Abstract
In species in which males and females exhibit different association patterns, the use of vocalizations that regulate interindividual distance may differ between the sexes. Spider monkey social groups are characterized by high fission–fusion dynamics and sex differences in association patterns; female–female associations have been described as more passive than those between philopatric males. Individuals of both sexes produce whinny vocalizations, which may allow callers and receivers to mediate interindividual spacing based on existing social relationships. As such, we hypothesized individuals of each sex would use whinny vocalizations at different rates and in different contexts. To investigate sex differences in the rate of whinnying across behavioral contexts, we collected focal animal samples on Yucatan spider monkeys (Ateles geoffroyi yucatanensis) over 8 mo at Runaway Creek Nature Reserve, Belize. In addition, we recorded all changes in subgroup composition to investigate whether a female’s likelihood of calling was influenced by the number of conspecifics joining, or leaving their subgroup. We found that females called at higher rates than males in most behavioral contexts, especially foraging. The probability that females would call increased during subgroup fissions and fusions, and correlated positively with the number of individuals joining or leaving their subgroup. Male calling rates did not differ across contexts, and males generally called less than females. Our results suggest that whinnying by females may allow callers to mediate interindividual spacing in contexts where proximity risks increasing feeding competition. In species in which the sexes associate in qualitatively different ways, vocalizations may play a role in maintaining these differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In species that exhibit high levels of sociality, mechanisms for the maintenance of social ties likely have important fitness consequences. Vocalizations may play a key role in this respect, as they travel quickly over long distances and are less affected by physical barriers than are other signaling modes (Kondo and Watanabe 2009). For species in which the frequency and quality of social interactions differ between the sexes, it is possible that the use of vocalizations by males and females may differ as well.
Sex differences in vocalizations can occur, for example, as the exclusive use of call types by males or females. This is exemplified in African elephants (Loxodonta africana), in which 19 of the 26 vocalizations in the species’ vocal repertoire are used exclusively by females (Poole 1994). Many of these female-specific vocalizations are used in the context of group coordination, including calls emitted by separated individuals, i.e., lost calls, calls associated with social greeting, and calls used to maintain group cohesion (Poole 1994). The exclusive use of these calls by females is likely to be related to the fact that females live in multitiered societies that consist of closely related kin units, bond groups, and clans. In contrast, males, which spend much of their time alone or in small groups with other males, produce only four sex-specific vocalizations, two of which are used as displays of dominance between individuals (Poole 1994).
Alternatively, sex differences in vocalization patterns may be more subtle, where males and females use the same calls, but at different rates or in differing contexts. For example, among New World ateline monkeys, male muriquis (Brachyteles hypoxanthus) emit “neigh” vocalizations at higher rates than females (Arnedo et al. 2010). This vocalization may facilitate group cohesion (Nishimura et al. 1988), and its elevated use by males is consistent with the higher rates of association exibited between the related males of a group (Arnedo et al. 2010). Conversely, female muriquis emit “staccato” vocalizations more frequently than males, and they appear to do so to increase interindividual distances (Arnedo et al. 2010). Capuchins (Cebus capucinus) may also mediate interindividual spacing through the use of “huh” vocalizations, which are used by females at elevated rates relative to males in foraging contexts (Gros-Louis 2004). Researchers have suggested these calls serve a spacing function, whereby callers influence the position of other individuals relative to themselves through advertising their own position. It has been suggested that both “staccato” and “huh” vocalizations serve to decrease feeding competition, as they are used at relatively high rates while feeding and are emitted more frequently by females, which experience higher energetic demands in comparison to males (Arnedo et al. 2010; Gros-Louis 2004).
Sex differences in association patterns may be reflected in the calling behavior of males and females, particularly with regard to vocalizations that are used to regulate interindividual distances via the attraction or repulsion of conspecifics (Arnedo et al. 2010; Boinski and Campbell 1996; Kondo and Watanabe 2009). One class of vocalization that merits investigation in this regard is the “contact” call. The term “contact call” has been applied to a broad spectrum of vocalizations that are used between visually separated individuals or parties (Kondo and Watanabe 2009). Responses to contact calls may also vary according to the individual identities of callers and receivers, or the behavioral contexts in which they are produced. As such, contact calls have been somewhat difficult to define operationally (Cheney and Seyfarth 1996; Kondo and Watanabe 2009; Rendall and Owren 2002). Nonetheless, sex differences in the use of contact calls have been observed in primate species characterized by male philopatry and high fission–fusion dynamics (Aureli et al. 2008). For example, male chimpanzees (Pan troglodytes) emit pant hoot vocalizations more often than females, often exchanging them between close associates in dispersed subgroups (Marler and Tenaza 1977; Mitani and Nishida 1993; Notman and Rendall 2005). As with the muriqui “neigh” vocalization, the pant hoot is thought to function as a contact call that facilitates group cohesion (Mitani and Nishida 1993). Although females are physiologically capable of producing pant hoots, they do so rarely as they are less motivated by the maintenance of social relationships with other individuals, and may even be socially inhibited from pant hooting in the presence of higher ranking males (Clark 1993).
Like chimpanzees, spider monkeys (Ateles sp.) are male-philopatric, ripe-fruit specialists whose high energy food resources are distributed unevenly both temporally and spatially (Chapman et al. 1995; Link and Di Fiore 2006; Symington 1990). This particular distribution pattern of fruit resources has been hypothesized to increase intragroup competition (Asensio et al. 2008; Chapman et al. 1995; Sterck et al. 1997; Symington 1990), particularly among females (Trivers 1972; Wrangham 1987), thus leading to a highly dispersed social grouping pattern in which subgroups exhibit temporal variation in size, composition, and cohesion (Chapman 1990; Chapman et al. 1995; Symington 1988, 1990; Wallace 2006). How male and female spider monkeys disperse and assort into subgroups, however, does not appear to be random; recent research suggests that males and females in at least one population of Yucatan spider monkeys (Ateles geoffroyi yucatanensis) rarely associate in the same subgroups, particularly during periods of high overall food availability, and may even avoid each other except during short, intermittent subgroup fusions (Hartwell et al. 2014). Female spider monkeys have also been described as being less gregarious than males (Aureli and Schaffner 2008; Chapman 1990; Symington 1987) and, although they do associate with other adult females, their rates of association as measured by subgroup membership suggest little selectivity with regard to which individuals associate together (Ramos-Fernandez et al. 2009). This is in stark contrast to the pattern observed among males, which associate at levels that suggest active companionship; while females may nonselectively associate with each other at feeding sites, males have preferred social partners (Ramos-Fernandez et al. 2009).
Of the approximately 14 spider monkey vocalizations, the whinny has been studied in most detail (Chapman and Lefebvre 1990; Chapman and Weary 1990; Ramos-Fernandez 2005, 2008; Santorelli et al. 2013; Teixidor and Byrne 1999). Like chimpanzee pant hoots, whinnies are medium- to long-range calls, and, as indicated by the observation that they tend to be produced immediately before or after a subgroup fusion, many studies have implicated whinnies in playing a “contact” function between spatially dispersed subgroups (Carpenter 1935; Eisenberg 1976; Ramos-Fernandez 2005, 2008). Early spider monkey research reported that whinnies are often produced by spider monkeys entering feeding trees, and that the call is emitted frequently by individuals as they forage (Chapman and Lefebvre 1990; Eisenberg 1976). The latter study (Chapman and Lefebvre 1990) also reported a positive relationship between the frequency of whinnying by foraging subgroups and the number of individuals that eventually joined those subgroups. The authors interpreted this to suggest that whinnies are “food calls,” used to attract conspecifics to a divisible food source. However, this interpretation has since been challenged (Ramos-Fernandez 2005, 2008), on the basis that the relatively low proportion of subgroups that were joined after calling in the foraging study was insufficient to warrant categorizing whinnies specifically as “food calls.”
More recent research has benefited from detailed acoustic analyses of whinnies, and these have revealed discernible differences in their acoustic structure between individuals, which may act as cues to a caller’s identity (Chapman and Weary 1990; Ramos-Fernandez 2005; Santorelli et al. 2013; Teixidor and Byrne 1999). Spider monkeys may therefore be able to determine the identity of a caller based on acoustic cues alone, allowing receivers to respond to whinnies based on their relationship with a particular caller. Playback experiments have shown that these cues are salient to receivers, as individuals are more likely to approach callers with which they have higher levels of association (Ramos-Fernandez 2005). This suggests that the composition of subgroups may at least in part be regulated by whinnies, as callers are approached by close associates and potentially avoided by others. In this way, the whinny vocalization is perhaps analogous to the chimpanzee pant hoot (Ramos-Fernandez 2005), which serves a similar function in the maintenance of contact between males that are close associates (Mitani and Nishida 1993).
Unlike chimpanzee pant hoots, however, whinnies appear to be used quite frequently by females (Fedigan and Baxter 1984; Ramos-Fernandez 2005), despite their lack of selectivity in association patterns, and research on whinnies to date has not identified whether there might be different patterns of whinny production and responses according to the sex of callers and receivers. Thus, although whinnies may serve a generalized contact function by allowing individuals of both sexes to adjust interindividual distances relative to other group members, it is also possible that the individually distinctive qualities of the call may result in an additional sexual dimension to whinnying that has not yet been explored. This is because the functional significance of the whinny may be determined by the social relationship between callers and receivers (Ramos-Fernandez 2005), and males and females show distinct association patterns (Chapman 1990; Ramos-Fernandez et al. 2009). A logical next step, then, is to determine whether females use whinnies at similar rates, and in similar contexts to those of males. Here, accordingly, we investigate sex differences in the use of the whinny vocalization by the black handed spider monkey (Ateles geoffroyi yucatanensis) at Runaway Creek Nature Reserve, Belize, in an effort to address the overarching question of whether whinnying might have sex-specific functional outcomes. In particular, we analyze differences in the rate of calling by male and female spider monkeys across several behavioral contexts. We then investigate the effects of subgroup composition, and changes in subgroup composition, on female calling behavior.
As males share “high-quality” relationships (Slater et al. 2009), and associate with one another at relatively high rates that suggest active companionship (Ramos-Fernandez et al. 2009), we hypothesize that their whinnies are used to monitor the whereabouts of other males with which they may preferentially associate. We therefore predict that males will call more often in contexts in which coordinated movements with close associates over larger distances is required, such as while traveling. Conversely, we hypothesize that calling by females may have the effect of spacing other individuals out relative to the caller, and should therefore occur more often in contexts where maintaining distance from others may be beneficial. Accordingly, we predict that females will call at elevated rates relative to males while foraging, when they are in larger subgroups containing females, and as subgroup size increases during fusions. By calling, female spider monkeys may be able to advertise their occupation of a food patch, thus keeping potential competitors at a distance. The ability to influence the position of other individuals while foraging may allow females to reduce feeding competition, while at the same time allowing callers to avoid aggressive interactions that may arise from that competition (Asensio et al. 2008). We base these latter two predictions concerning the effects of group size on calling in females on the assumption that, as subgroup size increases, so too does the risk of competition over resources (Asensio et al. 2009; Chapman et al. 1995), especially among other females. In addition, it has been shown that fusions are characterized by aggressive interactions, potentially as a result of conflicts between individuals from separate subgroups coming together (Aureli and Schaffner 2007). Whinnying may allow females, which experience elevated levels of aggression relative to males (Campbell 2003; Link et al. 2009; Slater et al. 2008), to determine the identity of approaching individuals, and assess risk before joining another subgroup. Finally, although we test whether any change to the composition of a female’s subgroup, i.e., fissions or fusions, will affect calling rates, we predict that only fusions will elicit whinnies from females, as their individually distinctive calls may allow arriving monkeys to determine subsequent courses of action, i.e., whether to approach, maintain distance, or to break away from the caller.
Methods
Study Site and Subjects
Runaway Creek Nature Reserve (RCNR) is situated in Central Belize (88°35ʹW and 17°22ʹN). RCNR is a karst landscape characterized by hills, low valleys, and seasonal swamps, and consists primarily of semideciduous, broadleaf tropical forest and pine savannah. It experiences a dry season from January through May, and a wet season from June through December. The mean annual rainfall in this area is estimated at 2000–2200 mm (Meerman 1999).
RCNR is home to two primate species, the black howler (Alouatta pigra) and the Yucatan, or black-handed, spider monkey (Ateles geoffroyi yucatanensis). We collected data from a resident group of habituated spider monkeys over an 8-mo period between January and August 2012. The home range of the study group is ca. 114 ha (Pavelka M unpubl. data), and borders the home range of a second group to the north. We identified all group members by their facial features, the coloration of their pelage, or through characteristics of their genitalia. We determined ages by body and (male) testes size, as well as behaviorally by relative independence from mothers. Since the onset of data collection in 2008, group size has varied from 33 to 37 individuals. During the study period, the community consisted of 34 individuals: 7 adult males, 11 adult females, 1 subadult male, 2 subadult females, 3 juvenile males, 5 juvenile females, and 5 infants.
General Data Collection Protocol
Our data collection protocol adhered to the requirements of Belize and complied with protocols approved by the Canadian Council on Animal Care and the Animal Care Committee of the University of Calgary. As spider monkeys typically exhibit fluid social grouping patterns and occupy large home ranges, we sampled monkey subgroups opportunistically throughout the day for as long as we could maintain contact. We collected scan samples every 30 min, recording the time, GPS location, composition and spread of the subgroup, as well as the identity and behavioral state of each of its members. Between scan samples, we used focal animal sampling (Altmann 1974) to collect behavioral data, including whinny vocalizations. Focal samples were 10 min in length, with a minimum 10-min interval between the end of one focal and the beginning of the next. We sampled the same sampled individual only once in a given 30-min period, to avoid biases in the data, and to increase the independence of each sample. Depending on the visibility and number of monkeys within a subgroup, one or two focal samples were collected in each 30-min period between scans.
Whinny vocalizations are a discrete call type that have been described as ranging from 0.3 to 1.5 s in length, and consisting of 2 to up to 12 repeating, frequency-modulated elements (Ramos-Fernandez 2005, 2008) (Fig. 1). We classified a call as a whinny if three minimum conditions were met: 1) if the call contained both a grunt-like element as well as a whistle-like element distinguishing it from the lower amplitude high whinny (Ramos-Fernandez 2005) and tee-hee vocalizations, which are characterized by only containing a whistle-like element; 2) if the call contained three or more elements, so as to distinguish further from a tee-hee, which has only two call elements; and 3) if the trained local field assistant accompanying the researcher who is familiar with the different spider monkey calls also agreed that a call was a whinny.
We chose focal subjects according to sampling representation; individuals that were underrepresented in the dataset were given priority. In some cases, we left subgroups containing overrepresented individuals to look for others. We recorded the age and sex of the focal animal, as well as the age/sex class of all other individuals within the focal animal’s subgroup at the onset of each 10-min sample. We operationally defined subgroups using a chain rule of 50 m; any individual within 50 m of any other individual within a subgroup was considered to be part of that same subgroup (Ramos-Fernandez 2005). In the event of subgroup fission, we attempted to follow the subgroup that contained the most undersampled individuals.
We associated all whinny vocalizations emitted by a focal animal with one of four behavioral contexts: foraging, socializing, inactivity, or traveling. We defined foraging as handling, inspecting, ingesting, or searching for a food item at the time of call production. We defined social contexts as sitting in body contact or within a 2-m radius of another individual, engaging in social play, or participating in social grooming. We defined traveling as moving within the canopy, excluding movements within the crown of a tree while foraging. The context “inactive” was assigned to calls emitted while an individual was sitting, lying down, or autogrooming outside the 2-m radius prescribed for the context “social.” If a focal animal changed behaviors at the onset of the whinny, we assigned the call to the behavior directly following the whinny.
We recorded subgroup fissions and fusions on an “all observed occurrence” basis. To capture the possible effects of subgroup fissions and fusions that may have immediately preceded or followed a focal sample, we classified focal samples as fission or fusion focals if individuals joined or left the subgroup within a 2-min time interval on either end of the sample, i.e., 2 min before or after the 10-min focal sample. This ensured that vocalizations that may have been associated with a subgroup composition change immediately before a focal sample were included as associated with that fission or fusion event. Similarly, we observed that the monkeys often called at the detection of approaching individuals before the researchers were aware that a fusion was occurring. We therefore considered all fissions or fusions that might have occurred within 2 min after the end of a focal sample to be connected with any calls that occurred during that sample. This allowed us to capture calls that may have been associated with the impending arrival of others that the monkeys detected, but that we did not initially detect during the 10-min sampling period. As we were interested in the effect that approaching or departing monkeys might have on vocalization rates, we counted, and treated as independent, all fissions and fusions that involved individuals moving outside or into the subgroup as defined by the 50-m chain rule, regardless of the duration that an individual remained in or out of the subgroup. We recorded the identity and age/sex classes of all individuals joining or leaving the subgroup.
Analysis
Effects of Sex and Behavioral Context
Of the 658 whinnies recorded between January and August 2012, we removed all those emitted during focal samples that were associated with a subgroup fission or fusion. We did this to control for the possible effects of subgroup composition changes on an individual’s rate of calling, while trying to isolate the possible effects of behavioral context on call production by each sex. Doing so left a total of 526 whinny vocalizations for the behavioral analysis.
We compared the rate of calling by male and female spider monkeys across all four behavioral contexts to determine the effect of sex and activity on an individual’s rate of calling. We determined rates of calling by each sex in each behavioral context by dividing the number of whinnies produced by each individual male and female by the total amount of time, in minutes, that each individual was observed in a particular context. We included only adult and subadult monkeys in the analyses. We only used calls that were clearly emitted by the focal animal; the identity of a caller could not always be known for certain if more than one monkey was in close proximity, and visibility of a focal animal partially obscured. These calls were not used in the analysis.
We used a two-way repeated measures ANOVA to analyze individual rates of calling by each sex in each behavioral context. We applied a Greenhouse–Geisser correction when data violated the assumption of sphericity. We performed post hoc simple effects analyses to compare the rates of calling between the four behavioral contexts within sexes and between sexes within each behavioral context. We used the Bonferroni correction to compensate for multiple comparisons.
Effects of Subgroup Characteristics
We restricted our analysis of the effects of subgroup size, composition, and changes to subgroup composition on the likelihood of calling to females only, as an initial inspection revealed insufficient samples of male whinnies across subgrouping variables. Although females called from 0 to 20 times per focal sample, we classified each focal sample as either a whinny focal or non-whinny focal for this analysis, regardless of how many times a focal animal actually called during that sample. In other words, whether a focal animal called once or 10 times, its sample was classified as a whinny focal. If it did not call at all, the sample was a non-whinny focal. We did this to decrease the risk of erroneously treating multiple whinnies within a single sample as independent events. Also, there was not enough variation in the number of whinnies in each focal sample to analyze the actual number of calls associated with a particular event. Of the 714 focal samples on females, 265 were classified as whinny focals. The average number of calls per whinny focal was 2.33, although focal animals emitted only a single whinny in 52.7% of these samples.
We constructed two generalized estimating equation (GEE) models to determine whether specific subgroup characteristics elicited a whinny response from females. GEEs are a type of generalized linear model (GLM) that controls for repeated measures on the same subjects over time (Ghisletta and Spini 2004). The first GEE model looked at the effects of subgroup composition on calling, while the second tested the effects of subgroup fissions and fusions (subgroup stability) on calling. In both models, the 10-min focal sampling periods were the subject, and we controlled for the identity of the focal female because of the correlated nature of measurements from repeated observations on the same individual.
In the first model, which we used to determine the effect of subgroup composition on a female’s likelihood of calling, the dependent variable was whether the focal animal emitted a whinny vocalization during the 10-min sampling period. The independent variables were the number of adult females and the number of adult males in the focal animal’s subgroup (excluding the focal animal). We looked at the number of males and females in the subgroup separately to determine whether subjects responded differently to the presence of either sex. We used only focal samples in which subgroup composition remained constant throughout the sample in the analysis, i.e., we did not include any focal samples in which either a fission or a fusion had occurred in the first GEE model.
The second model dealt with subgroup stability. As with the first model, the dependent variable was whether the focal female emitted a whinny vocalization during the sampling period. The independent variables were: the total number of females that left a focal animal’s subgroup (fission), the total number of males that left a focal animal’s subgroup (fission), the total number of females that joined (fusion) a focal animal’s subgroup, and the total number of males that joined (fusion) a focal animal’s subgroup during the focal sample. We considered the effects of males and females joining or leaving separately to determine whether subjects responded differently at fissions or fusions based on the sex of individuals leaving or joining their subgroup. This was done because field observers noted that fusions involving males joining an all-female subgroup were often qualitatively different than fusions involving only females. Males would often join the subgroup while moving quickly and noisily through the trees, which seemed to draw the attention of, and at times startle the females. Although not always the case, this would sometimes result in males chasing females, and seemed consistent with similar observations described by other researchers (Fedigan and Baxter 1984; Slater et al. 2008). We discarded focal samples in which both males and females left or joined the focal animal’s subgroup, allowing for the effects of females coming and going from a focal animal’s subgroup to be examined separately from males. We also discarded focal samples that were associated with both a fission and a fusion to separate the effects of fissions and fusions on the probability of calling. This left a total of 687 focal samples for this analysis.
For both GEE models, the direction of the relationship between an independent variable on the likelihood of calling is represented by the sign of coefficient B. The intensity of the effect is indicated by a Wald’s chi-square, with higher chi-square values indicating a stronger association. Our α level for all statistical analyses was 0.05.
Results
Sex and Individual Activity
The rate of whinny vocalizations differed significantly by sex (repeated measures ANOVA: F(1,18) = 5.41, P = 0.032), and by individual activity (ANOVA: F(1.72, 31.03) = 5.50, P = 0.012). Females called at a higher rate than males overall (females 4.21 ± SE 0.55 calls/h; males 2.18 ± SE 0.68 calls/h). There was a significant sex by activity interaction effect (ANOVA: F(1.724, 31.030) = 3.70, P = 0.042), indicating that the effect of an individual’s behavioral state on its rate of calling varied with the sex of the caller, or that the effect of sex on call rate varied between behavioral states. Each contrast within the interaction described previously is detailed in the text that follows.
Simple effects analysis of female whinnies in different behavioral contexts indicated a significant difference in the rates of calling between activities. Females whinnied at a significantly higher rate while foraging in comparison to any other activity (forage-social P = 0.006; forage-inactive P = 0.001; forage-travel P = 0.001; Fig. 2A). There was no significant difference in call rate by females across any other contexts (social-inactive P = 0.476; social-travel P = 0.935; inactive-travel P = 1.000). Males showed no significant differences in their rate of calling between any behavioral context (foraging-social P = 1.000; forage-inactive P = 0.675; forage-travel P = 0.578; social-inactive P = 1.000; social-travel P = 1.000; inactive-travel P = 1.000; Fig. 2A).
Rates of whinny vocalizations (calls/h) by Ateles geoffroyi yucatanensis by sex and activity. The significance levels of post hoc pairwise comparisons (a) between behavioral activities and (b) between sexes within behavioral activities are indicated by asterisks. Bars connected by an asterisk are significantly different (P < 0.05). The figure represents data collected over an 8-mo period from January to August 2012 at RCNR, central Belize.
Call rate was significantly higher for females in comparison to males while foraging (ANOVA: F = 8.24, d.f. = 1, P = 0.010), inactive (ANOVA: F = 6.27, d.f. = 1, P = 0.022), and while traveling (ANOVA: F = 7.46, d.f. = 1, P = 0.014; Fig. 2B). There was no significant difference in the rate of calling between males and females in social contexts (ANOVA: F = 0.69, d.f. = 1, P = 0.419; Fig. 2B).
Sex and Subgroup Characteristics
The Effects of Subgroup Size
The number of adult females in a focal female’s subgroup ranged from 0 to 7 individuals, with an average subgroup containing 1.12 females in addition to the female focal subject. The number of males in a female’s subgroup ranged from 0 to 3 individuals, with an average subgroup containing 0.17 adult males. Of the 714 focal samples on females, 124 were associated with either a fission, a fusion, or both, leaving a total of 590 focal samples on females for the group size analysis.
The results of the first GEE model showed no relationship between the likelihood of calling by females and the number of adult males or females in their subgroup (GEE: males: B = 0.038, χ2(1) = 0.047, P = 0.828; females: B = –0.016, χ2(1) = 0.014, P = 0.905).
The Effects of Subgroup Stability – Fissions and Fusions
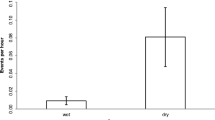
Results of the GEE model showed that the likelihood of a female calling during a focal sample was significantly influenced by the number of individuals joining (fusion) or leaving (fission) its subgroup. Females were significantly more likely to call as the number of adult males or adult females joining their subgroup increased (males joining: GEE: B = 1.510, χ2(1) = 21.488, P < 0.001; females joining: GEE: B = 0.930, χ2(1) = 11.448, P = 0.001; Fig. 3). Females were also significantly more likely to call as the number of adult males leaving their subgroup increased (GEE: B = 0.830, χ2(1) = 4.666, P = 0.031; Fig. 4). The likelihood of a female calling was not significantly influenced by the number of females leaving (fission) the subgroup (GEE: B = 0.356, χ2(1) = 1.956, P = 0.162; Fig. 4). The likelihood of a female calling was most influenced by the number of males joining, followed by the number of females joining, and finally by the number of males leaving her subgroup.
Discussion
The results of our analyses show that female spider monkeys whinny more often than males in almost all behavioral contexts, and that changes to subgroup composition, i.e., fissions and fusions, affect the likelihood of whinnying in females. Previous research concerning whinny vocalizations in spider monkeys has yielded an overarching hypothesis that the individually distinctive calls serve a generalized contact function by allowing individuals to monitor the whereabouts of others, and to adjust their relative positions based on existing relationships between callers and receivers (Ramos-Fernandez 2008). Our results support this broader hypothesis, while also providing some evidence for an additional dimension to call function based on sex differences. We hypothesized that females, which have been described as being the less gregarious sex (Aureli and Schaffner 2008), may use whinnies more often than males to achieve appropriate spacing, rather than as a means for maintaining contact with close associates. Subsequently, we predicted that females would call at elevated rates in behavioral contexts in which they were most likely to encounter competition, such as while foraging and during subgroup fusions. Our results support this hypothesis; female spider monkeys called at higher rates than males in almost all behavioral contexts, but especially while foraging. Their likelihood of calling also increased in association with subgroup fusions. Females called least often when engaged in social activities, and in this context their call rates did not differ significantly from those of males. The observation that females called more than males in all other behavioral contexts is somewhat surprising, as we had predicted that males would call more often while traveling to monitor other male associates with which they range frequently. That males did not appear to call more often in any behavioral context might reflect the possibility that closely associated males already maintain visual contact with each other as they travel in the same subgroup, and males therefore may not rely heavily on the use of the whinny to locate each other while traveling in the same direction. Future research would benefit from subgroup analyses that focus on interindividual distances within and between males and females in different behavioral contexts.
Our results also showed that female calling behavior was affected by changes in subgroup composition. Females were more likely to call as the number of males or females joining their subgroup increased. Similarly, females were more likely to call as the number of males leaving their subgroup increased. The tendency for female subjects to call during both fissions and fusions suggests an association between shifts in subgroup membership and the use of the whinny vocalization. Conversely, a female’s likelihood of calling was not affected by the number of individuals in its subgroup when subgroup composition was stable, regardless of the sex of the other subgroup members. This suggests that when subgroups are stable, i.e., there are no fissions or fusions occurring, neither the size nor the sex composition of a female’s subgroup affects its likelihood of whinnying. This latter finding contrasted with our prediction that females should call more often in larger subgroups, especially in subgroups composed of other females, as we hypothesized that whinnying is motivated by the presence of other female competitors. Although females may call in response to changes in subgroup composition, it is possible that once within a stable subgroup and in visual contact, spider monkeys do not rely heavily on the whinny vocalization to continually monitor the whereabouts of other individuals. Calling in association with changes in subgroup composition may allow individuals to space themselves out appropriately, such that it is not necessary to call again until subgroup members begin to move off (fission), or new individuals approach (fusion). Thus, the whinny may play a larger role in negotiating spacing between individuals in separate subgroups, rather than between individuals within the same subgroup; individuals in separate subgroups may use each other’s calls to decide whether or not they should, for example, approach, or move away from a caller’s subgroup, depending on their relationship with the caller.
Are Whinnies Cohesion Calls, Spacing Calls, or Both?
Previous research indicates that whinnies are often associated with subgroup fusions (Chapman and Lefebvre 1990; Eisenberg 1976; Ramos-Fernandez 2005). It is unclear, however, whether subgroup fusions are facilitated by calling, which acts to attract receivers, or whether it is the fusion that triggers calling. Although playback experiments have shown that spider monkeys may be attracted to each other’s whinnies, it was only receivers that were preferred social partners of the recorded caller that ever approach the speaker after a playback (Ramos-Fernandez 2005). Our data do not permit a direct analysis of the order in which whinnies and fusions occurred, but they do show that females whinny at relatively high rates in association with changes in subgroup composition, especially during fusions. As females have been described as less gregarious than males (Aureli and Schaffner 2008; Chapman 1990; Fedigan and Baxter 1984), and as less likely to be found in subgroups containing other individuals with which they regularly associate (Ramos-Fernandez et al. 2009), it is unclear as to whether their whinnies attract conspecifics. Further, if female whinnies do attract receivers, it is unclear as to what benefits might be gained given the potential for feeding competition that may arise as a product of two subgroups coming together.
Our analysis also revealed an unexpected contextual dimension in which spider monkeys (specifically females) are likely to call in association with subgroup fissions. Although the probability of whinnying was higher during subgroup fusions, the observed increase in probability of calling during fissions is difficult to interpret. One possibility is that female whinnies are, in fact, prompting other females to disperse from a subgroup, especially if the relationship between the caller and signaler is not affiliative, or is antagonistic. This scenario would lend support to the hypothesis that, at least in some contexts, female whinnies repel receivers rather than attract them. Another possibility, although not mutually exclusive to the former, is that the proximate trigger for whinnying in females is sensitive to heightened anxiety associated with any sudden changes to the social environment of the caller in which the outcomes are potentially uncertain. This possibility might explain why females whinny more generally during changes in subgroup composition than when subgroups are stable, particularly when the number of individuals joining or leaving a subgroup increases; it seems likely that the degree of uncertainty surrounding potentially imminent social interactions (or, conversely, at being abandoned by conspecifics) is commensurate with the number of other monkeys that are coming and going.
That whinnies may be associated with increased arousal or anxiety is also suggested by field observations of spider monkeys calling in response to sudden noises, such as breaking branches, falling trees, or loose rocks rolling down hillsides (Dubreuil C pers. obs.). These sudden noises may simulate the movement of conspecifics within audible range, yet out of sight of the caller, as would be the case during both fissions and fusions. Although both males and females face the risk of receiving aggression from other adults within their community (Rebecchini et al. 2011), there is evidence that females incur an elevated risk relative to males (Campbell 2003; Link et al. 2009; Slater et al. 2008), including the risk of male-directed infanticide (Alvarez et al. 2014). This is especially so during subgroup fusions (Aureli and Schaffner 2007). Females, then, may face a higher degree of social uncertainty surrounding the arrival of conspecifics, either in the form of potential female directed aggression or competition from other females, and any noise that presages an imminent fusion may trigger calling by nervous females, especially if they are alone or in small subgroups. Whinnying may decrease the risk of aggressive interactions by allowing non-associated females to locate each other, and space themselves out appropriately. This may also explain why females call while foraging; if other females are nearby, whinnying may allow a caller to influence interindividual distance within a subgroup when the potential for feeding competition, and associated risk of receiving aggression, is high (Asensio et al. 2008; Fedigan and Baxter 1984; Symington 1990). That both a caller’s associates and non-associates are equally likely to respond to a whinny by whinnying themselves (Ramos-Fernandez 2005), fits into this interpretation as well, as vocal responses to whinnies would allow both interactants (both the original caller and the individual that whinnies in response) to make decisions as to how to space themselves out relative to each other. Antiphonal responses would therefore allow non-associates to better monitor and avoid each other in contexts where dispersal is beneficial, while at the same time allowing close associates to better locate each other when out of visual contact (Ramos-Fernandez 2005).
That both whinny vocalizations and the muriqui staccato vocalization (Arnedo et al. 2010) are emitted at higher rates by females than by males, particularly while foraging, suggests a possible functional analogy between these signals; calling in both cases may advertise a female’s presence in a food patch, thereby deterring others from approaching. Although we suggest that whinnies allow for more flexibility in the social outcome between interactants, because of the call’s ability to convey cues to individual identity, the proposed functional analogy between staccatos and whinnies by females allows us to make testable predictions for future research. For example, female muriquis reportedly emit staccato vocalizations at higher rates when preferred food types are limited (Arnedo et al. 2010), and as such it may prove beneficial to explore the effect of food availability, or patch size, on whinnying. Spider monkey subgroup sizes have been noted to vary with food availability (Chapman et al. 1995; Shimooka 2003; Symington 1988), and it is possible that this change in subgroup size is mediated in part by variations in call rate. It may prove beneficial to explore receiver responses to whinnies in relation to food availability as well, as receivers may be more likely to avoid callers, for instance, when food availability is lower, and the cost of being in a larger subgroup is higher.
Our study offers further support to the broader consensus that contact calls used over long distances may be a mechanism by which many nonhuman primates in a social group regulate their positions relative to each other via an assessment of potential outcomes based on the current relationships that exist between callers and receivers. We add to this body of work the proposal that, whether contact calls attract or space receivers should include consideration of the different social and ecological fitness challenges faced by males and females, and how these might affect patterns of communication, specifically in the differential use of species-specific call types. High fission–fusion dynamic species are proposed to vary along dimensions of subgroup size, composition, and cohesion over time (Aurelli et al. 2008). In a recent investigation of sexual segregation in spider monkeys, it was proposed that variation in sexual segregation be considered as a sub-dimension of subgroup composition, as variation in this variable is constrained in sexually segregated species such as spider monkeys by a variety of socio-ecological factors, such as male philopatry (Hartwell et al. 2014). We add to this notion by proposing that vocalizations in sexually segregated species such as spider monkeys (and potentially, chimpanzees) may represent a means for achieving different functional outcomes for each sex. Future research on spider monkey whinnies would benefit from establishing how receivers respond to whinnies from different individuals, particularly from males vs. females. More broadly, research on other animal taxa should explore how species-specific call types might be used differently by males and females, and how these differences might relate to social organization and the particular social and ecological challenges faced by each sex.
References
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3), 227–267.
Alvarez, S., Di Fiore, A., Champion, J., Pavelka, M. S., Páez, J., & Link, A. (2014). Male-directed infanticide in spider monkeys (Ateles spp.). Primates, 1–9. doi:10.1007/s10329-014-0454-y.
Arnedo, L. F., Mendes, F. D. C., & Strier, K. M. (2010). Sex differences in vocal patterns in the northern muriqui (Brachyteles hypoxanthus). American Journal of Primatology, 72, 122–128.
Asensio, N., Korstjens, A. H., & Aureli, F. (2009). Fissioning minimizes ranging costs in spider monkeys: A multiple-level approach. Behavioral Ecology and Sociobiology, 63(5), 649–659.
Asensio, N., Korstjens, A. H., Schaffner, C. M., & Aureli, F. (2008). Intragroup aggression, fission-fusion dynamics and feeding competition in spider monkeys. Behaviour, 145, 983–1001.
Aureli, F., & Schaffner, C. M. (2007). Aggression and conflict management at fusion in spider monkeys. Biology Letters, 3, 147–149.
Aureli, F., & Schaffner, C. M. (2008). Social interactions, social relationships and the social system of spider monkeys. In C. J. Campbell (Ed.), Spider monkeys: Behavior, ecology and evolution of the genus Ateles (pp. 236–265). New York: Cambridge University Press.
Aureli, F., Schaffner, C., Boesch, C., Bearder, S. K., Call, J., Chapman, C. A., Connor, R., DiFiore, A., Dunbar, R. I. M., Henzi, P. S., Holekamp, K., Korstjens, A. H., Layton, R., Lee, P., Lehmann, J., Manson, J. H., Ramos-Fernandez, G., Strier, K. B., & van Schaik, C. P. (2008). Fission–fusion dynamics: New research frameworks. Current Anthropology, 49, 627–654.
Boinski, S., & Campbell, A. F. (1996). The huh vocalization of white-faced capuchins: A spacing call disguised as a food call? Ethology, 102, 826–840.
Campbell, C. J. (2003). Female-directed aggression in free-ranging Ateles geoffroyi. International Journal of Primatology, 24(2), 223–237.
Carpenter, C. R. (1935). Behavior of red spider monkey in Panama. Journal of Mammalogy, 16, 171–180.
Chapman, C. (1990). Association patterns of spider monkeys: The influence of ecology and sex on social organization. Behavioral Ecology and Sociobiology, 26, 409–414.
Chapman, C. A., & Lefebvre, L. (1990). Manipulating foraging group size: Spider monkey food calls at fruiting trees. Animal Behavior, 39, 891–896.
Chapman, C. A., & Weary, D. M. (1990). Variability in spider monkeys' vocalizations may provide basis for individual recognition. American Journal of Primatology, 22(4), 279–284.
Chapman, C. A., Wrangham, R. W., & Chapman, L. C. (1995). Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology, 36, 59–70.
Cheney, D. L., & Seyfarth, R. M. (1996). Function and intention in the calls of nonhuman primates. Proceedings of the British Academy, 88, 59–76.
Clark, A. P. (1993). Rank differences in production of vocalizations by wild chimpanzees as a function of social context. American Journal of Primatology, 31, 159–179.
Eisenberg, J. F. (1976). Communication mechanisms and social integration in the black spider monkey, Ateles fusciceps robustus, and related species. Smithsonian Contributions to Zoology., 213, 1–108.
Fedigan, L. M., & Baxter, M. J. (1984). Sex differences and social organization in free-ranging spider monkeys (Ateles geoffroyi). Primates, 25, 279–294.
Ghisletta, P., & Spini, D. (2004). An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals. Journal of Educational and Behavioral Statistics, 29(4), 421–437.
Gros-Louis, J. (2004). The function of food-associated calls in white-faced capuchin monkeys, Cebus capucinus, from the perspective of the signaller. Animal Behaviour, 67, 431–440.
Hartwell, K. S., Notman, H., Bonenfant, C., & Pavelka, M. S. (2014). Assessing the occurrence of sexual segregation in spider monkeys (Ateles geoffroyi yucatanensis), its mechanisms and function. International Journal of Primatology, 35(2), 425–444.
Kondo, N., & Watanabe, S. (2009). Contact calls: Information and social function. Japanese Psychological Research, 3, 197–208.
Link, A., & Di Fiore, A. (2006). Seed dispersal by spider monkeys and its importance in the maintenance of neotropical rain-forest diversity. Journal of Tropical Ecology, 22(3), 235–246.
Link, A., Di Fiore, A., & Spehar, S. N. (2009). Female directed aggression and social control in spider monkeys. In M. N. Muller & R. W. Wrangham (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 157–187). Cambridge, MA: Harvard University Press.
Marler, P., & Tenaza, R. (1977). Signaling behavior of apes with special reference to vocalization. In T. A. Sebeok (Ed.), How animals communicate (pp. 965–1033). Bloomington: Indiana University Press.
Meerman, J. C. (1999). Rapid ecological assessment of Runaway Creek. Belize: Zoological Society of Milwaukee.
Mitani, J. C., & Nishida, T. (1993). Contexts and social correlates of long-distance calling by male chimpanzees. Animal Behavior, 45, 735–746.
Nishimura, A., Fonseca, G. D., Mittermeier, R. A., Young, A. L., Strier, K. B., & Valle, C. M. C. (1988). The muriqui, genus Brachyteles. Ecology and Behavior of Neotropical Primates, 2, 577–610.
Notman, H., & Rendall, D. (2005). Contextual variation in chimpanzee pant hoots and its implications for referential communication. Animal Behaviour, 70, 177–190.
Poole, J. H. (1994). Sex differences in the behaviour of African elephants. In R. V. Short & E. Balaban (Eds.), The differences between the sexes (pp. 331–346). Cambridge, U.K.: Cambridge University Press.
Ramos-Fernandez, G. (2005). Vocal communication in a fission-fusion society: Do spider monkeys stay in touch with close associates? International Journal of Primatology, 26, 1077–1092.
Ramos-Fernandez, G. (2008). Communication in spider monkeys: The function and mechanisms underlying the use of the whinny. In C. J. Campbell (Ed.), Spider monkeys: Behavior, ecology, and evolution of the genus Ateles (pp. 221–235). New York: Cambridge University Press.
Ramos-Fernandez, G., Boyer, D., Aureli, F., & Vick, L. G. (2009). Association networks in spider monkeys (Ateles geoffroyi). BehavioralEcology and Sociobiology, 63(7), 999–1013.
Rebecchini, L., Schaffner, C. M., & Aureli, F. (2011). Risk is a component of social relationships in spider monkeys. Ethology, 117(8), 691–699.
Rendall, D., & Owren, M. J. (2002). Animal vocal communication: Say what? In M. Bekoff, C. Allen, & G. Burghardt (Eds.), The cognitive animal (pp. 307–313). Cambridge, MA: MIT Press.
Santorelli, C. J., Aureli, F., Ramos-Fernández, G., & Schaffner, C. M. (2013). Individual variation of whinnies reflects differences in membership between spider monkey (Ateles geoffroyi) communities. International Journal of Primatology, 34(6), 1172–1189.
Shimooka, Y. (2003). Seasonal variation in association patterns of wild spider monkeys (Ateles belzebuth belzebuth) at La Macarena, Colombia. Primates, 44(2), 83–90.
Slater, K. Y., Schaffner, C. M., & Aureli, F. (2008). Female-directed male aggression in wild Ateles geoffroyi yucatanensis. International Journal of Primatology, 29(6), 1657–1669.
Slater, K. Y., Schaffner, C. M., & Aureli, F. (2009). Sex differences in the social behavior of wild spider monkeys (Ateles geoffroyi yucatanensis). American Journal of Primatology, 71(1), 21–29.
Sterck, E. H., Watts, D. P., & van Schaik, C. P. (1997). The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology, 41(5), 291–309.
Symington, M. M. (1987). Sex ratio and maternal rank in wild spider monkeys: When daughters disperse. Behavioral Ecology and Sociobiology, 20(6), 421–425.
Symington, M. M. (1988). Food competition and foraging party size in the black spider monkey (Ateles paniscus chamek). Behaviour, 105, 117–134.
Symington, M. M. (1990). Fission-fusion social organization in Ateles and Pan. International Journal of Primatology, 11, 47–61.
Teixidor, P., & Byrne, R. W. (1999). The ‘whinny’ of spider monkeys: Individual recognition before situational meaning. Behaviour, 136, 279–308.
Trivers, R. L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man (pp. 1871–1971). Chicago: Aldine.
Wallace, R. (2006). Seasonal variations in black-faced spider monkey (Ateles chamek) habitat use and ranging behaviour in a southern Amazonian tropical forest. American Journal of Primatology, 68, 313–332.
Wrangham, R. W. (1987). Evolution of social structure. In B. B. Smuts, D. L. Cheney, R. M. Seyfart, R. W. Wrangham, & T. T. Struhsaker (Eds.), Primate societies (pp. 282–297). Chicago: University of Chicago Press.
Acknowledgments
This research was supported financially by the Natural Sciences and Engineering Research Council of Canada. Special thanks go to Gil and Lillian Boese from the Zoological Society of Milwaukee and the Foundation for Wildlife Conservation for permission to work at RCNR. Thanks to Birds without Borders for their assistance and support throughout this project. Thanks go out to the staff at the Tropical Education Center, Belize, for accommodation. This research would not have been possible without the many hours of field support and assistance in data collection by Gilroy Welch; thank you so much for your hard work and patience in the field. We would like to acknowledge Gabriel Ramos-Fernandez and an anonymous reviewer, as well as the editor-in-chief, Joanna Setchell, for their helpful feedback and suggestions on early drafts of this article. We also thank Tak Fung for his technical expertise and support with our statistical analysis. To Peter Henzi, many thanks for your helpful comments on earlier drafts of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubreuil, C., Notman, H. & Pavelka, M.S.M. Sex Differences in the Use of Whinny Vocalizations in Spider Monkeys (Ateles geoffroyi). Int J Primatol 36, 412–428 (2015). https://doi.org/10.1007/s10764-015-9832-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9832-6