Abstract
Hybridization is relatively well documented among Old World primates, but poorly investigated among New World monkeys. We investigated hybridization between the sexually dichromatic howlers Alouatta caraya and Alouatta guariba clamitans, whose lineages diverged ca. 5 million years ago. These taxa show allopatric distributions with a few recently discovered narrow contact zones. We collected 169 individual fecal samples of A. caraya and A. g. clamitans within (N = 121) and outside (N = 48) two contact zones in southern Brazil. We used mtDNA and Y-chromosome (SRY gene) sequences, and three diagnostic microsatellite loci to investigate their parental origin. We found 33 individuals (27%) with evidence of hybrid origin in the contact zones. Thirteen individuals presented mtDNA of A. caraya origin and Y-chromosome of A. g. clamitans origin and eight individuals have the opposite combination of markers. We assigned the hybrid origin of the remaining 12 individuals based on the discrepancy between their uniparental markers and microsatellite data, or a combination of diagnostic alleles of both species. This is the first evidence of hybridization between A. caraya and A. g. clamitans. We hypothesize that this hybridization is bidirectional, ancient, and geographically wider than previously thought. The confirmation of hybridization in our contact zones provides evidence that interspecific differences in male coat color do not function as prezygotic barriers. We concluded that sexual selection by means of mate recognition is a weak explanation for the evolution of sexual dichromatism in these species. Finally, our system highlights the great complexity of this mechanism and its potential for the evolution and diversification of primates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Natural hybridization is a widespread phenomenon in eukaryotes that seems to be more common in mammals than previously recognized (Arnold and Meyer 2006; Shurtliff 2013). Among primates, there is plenty of evidence of hybridization between congeneric strepsirrhines, haplorrhines and catharrhines species (Cercopithecus, Eulemur, Macaca, Papio, Tarsius, Hylobates, and Homo) (Arnold and Meyer 2006; Evans et al. 2001; Green et al. 2010; Merker et al. 2009; Noda et al. 2001; Phillips-Conroy and Jolly 1986; Samuels and Altmann 1986; Struhsaker et al. 1988; Tosi et al. 2002, 2003; Watanabe and Matsumura 1991; Wyner et al. 2002) and reports involving taxa belonging to different genera (Theropithecus and Papio, Hylobates and Symphalangus) (Dunbar and Dunbar 1974; Myers and Shafer 1979). In the New World, hybridization has been inferred from phenotype (Aguiar et al. 2008; Passamani et al. 1997) or confirmed by molecular data (Cortés-Ortiz et al. 2007; Kelaita and Cortés-Ortiz 2013; Malukiewicz et al. 2015; Silva et al. 1992) in a few species of Alouatta, Callithrix, Cebus, Saguinus, and Saimiri (Arnold and Meyer 2006; Cortés-Ortiz et al. 2007).
The nine currently recognized species of Alouatta (howlers) are allopatric through most of their ranges from southern Mexico to southern Brazil, but a few historically secondary contact zones have been reported (Cortés-Ortiz et al. 2015a, b). Molecular data have been used to confirm hybridization between Alouatta palliata and Alouatta pigra in a contact zone in the northern limit of the distribution of the genus in Mexico (Cortés-Ortiz et al. 2007; Kelaita and Cortés-Ortiz 2013). Hybridization between these species appears to be unidirectional. While a cross between a male A. palliata and a female A. pigra produces viable offspring, the opposite cross does not. This pattern is consistent with Haldane’s rule, according to which the heterogametic sex (males in mammals) is more likely to be inviable or sterile (Cortés-Ortiz et al. 2007, 2015a). The patterns of genetic variation found in hybrid/backcrossed individuals in Mexico suggest that the directionality results from chromosomal, cytonuclear, or genomic incompatibilities (Cortés-Ortiz et al. 2015a; Kelaita and Cortés-Ortiz 2013). Except for this single area, reports of hybridization in howlers are suggestive but not conclusive (Cortés-Ortiz et al. 2007, 2015a). The oldest known suggestion of mixed groups in howlers dates back to the nineteenth century when a female Alouatta caraya and a male Alouatta guariba clamitans were found living together (Isabelle 1983). The first mention of hybrids between these taxa comes from a morphological study based on museum specimens collected on the upper Paraná River in the 1940s, in which some individuals presented a mosaic pelage coloration pattern (Lorini and Persson 1990). Although putative hybrids between A. caraya and A. g. clamitans have been reported (Aguiar et al. 2007, 2008, 2014; Bicca-Marques et al. 2008; Dias et al. 2015; Gregorin 2006), there has been no genetic confirmation that they hybridize in the wild.
Contact zones between Alouatta caraya and Alouatta guariba clamitans have been reported in northeastern Argentina and southern Brazil (Agostini et al. 2008; Aguiar et al. 2007, 2008, 2014; Bicca-Marques et al. 2008; Gregorin 2006; Holzmann et al. 2015). The home ranges of syntopic monospecific groups can overlap extensively and mixed-species troops can arise (Agostini et al. 2010; Aguiar et al. 2008). The adults of these taxa are similar in size and appearance, and have both evolved biphasic sexual dichromatism in which male coat color changes during development (Bicca-Marques and Calegaro-Marques 1998; Van Belle and Bicca-Marques 2015). Therefore, there are four adult color phenotypes. In adult A. caraya males are fully black and adult females are yellowish or bridled-tawny, whereas adult A. g. clamitans individuals are reddish and dark brown, respectively (Bicca-Marques and Calegaro-Marques 1998; Gregorin 2006). The distinction in coat coloration within and between species enables the detection of intermediate and mosaic color patterns in contact zones that have been inferred to be hybrids (Agostini et al. 2008; Aguiar et al. 2007, 2008, 2014; Bicca-Marques et al. 2008). The observation of births of hybrid offspring of both sexes in captive howlers, from a male A. caraya and a female A. g. clamitans (Jesus et al. 2010), supports the link between intermediate/mosaic phenotypes and hybridization between these taxa. The survival of the male hybrid for 1.5 yr. is evidence of the viability of the heterogametic sex for this pair of parents. However, it died before reaching sexual maturity, so we do not know if it was fertile.
Alouatta caraya and Alouatta guariba clamitans are not sister species and belong to divergent clades that separated up to 5.1 million yr. ago (Cortés-Ortiz et al. 2003; Martins et al. 2011; Steinberg et al. 2014). Their contrasting sexual dichromatism is thought to have evolved independently because their sister taxa and other congeners do not show this trait. The first hypothesis developed to explain the evolution of sexual dichromatism in these taxa related coat color to sexual differences in energetic and thermal requirements (Thorington et al. 1979). However, studies of the thermoregulatory behavior of free-ranging A. caraya (Bicca-Marques and Calegaro-Marques 1998) and A. g. clamitans (Bicca-Marques and Azevedo 2004) found no support for this hypothesis. Alternative hypotheses focused on sociosexual selective pressures, namely signaling male fitness (e.g., fighting ability, maturity, health status; Crockett 1987; Darwin 1871; see also Bicca-Marques and Calegaro-Marques 1998) or sexual identity recognition (Crockett 1987; Paterson 1985; see also Bicca-Marques and Calegaro-Marques 1998). Whereas the first of these hypotheses relates to female mate choice or to the assessment of the strength of potential sexual competitors, the latter relates to the mate recognition concept. Irrespective of the selective advantage(s) that led to dichromatism, both hypotheses imply intraspecific recognition of potential sexual partners based on coat color. The standard trichromacy characteristic of Alouatta spp. enables them to physiologically distinguish both intra- and interspecific variations in adult color phenotypes (Kainz et al. 1998).
We investigated hybridization between the sexually dichromatic howlers Alouatta caraya and Alouatta guariba clamitans using uni- and biparental genetic markers assessed in biological samples collected in areas of sympatry and allopatry between these species. We also tested the hypothesis that A. caraya and A. g. clamitans avoid crossbreeding by recognizing their distinctly colored adults of each sex. If such mate recognition is the driving force for the evolution of sexual dichromatism in these taxa, then we predict the absence of hybrids in contact zones.
Methods
Study Areas
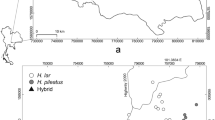
We conducted population surveys in two contact zones between Alouatta caraya and Alouatta guariba clamitans in Brazil (Fig. 1). Both regions are highly deforested and fragmented. The first study site is a single 150-ha, old growth, highly disturbed fragment of seasonal semideciduous forest in a matrix of pasture that is contiguous to the riparian forest of the left bank of the upper Paraná River (23°22′52.3′′S, 53°45′39.6′′W) in Icaraíma municipality, Paraná State (Aguiar et al. 2008). We surveyed a few additional small fragments in this region, but found no howlers. The second contact zone is a region located in the ecotone between the Atlantic forest and temperate grassland (29°35′0.0′′S, 54°59′0.0′′W) on the border of São Francisco de Assis and São Vicente do Sul municipalities, Rio Grande do Sul State (Bicca-Marques et al. 2008; Dias et al. 2015; Silva 2010). It is composed of several relatively small forest fragments spread over an undulating terrain dominated by rocky hills partially covered with grasslands, gallery forests, and secondary forests in different stages of regeneration (Bicca-Marques et al. 2008). These fragments are in a matrix of pasture and, in some cases, commercial pine tree plantations or annual cultures (e.g., soybean). They are 3–26 km away from each other. We also collected samples in presumed parental populations of both species in Alegrete (A. caraya) and Santa Maria (A. g. clamitans) municipalities, Rio Grande do Sul (Fig. 1). These parental populations are located ca. 80 km and 130 km from our studied contact zone, respectively.
Location of sampling sites in contact zones between Alouatta caraya and Alouatta guariba clamitans in the states of Paraná and Rio Grande do Sul, southern Brazil. Contact zones are indicated by open circles. Parental populations of A. caraya and A. g. clamitans are indicated by open squares and triangles, respectively.
Molecular Data Acquisition and Analysis
We surveyed at least 42 wild groups of howlers in contact zones in Paraná (N = 8) and Rio Grande do Sul (N = 34). Most groups (N = 33) were phenotypically monospecific (23 Alouatta caraya and 10 Alouatta guariba clamitans). Nine groups (five in Paraná and four in Rio Grande do Sul, corresponding to 63% and 12% of the surveyed groups in each area, respectively) were a mix of apparently purebred individuals of both species and/or apparently hybrid individuals. Nevertheless, our sampling was limited to a single 150-ha fragment in Paraná. We also surveyed at least five groups in each parental population in Rio Grande do Sul (Fig. 1).
We collected 169 individual fecal samples, 121 in the contact zones of Paraná (N = 19) and Rio Grande do Sul (N = 102), and the remaining 48 in the putative parental populations of Alouatta caraya (N = 30) and Alouatta g. clamitans (N = 18) in Rio Grande do Sul. We collected fecal samples immediately after defecation in most cases (N = 164) or while still fresh in latrines (N = 5) (Gilbert 1997). In the latter situation, we collected only one or two spatially separated samples from each latrine to minimize the risk of sampling feces from the same individual repeatedly. We stored samples in 50-mL tubes filled with ca. 25 mL of 70% ethanol at ambient temperature for a few hours to a few days in the field and then kept them frozen in the laboratory until extraction.
We extracted genomic DNA from 100 to 200 mg of feces using the QIAamp DNA Stool Mini Kit (QIAGEN), following the manufacturer’s protocol. We used negative controls within specialized workstations in an ancient DNA-type room to reduce the possibility of extract contamination (Surridge et al. 2002). We eluted these fecal sample extracts into a final 100 μL that we stored at −20°C for up to 24 mo before further processing.
To identify the maternal and paternal lineages of putative hybrids, we first tested for fixed (diagnostic) differences in the mtDNA control region and the Y-chromosome SRY gene of Alouatta caraya and Alouatta guariba clamitans. First, we used all available control region sequences from both species in GenBank, which consisted of an unpublished mtDNA genome (KY202428) for A. g. clamitans and 39 sequences of A. caraya (from Ascunce et al. 2003, 2007) and its whole mtDNA genome (Finstermeier et al. 2013). Although the aforementioned sequences clearly showed that the control regions of the mtDNA of the two species are quite different, they were limited in number and range, so we tested whether the differences were valid for a larger number of individuals in a broader geographical range using 263 sequences from both species from an ongoing phylogeographic study (Bonatto et al., unpubl. data). These sequences came from individuals from multiple locations in the distribution of each species, all ≥200 km away from our two study regions.
We used the primers How-S7 and How-RA1 (Ascunce et al. 2003) to amplify a fragment of ca. 713 bp of the mitochondrial control region via polymerase chain reaction (PCR). We used a volume of 20 μL containing 1 μL of genomic DNA, 0.6 μL of 1.5 mM of MgCl2, 2 μL of 0.2 mM dNTPs, 2 μL (1×) of 10× buffer, 2 μL of a 0.2 μM of each primer, 0.1 μL of Platinum Taq DNA Polymerase 5 U/μL (Invitrogen), 4 μL (1 M) of 5 M betaine, and 6.3 μL of ddH2O. After an initial denaturation period of 3 min at 94°C, amplifications proceeded with five cycles of denaturation at 94°C for 45 s, primer annealing at 55–50°C (in a touchdown approach decreasing 1°C in each cycle) for 45 s, and primer extension at 72°C for 90 s, followed by 35 cycles at 94°C for 45 s, 50°C for 45 s, and 72°C for 90 s. We used a final extension step at 72°C for 5 min.
No sequence was available for the Y-chromosome of these species. Thus, we amplified and sequenced ca. 782 bp of the SRY gene using primers described by Moreira (2002) using DNA from blood samples of 13 males (5 Alouatta caraya and 8 Alouatta guariba clamitans) collected in locations >500 km away from our study regions (as for mtDNA). We found five fixed SRY differences between our taxa. However, the amplification success of the fecal samples with these primers was low (<10%), probably owing to its large fragment size. We therefore used these sequences to design a new set of primers (SRY-F2–5′-AAAGTAACAACGAATTTGGTAGAA-3′ and SRY-R2: 5′- ATCACGAGACCACACAAGG-3′) that amplified a shorter fragment (ca. 300 bp) of the SRY gene, and contained two of the initial five interspecific polymorphisms. We performed PCRs as described previously with the following changes. After an initial denaturation for 4 min at 94°C, amplifications proceeded with 7 touchdown cycles at 94°C for 45 s, primer annealing at 60–53°C for 45 s, and primer extension at 72°C for 90 s, followed by 33 cycles at 94°C for 45 s, 53°C for 45 s, and 72°C for 90 s. We used a final extension at 72°C for 10 min. We amplified each sample twice to check for consistency of results.
We visually inspected allele size and quality of the PCR products of both uniparental markers in 1% agarose gel. We considered the reactions successful when DNA intensity was >30 ng/μL. Then, we purified sample products following the FasTap protocol. Samples were sequenced at Macrogen Inc. We inspected all sequences from both uniparental markers visually in Chromas Lite 2.1 (Technelysium Pty Ltd.). We aligned the sequences using the MUSCLE algorithm, implemented in AliView (Larsson 2014). Whenever necessary, we checked alignments visually and corrected them manually.
We also genotyped samples with three biparental microsatellite loci to help identify F1 hybrids. We initially tested eight microsatellite loci used previously in our laboratory for an ongoing population genetics study of both species (Bonatto et al., unpubl. data), five of which (AC14, AC17, D8S165, D17S804, 157 used for Alouatta caraya by Oklander et al. 2007) did not produce diagnostic alleles, or had reduced amplification efficiency because of the difficulties associated with fecal samples. The three microsatellite loci that we used (Ab7, Ab10, Ab17) were originally described for Alouatta belzebul (Gonçalves et al. 2004). Using these autosomal loci we found diagnostic alleles for the Rio Grande do Sul parental populations with a reasonable amplification success. Different alleles at these loci seem to be fixed in A. caraya and Alouatta guariba clamitans, allowing us to assign hybrids between these species with a reasonable level of confidence (see Results and Tables I and II for descriptions of loci and alleles). The simple assignment of hybrids is possible using only three or four diagnostic microsatellite markers (Boecklen and Howard 1997; Cortés-Ortiz et al. 2007). We conducted microsatellite PCR reactions with 0.2 mM of dNTP, 0.02 μM of forward primer, 0.2 μM of reverse primer, 1.5 mM of MgCl2, 1 M of Betaine, 0.2% Triton, 1× buffer, and 0.25 U of Platinum Taq DNA Polymerase (Invitrogen) in a final volume of 10 μL containing 3–5 μL genomic DNA. Following an initial denaturation period of 3 min at 94°C, amplifications of Ab7 proceeded with eight touchdown cycles at 94°C (45 s), 65–58°C (45 s), and 72°C (90 s), and 37 cycles at 94°C (45 s), 58°C (45 s), 72°C (90 s), and a final extension at 72°C (30 min). We followed a similar protocol to amplify Ab10 and Ab17, with differences in temperature and number of touchdown cycles, which included five cycles at 65–60°C (45 s), and 11 cycles at 60–50°C (45 s), respectively.
Fluorescently labeled primers were used to genotype the loci in an automated sequencer at Macrogen Inc. We reanalyzed all samples twice or three times per locus and found 100% concordance among replicates. We scored allele sizes using Peak Scanner Software 2 (Applied Biosystems). Both extraction and amplification reactions included negative controls.
Data Availability
Sry sequences are available in the following links.
Ethical Note
All research reported here complied with the appropriate national and institutional ethical guidelines and adhered to Brazilian legal requirements and to the Code of Best Practices for Field Primatology (https://www.asp.org/resources/docs/Code%20of_Best_Practices%20Oct%202,014.pdf). We declare no competing interests.
Results
Alouatta caraya and Alouatta guariba clamitans have divergent mtDNA sequences, including 46 diagnostic differences in the segment we studied (Table III). Of the 146 sequences obtained from both study regions, 101 (69%) were assigned to A. caraya, whereas the remaining 45 (31%) were assigned to A. g. clamitans. All 28 individuals from outside the contact zones had mtDNA haplotypes of the expected species (19 A. caraya and 9 A. g. clamitans) given their sampling location and phenotype (when available). Most of the 118 sequences from the contact zones were assigned to A. caraya (N = 82, 69%) and the rest to A. g. clamitans (N = 36, 31%).
Considering only the SRY sequences from individuals from regions outside our study regions, we identified two nucleotide sites that distinguish between Alouatta caraya and Alouatta guariba clamitans (Table IV, GenBank accession nos. MF289493–MF289494). Of the 79 SRY sequences from our study areas, 49 (62%) were assigned to A. caraya, whereas the remaining 30 (38%) were assigned to A. g. clamitans. Eleven (61%) of the 18 SRY sequences from the Alegrete and Santa Maria (Rio Grande do Sul) parental zones were assigned to A. caraya. The remaining seven sequences (39%) were assigned to A. g. clamitans. Although most of the SRY sequences found in the parental populations corresponded to the expected species, two individuals in the A. caraya population and three individuals in the A. g. clamitans population had the SRY sequence attributed to the other species. However, the mtDNA and autosomal microsatellites of these individuals were diagnostic of the expected parental species. Thirty-eight (62%) of the 61 Y-chromosome samples from the contact zones were assigned to A. caraya, while the remaining 23 (38%) were assigned to A. g. clamitans.
We successfully genotyped the three polymorphic loci containing diagnostic alleles for each species for 111 individuals from both parental populations and contact zones, with 14–19% missing data (Table I). We found no evidence of shared alleles for these loci between the two species in the putative parental populations (Table II). We found microsatellite evidence of hybridization in at least one locus (Table V) in 20 of 82 samples (24%) from the contact zones, but we could not determine the generation of interspecific mating. However, individuals from both parental populations presented only alleles diagnostic of the respective species, suggesting absence of hybridization in recent generations.
In total, 33 of the 121 individuals (27%) from the contact zones (7 of 19 individuals [37%] from Paraná and 26 of 102 individuals [26%] from Rio Grande do Sul) showed evidence of hybridization based on uniparental markers and/or biparental microsatellites. Six males were likely F1 hybrids because they had a combination of mtDNA and SRY haplotypes of both parental species and were interspecific heterozygous at all analyzed loci. Three putative females were also likely F1 hybrids because all their genotyped microsatellite loci were interspecific heterozygous. Three males with mtDNA from one species and SRY from the other are also putative hybrids, although the absence of microsatellite data prevents their assignment to any specific hybrid class. Some individuals are likely multigenerational backcrossed hybrids because they showed a single interspecific heterozygous locus or homozygous loci from different parental species or discordant mtDNA and SRY but monospecific autosomal loci.
In summary, 13 males showed Alouatta caraya mtDNA and Alouatta guariba clamitans Y-chromosome, and 8 males showed A. g. clamitans mtDNA and A. caraya Y-chromosome. We assigned 12 additional putative hybrids based on the discrepancy between their uniparental markers and microsatellites, or on a combination of diagnostic microsatellite alleles of both species (Table V).
Discussion
We provided the first genetic evidence of bidirectional hybridization between Alouatta caraya and Alouatta guariba clamitans. Both types of cross between these taxa (male A. caraya × female A. g. clamitans and male A. g. clamitans × female A. caraya) seemed to produce viable F1 hybrids as hypothesized based on field and captive data (Agostini et al. 2008; Cortés-Ortiz et al. 2015a; Jesus et al. 2010). Our hybrid system seems to be more complex than that reported between the northern congeners Alouatta palliata and Alouatta pigra. Only female F1 offspring resulting from female A. pigra and male A. palliata is capable of producing viable and fertile F2 offspring when crossing with a pure A. pigra, a pure A. palliata, or a backcrossed male with SRY of A. pigra. This system is compatible with the full operation of Haldane’s rule (Cortés-Ortiz et al. 2007). Some of our results, such as males with Y-chromosome from one species and mtDNA and all autosomal alleles from the other (Table V), suggest that at least some F1 male hybrids may be fertile in our A. caraya × A. g. clamitans system. Additional data on fertility and autosomal loci are needed to better assess whether Haldane’s rule also operates in our system. The occurrence of individuals with varying genotype patterns with alleles/haplotypes belonging to both species (Table V) is compatible with the presence of multigenerational hybrids (e.g., F2, backcrosses). Again, information from a larger set of loci is needed to fully investigate the matings that produced these patterns.
Although hybridization may positively influence speciation and evolution (Arnold 1997), it is more likely to have negative effects if its causes are anthropogenic (Seehausen et al. 2008). We could not assess whether the hybridization of our study species resulted from natural causes or from anthropogenic forest loss and fragmentation. However, the occurrence of individuals with monospecific microsatellite alleles and with a combination of mtDNA and Y-chromosome haplotypes of both species in one of our parental populations ≥80 km away from the nearest known contact zone points to an older hybridization process, over many generations, in which contraction and expansion of forest due to natural factors may have brought the two species together (Bicca-Marques et al. 2008). It is also uncertain if the acceleration of habitat (forest) loss and fragmentation occurring in these contact zones will increase or reduce the hybridization between these species. Arboreal primates depend on large, or at least highly connected, tracts of forest to maintain gene flow and for populations to persist long term. Although howlers can cope well with habitat spatial restriction at the individual level, long-term species viability is uncertain because small isolated populations face higher risks of deterministic (e.g., disease, hunting) or stochastic (e.g., genetic drift, biased sex ratios, extreme climatic events) effects resulting from forest loss and fragmentation (Arroyo-Rodriguez and Dias 2010; Bicca-Marques 2003). Therefore, the negative long-term effects of forest fragmentation may either result in population extirpations that decrease the probability of interspecific contact and hybridization in the fragmented landscape of the study regions, or force isolated and small mixed-species groups to hybridize as their only breeding option.
Finally, our findings suggest that sexual selection via mate recognition is a weak explanation for the evolution of sexual dichromatism in these taxa. The confirmation of hybridization in our contact zones provides evidence that the striking interspecific differences in male coat color do not function as prezygotic barriers, as hypothesized by Aguiar et al. (2007). Indeed, heterospecific mating appears to be common whenever Alouatta caraya and Alouatta guariba clamitans live in syntopy, as shown by the proportion of hybrid individuals in the study populations in our contact zones (26–37%), and the proportion of interspecific copulations in the forest fragment surveyed in Paraná (16%; Aguiar 2010). Females of both species do not avoid mating with males of the other species, despite being physiologically capable of distinguishing the coat colorations of conspecific and heterospecific males (Kainz et al. 1998). Hybridization between these taxa in the wild may indicate that even if sexual dichromatism evolved for long-distance sex recognition (Bicca-Marques and Calegaro-Marques 1998), this trait is not an effective behavioral or prezygotic barrier to hybridization in contact zones (Moynihan 1968). Future studies should evaluate color variation in these dichromatic howlers as a surrogate for fitness (signaling fighting ability and/or aggressiveness, health, maturity, or resource occupancy: Van Belle and Bicca-Marques 2015).
References
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2008). Infant hybrids in a newly formed mixed-species group of howler monkeys (Alouatta guariba clamitans and Alouatta caraya) in northeastern Argentina. Primates, 49, 304–307.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2010). Ranging patterns of two syntopic howler monkey species (Alouatta guariba and A. caraya) in northeastern Argentina. International Journal of Primatology, 31, 363–381.
Aguiar, L. M. (2010). Sistema social de grupos mistos de espécies de bugios (Alouatta caraya e A. clamitans) e potenciais híbridos no alto rio Paraná, sul do Brasil. Doctoral thesis, Curitiba: Universidade Federal do Paraná.
Aguiar, L. M., Mellek, D. M., Abreu, K. C., Boscarato, T. G., Bernardi, I. P., et al (2007). Sympatry between Alouatta caraya and Alouatta clamitans and the rediscovery of free-ranging potential hybrids in Southern Brazil. Primates, 48, 245–248.
Aguiar, L. M., Pie, M. R., & Passos, F. C. (2008). Wild mixed groups of howler species (Alouatta caraya and Alouatta clamitans) and new evidence for their hybridization. Primates, 49, 149–152.
Aguiar, L. M., Tonetto, J., & Bicca-Marques, J. C. (2014). Novas zonas de contato entre Alouatta caraya (Humboldt, 1812) e A. guariba clamitans Cabrera, 1940 no sul do Brasil. In F. C. Passos & J. M. Miranda (Eds.), A primatologia no Brasil (Vol. 15, pp. 338–344). Sociedade Brasileira de Primatologia: Curitiba.
Arnold, M. L. (1997). Natural hybridization and evolution. New York: Oxford University Press.
Arnold, M. L., & Meyer, A. (2006). Natural hybridization in primates: One evolutionary mechanism. Zoology (Jena, Germany), 109, 261–276.
Arroyo-Rodriguez, V., & Dias, P. A. (2010). Effects of habitat fragmentation and disturbance on howler monkeys: A review. American Journal of Primatology, 72, 1–16.
Ascunce, M. S., Cortes-Ortiz, L., & Mudry, M. D. (2003). The mitochondrial control region of the black howler monkey, Alouatta caraya (Primates, Platyrrhini), and the development of new primers. Molecular Ecology Notes, 3, 372–375.
Ascunce, M. S., Hasson, E., Mulligan, C. J., & Mudry, M. D. (2007). Mitochondrial sequence diversity of the southernmost extant New World monkey, Alouatta caraya. Molecular Phylogenetics and Evolution, 43, 202–215.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In L. K. Marsh (Ed.), Primates in fragments: Ecology and conservation (pp. 283–303). New York: Kluwer Academic/Plenum Press.
Bicca-Marques, J. C., & Azevedo, R. B. (2004). The ‘thermoregulation hypothesis’ does not explain the evolution of sexual dichromatism in the brown howler monkey (Alouatta guariba clamitans) [electronic version]. Folia Primatologica, 75, 236.
Bicca-Marques, J. C., & Calegaro-Marques, C. (1998). Behavioral thermoregulation in a sexually and developmentally dichromatic Neotropical primate, the black-and-gold howling monkey (Alouatta caraya). American Journal of Physical Anthropology, 106, 533–546.
Bicca-Marques, J. C., Prates, H. M., Aguiar, F. R., & Jones, C. B. (2008). Survey of Alouatta caraya, the black-and-gold howler monkey, and Alouatta guariba clamitans, the brown howler monkey, in a contact zone, State of Rio Grande do Sul, Brazil: Evidence for hybridization. Primates, 49, 246–252.
Boecklen, W. J., & Howard, D. J. (1997). Analysis of hybrid zones: Numbers of markers and power of resolution. Ecology, 78, 2611–2616.
Cortés-Ortiz, L., Bermingham, E., Rico, C., Rodríguez-Luna, E., Sampaio, I., & Ruiz-Garcia, M. (2003). Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Molecular Phylogenetics and Evolution, 26, 64–81.
Cortés-Ortiz, L., Duda Jr., T. F., Canales-Espinosa, D., Garcia-Orduna, F., Rodriguez-Luna, E., & Bermingham, E. (2007). Hybridization in large-bodied New World primates. Genetics, 176, 2421–2425.
Cortés-Ortiz, L., Agostini, I., Aguiar, L. M., Kelaita, M., Silva, F. E., & Bicca-Marques, J. C. (2015a). Hybridization in howler monkeys: Current understanding and future directions. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: Behavior, ecology, and conservation (pp. 107–131). New York: Springer Science+Business Media.
Cortés-Ortiz, L., Rylands, A. B., & Mittermeier, R. A. (2015b). The taxonomy of howler monkeys: Integrating old and new knowledge from morphological and genetic studies. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: Adaptive radiation, systematics, and morphology (pp. 55–84). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media.
Crockett, C. M. (1987). Diet, dimorphism and demography: Perspectives from howlers to hominids. In W. G. Kinzey (Ed.), The evolution of human behavior: Primate models (pp. 115–135). Albany: SUNY Press.
Darwin, C. (1871). The descent of man, and selection in relation to sex. London: Murray.
Dias, T. D., Conceição, M. S., Murer, L., & Fortes, V. B. (2015). Extension of the contact zone and probable hybridization between brown howler monkeys (Alouatta guariba clamitans) and black-and-gold howler monkeys (Alouatta caraya) (Primates, Atelidae) in southern Brazil. Mastozoología Neotropical, 22, 303–310.
Dunbar, R. I. M., & Dunbar, P. (1974). On hybridization between Theropithecus gelada and Papio anubis in the wild. Journal of Human Evolution, 3, 187–192.
Evans, B. J., Supriatna, J., & Melnick, D. J. (2001). Hybridization and population genetics of two macacque species in Sulawesi, Indonesia. Evolution, 55, 1686–1702.
Finstermeier, K., Zinner, D., Brameier, M., Meyer, M., Kreuz, E., et al (2013). A mitogenomic phylogeny of living primates. PLoS One, 8, e69504.
Gilbert, K. A. (1997). Red howling monkey use of specific defecation sites as a parasite avoidance strategy. Animal Behaviour, 54, 451–455.
Gonçalves, E. C., Silva, A., Barbosa, M. S. R., & Schneider, M. P. C. (2004). Isolation and characterization of microsatellite loci in Amazonian red-handed howlers Alouatta belzebul (Primates, Plathyrrini). Molecular Ecology Notes, 4, 406–408.
Green, R. E., Krause, J., Briggs, A. W., Maricic, T., Stenzel, U., et al (2010). A draft sequence of the Neandertal genome. Science, 328, 710–722.
Gregorin, R. (2006). Taxonomia e variação geográfica das espécies do gênero Alouatta Lacépède (Primates, Atelidae) no Brasil. Revista Brasileira de Zoologia, 23, 64–144.
Holzmann, I., Agostini, I., DeMatteo, K., Areta, J. I., Merino, M. L., & Di Bitetti, M. S. (2015). Using species distribution modeling to assess factors that determine the distribution of two parapatric howlers (Alouatta spp.) in South America. International Journal of Primatology, 36, 18–32.
Isabelle, A. (1983). Viagem ao Rio Grande do Sul, 1833–1834. Tradução e notas de Dante de Laytano. Porto Alegre: Martins Livreiro.
Jesus, A. S., Schunemann, H. E., Muller, J., da Silva, M. A., & Bicca-Marques, J. C. (2010). Hybridization between Alouatta caraya and Alouatta guariba clamitans in captivity. Primates, 51, 227–230.
Kainz, P. M., Neitz, J., & Neitz, M. (1998). Recent evolution of uniform trichromacy in a New World monkey. Vision Research, 38, 3315–3320.
Kelaita, M. A., & Cortés-Ortiz, L. (2013). Morphological variation of genetically confirmed Alouatta Pigra × A. palliata hybrids from a natural hybrid zone in Tabasco, Mexico. American Journal of Physical Anthropology, 150, 223–234.
Larsson, A. (2014). AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics, 30, 3276–3278.
Lorini, M. L., & Persson, V. G. (1990). A contribuição de Andre Mayer a historia natural no Parana (Brasil) II. Mamíferos do terceiro planalto paranaense. Arquivos de Biologia e Tecnologia, 33, 117–132.
Malukiewicz, J., Boere, V., Fuzessy, L. F., Grativol, A. D., de Oliveira, E. S. I., et al (2015). Natural and anthropogenic hybridization in two species of eastern Brazilian marmosets (Callithrix jacchus and C. penicillata). PLoS One, 10, e0127268.
Martins, F. M., Gifalli-Iughetti, C., Koiffman, C. P., & Harris, E. E. (2011). Coalescent analysis of mtDNA indicates Pleistocene divergence among three species of howler monkey (Alouatta spp.) and population subdivision within the Atlantic Coastal Forest species, A. guariba. Primates, 52, 77–87.
Merker, S., Driller, C., Perwitasari-Farajallah, D., Pamungkas, J., & Zischler, H. (2009). Elucidating geological and biological processes underlying the diversification of Sulawesi tarsiers. Proceedings of the National Academy of Sciences of the USA, 106, 8459–8464.
Moreira, M. A. M. (2002). SRY evolution in Cebidae (Platyrrhini: Primates). Journal of Molecular Evolution, 55, 92–103.
Moynihan, M. (1968). Social mimicry; character convergence versus character displacement. Evolution, 22, 315–331.
Myers, R. H., & Shafer, D. A. (1979). Hybrid ape offspring of a mating of gibbon and siamang. Science, 205, 306–310.
Noda, R., Kim, C. G., Takenaka, O., Ferrell, R. E., Tanoue, T., et al (2001). Mitochondrial 16S rRNA sequence diversity of hominoids. Journal of Heredity, 92, 490–496.
Oklander, L. I., Zunino, G. E., Di Fiore, A., & Corach, D. (2007). Isolation, characterization and evaluation of 11 autosomal STRs suitable for population studies in black and gold howler monkeys Alouatta caraya. Molecular Ecology Notes, 7, 117–120.
Passamani, M., Aguiar, L. M. S., Machado, R. B., & Figueiredo, E. (1997). Hybridization between Callithrix geoffroyi and C. penicillata in southeastern Minas Gerais, Brazil. Neotropical Primates, 5, 9–10.
Paterson, H. E. H. (1985). The recognition concept of species. In E. Vrba (Ed.), Species and speciation (pp. 21–29). Pretoria: Transvaal Museum.
Phillips-Conroy, J. E., & Jolly, C. J. (1986). Changes in the structure of the baboon hybrid zone in the Awash National Park, Ethiopia. American Journal of Physical Anthropology, 71, 337–350.
Samuels, A., & Altmann, J. (1986). Immigration of a Papio anubis male into a group of cynocephalus baboons and evidence for a anubis–cynocephalus hybrid zone in Amboseli, Kenya. International Journal of Primatology, 7, 131–138.
Seehausen, O., Takimoto, G., Roy, D., & Jokela, J. (2008). Speciation reversal and biodiversity dynamics with hybridization in changing environments. Molecular Ecology, 17, 30–44.
Shurtliff, Q. R. (2013). Mammalian hybrid zones: A review. Mammal Review, 43, 1–21.
Silva, F. E. (2010). Extensão da zona de contato e potencial hibridação entre Alouatta caraya e Alouatta guariba clamitans na região de São Fracisco de Assis, RS. MSc. thesis, Porto Alegre: Pontifícia Universidade Católica do Rio Grande do Sul.
Silva, B. T. F., Sampaio, M. I. C., Schneider, H., Schneider, M. P. C., Montoya, E., et al (1992). Natural hybridization between Saimiri taxa in the Peruvian Amazonia. Primates, 33, 107–113.
Steinberg, E. R., Nieves, M., & Mudry, M. D. (2014). Multiple sex chromosome systems in howler monkeys (Platyrrhini, Alouatta). Comparative Cytogenetics, 8, 43–69.
Struhsaker, T. T., Butynski, T. M., & Lwaga, J. S. (1988). Hybridization between redtail (Cercopithecus ascanius schmidti) and bue (C. mitis stuhlmanni) monkeys in the Kibale Forest, Uganda. In A. Gautier-Hion, F. Bourliere, J.-P. Gautier, & J. Kingdon (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 477–497). Cambridge: Cambridge University Press.
Surridge, A. K., Smith, A. C., Buchanan-Smith, H. M., & Mundy, N. I. (2002). Single-copy nuclear DNA sequences obtained from noninvasively collected primate feces. American Journal of Primatology, 56, 185–190.
Thorington Jr., R. W., Rudran, R., & Mack, D. (1979). Sexual dimorphism in Alouatta seniculus and observations on capture techniques. In J. F. Eisenberg (Ed.), Vertebrate ecology in the northern Neotropics (pp. 97–106). Washington, DC: Smithsonian Institution Press.
Tosi, A. J., Morales, J. C., & Melnick, D. J. (2002). Y-chromosome and mitochondrial markers in Macaca fascicularis indicate introgression with Indochinese M. mulatta and a biogeographic barrier in the Isthmus of Kra. International Journal of Primatology, 23, 161–178.
Tosi, A. J., Morales, J. C., & Melnick, D. J. (2003). Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution, 57, 1419–1435.
Van Belle, S., & Bicca-Marques, J. C. (2015). Insights into reproductive strategies and sexual selection in howler monkeys. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: Behavior, ecology, and conservation (pp. 57–84). Developments in Primatology: Progress and Prospects). New York: Springer Science+Business Media.
Watanabe, K., & Matsumura, S. (1991). The borderlands and possible hybrids between 3 species of macaques, M. nigra, M. nigrescens, and M. hecki in the northern peninsula of Sulawesi. Primates, 32, 365–369.
Wyner, Y. M., Johnson, S. E., Stumpf, R. M., & Desalle, R. (2002). Genetic assessment of a white-collared × red-fronted lemur hybrid zone in Andringitra, Madagascar. American Journal of Primatology, 67, 51–66.
Acknowledgments
We thank all landowners, and the staffs of Itaipu Binacional and Ilha Grande National Park for kindly granting us permits and logistics to collect fecal samples in their properties. We are also indebted to Anamélia S. Jesus, Elisa B. Decker, Fabiana Müller, Janaína P. Back, João C. Godoy, Karine G. D. Lopes, and Martín R. Herrera for their invaluable assistance during fieldwork. We wish to thank Amanda Kessler, Ana Paula Farro, Camila Magon, Cristine S. Trinca, Fernanda P. Valdez, Maísa C. Bertoldo, Manuel Escalona, Raiza C. Giacometti, and Tiago Ferraz for their lab help or advice. Fabrício Garcez, Pedro Ivo Simões, and Santiago Castroviejo-Fisher provided invaluable help with data analyses. The insightful commentaries and constructive criticisms of Liliana Cortés-Ortiz and two anonymous reviewers have greatly improved this article. We thank Joanna M. Setchell for her editorial comments and Liliana Cortés-Ortiz, Dietmar Zinner, and Christian Roos for the invitation to participate in this special volume on hybridization in primates. This study was supported by scholarships to I. Mourthe, L. M. Aguiar, and T. C. Trigo and a research grant to S. L. Bonatto from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES, Brazil) and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS, Brazil), and a scholarship to I. Mourthe from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (PDJ/CNPq, Brazil). J. C. Bicca-Marques also thanks CNPq for a research fellowship (#303306/2013-0). Idea Wild and Mohamed Bin Zayed Species Conservation Fund previously granted equipment to I. Mourthe that was used in this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Guest Editors of the Special Issue
Rights and permissions
About this article
Cite this article
Mourthe, I., Trindade, R.A., Aguiar, L.M. et al. Hybridization Between Neotropical Primates with Contrasting Sexual Dichromatism. Int J Primatol 40, 99–113 (2019). https://doi.org/10.1007/s10764-017-0011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-017-0011-9