Abstract
Natural hybridization has played various roles in the evolutionary history of primates. Its consequences range from genetic introgression between taxa, formation of hybrid zones, and formation of new lineages. Hylobates lar, the white-handed gibbon, and Hylobates pileatus, the pileated gibbon, are largely allopatric species in Southeast Asia with a narrow contact zone in Khao Yai National Park, Thailand, which contains both parental types and hybrids. Hybrid individuals in the zone are recognizable by their intermediate pelage and vocal patterns, but have not been analyzed genetically. We analyzed mitochondrial and microsatellite DNA of 52 individuals to estimate the relative genetic contributions of the parental species to each individual, and the amount of introgression into the parental species. We obtained fecal samples from 33 H. lar, 15 H. pileatus and four phenotypically intermediate individuals in the contact zone. Both mitochondrial and microsatellite markers confirmed distinct differences between these taxa. Both H. lar and H. pileatus contributed to the maternal lineages of the hybrids based on mitochondrial analysis; hybrids were viable and present in socially normal reproductive pairs. The microsatellite analysis identified ten admixed individuals, four F1 hybrids, which corresponded to phenotypic hybrids, and six H. lar-like backcrosses. All 15 H. pileatus samples were identified as originating from genetically H. pileatus individuals with no H. lar admixture; hence, backcrossing is biased toward H. lar. A relatively low number of phenotypic hybrids and backcrossed individuals along with a high number of parental types indicates a bimodal hybrid zone, which suggests relatively strong bias in mate selection between the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interspecific hybridization involves interbreeding between individuals of different species within the same genus, which results in the production of genetically mixed individuals. The viability of hybrids varies from none (sterility), to reduced viability, no influence, or rarely, to hybrid advantage over the parental species. The consequences of natural hybridization have been addressed with regard to various evolutionary outcomes (Arnold and Martin 2010; Zinner et al. 2011; Tung and Barreiro 2017). For example, the production of fertile hybrids can lead to the formation of a new lineage (e.g., Lamichhaney et al. 2018), admixed gene pools in the parental species, and reduced fitness of the parental species (Zinner et al. 2011). Adaptive introgression can occur if the admixed populations receive beneficial genes from the ancestral lineages (Salazar et al. 2010; Sams et al. 2016).
In primates, natural hybridization has been reported in all major lineages, for example in strepsirrhines (Gligor et al. 2009; Pastorini et al. 2009), in platyrrhines in America (Silva et al. 1992; Cortés-Ortiz et al. 2007), in cercopithecids in Asia and Africa (Detwiler 2019; Tosi et al. 2002; Tung et al. 2008), in hylobatids in Asia (Brockelman and Gittins 1984; Marshall and Sugardjito 1986), and in nonhuman hominids (great apes) (de Manuel et al. 2016). Natural hybridization in non-human primates has resulted in the appearance of new morphological variations (e.g., Brockelman and Gittins 1984; Cortés-Ortiz et al. 2007; Detwiler 2019; Gligor et al. 2009), altered vocal patterns (Brockelman and Schilling 1984; Mather 1992), changes in behavior (Ho et al. 2014), life history (Charpentier et al. 2008), and altered reproductive success (Charpentier et al. 2008). An important question raised by the appearance of hybrid characters is how they might affect fitness and alter gene flow across the contact zone.
Apes of the family Hylobatidae originated in Southeast Asia and diverged into four genera around 5–8.3 million years ago (MYA) (Thinh et al. 2010; Israfil et al. 2011; Carbone et al. 2014). The four genera within the Hylobatidae (Hylobates, Hoolock, Nomascus, and Symphalangus) contain 20 recognized species (Thinh et al. 2010; Anandam et al. 2013; Fan et al. 2017). Hylobates, a diverse genus containing nine species (Hylobates abbotti, Hylobates agilis, Hylobates albibarbis, Hylobates funereus, Hylobates klossii, Hylobates lar, Hylobates moloch, Hylobates muelleri, and Hylobates pileatus), underwent several radiations during the period between 1.5 and 4 MYA, based on molecular evidence (Thinh et al. 2010; Israfil et al. 2011; Chan et al. 2013). The genus likely originated in the Southeast Asian mainland and expanded southward (Whittaker et al. 2007; Thinh et al. 2010). The divergence of H. lar and H. pileatus, which may not be sister species, has been estimated at 3.65 MYA (CI 3.05–4.25) (Thinh et al. 2010).
Hylobates spp. are distributed allopatrically over the Sunda Shelf and adjacent mainland, and hybridization has been reported for three contact zones: between H. lar and H. pileatus in Khao Yai National Park, central Thailand (Marshall et al. 1972; Brockelman and Gittins 1984); between H. lar and H. agilis in northern West Malaysia (Gittins 1978; Brockelman and Gittins 1984); and between H. muelleri and H. agilis (later renamed H. albibarbis) in central Borneo (Marshall and Sugardjito 1986; Mather 1992). Ecological studies of H. lar, H. pileatus and their hybrids in Thailand (Brockelman 1978; Brockelman and Gittins 1984; Suwanvecho and Brockelman 2012; Asensio et al. 2017) have revealed that H. lar and H. pileatus in the contact zone have similar feeding behavior, habitat preferences and home range sizes, suggesting that they have virtually identical ecological niches. The two species were found to be interspecifically territorial and to have similar intra- and interspecific aggressive encounter rates (Suwanvecho and Brockelman 2012), and treat each other as ecological competitors (Asensio et al. 2017). The steep transition between H. lar and H. pileatus in the contact zone and the relatively low number of intermediate phenotypes (Brockelman and Gittins 1984) suggest that positive assortative mating might occur to create reproductive barriers between these species. Nevertheless, fertile hybrids and backcrossing to the parental species have been observed in the zone, as recognized by intermediate pelage and song patterns (Brockelman and Gittins 1984; Brockelman and Schilling 1984; Marshall and Brockelman 1986; Marshall and Sugardjito 1986; Asensio et al. 2017), and putative second generation female backcrosses could be recognized by the cadence of notes in their great calls (Brockelman and Schilling 1984). However, later-generation backcrosses may not be recognizable from phenotypic characters, and the extent of gene flow between the species is unknown.
Molecular markers such as mitochondrial DNA (mtDNA), sex-linked markers, and microsatellite DNA have been widely applied to determine hybrid individuals and investigate gene introgression in primates (e.g., Tosi et al. 2002; Cortés-Ortiz et al. 2007; Mourthe et al. 2019). Matsudaira et al. (2013) reported mitochondrial gene introgression between H. lar and H. pileatus in the H. lar side adjacent to the contact zone. The authors examined gibbons with H. lar phenotypes, and found that some individuals carried mitochondrial haplotypes of H. pileatus, suggesting introgression into H. lar. However, determining admixed individuals, especially backcrosses, and investigation of gene introgression have not yet been carried out in the contact zone and in the H. pileatus-dominated area.
In this study, we aimed to identify admixed individuals and investigate natural hybridization between H. lar and H. pileatus in the center of the contact zone, where both parental types coexist, by using mtDNA and nuclear microsatellite markers. mtDNA provides information on the phylogenetic relationship between the taxa and the genetic contribution from the maternal lineages. The nuclear microsatellite markers can determine the genetic proportion contributed by each parental species to each individual. In order to investigate the structure of the contact zone, and in particular the movement of genes across it, we tested the following hypotheses: (1) individuals identified as intermediate phenotypes share nuclear genes from both species; and (2) genetic introgression occurs in both parental species.
Methods
Study site
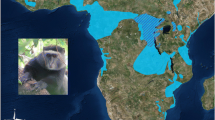
The study site is the narrow (less than 10 km wide) contact zone between H. lar and H. pileatus in the Khlong Sai area of Khao Yai National Park, Thailand (14°25′N, 101°23′E) (Fig. 1). The contact zone is in the area of the headwaters of the Takhong River, where the river is too narrow to form a barrier and tree canopies meet over the river. The forest canopy is continuous throughout most of the area except for some patches of fields and human developments. A few roads passing through the area are spanned by tree canopies that permit crossing by gibbons. There is no physical boundary to the species’ ranges, and H. lar, H. pileatus, and hybrid groups, intermingle in the contact zone (Asensio et al. 2017).
The sample collection sites in Khlong Sai study area, Khao Yai National Park, Thailand. a The hatched square shows the approximate extent of the contact zone (W. Y. Brockelman, unpublished data), and the circle shows our study site. b Distribution of the study individuals in the contact zone. Sampling locations are indicated by open circles (Hylobates lar), filled circles (Hylobates pileatus) and triangles [intermediate hybrids (Hybrid)]
Gibbon groups and phenotypes
All species of Hylobates live predominantly in small territorial groups consisting of a monogamous breeding pair of adults and their offspring (but not necessarily the offspring of both adults) (Brockelman et al. 1998; Bartlett 2011). The average group size of H. lar in Khao Yai is about 3.8 (range 2–7) individuals (Brockelman 2004). We identified four gibbon group types in the contact zone: H. lar groups, H. pileatus groups, mixed-species groups in which the main reproductive pair consisted of an adult male and adult female of the different species, and hybrid groups in which at least one of the paired adults was phenotypically hybrid-like (Brockelman and Gittins 1984; Asensio et al. 2017).
We recognized H. lar, H. pileatus and their hybrids by pelage and vocalizations (Brockelman and Gittins 1984; Brockelman and Schilling 1984). H. lar is asexually dichromatic as it has two color morphs: black (or dark brownish-black), and buff. Both morphs have a complete white face ring and whitish fur on the hands and feet. Body color in H. lar appears to be controlled by a single gene in which an allele for black is dominant over an allele for buff coloration (Brockelman 2004). Brockelman (2004) showed that reproductive pairing in H. lar is random with respect to color, and that pairs with different color combinations do not differ significantly with respect to their production of offspring. It is unclear how these alleles affect hybrid coloration. H. pileatus is sexually dichromatic in pelage coloration (Marshall et al. 1972). The duets of H. lar and H. pileatus are clearly distinct and easily recognizable from vocal patterns of both sexes (Marshall et al. 1972; Brockelman and Schilling 1984).
Sample collection
The gibbons, which were mostly unhabituated, were followed during daytime from February 2013 to September 2015 to collect fecal samples, whilst attempting not to disturb them by maintaining a cautious distance. The identity of the gibbons was recorded using pelage, color, age-class and sex, whenever possible, along with their group identification and composition following Asensio et al. (2017). In nearly all cases, the defecating individual was recognized (Table 1). For some samples (from two H. lar and six H. pileatus individuals), however, the collectors were not completely sure which animal had defecated. However, as these unknown samples belonged to groups classified as either H. lar or H. pileatus (as defined above), the exact identity of the individuals that produced them did not affect the results. We collected approximately 30 g of feces immediately after the gibbons defecated. The fecal samples were preserved in DET buffer (20% dimethyl sulfoxide, 250 mM ethylenediaminetetraacetic acid, 100 mM Tris, pH 7.5 saturated with NaCl) in 50-ml polypropylene tubes at ambient temperature in the field, and stored at 4 °C upon arrival at the laboratory until DNA extraction.
There were more H. lar than H. pileatus individuals at the study site. We obtained a total of 64 fecal samples comprising 40 from H. lar, 20 from H. pileatus, and four from hybrids that were determined phenotypically. We genotyped all of these samples using mtDNA and microsatellite DNA markers. Based on phenotyping, mtDNA sequencing and microsatellite genotype, ten of the gibbons were found to have yielded two to three samples each. All the duplicate samples yielded the same mitochondrial haplotypes and microsatellite genotypes and were later excluded from the data analyses. Thus, in total, 52 samples were included in the analyses, and comprised those from 33 H. lar, 15 H. pileatus, and four hybrid individuals.
DNA extraction and amplification
We extracted DNA from fecal samples using the QIAamp DNA Stool Mini Kit (Qiagen Hilden, Germany) following the manufacturer’s protocol, except for the elution steps. We extended the elution time to 30 min, and eluted the DNA in a 150-µl elution buffer. We amplified a 667- or 668-bp fragment of mitochondrial D-loop containing hypervariable segment I using the primers GIBDLF3 (5′-CTTCACCCTCAGCACCCAAAGC-3′) (Andayani et al. 2001) and HY16092R (5′-AAGACAGATACTGCGACATAGG-3′) (Matsudaira et al. 2013). We conducted the polymerase chain reactions (PCRs) in a 12.5-µl reaction, containing 1× PCR buffer, 1.25 µg BSA, 2.0 mM of each dNTP, 2.4 mM MgCl2, 0.32 µM of each primer, 0.5 U HotStar Taq DNA polymerase (Qiagen) and 2 µl DNA template. The cycling conditions were as follows: initial denaturation at 95 °C for 15 min, followed by 40 cycles of 95 °C for 1 min, 58 °C for 1 min and 72 °C for 1.5 min, and a final extension at 72 °C for 5 min. A negative control was included in each amplification to check for contamination during the process. The PCR products were purified by a restriction enzyme digestion method using Exonuclease I and Shrimp Alkaline Phosphatase. The purified products were sequenced from both directions by Macrogen (South Korea) using an ABI 3730XL DNA analyzer (Applied Biosystems).
Since there were no species-specific microsatellite primers for Hylobates spp., we selected nine human-derived microsatellite loci (Table 2) which were successfully amplified and polymorphic for both H. lar and H. pileatus. We amplified each locus separately in a 12.5-µl reaction containing 1× PCR buffer, 1.25 µg BSA, 2.0 mM of each dNTP, 2.4 mM MgCl2, 0.32 µM of fluorescent-labeled forward primer, 0.32 µM reverse primer, 0.5 U of HotStar Taq DNA polymerase (Qiagen) and 2 µl DNA template. The cycling conditions were as follows: initial denaturation at 95 °C for 15 min, followed by 40 cycles of 95 °C for 1 min, 52–57 °C for 1 min (Table 2) and 72 °C for 1 min, and a final extension at 72 °C for 15 min. A PCR negative control was included in each amplification. The PCR products were visualized on 2.5% agarose gel. The amplified PCR products from different loci with different dyes were multiplexed and sent for fragment length analysis using an ABI 3730XL DNA analyzer (Applied Biosystems) with a 400HD internal size standard. The allele sizes were analyzed using Peak Scanner Software v1.0 (Applied Biosystems). The procedure was repeated at least three times for each sample at each locus. The consensus genotypes were scored from at least two out of the three results in order to reduce errors caused by noninvasive sampling, such as allelic dropout. Additional repeats were performed if the samples did not show a consensus genotype. All microsatellite genotypes were repeatedly analyzed until two gave the same result. We also checked genotypes of parent–offspring pairs, and no mismatch was found.
Mitochondrial DNA analysis
The mitochondrial sequences were aligned using MUSCLE as implemented in MEGA7 (Kumar et al. 2016). We constructed phylogenetic trees using maximum likelihood (ML) and Bayesian inference (BI) approaches. The ML analysis was performed by using Treefinder (Jobb 2011) with 1000 bootstrap replications. The BI tree construction was performed by using MrBayes 3.2.6 (Ronquist et al. 2012) using four independent Markov Chain Monte Carlo (MCMC) runs for 1,000,000 generations with trees sampled every 200 generations. The first 25% of samples were discarded as burn-in. The sequences from the GenBank database for H. lar (accession no. X99256) and H. pileatus (AB504749 and HQ622787) were included in the tree construction. The sequences of Hoolock leuconedys (KY250071) and Hoolock hoolock (NC_033885) were used as outgroups. The BI tree was displayed with the posterior probabilities using FigTree v1.4.3 (Rambaut 2016). The haplotype diversity and nucleotide diversity of the H. lar and H. pileatus clades were separately analyzed using DnaSP v6 (Rozas et al. 2017). We constructed a haplotype network using PopART v1.7 (Leigh and Bryant 2015) to examine the intraspecific relationships among the haplotypes. We examined genetic differentiation between H. lar and H. pileatus by analysis of molecular variance (AMOVA) (Excoffier et al. 1992) using the pairwise FST calculated by conventional F-statistics based on haplotype frequencies and the distance method, implemented in Arlequin v3.5 (Excoffier and Lischer 2010).
Microsatellite DNA analysis
For the microsatellite analyses, we included only the samples that had been successfully genotyped for at least seven loci. The probability of identity (PID), indicating the probability of two random individuals containing the same genotype based on the nine microsatellite loci, was calculated using GenAlEx v.6.5 (Peakall and Smouse 2012). We evaluated the microsatellite diversity of H. lar and H. pileatus based on the number of alleles, observed heterozygosity and expected heterozygosity using Microsatellite Toolkit version 3.1 (Park 2001). The presence of null alleles and allelic dropout was examined using Micro-Checker v2.2.3 (van Oosterhout et al. 2004). Deviations from Hardy–Weinberg equilibrium (HWE) at each locus in each species, and linkage disequilibrium (LD) between pairs of loci, were assessed using Genepop v4.7.5 (Raymond and Rousset 1995; Rousset 2008). A sequential Bonferroni correction (Holm 1979) was applied to adjust for the significance levels of the HWE and LD tests for the multiple comparisons among loci at α = 0.05. We estimated the pairwise genetic distance between H. lar and H. pileatus by calculating Wright’s FST (Weir and Cockerham 1984) and Slatkin’s RST (Slatkin 1995; Michalakis and Excoffier 1996). Slatkin’s RST assumes a stepwise mutation model for microsatellite data. We examined genetic differentiation between the two species using AMOVA (Excoffier et al. 1992) implemented in Arlequin v3.5 (Excoffier and Lischer 2010).
Hybridization analysis
We analyzed the admixture model using a Bayesian MCMC method in Structure v2.3.4 (Pritchard et al. 2000). The number of populations (K) was determined as K = 2, representing the two parental species. Admixture analysis was performed with 1,000,000 MCMC generations, 20 independent simulations per K, and 100,000 generations burn-in. The estimates of admixture (q-value) were calculated for each individual. The q-value represented the proportion of genetic contribution from the parental lineages in the individual samples. Since we did not collect samples from parental populations outside the zone of contact, we could not determine the exact threshold q-value for distinguishing the pure species and hybrids. We sorted the q-value of the 47 samples from low to high and determined the cut-off q-value at 95%. Threshold values of 0.90–0.95 were reported and applied as in similar, previous, hybridization studies (e.g., Pastorini et al. 2009; Malukiewicz et al. 2015). The q-value ranged from 1.0 for H. lar to 0.0 for H. pileatus. Individuals with a q-value ≥ 0.95 were considered pure H. lar and were assigned to cluster 1, and those with q-values ≤ 0.05 were considered pure H. pileatus and were assigned to cluster 2. Individuals with intermediate q-values were considered admixed.
Results
Mitochondrial DNA
The phylogenetic trees constructed by using the ML and BI methods resulted in the same topology. Two distinct clades, H. lar and H. pileatus, were obtained with a high Bayesian posterior probability (presented as a percentage; 100%) and high ML bootstrap (100%) nodal supports. All H. lar-like samples were placed in the H. lar clade, and all H. pileatus-like samples were placed in the H. pileatus clade (Fig. 2). The three hybrid samples, A05_HB, A06_HB and A22_HB, were placed in the H. lar clade, and the hybrid sample A15_HB was placed in the H. pileatus clade.
Phylogenetic tree of H. lar and H. pileatus inferred from the D-loop gene based on maximum likelihood (ML) and Bayesian inference (BI). Bayesian posterior probabilities (presented as percentages) and maximum-likelihood bootstrap support values are presented on the branches (BI/ML). HL H. lar phenotype, HP H. pileatus phenotype, HB hybrid with intermediate pelage
A total of 15 haplotypes were determined among the 52 samples. Eight haplotypes (Hlar01–Hlar08) belonged to the H. lar clade (n = 36), and seven haplotypes (Hpil01–Hpil07) belonged to the H. pileatus clade (n = 16). The previously undocumented haplotypes (Hlar03, Hlar04, Hlar06, Hlar08 and Hpil01–Hpil07) were submitted to GenBank (accession nos. MT302850–MT302860). The haplotypes Hlar01, Hlar02, Hlar05 and Hlar07 corresponded to the haplotypes HKY2, HKY9, HKY1 and HKY7, respectively, which were previously identified in the H. lar population near the contact zone in Khao Yai National Park by Matsudaira et al. (2013). The haplotype diversity and nucleotide diversity of the H. lar clade were 0.5524 ± 0.0968 and 0.0026 ± 0.0017, respectively, and those of the H. pileatus clade were 0.8167 ± 0.0729 and 0.0065 ± 0.0038, respectively.
The haplotype network presented two distinct haplogroups, H. lar and H. pileatus (Fig. 3). The H. lar haplogroup showed a star-like shape in which Hlar01 was the most common haplotype, whereas the H. pileatus did not show this pattern. The intermediate hybrids contained mtDNA of both H. lar and H. pileatus. The three hybrids placed in the H. lar haplogroup carried haplotypes Hlar05 and Hlar08; the hybrid placed in the H. pileatus haplogroup carried haplotype Hpil01.
The haplotype network based on the mitochondrial D-loop of H. lar (Hlar; yellow) and H. pileatus (Hpil; blue). The proportion of hybrids sharing the haplotype is shown in white. The size of each circle is proportional to the number of samples carrying the haplotype. Black dots are connection points, representing missing haplotypes. Perpendicular bars on the connecting line represent the number of mutational steps between haplotypes
The pairwise FST between the H. lar and H. pileatus clades calculated based on both the conventional F-statistic (FST = 0.336) and the distance method (FST = 0.968) were high. Genetic differentiation at the species level based on both FST calculations was highly significant (P < 0.001).
Microsatellite DNA
We excluded five of the 33 H. lar samples from the microsatellite analysis because they were successfully genotyped for less than seven loci. Finally, we included 47 samples of 28 H. lar, 15 H. pileatus and the four intermediate hybrid individuals for the microsatellite analyses. The nine microsatellite loci were polymorphic and highly informative. The PIDs based on the nine loci used were as low as 2.2 × 10–9 in H. lar and 6.6 × 10–8 in H. pileatus, indicating that this microsatellite panel was suitable for individual identification and population genetics analyses. The microsatellite diversity of both species was considered moderate to high with the average number of alleles per locus at 7.1 ± 2.1 in H. lar and 5.6 ± 1.7 in H. pileatus. The average observed heterozygosity was 0.615 ± 0.031 and 0.644 ± 0.042 in H. lar and H. pileatus, respectively (Table 3). Overlapping allele ranges and shared alleles were observed for all loci, but the allele frequencies of the shared alleles were different between species. A significant deviation from the HWE was detected in locus D11S2002 for H. lar with a relatively high value of FIS. This was likely due to nonrandom sampling, since we obtained samples from close relatives in family groups. All loci were found in HWE for H. pileatus (Table 3). The presence of null alleles was detected for three loci (D4S243, D11S2002, D13S321) for H. lar, and one locus (D1S1656) for H. pileatus. We did not detect allelic dropout for any loci for either species. Thus, we included all loci for further analyses. No significant LD was found between pairs of loci in H. lar or in H. pileatus. The pairwise FST and RST between H. lar and H. pileatus were high. The AMOVA indicated significant genetic differentiation between the species (FST = 0.256, P < 0.001; RST = 0.579, P < 0.001).
The hybridization analysis revealed evidence of crossing between H. lar and H. pileatus. All H. pileatus phenotype samples were determined as pure species in cluster 2 with the q-values 0.009–0.038. Twenty-two H. lar samples were considered genetically H. lar in cluster 1 with q-values of 0.951–0.993. The results from program Structure identified ten admixed individuals, six H. lar-like and the four intermediate hybrids, with q-values of 0.410–0.939 (Fig. 4). The four hybrids had approximately equal proportions of genetic contribution (q-values 0.410–0.562) from each parental species. We observed a distinct cluster of H. pileatus, but did not observe a clear boundary in the H. lar cluster. We suspect some of the H. lar-like individuals might be backcrosses to H. lar, and therefore contain a greater genetic contribution from H. lar. The suspect backcrosses to H. lar were not recognizable by pelage.
Admixture analysis of 47 samples of H. lar, H. pileatus and intermediate hybrids from the contact zone based on microsatellite analysis for the number of populations (K = 2). Each bar represents one individual [H. lar genetic admixture (yellow), H. pileatus genetic admixture (blue)]. The y-axis shows the q-values: H. lar (≥ 0.95), H. pileatus (≤ 0.05), admixed (0.05 < q-value < 0.95). Ten admixed individuals were determined; the sample codes are shown above the bars. For abbreviations, see Fig. 2
Discussion
Our findings suggest a high degree of genetic differentiation between H. lar and H. pileatus. Gene flow between the species is limited, resulting in a narrow hybrid zone, which concords with previous findings in the study site (Brockelman and Gittins 1984; Asensio et al. 2017). Gene flow is asymmetrical in the hybrid zone, with greater introgression into the H. lar side than into H. pileatus.
Hybridization between H. lar and H. pileatus
The hybridization analysis confirmed that four individuals (8%) with intermediate pelage were hybrids or backcrosses. They were members of mixed-species and hybrid groups. Hybrids A22_HB and A15_HB were offspring of heterospecific groups M2 (female H. lar × male H. pileatus) and M3 (female H. pileatus × male H. lar) studied by Asensio et al. (2017), and are likely to be F1 hybrids. Hybrid A06_HB was the female of group H1 paired with a H. lar male. There is no information about the parents of this adult female. She has intermediate pelage and a great call pattern. The q-value of A06_HB is close to 0.5; thus, she is likely to be an F1 hybrid or an early generation backcross. Hybrid A05_HB is a juvenile; observations of her behavior and her pelage indicated that she is the offspring of female A06_HB, and hence a recent-generation backcross to H. lar. The q-values of A05_HB and A06_HB, respectively 0.422 and 0.434, are similar. These findings support our first hypothesis regarding a mixture of nuclear genes from both species in putative hybrids.
Interbreeding between female H. lar × male H. pileatus and the reciprocal cross produced viable F1 hybrids. Thus, females from both parental species could pass their mitochondrial genes to their hybrid offspring. Nevertheless, we did not find evidence of introgression of H. pileatus mitochondrial genes into the H. lar population, unlike Matsudaira et al. (2013), who did. All samples of the H. lar phenotypes contained H. lar mitochondrial haplotypes, as did all samples of the H. pileatus phenotypes. The fact that more hybrids had H. lar haplotypes (three individuals) than H. pileatus haplotypes (one individual) suggests that females of H. lar are more prone to mate with the other species than females of H. pileatus. This suggestion needs to be further tested with more samples from hybrids.
Matsudaira et al. (2013), who reported introgression into the H. lar population located at the Mo Singto study area approximately 5 km northwest of the contact zone, found nine out of 68 H. lar individuals carrying an H. pileatus haplotype (HKY11). However, we did not find the HKY11 haplotype in our H. pileatus samples from the contact zone. Matsudaira et al. (2013) suggested that these nine individuals were possibly third- or later-generation backcrosses. We determined that our four mitochondrial haplotypes of H. lar were the same as those reported at Mo Singto in the H. lar-dominated area, which suggests gene flow between the two areas. In addition, we observed a female H. pileatus in the H. lar range at Mo Singto during the period from 1997 to 2010 (she produced no offspring), and in May 2018 another female H. pileatus was seen (C. Kongrit, personal observation) but never formed a reproductive pair bond with any H. lar male. An adult female H. pileatus without a mate could not survive in an area populated by H. pileatus groups (to our knowledge, no group has ever been found to contain two adult H. pileatus females), but an adult H. pileatus female may share the territory of an H. lar group without being evicted by residents, even though she would have difficulty in establishing a pair bond. Brockelman and Gittins (1984) found more phenotypic hybrids at the edge of the zone than in the center of the zone. Occasionally, adults dispersing into the area occupied by the other species may pair and breed, but we believe that the most likely way that H. pileatus mitochondrial haplotypes move into the H. lar population is through repeated backcrossing, as suggested by Matsudaira et al. (2013). After the third or fourth generation backcross, the phenotypes will become indistinguishable from those of H. lar (making successive backcross daughter matings easier), while daughters will carry the full complement of maternally inherited H. pileatus haplotypes.
The presence of a low number of intermediate hybrids [6% (Brockelman and Gittins 1984); 7% (Asensio et al. 2017); 8% (this study)], and high abundance of parental species in the contact zone exemplify a bimodal hybrid zone (Jiggins and Mallet 2000). Previous studies provided clear evidence of a higher ratio of conspecific pairs than heterospecific pairs and hybrid groups in this contact zone [194 conspecific pairs, 20 heterospecific and hybrid groups (Brockelman and Gittins 1984); 29 conspecific pairs, three heterospecific pairs (Asensio et al. 2017)], resulting in a rarity of F1 hybrids. This contrasts greatly with reports on contact zones between well-marked, more closely related species, such as H. albibarbis and H. muelleri in Borneo (Mather 1992), and Eulemur fulvus rufus and Eulemur albocollaris (Wyner et al. 2002), in which the parental types are separated by a zone of intergrades or hybrid-like individuals. Several other contact zones between primate species have been reported to have > 20% hybrid individuals [Papio cynocephalus and Papio anubis (Tung et al. 2008); Alouatta caraya and Alouatta guariba clamitans (Mourthe et al. 2019)].
Although multigenerational backcrosses were found in the present study, F1 hybrids were relatively rare. The rarity of recent-generation hybrids, especially F1, and the existence of the parental species in contact with one another in the center of the hybrid zone indicate highly nonrandom mating and strong reproductive isolation between the species. Such a pattern has been also reported in other primate hybrid zones (e.g., Cortés-Ortiz et al. 2019). The narrow hybrid zone between H. lar and H. pileatus is likely to be maintained as a tension zone under a dispersal and selection balance, in which parental species disperse into a contact zone and selection acts against hybrids (Barton and Hewitt 1985). As gibbon social groups are highly sedentary, movement into the zone should occur by dispersal of single individuals in search of mates. Hence, all groups within the zone would be the result of pairing of such individuals within the zone and not of the displacement of newly mated pairs or whole groups. Natal dispersal distance is typically only one or two territories in the H. lar population in the Mo Singto study site about 5 km from the contact zone (Brockelman et al. 1998). However, Asensio et al. (2017), comparing successfully mated pairs in homospecific, mixed species and hybrid groups, found no significant differences in the number of offspring present per group. However, we need to determine whether hybrids are as successful at finding mates as the parental species, and because every hybrid is genetically unique, we cannot assume that they are uniform in this regard (Arnold 1997).
Microsatellite analysis revealed six admixed H. lar-like individuals, suggesting the direction of backcrossing towards H. lar. The presence of such backcrosses indicates that a larger sample size would yield more later-generation backcrosses not recognizable by pelage alone. Hence, our second hypothesis is only partially supported as we found no evidence of introgression into H. pileatus.
In addition, Brockelman and Gittins (1984) observed that four female H. lar had more notes in their great calls than those typical of female H. lar, and suspected that they might have been backcrosses. Admixed individuals can show a wide variety of pelage patterns, but quantitative variation in the highly stereotyped great calls appears to be a more sensitive indication of genetic parentage than pelage (Brockelman and Schilling 1984). Whatever its cause, this finding indicates that the hybrid zone might be larger if acoustic cues are taken into account, particularly as song pattern differences are the clearest way of distinguishing biological species of gibbons (Marshall and Marshall 1976).
Unidirectional gene flow into H. lar could be driven by female choice in pair bonding (Wirtz 1999). The duetted song patterns of H. lar and H. pileatus are species specific (Marshall and Marshall 1976; Brockelman and Schilling 1984; Marshall and Sugardjito 1986), and are related to the maintenance of pair bonds (Geissmann and Orgeldinger 2001). In the H. pileatus duet, males wait during the long bubbly trill of the female and add their “coda” during the last part of the trill (Marshall et al. 1972). In contrast, the male H. lar adds his coda a second or two after the female great call ends (Marshall et al. 1972; Geissmann 1984; Raemaekers et al. 1984). We speculate that female hybrids that produce an intermediate song type without the H. pileatus-like bubbly trill may have a lower chance of coordinating the duet with H. pileatus males, but may sing more easily with H. lar males. Asensio et al. (2017) found that all adult female intermediate hybrids in the study area were paired with H. lar males. It is also possible that the direction of gene flow in the contact zone could be affected by the patterns of dichromatism in the species. For example, H. pileatus females might discriminate against light-colored H. lar morphs (W. Y. Brockelman, unpublished data).
To summarize our main findings, natural hybridization between H. lar and H. pileatus produced viable and fertile F1 hybrids. The intermediate phenotypes of the hybrids reflected the contribution of nuclear genes from both parental species. The relative abundance of parental types versus the low number of F1 hybrids and admixed individuals indicates the presence of reproductive barriers between these biological species [sensu Mayr (1963)]. Our findings provide evidence for asymmetrical introgression of nuclear genes into H. lar; however, further investigation of the genetic structure of H. lar and H. pileatus in their allopatric areas is recommended, as this would provide a better understanding of the directions of gene flow between these two parental species.
References
Anandam MV, Groves CP, Molur S, Rawson BM, Richardson MC, Roos C, Whittaker DJ (2013) Species accounts of Hylobatidae. In: Mittermeier RA, Rylands AB, Wilson DE (eds) Handbook of the mammals of the world. Lynx, Barcelona, pp 778–791
Andayani N, Morales JC, Forstner MRJ, Supriatna J, Melnick DJ (2001) Genetic variability in mtDNA of the silvery gibbon: implications for the conservation of a critically endangered species. Conserv Biol 15:770–775
Arnold ML (1997) Natural hybridization and evolution. Oxford University Press, Oxford
Arnold ML, Martin NH (2010) Hybrid fitness across time and habitats. Trends Ecol Evol 25:530–536
Asensio N, José-Domínguez JM, Kongrit C, Brockelman WY (2017) The ecology of white-handed and pileated gibbons in a zone of overlap and hybridization in Thailand. Am J Phys Anthropol 163:716–728
Barelli C, Matsudaira K, Wolf T, Roos C, Heistermann M, Hodges K, Ishida T, Malaivijitnond S, Reichard UH (2013) Extra-pair paternity confirmed in wild white-handed gibbons. Am J Primatol 75:1185–1195
Bartlett TQ (2011) Hylobatidae: the small apes of Asia. In: Campball CJ, Fuentes A, MacKinnon KC, Stumph RM, Bearder SK (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford, pp 300–312
Barton NH, Hewitt GM (1985) Analysis of hybrid zones. Annu Rev Ecol Syst 16:113–148
Bradley BJ, Boesch C, Vigilant L (2000) Identification and redesign of human microsatellite markers for genotyping wild chimpanzee (Pan troglodytes verus) and gorilla (Gorilla gorilla gorilla) DNA from faeces. Conserv Genet 1:289–292
Brockelman WY (1978) Preliminary report on relations between the gibbons Hylobates lar and H. pileatus in Thailand. In: Chivers DJ, Joysey KA (eds) Recent advances in primatology. Academic Press, London, pp 315–318
Brockelman WY (2004) Inheritance and selective effects of color phase in white-handed gibbons (Hylobates lar) in central Thailand. Mamm Biol 69:73–80
Brockelman WY, Gittins SP (1984) Natural hybridization in the Hylobates lar species group: implications for speciation in gibbons. In: Preuschoft H, Chivers DJ, Brockelman WY, Creel N (eds) The lesser apes: evolutionary and behavioural biology. Edinburgh University Press, Edinburgh, pp 498–532
Brockelman WY, Schilling D (1984) Inheritance of stereotyped gibbon calls. Nature 312:634–636
Brockelman WY, Reichard U, Treesucon U, Raemaekers JJ (1998) Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behav Ecol Sociobiol 42:329–339
Carbone L, Alan Harris R, Gnerre S et al (2014) Gibbon genome and the fast karyotype evolution of small apes. Nature 513:195–201
Chan YC, Roos C, Inoue-Murayama M, Inoue E, Shih CC, Pei KJC, Vigilant L (2013) Inferring the evolutionary histories of divergences in Hylobates and Nomascus gibbons through multilocus sequence data. BMC Evol Biol 13:82. https://doi.org/10.1186/1471-2148-13-82
Charpentier MJE, Tung J, Altmann J, Alberts C (2008) Age at maturity in wild baboons: genetic, environmental and demographic influences. Mol Ecol 17:2026–2040. https://doi.org/10.1111/j.1365-294X.2008.03724.x
Cortés-Ortiz L, Duda TF, Canales-Espinosa D, García-Orduña F, Rodríguez-Luna E, Bermingham E (2007) Hybridization in large-bodied New World primates. Genetics 176:2421–2425
Cortés-Ortiz L, Nidiffer MD, Hermida-Lagunes J, García-Orduña F, Rangel-Negrín A, Kitchen DM, Bergman TJ, Dias PAD, Canales-Espinosa D (2019) Reduced introgression of sex chromosome markers in the Mexican howler monkey (Alouatta palliata × A. pigra) hybrid zone. Int J Primatol 40:114–131
de Manuel M, Kuhlwilm M, Frandsen P et al (2016) Chimpanzee genomic diversity reveals ancient admixture with bonobos. Science 354:477–481
Detwiler KM (2019) Mitochondrial DNA analyses of Cercopithecus monkeys reveal a localized hybrid origin for C. mitis doggetti in Gombe National Park. Tanzania Int J Primatol 40:28–52
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Fan PF, He K, Chen X, Ortiz A, Zhang B, Zhao Z, Li YQ, Zhang HB, Kimock C, Wang WZ, Groves C, Turvey ST, Roos C, Helgen KM, Jiang XL (2017) Description of a new species of Hoolock gibbon (Primates: Hylobatidae) based on integrative taxonomy. Am J Primatol. https://doi.org/10.1002/ajp.22631
Geissmann T (1984) Inheritance of song parameters in the gibbon song, analysed in 2 hybrid gibbons (Hylobates pileatus × H. lar). Folia Primatol 42:216–235
Geissmann T, Orgeldinger M (2001) The relationship between duet songs and pair bonds in siamangs, Hylobates syndactylus. Anim Behav 60:805–809
Gittins SP (1978) The species range of the gibbon Hylobates agilis. In: Chivers DJ, Joysey KA (eds) Recent advanced in primatology. Academic Press, London, pp 319–321
Gligor M, Ganzhorn JU, Rakotondravony D, Ramilijaona OR, Razafimahatratra E, Zischler H, Hapke A (2009) Hybridization between mouse lemurs in an ecological transition zone in southern Madagascar. Mol Ecol 18:520–533
Ho L, Cortés-Ortiz L, Dias PAD, Canales-Espinosa D, Kitchen DM, Bergman TJ (2014) Effect of ancestry on behavioral variation in two species of howler monkeys (Alouatta pigra and A. palliata) and their hybrids. Am J Primatol 76:855–867
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Israfil H, Zehr SM, Mootnick AR, Ruvolo M, Steiper ME (2011) Unresolved molecular phylogenies of gibbons and siamangs (family: Hylobatidae) based on mitochondrial, Y-linked, and X-linked loci indicate a rapid Miocene radiation or sudden vicariance event. Mol Phylogen Evol 58:447–455
Jiggins C, Mallet J (2000) Bimodal hybrid zones and speciation. Trends Ecol Evol 15:250–255
Jobb G (2011) TREEFINDER version March 2011. http://www.treefinder.de. Accessed 19 May 2021
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR (2018) Rapid hybrid speciation in Darwin’s finches. Science 359:224–228
Leigh JW, Bryant D (2015) popart: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116
Malukiewicz J, Boere V, Fuzessy LF, Grativol AD, de Silva OI, Pereira LCM, Ruiz-Miranda CR, Valença YM, Stone AC (2015) Natural and anthropogenic hybridization in two species of eastern Brazilian marmosets (Callithrix jacchus and C. penicillata). PLOS ONE 10(6):e0127268. https://doi.org/10.1371/journal.pone.0127268
Marshall JT, Brockelman WY (1986) Pelage of hybrid gibbons (Hylobates lar × H. pileatus) observed in Khao Yai National Park. Thailand Nat Hist Bull Siam Soc 34:145–157
Marshall JT, Marshall ER (1976) Gibbons and their territorial songs. Science 193:235–238
Marshall JT, Sugardjito S (1986) Gibbon systematics. In: Swindler DR, Erwin J (eds) Comparative primate biology: systematics, evolution, and anatomy, vol 1. Liss, New York, pp 137–185
Marshall JT, Ross BA, Chantharojvong S (1972) The species of gibbons in Thailand. J Mammal 53:479–486
Mather R (1992) A field study of hybrid gibbons in central Kalimantan, Indonesia. PhD thesis, Cambridge University
Matsudaira K, Reichard UH, Malaivijitnond S, Ishida T (2013) Molecular evidence for the introgression between Hylobates lar and H. pileatus in the wild. Primates 54:33–37
Mayr E (1963) Animal species and evolution. Belknap, Cambridge, MA
Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics 142:1061–1064
Mourthe I, Trindade RA, Aguiar LM, Trigo TC, Bicca-Marques JC, Bonatto SL (2019) Hybridization between Neotropical primates with contrasting sexual dichromatism. Int J Primatol 40:99–113
Nürnberg P, Sauermann U, Kayser M, Lanfer C, Manz E, Widdig A, Berard J, Bercovitch FB, Kessler M, Schmidtke J, Krawczak M (1998) Paternity assessment in rhesus macaques (Macaca mulatta): multilocus DNA fingerprinting and PCR marker typing. Am J Primatol 44:1–18
Park SDE (2001) Trypanotolerance in west African cattle and the population genetic effects of selection. PhD thesis, University of Dublin
Pastorini J, Zaramody A, Curtis DJ, Nievergelt CM, Mundy NI (2009) Genetic analysis of hybridization and introgression between wild mongoose and brown lemurs. BMC Evol Biol 9:32. https://doi.org/10.1186/1471-2148-9-32
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raemaekers JJ, Raemaekers PM, Haimoff EH (1984) Loud calls of the gibbon (Hylobates lar): repertoire, organization and context. Behaviour 91:146–189
Rambaut A (2016) FigTree v1.4.3. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 19 May 2021
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rousset F (2008) Genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302
Salazar C, Baxter SW, Pardo-Diaz C, Wu G, Surridge A, Linares M, Bermingham E, Jiggins CD (2010) Genetic evidence for hybrid trait speciation in Heliconius butterflies. PLoS Genet 6:e1000930. https://doi.org/10.1371/journal.pgen.1000930
Sams AJ, Dumaine A, Nédélec Y, Yotova V, Alfieri C, Tanner JE, Messer PW, Barreiro LB (2016) Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome Biol 17:246. https://doi.org/10.1186/s13059-016-1098-6
Silva BTF, Sampaio MIC, Schneider H, Schneider MPC, Montoya E, Encarnación F, Salzano FM (1992) Natural hybridization between Saimiri taxa in the Peruvian Amazonia. Primates 33:107–113
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Suwanvecho U, Brockelman WY (2012) Interspecific territoriality in gibbons (Hylobates lar and H. pileatus) and its effects on the dynamics of interspecies contact zones. Primates 53:97–108
Thinh VN, Mootnick AR, Geissmann T, Li M, Ziegler T, Agil M, Moisson P, Nadler T, Walter L, Roos C (2010) Mitochondrial evidence for multiple radiations in the evolutionary history of small apes. BMC Evol Biol 10:74. https://doi.org/10.1186/1471-2148-10-74
Tosi AJ, Morales JC, Melnick DJ (2002) Y-chromosome and mitochondrial markers in Macaca fascicularis indicate introgression with Indochinese M. mulatta and a biogeographic barrier in the Isthmus of Kra. Int J Primatol 23:161–178
Tung J, Barreiro LB (2017) The contribution of admixture to primate evolution. Curr Opin Genet Dev 47:61–68
Tung J, Charpentier MJE, Garfield DA, Altmann J, Alberts SC (2008) Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Mol Ecol 17:1998–2011
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Whittaker DJ, Morales JC, Melnick DJ (2007) Resolution of the Hylobates phylogeny: congruence of mitochondrial D-loop sequences with molecular, behavioral, and morphological data sets. Mol Phylogen Evol 45:620–628
Wirtz P (1999) Mother species–father species: unidirectional hybridization in animals with female choice. Anim Behav 58:1–12
Wyner YM, Johnson SE, Stumpf RM, Desalle R (2002) Genetic assessment of a white-collared × red-fronted lemur hybrid zone at Andringitra, Madagascar. Am J Primatol 57:51–66
Zinner D, Arnold ML, Roos C (2011) The strange blood: natural hybridization in primates. Evol Anthropol 20:96–103
Acknowledgments
We would like to express our special thanks to Juan Manuel José Dominguez, Jakkrit Kachanun, Sukanda Arunyawasri and Somsak Sarabua for their invaluable assistance in the field. We are grateful to the Department of National Parks, Wildlife and Plant Conservation for permission to conduct this research in Khao Yai National Park, and to the National Research Council of Thailand for their permission to conduct the research. This research was supported by Mahidol University and a Science Achievement Scholarship of Thailand.
Author information
Authors and Affiliations
Contributions
NA, WYB and CK contributed to the research idea, study design and field data collection. The laboratory work and data analysis were performed by DM, CK and EJ. The first draft of the manuscript was written by DM. All the authors commented on and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflict of interest.
Ethical statement
This research was carried out under animal ethics protocol no. MUSC61-040-442 authorized by Mahidol University-Institute Animal Care and Use Committee (MU-IACUC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Markviriya, D., Asensio, N., Brockelman, W.Y. et al. Genetic analysis of hybridization between white-handed (Hylobates lar) and pileated (Hylobates pileatus) gibbons in a contact zone in Khao Yai National Park, Thailand. Primates 63, 51–63 (2022). https://doi.org/10.1007/s10329-021-00958-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-021-00958-y