Abstract
Hybridization, or the process by which individuals from genetically distinct populations (e.g., species, subspecies) mate and produce at least some offspring, is of great relevance to understanding the basis of reproductive isolation and, in some cases, the origins of biodiversity. Natural hybridization among primates has been well documented for a few taxa, but just recently the genetic confirmation of hybridization for a number of taxa has produced new awareness of the prevalence of this phenomenon within the order and its importance in primate evolution. The study of hybridization of Alouatta pigra and A. palliata in Mexico was among the first to genetically confirm the current occurrence of hybridization in primates. Following this study, other reports of hybridization have shown that this phenomenon is more widespread among primates than previously anticipated. Within the genus Alouatta, there have been reports on the presence of hybridization between A. caraya and A. guariba in a number of contact zones in Brazil and Argentina, and various studies are currently ongoing in some of these sites to understand the extent and patterns of hybridization between these species. In this chapter, we evaluate the extent of hybridization in the genus Alouatta, revise the current knowledge of the genetic and morphological aspects of these hybrid systems, and identify future directions in the study of hybridization within this genus, to understand the possible implications of the hybridization process in the evolutionary history of howler monkeys.
Resumen
Hibridación, o el proceso mediante el cual individuos de poblaciones genéticamente distintas (especies o subespecies) se aparean y producen descendencia, tiene gran relevancia en la comprensión de las bases para el aislamiento reproductivo entre distintos taxa y, en algunos casos, para entender el origen de la biodiversidad. La hibridación natural entre primates ha sido bien conocida para unas cuantas especies, pero sólo recientemente la confirmación genética de hibridación entre numerosos taxa de primates ha sido posible y ha conducido a una nueva percepción de la prevalencia de este fenómeno entre los primates y su importancia en la evolución de este grupo. El estudio de la hibridación entre Alouatta pigra and A. palliata en México fue uno de los primeros que confirmó con evidencia genética la ocurrencia de hibridación en primates. Después de este estudio, otros reportes de hibridación en distintos taxa de primates han puesto de manifiesto que este fenómeno es más común en el orden Primates de lo que inicialmente se pensaba. Dentro del género Alouatta, también han habido reportes de hibridación entre A. caraya y A. guariba en distintas zonas de contacto en Brasil y Argentina, y varios estudios actualmente están en curso en algunas de estas áreas para entender la magnitud de este fenómeno y los patrones de hibridación entre estas especies. En este capítulo evaluamos la presencia de hibridación en el género Alouatta, revisamos lo que se conoce sobre los aspectos genéticos y morfológicos en estos sistemas híbridos y planteamos direcciones futuras en el estudio de la hibridación en este género, para entender las implicaciones del proceso de hibridación en la historia evolutiva de los monos aulladores.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hybridization is the crossing of genetically distinct taxa that produces some viable offspring (Arnold 1997; Mallet 2005). Crosses of pure individuals from different genetic lineages result in first-generation hybrids (F1s), but hybrid individuals can backcross with pure individuals of one of the parental species or crossbreed with other hybrid individuals, producing offspring with variable levels of genetic admixture. Although hybridization was initially considered a process mainly occurring among plants, and with limited representation in animals, a variety of genetic studies in the past few decades have shown that this phenomenon is rather common among sexually reproducing animals, especially between closely related species (Dowling and Secor 1997; Mallet 2005).
In primates, hybridization has been reported in captivity for a number of taxa (e.g., Chiarelli 1973; Tenaza 1984; Coimbra-Filho et al. 1984; Jolly et al. 1997); however, only few cases of natural hybridization in primates were known and studied before the twenty-first century, and most of these involved cercopithecine monkeys (Bernstein 1966; Struhsaker 1970; Nagel 1973; Dunbar and Dunbar 1974; Samuels and Altmann 1986). Identification of hybrids in these studies primarily relied on behavioral and morphological features of individuals that showed mixed characteristics typical of each parental taxon.
The widespread use of molecular techniques to address different aspects of primate systematics, behavior, and ecology during the last two decades has allowed the detection of an increased number of cases of hybridization in different primate taxa (e.g., Merker et al. (2009) in tarsiers; Cortés-Ortiz et al. (2007) in howler monkeys; Wyner et al. (2002) in lemurs; da Silva et al. (1992) in squirrel monkeys), including those in our own lineage (Green et al. 2010). However, there are still large gaps in our understanding of the genetic and morphological outcomes of hybridization at the individual and population levels, as well as their implications for the evolutionary trajectories of primate lineages.
In this chapter we review our current understanding of the prevalence of hybridization among howler monkeys. Alouatta is among of the first Neotropical primate genera for which genetic confirmation of hybridization is available (Cortés-Ortiz et al. 2007). We summarize demographic, morphological, behavioral, and genetic studies currently available, and make recommendations on future directions in the study of Alouatta hybrid zones and the implications of hybridization in primate evolution.
2 Distribution of Howler Monkey Contact Zones
As illustrated throughout this volume, howler monkeys are distributed across the Neotropics and have the broadest distribution of any Neotropical primate genus (Fig. 3.1). Phylogenetic studies have identified between 10 and 14 species and 22 taxa (species and subspecies), but there are a number of poorly known forms that still remain to be studied to allow an adequate evaluation of their taxonomic status (e.g., Peruvian species/subspecies) (see Cortés-Ortiz et al. 2014).
Although howler monkey species maintain allopatric/parapatric distributions in most of their range, small areas of overlap have been reported for some species (Fig. 5.1), including contact between A. palliata and A. pigra in Mexico (Smith 1970; Horwich and Johnson 1986; Cortés-Ortiz et al. 2007), A. palliata and A. seniculus in northwestern Colombia (Hernández-Camacho and Cooper 1976; Defler 1994), A. caraya and A. guariba in northern Argentina (Di Bitetti 2005; Agostini et al. 2008), A. caraya and A. guariba in southern Brazil (Gregorin 2006; Aguiar et al. 2007, 2008, 2014; Bicca-Marques et al. 2008), A. g. guariba and A. g. clamitans in Brazil (Kinzey 1982), A. discolor and A. s. puruensis in Brazil (Pinto and Setz 2000), A. caraya and A. sara in Bolivia (Wallace et al. 2000; Büntge and Pyritz 2007) and Brazil (Iwanaga and Ferrari 2002), and A. macconnelli and A. nigerrima in Brazil (Napier 1976 and Cruz Lima 1945, cited in Gregorin 2006). It is likely that these areas of sympatry are due to secondary contact as a consequence of range expansions after periods of isolation (Cortés-Ortiz et al. 2003; Ford 2006; Gregorin 2006), and therefore, many other areas of contact among different Alouatta species may also exist. However, few surveys have been conducted in areas of potential contact within the limits of the distribution of parapatric howler monkey species, and those that exist show that sympatry is rare, but more common than previously anticipated. In some of the areas of sympatry among howler monkeys, individuals with intermediate or mosaic features have been observed (Cortés-Ortiz et al. 2003, 2007; Gregorin 2006; Aguiar et al. 2007; Agostini et al. 2008; Bicca-Marques et al. 2008; Silva 2010), suggesting at least some degree of crossbreeding between taxa and the formation of hybrid zones.
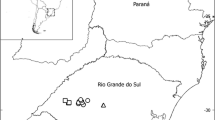
Approximate location of the reported areas of contact between howler monkey species. (1) A. palliata and A. pigra in Mexico (Horwich and Johnson 1986; Smith 1970), (2) A. palliata and A. seniculus in northwestern Colombia (Defler 1994; Hernández-Camacho and Cooper 1976), (3) A. caraya and A. guariba clamitans in northern Argentina (Agostini et al. 2008; Di Bitetti 2005), (4) A. caraya and A. g. clamitans in southern Brazil (Bicca-Marques et al. 2008), (5) A. caraya and A. g. clamitans in southern Brazil (Aguiar et al. 2007; 2014; Gregorin 2006), (6) A. g. guariba and A. g. clamitans in Brazil (Kinzey 1982), (7) A. discolor and A. s. puruensis in Brazil (Pinto and Setz 2000), (8) A. caraya and A. sara in Bolivia (Büntge and Pyritz 2007), (9) A. caraya and A. sara in Bolivia (Wallace et al. 2000), (10) A. caraya and A. sara in Brazil (Iwanaga and Ferrari 2002), (11) A. macconnelli and A. nigerrima in Brazil (Napier 1976 and Cruz Lima 1945, cited in Gregorin 2006)
3 Studies of Hybridization in Howler Monkeys: Mixed Groups and Demographic Features of Syntopic Hybridizing Species
Evidence of hybridization has been reported for only two pairs of species of howler monkeys: A. palliata × A. pigra and A. caraya × A. guariba. These species are distinguishable on the basis of both morphological (Hill 1962; Groves 2001; Gregorin 2006) and genetic (de Oliveira et al. 2002; Cortés-Ortiz et al. 2003; Steinberg et al. 2008) features. The hybridizing species of each of these pairs diverged at approximately 3 and 5 MA, respectively (Cortés-Ortiz et al. 2003). Reports of possible hybridization were initially based on morphological and behavioral observations of individuals living in proximity or in mixed species groups. Later, demographic, behavioral, and genetic studies confirmed or strongly suggested the presence of hybrid offspring in the wild (Cortés-Ortiz et al. 2007; Agostini et al. 2008; Aguiar et al. 2008; Bicca-Marques et al. 2008) and in captivity (de Jesus et al. 2010).
3.1 A. palliata × A. pigra Hybrid Zone in Tabasco, Mexico
Smith (1970) first reported a possible area of sympatry of A. palliata and A. pigra in Tabasco, Mexico, based on museum specimens collected ~8 km SE of Macuspana (17°45′40″N, 92°35′35″W). More than a decade later, Horwich and Johnson (1986) surveyed the area where the specimens studied by Smith were collected as well as other nearby areas, but failed in finding direct evidence of the presence of howler monkeys. Nonetheless, through interviewing of local people, they identified a possible area of sympatry in the vicinity of Teapa (17°33′25″N, 92°56′50″W), about 40 km SE of Macuspana. In the early 1990s, Francisco García Orduña, Domingo Canales Espinosa, and Ernesto Rodríguez Luna from the Universidad Veracruzana (UV) in Mexico surveyed several areas across the state of Tabasco and found groups of A. palliata and A. pigra living in close proximity, as well as mixed groups composed of individuals of both species, and groups with individuals that emitted distinct vocalizations that sounded “intermediate” between the calls of either species (García-Orduña et al. unpubl. data; see also Kitchen et al. 2014). Later excursions to the area with the aim of collecting biological samples for genetic studies revealed that a number of individuals possessed mixed morphological features distinctive of each species (mainly subtle facial features, as well as pelage coloration) (Cortés-Ortiz unpubl. data; see Fig. 5.2 for an example of differences in facial features). Cortés-Ortiz and collaborators sampled 44 groups within this contact zone between 1998 and 2010 (Table 5.1). Most groups (N = 28) were phenotypically monospecific (17 A. pigra and 11 A. palliata), but three groups were mixed with individuals phenotypically resembling either species living together, and the remaining 13 groups included individuals with intermediate/mosaic features (detected via either morphology or vocalizations; see Figs. 14.1 and 14.2 in da Cunha et al. 2014 for differences in vocalizations) suggestive of a hybrid origin (but see Sect. 5.4 for a better understanding of the complex relationship between morphology and genetics in this system). Based on these surveys and data, we now know that the A. palliata × A. pigra hybrid zone in Tabasco is about 20 km wide and covers at least 67 km2, with a patchwork of pure, mixed, and hybrid groups (Cortés-Ortiz et al. 2003, 2007) (see Table 5.1 for details on group composition).
Example of facial differences between A. pigra and A. palliata females and mixed features in a hybrid female. All pictures are from adult females: (1) nostrils more frontal in A. pigra and nasal alar walls more prominent in A. palliata, (2) prominent ridge of the nasal bone in A. palliata and not apparent in A. pigra, (3) hair covering a larger area of the cheeks in A. pigra than in A. palliata, and (4) longer beard in A. pigra than in A. palliata. Black arrows denote A. pigra features, white arrows denote A. palliata features, and dashed arrows denote intermediate features in the hybrid. Weight averages from Kelaita et al. (2011)
3.2 A. caraya × A. guariba Hybrid Zones in Brazil
Records of mixed groups formed by A. caraya and A. guariba can be traced back to the beginning of the nineteenth century in the State of Rio Grande do Sul in Brazil (Isabelle 1983). However, Lorini and Persson (1990) were the first to report possible hybridization between these species in Brazil based on morphological studies of museum specimens collected in the 1940s by A. Meyer in the region of the Upper Parana River in the northwestern extreme of the State of Paraná. These specimens had a mosaic pelage coloration pattern representing a mixture of the typical patterns of the two parental species. In his comprehensive review of Brazilian howler monkeys, Gregorin (2006) analyzed the same specimens and also concluded that they represented hybrid individuals. Aguiar et al. (2007, 2008) surveyed a nearby area in the surroundings of the Ilha Grande National Park (23°24′S, 53°49′W) and found both monospecific groups of A. caraya and A. guariba living in sympatry and groups containing individuals with mosaic coloration patterns (see Fig. 2 in Aguiar et al. 2007 and Fig. 1 in Aguiar et al. 2008). They reported a total of 11 groups living within the boundaries of a 150 ha forest fragment (two monospecific groups of each species, two groups with A. guariba + putative hybrids, and five polyspecific groups of A. caraya + A. guariba + putative hybrids), as well as five A. caraya and two A. guariba groups living along a 17 km stretch of riverine forest and two monospecific groups (one of each species) living in sympatry in a near forest fragment (“Paredão das Araras,” 23°21′10.1″S, 53°44′08.5″W). They found A. guariba as the most abundant species in the area, perhaps as a consequence of the prevalence of Atlantic Forest in the area, which is a type of habitat usually inhabited by this species rather than by A. caraya. The proportion of putative hybrids was similar to the proportion of A. caraya individuals in the area.
Another area of sympatry and hybridization between these taxa in Brazil occurs in the region of São Francisco de Assis, State of Rio Grande do Sul (Bicca-Marques et al. 2008; Silva 2010). Between 2006 and 2009 the team of primatologists and students headed by Bicca-Marques surveyed six localities within an area of approximately 600 km2 in this region (29°33′50″–29°35′10″S, 54°58′40″–54°59′50″W), finding a total of 43 groups, 22 of which included at least one potential hybrid individual (i.e., with a mosaic phenotype) (Silva 2010). Interestingly, the distribution of phenotypically A. guariba groups decreased westwards and the opposite trend was observed for A. caraya groups. The westernmost locality surveyed contained only A. caraya groups, and a high percentage of hybrid individuals (42 %) was still present in the easternmost surveyed locality, suggesting that the area of contact and hybridization between these taxa may extend beyond the approximately 20 km wide strip surveyed.
3.3 A. caraya × A. guariba Hybrid Zones in Argentina
In Argentina, A. guariba and A. caraya have overlapping distributions in a small region in the province of Misiones, where syntopic populations have been detected in the strictly protected area of El Piñalito Provincial Park (Agostini et al. 2008). In a survey of approximately 800 ha, Agostini et al. (2008) detected three groups of A. caraya, five of A. guariba, and one mixed group composed of one adult A. guariba male, two A. guariba females, and one A. caraya female. The latter female was observed copulating with A. guariba males and giving birth twice to individuals with mosaic phenotypes, similar to those reported in Brazil (see Sect. 5.3.2). The extent of hybridization in this area is still unknown, but the absence of adults with mosaic pelage coloration patterns suggests that hybridization may be less common in this site than in the Brazilian contact zones. More recent surveys in the State of Misiones (one by I. Holzmann during and immediately after a yellow fever outbreak in 2008 [Holzmann 2012] and one by Agostini in 2010 [Agostini unpubl. data]) found no morphological or demographic evidence of hybridization. However, without extensive surveys in other localities within this contact zone, any statement about the lack of hybridization in this region would be premature.
4 Morphological Signals of Hybridization
The finding of individuals with intermediate phenotypes (i.e., diagnostic traits of each parental species co-occurring in the same individual) is often seen as evidence of hybridization. However, our understanding of the effects of hybridization on the morphological development of an individual is rather poor. On the one hand, we lack a clear understanding of the extent of phenotypic variation in hybrid individuals (Ackermann 2010), and on the other, many studies have only been able to detect hybridization when genetic markers are used (i.e., when hybridization is cryptic; e.g., Jasinska et al. (2010) in plants; Neaves et al. (2010) in marsupials; Gaubert et al. (2005) in carnivores). The slowly increasing number of studies incorporating genetic and morphological data in the study of the hybridization process suggests that morphologically intermediate and cryptic hybrids are the extremes of a continuum in the morphological expression of hybridization (e.g., Ackermann et al. 2006; Ackermann and Bishop 2010; Kelaita and Cortés-Ortiz 2013).
Much of what it is known about primate hybrid morphology comes from studies of Old World monkeys such as baboons (e.g., Jolly 2001; Ackermann et al. 2006), macaques (e.g., Bynum 2002; Schillaci et al. 2005), and some cercopithecine species (Detwiler 2002). Only a handful of studies addressing the morphology of hybrid New World monkeys have been carried out (e.g., Cheverud et al. 1993 and Kohn et al. 2001 for captive tamarins; Peres et al. 1996 for wild saddled back tamarins). In this section we discuss patterns of morphological variation observed in both presumed (based on phenotype) and genetically confirmed howler monkey hybrids, and discuss the reliability of using morphological cues to identify hybrid individuals.
Howler monkey species differ in numerous phenotypic attributes. Among the most conspicuous are the pelage color patterns that distinguish parapatric species. This is particularly true for the four species that are known to hybridize: A. caraya, A. guariba, A. palliata, and A. pigra. In A. caraya, adult males are completely black and adult females are pale yellowish-brown, whereas males of A. guariba are red and females are dark brown (Gregorin 2006). Coat coloration of A. palliata adults is black with light golden hairs on the flanks, whereas the pelage coloration of A. pigra is completely black and hairs have a softer texture than in A. palliata (Smith 1970). Intermediate pelage coloration between A. caraya and A. guariba was the trait used by Lorini and Persson (1990) to recognize some of the museum specimens of their study as putative hybrids. This identification generated expectations of hybrid morphotypes represented by mosaic combinations of coat color polymorphisms (Gregorin 2006), which were later used to classify putative A. caraya × A. guariba hybrids in the wild (Aguiar et al. 2007, 2008; Agostini et al. 2008; Bicca-Marques et al. 2008; Silva 2010). The distinctive pelage coloration of adult males and females of the sexually dichromatic A. guariba and A. caraya presumably results in easily distinguishable mosaic and/or intermediate features in the hybrid individuals, with up to 20 morphotypes identified in the wild (Aguiar et al. 2008; Silva 2010).
While the detection of A. caraya × A. guariba hybrids may be possible based on pelage coloration (at least to a certain extent), the recognition of the more similarly colored A. palliata × A. pigra hybrids using the same methods is not always possible. Alouatta palliata and A. pigra display some cranial and facial shape differences that can be used to distinguish individuals of each species in the field (see the example in Fig. 5.2). However, these traits show considerable intraspecific variation, and the intermixing of these features produces a broad range of hybrid morphotypes that compromised attempts to generate a clear criterion to accurately distinguish genetically confirmed hybrid and non-hybrid individuals (Kelaita and Cortés-Ortiz 2013).
Morphometric data, in contrast, have shown several quantifiable size differences between A. palliata and A. pigra for several variables (Kelaita et al. 2011), but analyses of morphological variation based on quantitative (metric) measurements of body size also showed a great variation in the hybrid phenotypes in Mexico (Kelaita and Cortés-Ortiz 2013). Kelaita and Cortés-Ortiz (2013) confirmed the hybrid status of individuals using diagnostic genetic markers (see Sect. 5.5 for details). The genetic data revealed that only 12 % of 128 identified hybrids had similar portions of their genome coming from each parental species. Although none of these individuals were F1 individuals, they were classified as “intermediate” and likely represent early-generation hybrids. The majority of identified hybrids were multigenerational backcrosses, probably resulting from the crossing of first-generation hybrids and their descendants with purebred individuals of one or the other parental species, or from the continued mating among hybrids during multiple generations. Depending on the number of diagnostic alleles of each species present in hybrid individuals, they were classified as A. palliata-like or A. pigra-like multigenerational backcrossed hybrids. A comparison of 14 morphometric variables among purebred and hybrid adult individuals showed that genetically intermediate hybrids exhibited great variation in morphometric characters. Both male and female intermediates ran the gamut of potential states for each variable, in some cases resembling A. palliata, while in others resembling A. pigra, or exhibiting values intermediate between or overlapping with the two parental species. On the other hand, multigenerational backcrossed hybrids only resembled the parental species with which they shared most of their alleles (Kelaita and Cortés-Ortiz 2013), compromising their accurate identification as hybrids.
These results indicate that instances of hybridization between well-established taxonomic groups can be underestimated if only a morphological criterion is utilized to identify hybrids. In the case of A. palliata × A. pigra hybrids, the majority of hybrid individuals are morphologically indistinguishable from parental species. The A. guariba × A. caraya hybrid studies revealed that hybrid individuals, identified based on pelage coloration patterns, comprised between 14 % (Aguiar et al. 2008) and 25 % (Silva 2010) of all individuals sampled from the respective hybrid zones. Considering that in the howler monkey hybrid zone in Mexico genetically intermediate hybrids comprise 12 % of all sampled individuals, it is likely that the purported A. guariba × A. caraya hybrids may also represent genetically intermediate individuals. The incorporation of molecular methods will help to test this prediction in the A. guariba × A. caraya hybrid zones.
5 Genetic Studies in the Howler Monkey Hybrid Zones
Genetic confirmation of hybridization in howler monkeys only exists for the A. palliata × A. pigra hybrid system. An initial study by Cortés-Ortiz et al. (2007) in Tabasco, Mexico determined the hybrid status of 13 individuals based on mitochondrial (mtDNA) and Y-chromosome (SRY gene) sequence data that, respectively, track the maternal and paternal lineages of hybrids, as well as on eight bi-paternally inherited microsatellite loci (three of which had diagnostic alleles for the parental species). Individuals were considered “hybrids” whenever discordance between mtDNA, SRY, and/or microsatellites occurred or when microsatellite loci in the same individual contained a combination of alleles diagnostic of each species. This study suggested unidirectional hybridization in this population, in which the cross of A. palliata males and A. pigra females only produced F1 fertile females, but the cross of A. pigra males and A. palliata females appeared to fail in producing fertile offspring. This result is consistent with the prediction of Haldane’s rule, which establishes that it is more likely for the heterogametic sex (i.e., males for mammals) to be inviable or sterile (Haldane 1922). Nonetheless, the genetic variability at the uni- and bi-parentally inherited loci found among hybrids showed that backcrossing was occurring and that the production of fertile multigenerational backcrossed males was possible (Cortés-Ortiz et al. 2007). Preliminary results of an ongoing study based on a larger sample size of individuals (N = 178) from the same hybrid zone and using 15 diagnostic microsatellite loci (which have a higher power to detect mixed ancestry) give support to the directional bias in hybridization and subsequent backcrossing. These new results also show novel genetic combinations (see Fig. 5.3) and a much higher percentage of hybrid individuals in the area of contact than initially recognized (Cortés-Ortiz unpubl. data). Most hybrids in the area are multigenerational, and only a handful of individuals are likely the product of crosses between purebreds and recent generation hybrids. Figure 5.4 summarizes the individual genetic variation found in this contact zone. Interestingly, when analyzing the genetic composition of hybrids, it is apparent that mtDNA haplotypes from A. pigra are more likely to be present in individuals with most of their nuclear genome (represented by the microsatellite alleles) of the A. palliata type, but only one hybrid with mostly A. pigra nuclear background has an A. palliata mtDNA haplotype. It is also remarkable that all male hybrids have the SRY gene type (reflecting paternal lineage) coincident with the majority of their nuclear background. These observations also support the predictions of Haldane’s rule in the A. palliata × A. pigra hybrid system (Cortés-Ortiz et al. 2007), in which only females are produced in the first generation of crossing, and viable or fertile males appear in the population only after extensive backcrossing among multigenerational hybrids or between hybrids and purebred individuals (see Fig. 5.3C). The patterns of genetic variation observed among hybrid/backcrossed individuals suggest that the directionality in hybridization may be due to chromosomal, cytonuclear, or genomic incompatibilities. Steinberg et al. (2008) studied the chromosomal arrangements of Mesoamerican howler monkeys (see also Mudry et al. 2015) and found that A. pigra and A. palliata have different modal chromosome numbers (2n = 58 for A. pigra and 2n = 53 and 54 for A. palliata males and females, respectively), and males have different sex determination systems (X1X2Y1Y2 quadrivalent in A. pigra and X1X2Y trivalent in A. palliata). Whether the apparent lack of early-generation male hybrids is a consequence of chromosomal incompatibilities due to these chromosomal differences is still an open question.
Possible outcomes of crosses between A. palliata, A. pigra and hybrid individuals based on genotypic data of individuals from the Mexican hybrid zone. (a) Crosses between A. pigra females and A. palliata males only produce fertile females. These F1 females may mate with either A. pigra males or backcrossed males with Api SRY type and produce female offspring. It is unknown whether males with Api SRY type may be produced in this or only in later generations of backcrossing. (b) Crosses between A. palliata females and A. pigra males either do not occur, do not produce offspring, or rarely occur and produce unfertile offspring. (c) Further generation hybrids may continue to backcross with either purebred or backcrossed individuals and eventually produce males with Apa SRY type (Modified from Fig. 3 of Cortés-Ortiz et al. 2007)
Genetic composition of individuals from the Mexican hybrid zone. The X-axis represents the number of A. palliata diagnostic alleles. Individuals with 0 A. palliata diagnostic alleles represent pure A. pigra individuals whereas those with 30 A. palliata diagnostic alleles represent pure A. palliata individuals. (a) Variation based on 15 diagnostic microsatellite loci, (b) composition of mitochondrial DNA (mtDNA) haplotypes, and (c) composition of sex determination gene (SRY) haplotypes
Although molecular data for the A. caraya × A. guariba hybrid zones are not yet available, the demographic and morphological patterns observed in their contact zones allow some inferences based on the knowledge generated from the A. palliata × A. pigra genetic studies. First, the presence of mosaic coat color features in putative hybrid males (one subadult male in Aguiar et al. (2007), one infant male in Agostini et al. (2008), four in Bicca-Marques et al. (2008), one in Jesus et al. (2010), and eight adult males in Silva (2010)) suggests that at least some male hybrids are viable. This inference is supported by a case of hybridization in captivity between putatively purebred individuals (Jesus et al. 2010). Second, if the mosaic individuals represent early-generation hybrids, as found in the A. palliata × A. pigra hybrid zone (Kelaita and Cortés-Ortiz 2013), it is possible that Haldane’s rule is not operating in the A. caraya × A. guariba system. However, the absence of information on the longevity of the morphological signal of hybridization and the lack of molecular data makes it impossible to come to strong conclusions on this respect. Third, A. caraya and A. guariba also have different modal chromosome numbers (2n = 52 for A. caraya and 2n = 45–52 for A. guariba; de Oliveira et al. 2002) and males have different sex determination systems (X1X2Y1Y2 quadrivalent in A. caraya and X1X2 X3 Y1Y2 pentavalent in A. guariba clamitans; de Oliveira et al. 2002); therefore, the production of one viable F1 male hybrid in captivity (Jesus et al. 2010) is at least unexpected. Comparative genetic studies in the hybrid zones will provide an outstanding opportunity to explore whether molecular and/or cytogenetic mechanisms (or both) are responsible for the observed levels of reproductive isolation and the maintenance of species integrity despite hybridization.
6 Future Directions in the Study of Hybridization of Howler Monkeys
It has been recently recognized that hybridization is a powerful force that has shaped the evolutionary trajectory of a wide range of animal taxa (Dowling and Secor 1997; Arnold 1997; Grant et al. 2004; Mallet 2005). When hybridization occurs, genetic material of one lineage may enter the genetic pool of another, introducing genetic novelty to the latter (a process known as genetic introgression) (Rheindt and Edwards 2011). If this introduction of genetic novelty is advantageous to the recipient individuals, it may influence the evolutionary trajectory of the hybrid population or of one or both of the parental lineages (e.g., Grant and Grant 2010). Therefore, instances of hybridization may contribute to the adaptive radiation and diversification of species.
Several historical, demographic, behavioral, and ecological processes are involved in the origin and maintenance of hybrid zones, and a number of different mechanisms may operate together to maintain the hybridization process. Most of our future research is directed towards understanding the mechanisms that influence the hybridization process in howler monkeys, as well as the effect of hybridization in the ecology and behavior of the interacting taxa.
6.1 Endogenous and Exogenous Selective Forces in Hybridization
In general, hybridization may influence evolution in a variety of ways, and it mostly depends on endogenous and exogenous selective forces operating on each hybrid system (Barton 2001). When there is an intrinsic loss of fitness in hybrids, due, for example, to genetic incompatibilities between the two parental genomes (endogenous selection), it is likely that the hybrid zone will constitute a barrier preventing gene flow between the parental taxa. On the other hand, it has been argued that hybrid zones may be maintained by adaptation to different environments (exogenous selection), in which hybrid individuals may be more adapted to fluctuating or intermediate environments (e.g., Cruzan and Arnold 1993). In this case, individuals within the hybrid zone may exhibit a greater variance in fitness. Hybrids with higher fitness will contribute to adaptation either by introgression of alleles to parental taxa or by the establishment of recombinant genotypes (Barton 2001). However, these two selective forces (endogenous and exogenous) are not mutually exclusive and can operate together in the same system: whereas hybrid zones can be maintained by the selection against hybrids and represent barriers to gene flow, the divergence between interacting populations may be generated by adaptation to fluctuating environments (Barton 2001).
Studies have only recently been directed to understanding the effects of hybridization and gene introgression in the evolutionary history of primates (e. g., Arnold and Meyer 2006; Arnold 2009; Ackermann 2010; Green et al. 2010; Zinner et al. 2011). In addition, only a few examples have actually provided some insight into the patterns of hybridization among primate taxa using genetic data (Cortés-Ortiz et al. 2007; Tung et al. 2008; Zinner et al. 2009; Merker et al. 2009; Ackermann and Bishop 2010).
In the case of the hybridization of howler monkeys in Mexico, there is some support for the operation of endogenous selection (e.g., Haldane’s Rule effect), and there are no current environmental differences between the habitats of A. palliata and A. pigra throughout their distribution range that suggest strong influence of exogenous selection in this hybrid system. The responsible mechanisms for the partial reproductive isolation between the two species remain unknown, but genetic analyses suggest that some of these mechanisms could be attributed to chromosomal differences or to incompatibilities between nuclear and mitochondrial genomes (see Sect. 5.5). Cytogenetic and molecular studies comparing chromosomal and genomic regions associated with hybrid incompatibility should be a next step in our attempts to understand the endogenous mechanisms driving distinct levels of reproductive isolation in howler monkey hybrid zones.
On the other hand, exogenous selection may be strongly influencing the A. caraya × A. guariba hybrid zones. The currently known hybrid zones between these species in Brazil are located within regions of contact between two biomes (the Atlantic Forest and the Pampas, Bicca-Marques et al. 2008; and the Atlantic Forest, the Pantanal, and the Cerrado, Aguiar et al. 2007, 2008), with forests that are typically inhabited by each species (the Atlantic Forest by A. guariba and the Pantanal, the Pampas and the Cerrado by A. caraya). In Argentina, the hybrid zone lies within the Atlantic Forest ecoregion, for which A. guariba is endemic, but it is not a typical habitat for A. caraya. However, both species have very similar trophic niches (Bicca-Marques et al. 2008; Agostini et al. 2010) and are quite tolerant to habitat disturbance (Zunino et al. 2007; Bicca-Marques et al. 2008), which has been recently occurring in this area (Agostini et al. 2008). Therefore, the presence of both species in the area is likely the result of relatively recent secondary contact, with A. caraya individuals spreading into areas typically inhabited by A. guariba, as a consequence of forest disturbance. These incursions may occur infrequently generating an asymmetrical proportion of individuals of both species. The demography (i.e., abundance, sex ratios, rates of dispersal, etc.) and behavior of hybridizing taxa can affect levels and patterns of gene introgression in hybrid zones (Barton and Hewitt 1989; Wirtz 1999; Rohwer et al. 2001; Field et al. 2011; Gompert et al. 2012), generating different outcomes in the distribution of genetic backgrounds among hybrid zones with different ecological conditions. The availability of multiple contact zones between A. caraya and A. guariba with important ecological differences among them, as well as differences in the demographic composition of the two hybridizing species, offers a rare opportunity for testing the role that these factors may play on the occurrence and maintenance of the hybrid zones and the patterns of gene introgression. Comparative ecological studies within and outside these three hybrid zones between A. caraya and A. guariba would provide the grounds to understand the effect of exogenous selection in the fitness of hybrid individuals with distinct genetic architectures, and the differential effects of exogenous versus endogenous selection in the hybridization of howler monkeys.
6.2 Habitat Fragmentation and Its Effect on the Hybridization of Howler Monkeys
All howler monkey hybrid zones currently known are located in or surrounded by highly fragmented environments. It has been suggested that human-induced activities may play an important role in promoting hybridization in primates (Detwiler et al. 2005). Based on paleoecological data from the São Francisco de Assis region, Bicca-Marques et al. (2008) suggested that the contact between A. caraya and A. guariba is a recent consequence of the expansion of the two forests biomes in the past 2,000 years. Similarly, the current contact zone of A. palliata and A. pigra in Mexico seems to be the result of a secondary contact due to a two-wave colonization process (Cortés-Ortiz et al. 2003; Ford 2006) with a recent northward expansion of A. palliata (Cortés-Ortiz 2003). Therefore, it is likely that the origins of these howler monkey hybrid zones are due to paleoecological processes and not to habitat fragmentation. However, howler monkeys are strictly arboreal primates that only descend to the ground to cross canopy gaps or to disperse between fragments (Bicca-Marques and Calegaro-Marques 1995; Pozo-Montuy and Serio-Silva 2007), a task strongly compromised when inter-patch distances are longer than 200 m (Mandujano and Estrada 2005). Therefore, it is possible that habitat disturbance and fragmentation may influence the hybridization process in howler monkeys either by isolating their populations and reducing contact between hybridizing species, or by confining individuals of different species within particular fragments and promoting interbreeding. Dias et al. (2013) analyzed habitat configuration in fragmented landscapes both within the hybrid zone in Tabasco Mexico and in nearby areas where only purebred individuals occur. They concluded that hybridization between Mexican howler monkeys is facilitated in fragmented landscapes where there is a larger number of small, though less isolated, fragments. Testing hypothesis regarding the actual role of fragmentation in promoting or preventing hybridization requires the study of syntopic populations in both fragmented and extensive forest. The A. caraya × A. guariba hybrid zones portrayed here may provide a unique opportunity within primates, with cases of natural hybridization occurring in both highly fragmented areas of Brazil and the mostly pristine Atlantic Forest of Argentina.
6.3 Effect of Hybridization in the Vocal Communication of Hybridizing Species
One characteristic feature of howler monkeys is their conspicuous, loud vocalizations (Whitehead 1995). Although nonhuman primate vocalizations have long been considered genetically determined, some studies have questioned this assumption based on the existent variation among individuals and populations within taxa (Sun et al. 2011). This question can be addressed by analyzing vocalizations from purebred and hybrid individuals with different levels of admixture in the hybrid zone. During a study on social behavior in one of the Brazilian hybrid zones, Aguiar (2010) detected that loud vocalizations tended to occur more frequently between conspecific males than during heterospecific interactions (including interactions with hybrids). A similar observation has been reported in Argentina (Holzmann et al. 2012) for syntopic A. caraya and A. guariba. These observations may support the argument of a genetic basis of vocalizations. However, Aguiar (2010) also observed that one hybrid female modified her vocalizations according to the species that she was interacting with. This plasticity could be either ecologically or genetically determined. An ongoing study of vocalizations integrating genetic, behavioral, and morphological data (Kitchen et al. unpubl. data; see also Kitchen et al. 2015) in the Mexican hybrid zone is starting to provide insights into the influence of genetics on the vocalizations of howler monkeys.
6.4 Interaction Between Social Dynamics and Hybridization
Hybrid zones have been considered natural laboratories for the study of the characters and processes leading to divergence and speciation (Hewitt 1988), which include behavioral strategies to acquire mates by the two parental populations and their hybrid offspring. However, despite a continuously growing number of studies dedicated to understanding the social and reproductive dynamics in primates, very little work has been focused on reproductive strategies of individuals within primate hybrid zones (e.g., Bergman and Beehner 2004; Bergman et al. 2008). Hybrid zones provide the opportunity to explore reproductive strategies of individuals with very different genetic backgrounds (both pure and admixed) in the same ecological and social context (Bergman et al. 2008). Ongoing studies on the social dynamics in the Mexican hybrid zone (e.g., Ho et al. 2014) will allow us to evaluate the competitive abilities of hybrid versus purebred individuals. In Brazil, Aguiar (2010) conducted a study on social interactions in two mixed groups composed of pure A. caraya, A. guariba and putative hybrids. Although the two groups were very different in composition, his analyses suggested that heterospecific associations confer some competitive advantages when facing other groups. He also found that affiliative and sexual interactions mostly included putative hybrids and were less frequent between apparently pure heterospecific individuals. Furthermore, he found that one hybrid female had a higher rank in the group than the putatively purebred A. caraya female. Although the sample size in his study is very small, these observations suggest the presence of assortative mating and a possible reproductive advantage in hybrids (Aguiar 2010). Behavioral studies comparing social interactions and dynamics of a larger number of groups with different compositions (A. caraya and A. guariba, as well as mixed and hybrid groups) within the area of contact between these species may allow the understanding of the interaction between hybridization and social dynamics.
Furthermore, the integrated genetic and behavioral study of primate populations in different hybrid zones can provide important information on the genetic composition of reproductively successful individuals and inform the relative effects of genetics and social dynamics on the overall fitness of hybrid versus purebred individuals. The study of social dynamics in the howler monkey hybrid zones would be especially insightful given the relatively good knowledge of different aspects of the social systems of the hybridizing species, due to a large and growing number of basic studies on social and sexual behavior of these taxa (see Van Belle and Bicca-Marques 2015). These studies can serve as a basis to conduct comparative observations between purebred and hybrid individuals in the same ecological and social context. There are important differences in social structure and mating systems between the hybridizing taxa. For example, while A. pigra has an average group size of ~6.3 individuals (range 2–16) with an adult sex ratio between 0.7 and 1.3 females per male, A. palliata has an average group size of ~15 individuals (range 2–45) with an adult sex ratio between 1.2 and 4.2 females per male (Di Fiore et al. 2010). In both species there is bisexual dispersal, but it is reported that A. pigra females commonly stay in natal groups (Van Belle et al. 2011) whereas most A. palliata females disperse (Glander 1992). Immigration of A. pigra females in well-established groups is rarely observed (Brockett et al. 2000), and females aggressively chase away extragroup females (Brockett et al. 2000; Van Belle et al. 2011). In contrast, A. palliata females regularly join established groups, first as low-ranking individuals, and gradually become dominant (Glander 1992). In A. pigra alpha or “central” males have almost exclusive access to fertile females, whereas “noncentral” males have few or no mating opportunities (Van Belle et al. 2008), but in A. palliata mating opportunities among group males are more evenly distributed (Jones and Cortés-Ortiz 1998; Ellsworth 2000; Milton et al. 2009).
These and other differences in social systems between the two parental species likely affect the genetic structure of individuals within the hybrid zone and will enable evaluations of the success of reproductive strategies of pure versus admixed individuals. These studies would require systematic long-term data collection on behavior, demography, and genetics for a large number of groups with distinct compositions within and outside the hybrid zone, using concordant methodologies. Despite the inherent difficulties of maintaining long-term studies given the costs and demands of field work (Strier 2010), the maintenance of long-term research in primate hybrid zones and the comparative studies across primate hybrid systems is critical to develop a holistic understanding of the evolutionary consequences of hybridization in primates.
6.5 Studies of Hybridization in the Genomic Era
The advent of the newer technologies to sequence entire genomes opens an exciting possibility in the genetic study of primate hybrid zones. Currently, there is a number of primate genome sequencing projects underway and within the next several years it is likely that genome sequence data will become available for most, if not all, primate genera (Bradley and Lawler 2011). The use of a larger number of genetic markers across the genome that characterize parental taxa will dramatically increase the power and accuracy of detecting admixed individuals. Also, polymorphism of these markers in conjunction with behavioral observations will allow us to establish kin relationships in hybrid populations to evaluate aspects such as individual reproductive success, and the possible effect of kinship in structuring social relationships within a hybrid zone. Furthermore, the understanding of patterns of introgression of different regions of the genome of each of the parental species will potentially enable the identification of genes that contribute to various levels of reproductive isolation, such as those observed in the A. palliata × A. pigra hybrid system, and the maintenance of species boundaries despite gene flow.
References
Ackermann RR (2010) Phenotypic traits of primate hybrids: recognizing admixture in the fossil record. Evol Anthropol 19:258–270
Ackermann RR, Bishop JM (2010) Morphological and molecular evidence reveals recent hybridization between gorilla taxa. Evolution 64:271–290
Ackermann RR, Rogers J, Cheverud JM (2006) Identifying the morphological signatures of hybridization in primate and human evolution. J Hum Evol 51:632–645
Agostini I, Holzmann I, Di Bitetti MS (2008) Infant hybrids in a newly formed mixed-species group of howler monkeys (Alouatta guariba clamitans and Alouatta caraya) in northeastern Argentina. Primates 49:304–307
Agostini I, Holzmann I, Di Bitetti MS (2010) Are howler monkeys ecologically equivalent? Trophic niche overlap in syntopic Alouatta guariba clamitans and Alouatta caraya. Am J Primatol 72:173–186
Aguiar LM (2010) Sistema social de grupos mistos de espécies de bugios (Alouatta caraya e A. clamitans) e potenciais híbridos no Alto Rio Paraná, sul do Brasil. Ph.D. thesis. Universidade Federal do Paraná, Curitiba
Aguiar LM, Mellek DM, Abreu KC, Boscarato TG, Bernardi IP, Miranda JMD, Passos FC (2007) Sympatry of Alouatta caraya and Alouatta clamitans and the rediscovery of free-ranging potential hybrids in Southern Brazil. Primates 48:245–248
Aguiar LM, Pie MR, Passos FC (2008) Wild mixed groups of howler species (Alouatta caraya and Alouatta clamitans) and new evidence for their hybridization. Primates 49:149–152
Aguiar LM, Tonetto J, Bicca-Marques JC (2014) Novas zonas de contato entre Alouatta caraya e A. guariba clamitans no sul do Brasil. In: Miranda JMD, Passos FC (eds) A primatologia no Brasil, vol. 13. Sociedade Brasileira de Primatologia, Curitiba
Arnold ML (1997) Natural hybridization and evolution. Oxford University Press, Oxford
Arnold ML (2009) Reticulate evolution and humans origins and ecology. Oxford University Press, Oxford
Arnold M, Meyer A (2006) Natural hybridization in primates: one evolutionary mechanism. Zoology 109:261–276
Barton NH (2001) The role of hybridisation in evolution. Mol Ecol 10:551–568
Barton NH, Hewitt GM (1989) Adaptation, speciation and hybrid zones. Nature 341:497–503
Bergman TJ, Beehner JC (2004) The social system of a hybrid baboon group (Papio hamadryas anubis × P. h. hamadryas). Int J Primatol 25:1313–1330
Bergman TJ, Phillips-Conroy JE, Jolly CJ (2008) Behavioral variation and reproductive success of male baboons (Papio anubis × Papio hamadryas) in a hybrid social group. Am J Primatol 70:136–147
Bernstein IS (1966) Naturally occurring primates hybrid. Science 154:1559–1560
Bicca-Marques JC, Calegaro-Marques C (1995) Locomotion of black howlers in a habitat with discontinuous canopy. Folia Primatol 64:55–61
Bicca-Marques JC, Prates HM, Aguiar FRC, Jones CB (2008) Survey of Alouatta caraya, the black-and-gold howler monkey, and Alouatta guariba clamitans, the brown howler monkey, in a contact zone, State of Rio Grande do Sul, Brazil: evidence for hybridization. Primates 49:246–252
Bradley BJ, Lawler RR (2011) Linking genotypes, phenotypes and fitness in wild primate populations. Evol Anthropol 20:104–119
Brockett RC, Horwich RH, Jones CB (2000) Female dispersal in the Belizean black howling monkey Alouatta pigra. Neotrop Primates 8:32–34
Büntge ABS, Pyritz LW (2007) Sympatric occurrence of Alouatta caraya and Alouatta sara at the Río Yacuma in the Beni Department, Northern Bolivia. Neotrop Primates 14:82–83
Bynum EL (2002) Morphological variation within a macaque hybrid zone. Am J Phys Anthropol 118:45–49
Cheverud J, Jacobs S, Moore A (1993) Genetic differences among subspecies of the saddle-back tamarin (Saguinus fuscicollis): evidence from hybrids. Am J Primatol 31:23–39
Chiarelli B (1973) Check-list of catarrhina primate hybrids. J Hum Evol 2:301–305
Coimbra-Filho AF, Silva RR, Pissinatti A (1984) Heterose em fêmea híbrida de Callithrix (Callitrichidae—Primates). In: Mello MT (ed) A primatologia no Brasil 1. Sociedade Brasileira de Primatologia, Belo Horizonte
Cortés-Ortiz L (2003) Evolution of howler monkeys, Genus Alouatta. Ph.D. dissertation. University of East Anglia, Norwich, Norfolk
Cortés-Ortiz L, Bermingham E, Rico C, Rodríguez-Luna E, Sampaio I, Ruiz-García M (2003) Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol Phylogenet Evol 26:64–81
Cortés-Ortiz L, Duda TJ Jr, Canales- Espinosa D, García-Orduna F, Rodríguez-Luna E, Bermingham E (2007) Hybridization in large-bodied New World primates. Genetics 176:2421–2425
Cortés-Ortiz L, Rylands AB, Mittermeier R (2014) The taxonomy of howler monkeys: integrating old and new knowledge from morphological and genetic studies. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
Cruz Lima E (1945) Mammals of Amazônia. I. General Introduction and Primates. Contrib. Mus. Paraense Emílio Goeldi Hist. Nat., Ethnogr., Belém do Pará, Rio de Janeiro
Cruzan MB, Arnold ML (1993) Ecological and genetic associations in an Iris hybrid zone. Evolution 47:1432–1445
da Cunha RGT, Oliveira DAG, Holzmann I, Kitchen DM (2015) Production of loud and quiet calls in howler monkeys. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
da Silva BTF, Sampaio MIC, Schneider H, Schneider MPC, Montoya E, Encarnacion F, Salzano FM (1992) Natural hybridization between Saimiri taxa in the Peruvian Amazonia. Primates 33:107–113
Jesus AS, Schunemann HE, Müller J, da Silva MA, Bicca-Marques JC (2010) Hybridization between Alouatta caraya and Alouatta guariba clamitans in captivity. Primates 51:227–230
de Oliveira EHC, Neusser M, Figueiredo WB, Nagamachi CY, Pieczarka JC, Sbalqueiro IJ, Wienberg J, Müller S (2002) The phylogeny of the howler monkeys, (Alouatta, Platyrrhini): reconstruction by multicolor or cross species chromosome painting. Chromosome Res 10:669–683
Defler TR (1994) La conservación de primates en Colombia. Trianea (Act Cien INDERENA) 5:255–287
Detwiler KM (2002) Hybridization between red-tailed monkeys (Cercopithecus ascanius) and blue monkeys (C. mitis) in East African forests. In: Glenn ME, Cords M (eds) The guenons: diversity and adaptation in African monkeys. Plenum, New York
Detwiler KM, Burrell AS, Jolly CJ (2005) Conservation implications of hybridization in African cercopithecine monkeys. Int J Primatol 26:661–684
Di Bitetti MS (2005) Perspectivas para a conservacao de primatas em Misiones. In: Galindo-Leal C, Camara IG (eds) Mata Atlantica: biodiversidade, ameacas e perspectivas. Fundacao SOS Mata Atlantica, Sao Paulo, Conservacao Internacional, Belo Horizonte
Di Fiore A, Link A, Campbell CJ (2010) The Atelines: behavior and socioecological diversity in a New World monkey radiation. In: Campbell CJ, Fuentes AF, MacKinnon KC, Stumpf R, Bearder S (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford
Dias PAD, Alvarado-Serrano D, Rangel-Negrín A, Canales-Espinosa D, Cortés-Ortiz L (2013) Landscape attributes affecting the natural hybridization of Mexican howler monkeys. In: Marsh L, Chapman C (eds) Primates in Fragments II. Springer, New York.
Dowling TE, Secor CL (1997) The role of hybridization in the evolutionary diversification of animals. Annu Rev Ecol Syst 28:593–619
Dunbar RIM, Dunbar P (1974) On hybridization between Theropithecus gelada and Papio anubis in the wild. J Hum Evol 3:187–192
Ellsworth JA (2000) Molecular evolution, social structure and phylogeography of the mantled howler monkey (Alouatta palliata). Ph.D. dissertation, University of Nevada. Reno, Nevada
Field DL, Ayre DJ, Whelan RJ, Young AG (2011) The importance of pre-mating barriers and the local demographic context for contemporary mating patterns in hybrid zones of Eucalyptus aggregata and Eucalyptus rubida. Mol Ecol 20:2367–2379
Ford SM (2006) The biogeographic history of Mesoamerican primates. In: Estrada A, Garber PA, Pavelka MSM, Luecke L (eds) New perspectives in the study of Mesoamerican primates: distribution, ecology, behavior and conservation. Developments in primatology: progress and prospects. Tuttle RA (series ed). Kluwer/Springer, New York
Gaubert P, Taylor PJ, Fernandes CA, Bruford MW, Veron G (2005) Patterns of cryptic hybridization revealed using an integrative approach: a case study on genets (Carnivora, Viverridae, Genetta spp.) from the southern African subregion. Biol J Linn Soc 86:11–33
Glander KE (1992) Dispersal patterns in Costa Rican mantled howling monkeys. Int J Primatol 13:415–436
Gompert Z, Parchman TL, Buerkle CA (2012) Genomics of isolation in hybrids. Phil Trans R Soc B 367:439–450
Grant PR, Grant BR (2010) Conspecific versus heterospecific gene exchange between populations of Darwin’s finches. Philos Trans R Soc B Biol Sci 365:1065–1076
Grant PR, Grant BR, Markert JA, Keller LF, Petren K (2004) Convergent evolution of Darwin’s finches caused by introgressive hybridization and selection. Evolution 58:1588–1599
Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MHY, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Z, Gusic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, De La Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PLF, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Pääbo S (2010) A draft sequence of the Neandertal genome. Science 328:710–722
Gregorin R (2006) Taxonomia e variacao geografica das especies do genero Alouatta Lacepede (Primates, Atelidae) no Brasil. Rev Bras Zool 23:64–144
Groves CP (2001) The taxonomy of primates. Smithsonian Institution Press, Washington, DC
Haldane JBS (1922) Sex ratio and unisexual sterility in hybrid animals. J Genet 12:101–109
Hernández-Camacho J, Cooper R (1976) The nonhuman primates of Colombia. In: Thorington R Jr, Heltne P (eds) Neotropical primates. National Academy of Sciences, Washington, DC
Hewitt G (1988) Hybrid zones—natural laboratories for evolution studies. Trends Ecol Evol 3:158–166
Hill WCO (1962) Primates comparative anatomy and taxonomy IV—Cebidae, Part B. Edinburgh University Pubs Science & Maths, Edinburgh
Ho L, Cortés-Ortiz L, Dias PA, Canales-Espinosa D, Kitchen DM, Bergman TJ (2014) Effect of ancestry on behavioral variation in two species of howler monkeys (Alouatta pigra and A. palliata) and their hybrids. Am J Primatol 76:855–867
Holzmann I (2012) Distribución geográfica potencial y comportamiento vocal de dos especies de mono aullador (Alouatta guariba clamitans y Alouatta caraya). Ph.D. dissertation. Universidad Nacional de La Plata, Argentina
Holzmann I, Agostini I, Di Bitetti M (2012) Roaring behavior of two syntopic howler species (Alouatta caraya and A. guariba clamitans): evidence supports the mate defense hypothesis. Int J Primatol 33:338–355
Horwich RH, Johnson EW (1986) Geographic distribution of the black howler monkey (Alouatta pigra) in Central America. Primates 27:53–62
Isabelle A (1983) Viagem ao Rio Grande do Sul, 1833-1834. Tradução e notas de Dante de Laytano. Martins Livreiro, Porto Alegre
Iwanaga S, Ferrari SF (2002) Geographic distribution of red howlers (Alouatta seniculus) in Southwestern Brazilian Amazonia, with notes on Alouatta caraya. Int J Primatol 23:1245–1256
Jasinska AK, Wachowiak W, Muchewicz E, Boratyn´ Ska K, Montserrat JM, Boratyn´ Ski A (2010) Cryptic hybrids between Pinus uncinata and P. sylvestris. Bot J Linn Soc 163:473–485
Jolly CJ (2001) A proper study for mankind: analogies from the Papionin monkeys and their implications for human evolution. Yearb Phys Anthropol 44:177–204
Jolly CJ, Woolley-Barker T, Beyene S, Disotell TR, Phillips-Conroy JE (1997) Intergeneric hybrid baboons. Int J Primatol 18:597–627
Jones CB, Cortés-Ortiz L (1998) Facultative polyandry in the howling monkey (Alouatta palliata): Carpenter was correct. Bol Primatol Lat 7:1–7
Kelaita M, Cortés-Ortiz L (2013) Morphology of genetically-confirmed Alouatta pigra × A. palliata hybrids from a natural hybrid zone in Tabasco, Mexico. Am J Phys Anthropol 150:223–234
Kelaita MA, Dias PAD, Aguilar-Cucurachi MS, Canales-Espinosa D, Cortés-Ortiz L (2011) Impact of intrasexual selection on sexual dimorphism and testes size in the Mexican howler monkeys Alouatta palliata and A. pigra. Am J Phys Anthropol 146:179–187
Kinzey WG (1982) Distribution of primates and forest refuges. In: Prance GT (ed) Biological diversification in the tropics. Columbia University Press, New York
Kitchen DM, da Cunha RGT, Holzmann I, Oliveira DAG (2015) Function of loud calls in howler monkeys. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
Kohn L, Langton L, Cheverud J (2001) Subspecific genetic differences in the saddle-back tamarin (Saguinus fuscicollis): postcranial skeleton. Am J Primatol 54:41–56
Lorini ML, Persson VG (1990) A contribuicao de Andre Mayer a historia natural no Parana (Brasil) II. Mamıferos do terceiro planalto paranaense. Arq Bras Biol Tecnol 33:117–132
Mallet J (2005) Hybridization as an invasion of the genome. Trends Ecol Evol 20:229–237
Mandujano S, Estrada A (2005) Detección de umbrales de área y distancia de aislamiento para la ocupación de fragmentos de selva por monos aulladores, Alouatta palliata, en Los Tuxtlas, Mexico. Universidad y Ciencia Número Especial II 11–21
Merker S, Driller C, Perwitasari-Farajallah D, Pamungkas J, Zischler H (2009) Elucidating geological and biological processes underlying the diversification of Sulawesi tarsiers. Proc Natl Acad Sci U S A 106:8459–8464
Milton K, Lozier JD, Lacey EA (2009) Genetic structure of an isolated population of mantled howler monkeys (Alouatta palliata) on Barro Colorado Island, Panama. Conserv Genet 10:347–358
Mudry MD, Nieves M, Steinberg ER (2015) Cytogenetics of howler monkeys. In: Kowalewski M, Garber PA, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
Nagel U (1973) A comparison of anubis baboons, hamadryas baboons and their hybrids at a species border in Ethiopia. Folia Primatol 19:104–165
Napier PH (1976) Catalogue of primates in the British Museum (Natural History), part 1: families Callitrichidae and Cebidae. British Museum (Natural History), London
Neaves LE, Zenger KR, Cooper DW, Eldridge MDB (2010) Molecular detection of hybridization between sympatric kangaroo species in south-eastern Australia. Heredity 104:502–512
Peres CA, Patton JL, Da Silva MNF (1996) Riverine barriers and gene flow in Amazonian saddle-back tamarin monkeys. Folia Primatol 67:113–124
Pinto LP, Setz EZF (2000) Sympatry and new locality for Alouatta belzebul discolor and Alouatta seniculus in the Southern Amazon. Neotrop Primates 8:150–151
Pozo-Montuy G, Serio-Silva JC (2007) Movement and resource use by a group of Alouatta pigra in a forest fragment in Balancán, México. Primates 48:102–107
Rheindt FE, Edwards SV (2011) Genetic introgression: an integral but neglected component of speciation in birds. Auk 128:620–632
Rohwer S, Bermingham E, Wood C (2001) Plumage and mitochondrial DNA haplotype variation across a moving hybrid zone. Evolution 55:405–422
Samuels A, Altmann J (1986) Immigration of a Papio anubis male into a group of cynocephalus baboons and evidence for anubis-cynocephalus hybrid zone in Amboseli, Kenya. Int J Primatol 7:131–138
Schillaci MA, Froehlich JW, Supriatna J, Jones-Engel L (2005) The effects of hybridization on growth allometry and craniofacial form in Sulawesi macaques. J Hum Evol 49:335–369
Silva FE (2010) Extensão da zona de contato e potencial hibridação entre Alouatta caraya e Alouatta guariba clamitans na região de São Francisco de Assis, RS. M.Sc. dissertation. Porto Alegre, Brazil
Smith JD (1970) The systematic status of the black howler monkey, Alouatta pigra Lawrence. J Mammal 51:358–369
Steinberg ER, Cortés-Ortiz L, Nieves M, Bolzán AD, García- Orduña F, Hermida-Lagunes J, Canales-Espinosa D, Mudry MD (2008) The karyotype of Alouatta pigra (primates: platyrrhini): mitotic and meiotic analyses. Cytogenet Genome Res 122:103–109
Strier KB (2010) Long-term field studies: positive impacts and unintended consequences. Am J Primatol 72:772–778
Struhsaker TT (1970) Phylogenetic implications of some vocalizations of Cercopithecus monkeys. In: Napier JR, Napier PH (eds) Old World monkeys: evolution, systematics, and behavior. Academic, London
Sun GZ, Huang B, Guan ZH, Geissmann T, Jiang XL (2011) Individuality in male songs of wild black crested gibbons (Nomascus concolor). Am J Primatol 73:431–438
Tenaza R (1984) Songs of hybrid gibbons (Hylobates lar × H. muelleri). Am J Primatol 8:249–253
Tung J, Charpentier M, Garfield D, Altmann J, Alberts S (2008) Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Mol Ecol 17:1998–2011
Van Belle S, Bicca-Marques JC (2015) Insights into reproductive strategies and sexual selection in howler monkeys. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: behavior, ecology and conservation. Springer, New York
Van Belle S, Estrada A, Strier KB (2008) Social relationships among male Alouatta pigra. Int J Primatol 29:1481–1498
Van Belle S, Estrada A, Strier KB (2011) Insights into social relationships among female black howler monkeys Alouatta pigra at Palenque National Park, Mexico. Current Zool 57:1–7
Wallace RB, Painter RLE, Rumiz DI, Taber AB (2000) Primate diversity, distribution and relative abundances in the Rios Blanco y Negro Wildlife Reserve, Santa Cruz Department, Bolivia. Neotrop Primates 8:24–28
Whitehead JM (1995) Vox Alouattinae—a preliminary survey of the acoustic characteristics of long distance calls of howling monkeys. Int J Primatol 16:121–144
Wirtz P (1999) Mother species—father species: unidirectional hybridization in animals with female choice. Anim Behav 58:1–12
Wyner YM, Johnson SE, Stumpf RM, DeSalle R (2002) Genetic assessment of a white-collared x red-fronted lemur hybrid zone at Andringitra. Madagascar. Am J Primatol 67:51–66
Zinner D, Groeneveld L, Keller C, Roos C (2009) Mitochondrial phylogeography of baboons (Papio spp.): indication for introgressive hybridization? BMC Evol Biol 9:83
Zinner D, Arnold ML, Roos C (2011) The strange blood: natural hybridization in primates. Evol Anthropol 20:96–103
Zunino G, Kowaleski M, Oklander L, Gonzalez V (2007) Habitat fragmentation and population trends of the black and gold howler monkey (Alouatta caraya) in a semideciduous forest in northern Argentina. Am J Primatol 69:966–975
Acknowledgments
This work has been supported by NSF grant # BCS-0962807 to LCO. Genetic, behavioral, and morphologic work presented in this study for the Mexican hybrid zone was supported by the University of Michigan (OVPR #U014374), Universidad Veracruzana, PROMEP UVER 98-11-019 and 103.5/03/1154EXB-9, and NSF grants DEB-0640519 and BCS-0962807 to LCO. Research in Argentina was supported by a Conservation Grant of the International Primatological Society to IA and from Idea Wild. Behavioral and morphological studies in Paraná Brazil by LMA were supported by CNPq and Universidade Federal do Paraná (UFPR). Research in the State of Rio Grande do Sul, Brazil, was supported by a CAPES fellowship to FES and by CNPq research grants (#306090/2006-6 and 303154/2009-8) to JCBM.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cortés-Ortiz, L., Agostini, I., Aguiar, L.M., Kelaita, M., Silva, F.E., Bicca-Marques, J.C. (2015). Hybridization in Howler Monkeys: Current Understanding and Future Directions. In: Kowalewski, M., Garber, P., Cortés-Ortiz, L., Urbani, B., Youlatos, D. (eds) Howler Monkeys. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1957-4_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1957-4_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1956-7
Online ISBN: 978-1-4939-1957-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)