be used for fluid 1 (\(x_1 \rightarrow 1\)) and fluid 2 (\(x_1 \rightarrow 0\)), or the expression proposed by Carey et al. [5, 6] for pure fluids.
-
(iv)
Two approaches will be used:
-
Predictive approach: Set \(\beta _{12}=0\), and study: Case 1: \(\chi _i\) is a value constant. Case 2: \(\chi _i\) is a linear function of temperature.
-
Fitted approach: Study the following cases: Case 1: \(\chi _i\) is a value constant and \(\beta _{12}\) is a value constant, which can be obtained by fitting experimental information of the tension of the mixture. Case 2: \(\chi _i\) and \(\beta _{12}\) are linear functions of temperature. The adjustable parameters present in the symmetric parameter correlation can be obtained by fitting experimental information of the tension of the mixture.
-
4 Results and Discussions
The experimental surface tensions of pure components at working temperatures and at atmospheric pressure along with previously published data are collected in Table 2. The agreement between the experimental and literature data for n-alkanols is satisfactory. For alkyl levulinates, we have only found three previous references [2, 19, 20]. Two references from our laboratory, the agreement is excellent, and the other one from Ramli and Abdullah [2] being our experimental surface tensions a little higher.
Experimental surface tensions, \(\sigma\), together with calculated surface tension deviations, \(\Delta \sigma\), are collected in the supplementary material (see Table S1). The surface tension deviations are plotted in Figs. 1, 2, 3, and 4.
Surface tension deviations, \(\Delta \sigma\), as a function of the molar fraction, \(x_1\), at working temperatures and at \(P=0.1\) MPa for the binary mixtures alkyl levulinate (1) + methanol (2). Symbols: experimental data obtained in this work; (circle) methyl levulinate (1) + methanol (2) mixture, (triangle) ethyl levulinate (1) + methanol (2) mixture, (black) 283.15 K, (blue) 298.15 K, (red) 313.15 K. Redlich–Kister equation; (——–) methyl levulinate (1) + methanol (2) mixture, (- - - - - -) ethyl levulinate (1) + methanol (2) mixture (Color figure online)
Surface tension deviations, \(\Delta \sigma\), as a function of the molar fraction, \(x_1\), at working temperatures and at \(P=0.1\) MPa for the binary mixtures alkyl levulinate (1) + ethanol (2). Symbols: experimental data obtained in this work; (circle) methyl levulinate (1) + ethanol (2) mixture, (triangle) ethyl levulinate (1) + ethanol (2) mixture, (black) 283.15 K, (blue) 298.15 K, (red) 313.15 K. Redlich–Kister equation; (——–) methyl levulinate (1) + ethanol (2) mixture, (- - - - - -) ethyl levulinate (1) + ethanol (2) mixture
Surface tension deviations, \(\Delta \sigma\), as a function of the molar fraction, \(x_1\), at working temperatures and at \(P=0.1\) MPa for the binary mixtures alkyl levulinate (1) + 1-propanol (2). Symbols: experimental data obtained in this work; (circle) methyl levulinate (1) + 1-propanol (2) mixture, (triangle) ethyl levulinate (1) + 1-propanol (2) mixture, (black) 283.15 K, (blue) 298.15 K, (red) 313.15 K. Redlich–Kister equation; (——–) methyl levulinate (1) + 1-propanol (2) mixture, (- - - - - -) ethyl levulinate (1) + 1-propanol (2) mixture
Surface tension deviations, \(\Delta \sigma\), as a function of the molar fraction, \(x_1\), at working temperatures and at \(P=0.1\) MPa for the binary mixtures alkyl levulinate (1) + 1-butanol (2). Symbols: experimental data obtained in this work; (circle) methyl levulinate (1) + 1-butanol (2) mixture, (triangle) ethyl levulinate (1) + 1-butanol (2) mixture, (black) 283.15 K, (blue) 298.15 K, (red) 313.15 K. Redlich–Kister equation; (——–) methyl levulinate (1) + 1-butanol (2) mixture, (- - - - - -) ethyl levulinate (1) + 1-butanol (2) mixture
The surface tension deviation with respect to a linear dependence on the mole fraction, \(\Delta \sigma\), was calculated from Eq. 10:
where \(\sigma\), \(\sigma _i\), and \(x_i\) are the surface tension of the mixture, the surface tension of component i, and the mole fraction of component i, respectively.
For each binary mixture, the surface tension deviation as a function of mole fraction was correlated with the Redlich–Kister equation [21], i.e., by Eq. 11:
being \(A_i\) the adjustable parameters. The number of these coefficients was chosen in order to minimize the standard deviations. The obtained parameters, \(A_i\), along with the corresponding standard deviations, \(\sigma (\Delta \sigma )\), are given in Table 3.
The binary mixtures methyl levulinate + methanol and ethyl levulinate + methanol present symmetrical curves of positive surface tension deviation over the whole composition range and at the three working temperatures. An increase in temperature leads to an increase in the surface tension deviation values. The binary mixtures containing ethanol present a sigmoidal behavior against composition of the surface tension deviations: for the system methyl levulinate + ethanol, this behavior is observed at temperatures 298.15 K and 313.15 K, and at \(T = 283.15\) K, the surface tension deviations are negative. While for the system ethyl levulinate + ethanol, the sigmoidal behavior is presented only at \(T = 283.15\) K; at the rest of temperatures 298.15 K and 313.15 K, the surface tension deviations are positive. That is, for these systems containing ethanol, the surface tension deviations tend to be more positive with temperature. The binary mixtures containing 1-propanol show negative surface tension deviations and less symmetrical curves over the entire composition range and at different temperatures, with the exception of the system ethyl levulinate + 1-propanol which shows at \(T = 313.15\) K a sigmoidal behavior positive surface tension deviation. The surface tension deviations for these systems tend to less negative or even positive with temperature. Finally, the binary mixtures studied which contain 1-butanol have negative \(\Delta \sigma\) values. It can be observed that increasing the temperature the deviations become less negative.
For a given alkyl levulinate, at the three temperatures, \(\Delta \sigma\) decreases in the sequence: methanol > ethanol > 1-propanol > 1-butanol,, although for ethyl levulinate at \(T = 313.15\) K, and at high molar fractions of ethyl levulinate, the surface tension deviations for ethanol and 1-propanol are similar. On the other hand, the binary mixtures containing ethyl levulinate show more elevated positive surface tension deviations or less negative \(\Delta \sigma\) values than the systems with methyl levulinate. Except in a couple of cases: for the binary mixtures containing methanol at \(T = 313.15\) K for which the two alkyl levulinates present similar \(\Delta \sigma\) values; and for the systems alkyl levulinate with ethanol at \(T = 313.15\) K in which only at low molar fractions the ethyl levulinate presents higher surface tension deviations values.
The graphical comparison between our surface tension results and those of Ramli and Abdullah [2] is presented in Figs. 5 and 6. For the system methyl levulinate + methanol at \(T = 298.15\) K and 313.15 K, the average deviation is around 1 mN\(\cdot\)m\(^{-1}\) (\(\approx\) 3 %), this deviation is higher and greater from \(x_1 = 0.3\). While for the mixture ethyl levulinate + ethanol at the two temperatures, the average deviation is around 0.6 mN\(\cdot\)m\(^{-1}\) (\(\approx\) 2 %), being also this deviation bigger from \(x_1 = 0.3\)
Comparison between experimental and literature surface tensions, \(\sigma\), as a function of the molar fractions, \(x_1\), at \(T=298.15\) K and 313.15 K, and \(P=0.1\) MPa for the binary mixture methyl levulinate (1) + methanol (2). Symbols: (circle) Experimental data obtained in this work, (triangle) Experimental data obtained from literature [2], (blue) 298.15 K, (red) 313.15 K.
Comparison between experimental and literature surface tensions, \(\sigma\), as a function of the molar fractions, \(x_1\), at \(T=298.15\) K and 313.15 K, and \(P=0.1\) MPa for the binary mixture ethyl levulinate (1) + ethanol (2). Symbols: (circle) Experimental data obtained in this work, (triangle) Experimental data obtained from literature [2], (blue) 298.15 K, (red) 313.15 K
Linear gradient theory coupled with Peng–Robinson–Stryjek–Vera equation of state was used to model the behavior of surface tension in mixtures. First of all, it was necessary to obtain the parameters of the pure fluids that form the binary mixtures, which are shown in Tables 4 and 5. Critical temperature and critical pressure for all alcohols were obtained from DIPPR [22], while for the n-alkyl levulinates, it was obtained from Nikitin et al. [23]. The \(c_1\) and \(c_2\) parameters were calculated by fitting the experimental vapor pressure [22, 23] from the minimization of the objective function defined by Eq. 12:
where N represents the number of experimental points, exp and calc are experimental and calculated value, respectively.
According to Table 4, good results in vapor pressure were obtained for all fluids (overall deviation = 0.42 %); the smallest deviation was for methanol (0.00 %). The statistical deviation in vapor pressure was obtained by Eq. 13:
To obtain the adjustable parameters required in the computation of the surface tension of pure fluids, the objective function given by Eq. 14 was used, and the deviation between the theoretical and experimental surface tension data was obtained through the Eq. 15,
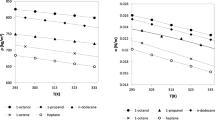
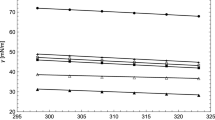
The results of the deviation in surface tension are published in Table 5. For all fluids (except methanol), the correlation allowed modeling the experimental surface tension with a smaller deviation (0.22 %) than that obtained with a temperature-independent influence parameter (1.07 %). In the case of methanol, the approximately null value for the slope implies that a linear dependence between the temperature and the influence parameter does not improve the results for the temperature range of 283.15 K to 313.15 K. On the other hand, Figs. 7 and 8 show the variation of surface tension with temperature for alkyl levulinate and alcohols, respectively. According to Fig. 7, both cases show a good agreement in the theoretical and experimental data, while in Fig. 8, the theoretical surface tension is close to the experimental one only when the linear dependence was considered for the influence parameter.
To model the experimental surface tension of the n-alkyl levulinate (1) + alcohol (2) mixtures, it was necessary to previously obtain the phase equilibria. According to the literature related to the gradient theory, to guarantee consistency in the results of surface tension, the phase equilibrium must be correctly modeled. Therefore, it is necessary to adjust the adjustable parameter of the mixing rule with experimental information of the phase equilibria. Unfortunately, after a literature search, only the phase equilibria of ethyl levulinate (1) + ethanol (2) at 333.15 K were obtained [7]. Therefore, as no data are available for all mixtures in the temperature range of 283.15 K to 313.15 K, a predictive equation of state approach to phase equilibria modeling was used in this manuscript, i.e., \(k_{12} = 0\).
Figures S1 to S8 illustrate the isothermal phase equilibrium at 283.15 K, 298.15 K, and 313.15 K in the plane \(P-x_1-y_1\), which was obtained by pressure bubble computation, i.e., given the temperature and the liquid phase mole fraction, the vapor phase mole fraction, pressure, and densities can be calculated. In all Figs. S1 to S8, it is observed that the bubble curve is above the dew curve. Next, attention will be paid to Fig. S6. In this figure, in addition to the predictions, the result of the adjustment to 333.15 K is shown. Clearly, although \(k_{12}=0\) under-predicts the pressure of the mixture, PRSV-EoS qualitatively predicts the mixture pressure. On the other hand, it was found that a binary interaction parameter equal to 0.0251 correctly adjusts the phase equilibria for the ethyl levulinate (1) + ethanol (2) mixture at 333.15 K [7]. However, even though optimal \(k_{12}\) differs from zero, the fact that \(k_{12} = 0\) qualitatively represents phase equilibria is a good idea to consider in the absence of experimental data.
Two approaches were used to study the surface tension obtained with LSGT + PRSV-EoS, i.e., an predictive approach (set \(\beta _{12} = 0\)) and fitted approach (\(\beta _{12} =\) constant and \(\beta _{12}= f(T)\), where the adjustable parameters are obtained by regression of experimental tension data of the mixture). Table 6 shows the statistical deviation using Eq. 15 and the impact of using constant influence parameter or linear functions of temperature. According to the table, a slight improvement in the prediction of surface tension (4.95 % and 2.76 % obtained for methyl levulinate (1) + alcohol (2) mixtures and ethyl levulinate (1) + alcohol (2) mixtures, respectively) is obtained when a linear dependence between temperature and the influence parameter has been considered, compared to 5.07 % and 2.87 % for the same mixtures when a constant influence parameter was used. Also, for both cases, the predictions were better for the mixtures of alcohol with ethyl levulinate. The lowest deviation (with both models, \(\le\) 1.42%) was obtained for the ethyl levulinate + methanol mixture; this can be explained because a linear dependence between the fluid density in the mixture and the position in the interface does not significantly affect the surface tension calculations when the symmetric parameter has a null value. In order to improve the results obtained predictively, LSGT was also applied with a fitted approach using two cases, constant values for the pure influence parameters and the symmetric parameter, and using linear functions of temperature for the pure influence parameter and symmetric parameter. The objective function used in this investigation was that of Weiland et al. [24, 25], which is given by Eq. 16:
Smith et al. [26] also used LSGT and recommended using Eq. 16 because weights all the surface tension (high and low) equally.
Table 7 shows the results of the fitted approach, when a constant symmetric parameter and a linear correlation with temperature were used. Applying the two cases of the fitted approach, the deviation in surface tension was lower for the ethyl levulinate + alcohol mixtures. On the other hand, the use of the influence and symmetric parameters depending on the temperature allowed a better representation of the surface tensions of all the mixtures (overall deviation = 1.43 % for methyl levulinate + alcohol mixtures and overall deviation = 0.72 % for ethyl levulinate + alcohol mixtures). Also, according to Table 7, the symmetric parameter decreases with increasing temperature. The smallest deviation was for the methyl levulinate + methanol mixture (0.60 %, obtained using linear correlations for the influence parameter and for the symmetric parameter) and the highest deviation was for the methyl levulinate + 1-propanol (2.35 %, obtained using constant values for influence parameter and symmetric parameter). Therefore, using case 2 of this fitted approach, at 32 % and 50 %, decrease in surface tension deviation is achieved with respect to case 1.
To illustrate the variation of the surface tension with the liquid mole fraction of the alkyl levulinate, two temperatures were selected (283.15 K and 313.15 K) and both approaches with both cases were used. The results are shown in Figs. 9, 10, 11, 12, 13, 14, 15, and 16. From these figures, it is seen that as the liquid mole fraction of the alkyl levulinate increases, the surface tension of the mixture increases. The graphical results for the surface tension of the mixtures indicate that the predictions obtained with fitted parameters (constant, or part of a correlation) give similar results, which is according to reported results in Table 6. Figures 9 and 13 show that the best predictions were for methyl levulinate (1) + methanol (2) a 313.15 K and for ethyl levulinate (1) + methanol (2) at 283.15 K and 313.15 K, respectively. On the other hand, for all the mixtures, it can be seen graphically that the fitted approach greatly improves the representation of the experimental surface tension data. Finally, it is relevant to mention that using linear density profiles reduces the computational cost compared to SGT, and good results can be obtained for surface tension despite the assumption of linearity. The disadvantage of the linear model used in this work is that is not possible to determine interfacial properties such as density profile, interfacial thickness, and adsorption/desorption of the components. For this reason, null symmetric parameters in LSGT + PRSV-EoS do not guarantee good results (overall deviation considers all mixtures = 3.97 % and 3.86 % using constant values and correlations for influence parameters), because clearly the density profile is not linear (according to SGT), so the fitted approach (overall deviation considers all mixtures = 1.77 % and 1.08 % using constant values and correlations for influence and symmetric parameters) forces the model to improve the prediction although linear density profiles are considered.
Surface tension for the methyl levulinate (1) + methanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the methyl levulinate (1) + ethanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the methyl levulinate (1) + 1-propanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the methyl levulinate (1) + 1-butanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the ethyl levulinate (1) + methanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the ethyl levulinate (1) + ethanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the ethyl levulinate (1) + 1-propanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K
Surface tension for the ethyl levulinate (1) + 1-butanol (2) mixture at 283.15 K and 313.15 K using LGT + PRSV-EoS. (- - - - -) predictive approach and \(\chi _i\) = constant, (——–) predictive approach and \(\chi _i = f(T)\), (— — —) fitted approach, \(\beta _{12}\) = constant, and \(\chi _i\) = constant, (— - —) fitted approach, \(\beta _{12} = f(T)\), and \(\chi _i=f(T)\). Symbols: experimental data obtained in this work; (black) \(T=283.15\) K, (red) \(T=313.15\) K (Color figure online)
5 Conclusions
In this study we present the surface tensions of binary mixtures of methyl levulinate and ethyl levulinate with methanol, ethanol, 1-propanol, and 1-butanol at several temperatures (283.15, 298.15, and 313.15 K) and at \(P = 0.1\) MPa. The surface tension deviations from a linear dependence of mole fraction were calculated. These surface tension deviations change from positive values to negative ones with increasing alkanol chain length. With respect to differences between alkyl levulinates, mixtures containing ethyl levulinate present higher surface tension deviation positive values or less negative ones than mixtures involving methyl levulinate.
Peng–Robinson–Stryjek–Vera equation of state (PRSV-EoS) was able to predict the vapor-liquid equilibrium for the alkyl levulinate (1) + alcohol (2) mixtures. These results can be used as a reference for future works where the phase equilibria for alkyl levulinate (1) + alkanol (2) systems are measured experimentally, because only one work with experimental data is available in the literature [7].
For the modeling of the surface tension, the linear square gradient theory (LSGT) was used plus the equation of state (PRSV-EoS). The linear dependence of the influence parameter with the temperature allowed to correctly fit the surface tension data of the pure fluids (overall deviation = 0.22 %). The theoretical model LSGT + PRSV-EoS was used as predictive and fitted in order to model the experimental data obtained in this work. A null symmetric parameter was not able to correctly predict the experimental data of all the mixtures (overall deviation = 3.97 % and 3.86 %), except for ethyl levulinate (1) + methanol (2) mixture (deviation = 1.42 % and 1.38 %). The predictive results were improved by correlating the symmetric parameter with temperature. The fitted approach allowed to reduce the deviation (obtained predictively) by 72 %. Therefore, the assumption of linear density profiles and the use of two adjustable parameters of the mixture in LSGT correctly modeled the surface tension for all mixtures (overall deviation = 1.08 %).
Data Availability
Data availability is not applicable.
Abbreviations
- \(\chi\) :
-
Influence parameter
- \(\chi _{12}\) :
-
Cross-influence parameter
- A :
-
Adjustable parameter of the influence parameter
- a :
-
Cohesion parameter in the PRSV-EoS
- AAD :
-
Statistical deviation
- B :
-
Adjustable parameter of the influence parameter
- b :
-
Covolume parameter in the PRSV-EoS
- \(c_1\) :
-
Adjustable parameter of the cohesive parameter
- \(c_2\) :
-
Adjustable parameter of the cohesive parameter
- \(f_0\) :
-
Helmholtz energy density
- \(k_{12}\) :
-
Interaction parameter for the quadratic mixing rule
- N :
-
Number of experimental points
- n :
-
Number of points in the linear gradient theory
- \(n_c\) :
-
Number of components of the mixture
- P :
-
Absolute pressure
- R :
-
Universal gas constant
- T :
-
Absolute temperature
- w :
-
Mole fraction
- x :
-
Liquid mole fraction
- y :
-
Vapor mole fraction
- z :
-
Interfacial position
- \(\beta _{12}\) :
-
Symmetric parameter of the linear gradient theory
- \(\mu\) :
-
Chemical potential
- \(\rho\) :
-
Molar density
- \(\sigma\) :
-
Surface tension
- c :
-
Critical condition
- i, j :
-
Species
- ref :
-
Reference
- 0:
-
Equilibrium condition
- calc :
-
Calculated
- exp :
-
Experimental
- L :
-
Liquid phase
- V :
-
Vapor phase
References
H. Ariba, Y. Wang, C. Devouge-Boyer, R.P. Stateva, S. Leveneur, Physicochemical properties for the reaction systems: levulinic acid, its esters, and \(\gamma\)-valerolactone. J. Chem. Eng. Data 65(6), 3008–3020 (2020)
N.A.S. Ramli, F. Abdullah, Study of density, surface tension, and refractive index of binary mixtures containing alkyl levulinate and n-alcohol from 298.15 to 323.15 K. J. Chem. Eng. Data 66(5), 1856–1876 (2021)
Abidi, R.: Contribution à l’étude des systèmes binaires contenant des lévulinates d’alkyle et d’alcools. PhD thesis, Universidad de Zaragoza (2022)
R. Abidi, M. Artal, M. Hichri, C. Lafuente, Experimental and modelled thermophysical behaviour of methyl levulinate (methyl 4-oxopentanoate) and n-alkanol systems. J. Mol. Liq. 339, 116739 (2021)
B.S. Carey, L.E. Scriven, H.T. Davis, Semiempirical theory of surface tensions of pure normal alkanes and alcohols. AIChE J. 24(6), 1076–1080 (1978)
B.S. Carey, L.E. Scriven, H.T. Davis, Semiempirical theory of surface tension of binary systems. AIChE J. 26(5), 705–711 (1980)
A.J. Resk, L. Peereboom, A.K. Kolah, D.J. Miller, C.T. Lira, Phase equilibria in systems with levulinic acid and ethyl levulinate. J. Chem. Eng. Data 59(4), 1062–1068 (2014)
Y.-X. Zuo, E.H. Stenby, Calculation of surface tensions of polar mixtures with a simplified gradient theory model. J. Chem. Eng. Jpn. 29(1), 159–165 (1996)
Y.-X. Zuo, E.H. Stenby, A linear gradient theory model for calculating interfacial tensions of mixtures. J. Colloid Interface Sci. 182(1), 126–132 (1996)
D.-Y. Peng, D.B. Robinson, A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15(1), 59–64 (1976)
R. Stryjek, J.H. Vera, Prsv: An improved peng-robinson equation of state for pure compounds and mixtures. Can. J. Chem. Eng. 64(2), 323–333 (1986)
A. Hernández, M. Cartes, A. Mejía, Measurement and modeling of isobaric vapor-liquid equilibrium and isothermal interfacial tensions of ethanol+ hexane+ 2, 5-dimethylfuran mixture. Fuel 229, 105–115 (2018)
A. Hernández, Interfacial behavior prediction of alcohol+ glycerol mixtures using gradient theory. Chem. Phys. 534, 110747 (2020)
A. Hernández, Modeling vapor–liquid equilibria and surface tension of carboxylic acids + water mixtures using Peng–Robinson equation of state and gradient theory. Int. J. Thermophys. 42(13), 1–27 (2021)
A. Hernández, R. Tahery, Modeling of phase equilibria and surface tension for n, n-dimethylcyclohexylamine + alcohol mixtures at different temperatures. Int. J. Thermophys. 42(67), 1–27 (2021)
A. Hernández, R. Tahery, Modeling of surface tension and phase equilibria for water+ amine mixtures from (298.15 to 323.15) K using different thermodynamic models. J. Solut. Chem. 51, 31–57 (2022)
I. Cachadiña, A. Hernández, À. Mulero, Surface tension of esters. Temperature dependence of the influence parameter in density gradient theory with Peng–Robinson equation of state. Case Stud. Therm. Eng. 36, 102193 (2022)
J.M. Prausnitz, R.N. Lichtenthaler, E.G. De Azevedo, Molecular Thermodynamics of Fluid-Phase Equilibria (Prentice Hall, Upper Saddle River, 1998)
L. Lomba, M. Carlos Lafuente, I. Gascón. García-Mardones, B. Giner, Thermophysical study of methyl levulinate. J. Chem. Thermodyn. 65, 34–41 (2013)
L. Lomba, B. Giner, I. Bandrés, C. Lafuente, M.R. Pino, Physicochemical properties of green solvents derived from biomass. Green Chem. 13(8), 2062–2070 (2011)
O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40(2), 345–348 (1948)
T.E. Daubert, R.P. Danner, Danner, Data Compilation. Tables of Properties of Pure Compounds (DIPPR, New York, 1985)
E.D. Nikitin, A.P. Popov, N.S. Bogatishcheva, M.Z. Faizullin, Critical temperatures and pressures, heat capacities, and thermal diffusivities of levulinic acid and four n-alkyl levulinates. J. Chem. Thermodyn. 135, 233–240 (2019)
R.H. Weiland, T. Chakravarty, A.E. Mather, Solubility of carbon dioxide and hydrogen sulfide in aqueous alkanolamines. Ind. Eng. Chem. Res. 32(7), 1419–1430 (1993)
R.H. Weiland, T. Chakravarty, and A.E. Mather. Solubility of carbon dioxide and hydrogen sulfide in aqueous alkanolamines.[erratum to document cited in ca119: 35070]. Ind. Chem. Res. 34(9), 3173 (1995)
K.A.G. Schmidt, G.K. Folas, B. Kvamme, Calculation of the interfacial tension of the methane–water system with the linear gradient theory. Fluid Phase Equilib. 261(1–2), 230–237 (2007)
C.M. Kinart, W.J. Kinart, A. Bald, A. Szejgis, Study of the intermolecular interactions in liquid n,n-dimethylacetamide–water mixtures. Phys. Chem. Liquids 30(3), 151–157 (1995)
R. Gopal, S.A. Rizvi, Physical properties of some mono-and dialkyl-substituted amides at different temperatures. J. Indian Chem. Soc. 43, 179–182 (1966)
Y.V. Efremov, Density surface tension vapour pressure and critical parameters of alcohols. Russ. J. Phys. Chem. USSR 40(6), 667 (1966)
J.A. Dean, N.A. Lange, Lange’s Handbook of Chemistry, 13th edn (McGraw-Hill, New York, 1985)
B.Y. Teitel’baum, T.A. Gortalova, E.E. Sidorova, Polithermic study of surface tension of aqueus solutions of alcohols. Zh. Fiz. Khim. 25, 911–919 (1951)
A.E. Andreatta, E. Rodil, A. Arce, A. Soto, Surface tension of binary mixtures of 1-alkyl-3-methyl-imidazolium bis (trifluoromethylsulfonyl) imide ionic liquids with alcohols. J. Solut. Chem. 43(2), 404–420 (2014)
Z. Chen, S. Xia, P. Ma, Measuring surface tension of liquids at high temperature and elevated pressure. J. Chem. Eng. Data 53(3), 742–744 (2008)
A. Watanabe, S. Sugiyama, Temperature coefficient of surface tension for organic liquids of homologous series. Nippon Kagaku Kaishi 11, 2047–2051 (1973)
K.-D. Chen, Y.-F. Lin, C.-H. Tu, Densities, viscosities, refractive indexes, and surface tensions for mixtures of ethanol, benzyl acetate, and benzyl alcohol. J. Chem. Eng. Data 57(4), 1118–1127 (2012)
N.G. Tsierkezos, Application of the extended langmuir model for the determination of lyophobicity of 1-propanol in acetonitrile. Int. J. Thermophys. 30(3), 910–918 (2009)
E. Álvarez, A. Correa, J.M. Correa, E. García-Rosello, J.M. Navaza, Surface tensions of three amyl alcohol+ ethanol binary mixtures from (293.15 to 323.15) K. J. Chem. Eng. Data 56(11), 4235–4238 (2011)
H. Yue, Z. Liu, Surface tension of binary mixtures of 2,2,4-trimethylpentane + 1-alkanols from 298.15 to 323.15 K. J. Chem. Eng.Data 61(3), 1270–1279 (2016)
Acknowledgements
We would like to express our acknowledgment for financial assistance from the “Ministère de l’Enseignement Supérieur et de la Recherche Scientifique de la Tunisie” and Diputación General de Aragón and Fondo Social Europeo “Construyendo Europa desde Aragón” (E31_20R).
Funding
“Ministère de l’Enseignement Supérieur et de la Recherche Scientifique de la Tunisie” and Diputación General de Aragón and Fondo Social Europeo “Construyendo Europa desde Aragón” (E31_20R).
Author information
Authors and Affiliations
Contributions
RA: Investigation. MH: Investigation and supervision. CL: Investigation, writing, supervision. AH: Surface tension and phase equilibria modeling, writing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abidi, R., Hichri, M., Lafuente, C. et al. Surface Tensions for Binary Mixtures of Alkyl Levulinate + Alkanol: Measurement and Modeling. Int J Thermophys 44, 33 (2023). https://doi.org/10.1007/s10765-022-03142-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-022-03142-6