Abstract
Sexual segregation is a recognized dimension of the socioecology of many vertebrates, but it has not been systematically examined in primates. We investigated temporal patterns of sexual segregation in spider monkeys (Ateles geoffroyi yucatanensis) using a test that distinguishes sexual segregation from aggregation and random association between the sexes. We further investigated how sexual segregation varies over time as a function of food availability, and then tested other possible factors that might be causally linked to sexual segregation in spider monkeys. We predicted that male philopatry and cooperative territorial defence leads to sexual dimorphism in behavior, which in turn creates different optimal energetic requirements for males and females as reflected in differing activity budgets and diet. We investigated sexual segregation in a group of 33–35 spider monkeys at Runaway Creek Nature Reserve in Belize over 23 mo in 2008–2009. We used the sex compositions of subgroups recorded in scan samples to test the occurrence of sexual segregation at monthly and biweekly time scales.We found that males and females were significantly segregated in 15 out of the 23 mo of the study, and that periods of nonsegregation coincided with months of low food availability. The sexes differed significantly in activity and diet; males spent more time traveling, and less time resting and feeding than females, and they had a higher proportion of ripe fruits in their diets than did females. We propose that sexual segregation in spider monkeys is primarily a form of social segregation that results from males and females pursuing optimal dietary and behavioral strategies to satisfy sex-specific energetic demands. We further suggest that sexual segregation represents an important constraint on fission–fusion dynamics that should be considered when assessing the potential for variability in subgroup composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among many animals, males and females live separately and associate only to mate, either opportunistically or during a mating season (McCullough et al. 1989). Sexual segregation is broadly defined as the separation of males and females outside mating periods, but it is more specifically defined in terms of social groupings or habitat choice (Conradt 1999). Sexual segregation of one kind or another has been described in a wide range of vertebrates (Ruckstuhl and Neuhaus 2005), but most thoroughly among ungulate species (Ovis canadensis: Ruckstuhl 1998; Cervus elaphus: Conradt et al. 2000; Capra ibex: Villaret and Bon 1995). In the large majority of these studies, sexual segregation is described in proximate terms as resulting from differences in feeding behavior and habitat use by sexually dimorphic males and females, or from social preferences for same-sex associates (Ruckstuhl and Neuhaus 2000).Because sexual segregation appears most prevalent in seasonally breeding, sexually dimorphic species, functional explanations for its occurrence have largely derived from sexual selection (Darwin 1871) and parental investment (Trivers 1972) theories, which emphasize the relative effects of inter- and intrasexual competition on male and female morphology and behavior (Ruckstuhl 2007; Ruckstuhl and Neuhaus 2002).For instance, males may forage for longer and on higher quality foods than females to meet the energetic demands required for the development and maintenance of secondary sexual characteristics (Clutton-Brock 1983; Conradt 1998; Ruckstuhl 1998), while females with young may select habitats safer from predation at the expense of food quality (Main et al. 1996; Ruckstuhl and Neuhaus 2000).Alternatively, females may exhibit a preference for habitats with higher quality food because of their smaller size, lower digestive capabilities, and the higher nutritional demands of pregnancy and lactation (Ruckstuhl 2007; Ruckstuhl and Neuhaus 2000).
Studies of a variety of mammalian taxa have shown that association patterns between males and females exhibit temporal variation as a function of a number of factors, including mating and birthing seasons, food abundance, and predator pressure (Bowyer 2004; Loe et al. 2006; Ruckstuhl 2007).Although variation in food availability might represent a constraint on sexual segregation, it does not explain why, from a functional perspective, males and females may segregate in the first place. According to the “activity budget” hypothesis, spatial cohesion in social groups requires activity synchronization of group members, but these groups become unstable when males and females have differing demands on the optimal allocation of time to various activities such as foraging and resting (Conradt 1998; Conradt and Roper 2000; Ruckstuhl 1998).In addition, different energetic demands on males and females may necessitate reliance on different types of food resources (Cook et al. 2007; Stokke 1999; Whitehead et al. 1991).
Another factor that may influence the likelihood of mixed-sex association patterns is the presence of estrous females, and/or the presence of newborn infants. For example, in chimpanzees (Pan troglodytes), the number of males in a subgroup positively correlates with the presence of estrous females (Boesch 1996; Matsumoto-Oda et al. 1998; Sakura 1994). In other species, such as bonnet macaques (Macaca radiata) and spider monkeys (Ateles geoffroyi), the presence of newborn infants may attract both males and females into the same subgroups (Evans et al. 2012; Silk 1999; Slater et al. 2007).
Although not unique among social living mammals, primates are often considered unusual in that males and females typically maintain stable, year-round associations in cohesive social groups that contain at least one adult male and one or more adult females (Fedigan 1992; Watts 2005), even in species that are highly sexually dimorphic, such as gorillas (Gorilla spp.), and highly seasonal breeders, such as lemurs. However, as with other mammals, association patterns between primate males and females may exhibit temporal variation as a function of variation in food availability and distribution by influencing subgroup size, which may in turn affect the patterns of association between sexes. Although the exact relationship between subgroup size and composition, on the one hand, and patch size and overall food availability on the other is unclear, e.g., Pan troglodytes schweinfurthii (Newton-Fisher et al. 2000), Pongo pygmaeus (Knott 1999), and Rhinopithecus bieti (Ren et al. 2012), there is some evidence to suggest that lower food availability may increase the likelihood of mixed-sex associations if larger groups form at fewer available food patches. For example, Stanford et al. (1994) found that chimpanzee subgroup size was largest during the dry season when food supply was restricted, suggesting that more individuals are likely to coincide at fewer food patches.
Although sustained, mixed-sex associations have generally been considered the norm in primates, chimpanzees and spider monkeys were identified early on as exceptions to this pattern owing to the highly fluid nature of their grouping patterns in which subgroups containing just males or females are frequently observed (Fedigan and Baxter 1984; Wrangham and Smuts 1980). Both species live in large, territorial, multimale/multifemale communities characterized by male philopatry and female dispersal (Di Fiore et al. 2009; Wrangham 1979). Unlike most species for which sexual segregation is typically described, however, neither chimpanzees or spider monkeys exhibit marked breeding seasons (Campbell and Gibson 2008; Goodall 1986), and both show only moderate sexual dimorphism in body size (Di Fiore and Campbell 2007; Stumpf 2007).At the same time, these two primate species for which sexual segregation is most often described are also those that exhibit high fission–fusion dynamics, i.e., high levels of temporal variation in the size, composition, and spatial cohesion of subgroups, relative to other species, likely in response to local ecological conditions such as food availability and intragroup competition (Aureli et al. 2008).
Although the term “sexually segregated” was introduced conceptually early on in the primate literature to describe the social organization of spider monkeys (Fedigan and Baxter 1984), there have been no empirical studies that provide an operational measure —along with a statistical test procedure— to determine the actual occurrence of sexual segregation and whether it varies consistently over time as a function of ecological or social factors. One approach that has recently emerged to characterize association patterns in nonhuman primates statistically is social network analysis (SNA) (Ramos-Fernandez et al. 2009; reviewed in Sueur et al. 2011).SNA can use various measures of dyadic associations between known individuals, e.g., grooming frequencies, affiliation, subgroup membership, to display the relative strengths of different relationships between all known individuals in a social group.However, the use of SNA requires repeated interactions between recognizable individuals to construct an association matrix.
As an alternative method, the Sexual Segregation and Aggregation Statistic (SSAS;Bonenfant et al. 2007) is a statistical index that has been used to test for the occurrence of sexual segregation and aggregation in other taxa, e.g., Ovis ammon hodgsoni (Singh et al. 2010). SSAS uses the sex composition of subgroups of variable sizes over specified time intervals to distinguish periods of sexual segregation, i.e., when the sex ratio of each subgroup differs greatly from the population sex ratio; aggregation, i.e., the sex ratio of each subgroup is similar to the population sex ratio; and random association, i.e., males and females associate at rates expected by chance, given the population sex ratio). Unlike SNA, SSAS requires only a large enough sample of subgroups in which the sexes of the individuals can be identified, and it is thus ideally suited to analyzing subgroup compositions from newer study sites in which individual identities may not yet be known. In addition, SSAS can be used to determine whether a population or species is sexually segregated year-round, and whether temporal variation in segregation correlates to known social and/or ecological variables. In this manner, the occurrence of sexual segregation can also be detected across populations within species that occur in different ecological conditions across a geographic range.
In this study we use SSAS (Bonenfant et al. 2007) to test the occurrence of sexual segregation in spider monkeys by distinguishing sexual segregation from aggregation and random association between the sexes, and in so doing we provide the first study to explicitly test sexual segregation in a nonhuman primate species. Based on data we collected over 23 mo on a group of wild spider monkeys in Belize, we use SSAS to determine whether spider monkeys live in sexually segregated societies and whether they exhibit temporal variation in their patterns of segregation or aggregation. We then extend our analysis to investigate possible causal mechanisms that might underlie sexual segregation in spider monkeys, including variation in food availability, sex differences in activity budgets, dietary profiles, and estimated periods of fertility in females. Specifically, we test whether there is a correlation between sexual segregation and temporal variation in food availability. We predict that during periods of relatively low food availability males and females will be less likely to segregate as fewer patch options may concentrate more monkeys into larger, mixed-sex subgroups.
We then test for differences in the dietary profiles, activity budgets, and behavioral synchrony between males and females during periods of sexual segregation and nonsegregation. Male and female spider monkeys show only moderate sexual dimorphism in body size (1.12 male-to-female body weight: Di Fiore and Campbell 2007), which is below the empirical threshold of a 20 % difference for sexual segregation to occur (Ruckstuhl 2007).However, male philopatry and cooperative polygyny are predicted to require a greater sustained mating effort on the part of males as they foster social bonds to cooperatively patrol and defend a territory containing numerous females (Key and Ross 1999). If sexual segregation in spider monkeys is the result of necessary differences in the allocation of energy to specific activities, we would expect to observe different amounts of time allocated to different activities in males and females when they are segregated, as well as lower intrasubgroup behavioral synchrony. Similarly, we would expect that males and females might allocate different amounts of time to foraging on specific food types, as represented in the different dietary profiles of each sex.
We also test the possibility that estimated times of conception of infants and/or the 2-wk time periods surrounding infant births coincide with periods of nonsegregation in spider monkeys to determine whether female reproductive cycle has an effect on patterns of sexual segregation and aggregation. We predict that periods of random association or aggregation may coincide with estimated periods of maximum fertility in females, and/or with infant births, as newborn infants may attract adult members of both sexes to a subgroup.
Methods
Study Site
Runaway Creek Nature Reserve (RCNR) is a 2469-ha private reserve in central Belize (88°35′W and 17°22′N) comprising two main vegetative zones: pine savannah and semideciduous broadleaf tropical forest. It is part of a much larger area of continuous forest. At 20–120 m above sea level, RCNR is made up of a series of steep limestone karst hills, low valleys, and seasonal swamps. This area of Belize has two seasons, a dry season from January to May and a wet season from June to December in which it receives an estimated 2000–2200 mm of rain annually (Meerman 1999).We have collected data on the behavior and ecology of the spider monkeys at RCNRsystematically since June 2007. There are at least three groups (communities) of spider monkeys within the property boundaries of RCNR, which is connected to a larger population to the east and south of the reserve.
Focal Group
We studied one groupof spider monkeys over 23 mo from January 2008 to December 2009 (except for August 2008). All group members were habituated and rangedin an area of ca. 114 ha.Although there were some changes in individual age classifications over the 23 mo of data collectionas younger individuals matured and older ones disappeared, the number of adult males and females remained similar, with total group size varying between 31 and 35 individuals. In 2008 there were 11 adult females and 3 adult males, and in 2009 12 adult females and 3 adult males. Thus, throughout the period of data collection the focal group had a sex ratio of ca. 1:4 males to females. We identified all group members individually by differences in facial markings and characteristics of the anogenital region, and we distinguished males from females based on easily observable differences in genitalia. We distinguished adults from subadults by their larger, more robust body size, darker faces, and sexual maturity, i.e., adult males had fully descended testes and females had given birth to one or more infants (also indicated by fuller breasts and longer nipples).
Behavioral Data Collection
Our data collection protocol adhered to the legal requirements of Belize and complied with the protocols approved by the Canadian Council on Animal Care and the Animal Care Committee at the University of Calgary. We conducted full- or part-day follows of spider monkey subgroups and spent a total of 2352 h in the forest, during which we were in contact with monkeys for a total of 876 h.The majority of subgroup follows were part-day because maintaining contact with a subgroup for entire days was challenging because of the steep karst hills, cliffs, and valleys that characterize the terrain at RCNR.This was particularly true of subgroups containing male spider monkeys, which travel further and faster than females (Chapman 1990; Shimooka 2005; Spehar et al. 2010), and are thus more difficult to follow. As a result, we encountered and followed all-male subgroups less than all-female subgroups (which is not surprising given the sex ratio of 1:4 males to females); however, our analysis accounted for this bias by testing whether the sex compositions of subgroups for a given time period reflected the sex ratio of the population.
If subgroups containing males were more likely to be lost to observers, this could also bias data against mixed-sex subgroups, and therefore potentially bias results in favor of sexual segregation. However, we did not undersamplemixed-sex subgroups relative to same-sex subgroups owingto the difficulty in following males. Mixed-sex subgroups constituted only 16.4 % of the total number of subgroups lost to observers. The majority of subgroups lost were either all-male (20.3 %) or all-female (63.3 %), which is close to the expected sex ratio of the population.
We employed a “chain rule” (Ramos-Fernandez 2005) to define subgroups. We included any individual that was <50 m from another monkey as part of a subgroup.We arrived at this operational definition of a subgroup after observing that coordinated subgroup movement and vocal and visual communication occurred between individuals that were roughly a maximum of 50 m apart in most instances. We defined a fission as the point at which an individual moved farther than 50 m from any other subgroup member for >30 min, and a fusion as when an individual moved within 50 m of another subgroup member.
During full- or part-day follows of subgroups, we recorded the timeat first contact, as well as subgroup size, composition, and identity of recognizable individuals. At 30-min intervals, we conducted an instantaneous scan of all members of the subgroup, recording the subgroup size and composition, as well as the identity (or age/sex class) and behavior (feed, travel, inactive, social, or other) of each monkey present. When a monkey was feeding, we recorded the plant part and species. We collected a total of 1691 subgroup scans over the 2-yr study.We used 1492 of these in our analysis after omitting scans that did not include at least one fully mature adult.
Food Availability Sampling
We sampled a total of 21 40 m × 40 m (1600 m2) vegetation plots in the range of the focal group and in all habitat types used by the monkeys. To track temporal variation in ripe fruit availability, we monitored a phenology trail on a biweekly basis in 2009 (we did not collect phenological data in the first year of the study in 2008). The trail included 135 trees from 27 food tree species, each represented by 5 individual trees. We chose species for the phenology trail based on the top fruit species fed on by the spider monkeys at RCNR (not including vines) and that constituted a minimum of 1 % of their diet. We scored each tree with an estimate of ripe fruit on the crown as one of: 0 %, 25 %, 50 %, 75 %, or 100 %. We then took the mean percentage of fruit coverage for each tree species and multiplied it by the dominance of that species in the area. To provide a biweekly food availability score, we summed all scores across species for each biweekly period. The resulting scores are unitless measures of relative food availability for the year 2009. We used these to identify 2-wkperiods when food availability was relatively high compared to low, given the food tree species’ dominance and fruit phenology.
Data Analysis
Testing Sexual Segregation Using SSAS
Using the number of adult males and adult females recorded in subgroup scans (nonadults, such as subadults, could not be considered fully independent of their mothers), we used the SASS (Bonenfant et al. 2007) to test for random association between males and females against sexual segregation or aggregation at both monthly and biweekly time scales. The SSAS calculation uses all subgroup composition values, i.e., the number of adult males and females, collected over each specified time period.A monthly time scale was broad enough to capture larger temporal patterns, such as seasonal variation, and we used a biweekly scale to correlate sexual segregation with changes in food availability (Bowyer et al. 1996; Wiens 1989).The SSAS analysis accounts for biases in the data against specific types of subgroups, e.g., subgroups containing males, by testing whether the sex compositions of subgroups for a given time period deviate from the average sex ratio among all observed subgroups in the population, i.e., the data are not biased as long as the observed sex ratio of subgroups within biweekly or monthly time periods reflect the sex ratio of the population.
The SSAS equation is derived from the χ2 statistic and calculates an index value ranging from 0 (complete aggregation) to 1 (complete segregation). ASSAS value itself is not a meaningful continuous variable because it is dependent on subgroup size (Bonenfant et al. 2007). Because subgroup size cannot be held constant over time, SSAS values must be treated as categorical, e.g., sexually segregated, aggregated, or randomly associated, or binary (segregated or nonsegregated). The formulation of the SSAS is
where N = the total number of observed individuals, i.e., X + Y, for the specified time period, e.g., 1 mo); X = the total number of females observed per time period; Y = the total number of males per time period; XY is the number of males multiplied by the number of females sampled in the time period; k is the total number of subgroups sampled; i is the selected subgroup; N i is the subgroup size; and X i Y i is the number of males multiplied by the number of females in the subgroup. In cases in which we observed single individuals, we assigned a “group” score of 1 to the sex of the observed individual, and 0 to the other sex (Bonenfant et al. 2007).
To test the significance of the observed segregation/aggregation patterns, we ran a series of permutation tests using the same dataset to create an expected distribution of SSAS under the null hypothesis of random association between the sexes. The permuted contingency table retained the same row and column totals as the original contingency table in the data set so that individuals of a given subgroup were randomly assigned as male or female (Bonenfant et al. 2007).As with most ecological processes, sexual segregation changes with the observed spatio-temporal scale (Bowyer et al. 1996; Loe et al. 2006). We determined the time scale at which to test sexual segregation based on the amount of data available and we performed the permutations within biweekly and monthly time periods using the whole dataset. Although a shorter time scale, e.g., biweekly versus monthly, contains fewer samples within each time period, the SSAS permutation is still appropriate for testing sexual segregation because a SSAS value is not itself a meaningful continuous variable and it only interprets the test significance.
We permuted the contingency table 999 times to simulate an empirical distribution of SSAS to yield the upper and lower limits of random association between the sexes at the 5 % level. We identified time periods of sexual segregation or aggregation when the observed biweekly or monthly SSAS values fell above the upper limit (segregation) or below the lower limit (aggregation) of random association between the sexes. Male and female spider monkeys grouped at random when the observed SSAS fell within the upper and lower limits.
Sexual Segregation and Food Availability
Because association patterns between males and females might exhibit temporal variation as a function of food availability, we applied the SSAS at biweekly time intervals to test for associations between sexual segregation values and overall food availability (for the year 2009 only). We then used a logistic regression to determine whether biweekly variation in food availability predicted whether or not males and females segregated during particular biweekly time periods. We treated “segregated” vs. “nonsegregated” as the binary variable because SSAS values cannot be treated as continuous without holding subgroup size constant in time and food availability scores as the explanatory variable.
To test whether periods of nonsegregation were the result of larger subgroups associated with limited food supply, we performed an additional regression to determine if biweekly food availability scores correctly predicted (adult) mean subgroup size over the same biweekly periods.
Sex Differences in Activity and Diet
From the subgroup scans, we calculated the percent of time spent in each of the following five activities: feed, travel, inactive, social (allogroom, pectoral sniff and embrace, sit in body contact, and sexual and aggressive interactions), or other (place sniff, chest rub, and other scent marking behavior), and compared the activity budgets of males and females during biweekly periods of sexual segregation and nonsegregation, i.e., aggregation or random association. We first computed Cohen’s κ coefficient to examine activity agreement between males and females for all subgroups within a biweekly period. Cohen’s κ takes on positive values when animals engage in similar activities within a subgroup and negative values if they behave differently. We measured synchrony in behavior by comparing κ values of all subgroups within a given time period, e.g., if we observed 20 subgroups within a biweekly period, then we measured the κ coefficient forsynchrony in behavior for those 20 subgroups. We compared mean κ values across all subgroups of a given time period to zero (the theoretical value for random agreement) to determine whether subgroups were less synchronous in behavior when sexual segregation occurred than when males and females were aggregated or grouped at random.
We also calculated male and female dietary profiles from subgroup scans and compared the diets of males and females during periods of segregation and nonsegregation. We categorized plant parts as ripe fruit, unripe fruit, flowers, or leaves. We tested whether the proportion of plant parts eaten by males and females differed between periods of segregation and nonsegregation using a χ2 test.
We considered the use of general linear models (GLMs) to account for the repeated measures of diet and activity on the same individuals, but decided that theχ2was the most appropriate test. Although we obtained identical results with the mixed GLMas with the χ2 test, we lost ca. 10 % of data because the identities beyond age/sex class of some individuals were not known. In addition, the random effect was virtually absent (estimated to 0), which is consistent with the high variability in activity and foraging behavior of individuals over time, i.e., one particular monkey does not feed exclusively on flowers and never ripe fruit.
Female Fertility and Sexual Segregation
We used estimated conception times for 12 infants born over the course of the study to infer periods of maximum fertility in females. We estimated conception times using the exact birth dates of infants for two infants, and approximated birth dates based on the last time a female was seen without an infant and the first time she was seen with an infant for a further 10 infants. We identified newborn infants by their sparse pelage, almost entirely pink faces, and limp hanging tails which have limited grasping ability (Symington 1988). Infants also ride ventrally for the first few months after birth, staying close to the mother’s breast and nursing frequently. Female spider monkeys experienceca. 7 moof gestation (Eisenberg 1973; Milton 1981), so we estimated conception at 7 mo before the birth of an infant to within a 2-wk time period. Although this is a rough estimate of female fertility, more exact measures, such as hormonal data or observed copulations, were not available. We used a simple logistic regression to determine whether birth and/or conception periods predicted biweekly periods of nonsegregation, i.e., periods of aggregation or random association, treating “segregated” vs. “nonsegregated” as the binary variable with times of birth and conception as the explanatory variable.
We conducted all statistical analyses in Software R version 2.10.1. We set statistical significance at P = 0.05 and all tests were two-tailed.
Results
Monthly Variation in Sexual Segregation
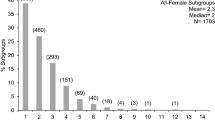
Subgroup size (adults only) ranged 1–11 individuals (mean = 3.5 ± SD 1.83 individuals). Of the 1492 scan samples we used in the SSAS analysis for 2008–2009, 76 % were all-female (N = 1129), 10 % were all-male (N = 142), and 15 % were a mix of adult males and females (N = 221).Based on the analysis of monthly sex-specific subgroup composition, we found that spider monkey subgroups were significantly sexually segregated in 15 of the 23 mo (65 % of mo; Fig. 1). For the remaining 8 mo, males and females grouped as predicted by random association. We never observed significant aggregation of males and females.
Males and females were segregated during 9 of 12 mo in 2009 (75 % of months) and only 6 of 11 study months in 2008 (55 % of months), but this difference was not significant (chi-square test: χ2 = 0.02, df = 1, P = 0.88). The timing of sexual segregation showed some consistency between the 2 yr. Males and females were segregated primarily from February to April and in September and October. The association of the sexes was random as opposed to segregated during May and June in both years.
Biweekly Variation in Food Availability and SSAS in 2009
Food availability scores ranged 2.3–15 (mean food score = 7.9 ± SD 4.1) and were highest in April–May and August–November, and lowest January, June, and July in 2009.Sexes were more likely to segregate when food availability was high (logistic regression: Waldχ2 = 4.56, df = 1, P = 0.033; Fig. 2). Food availability correctly predicted whether males and females would segregate in 76.5 % of all cases. There was no significant association between food scores and mean subgroup size (linear regression: r 2 = 0.029, df = 17, P = 0.487).
Biweekly variation in food availability and sexual segregation in spider monkeys at RCNR in 2009. Black and open circles indicate significant vs. nonsignificant sexual segregation, respectively, for the time period during which food availability was measured. Missing food scores are marked with an X.
Sex Differences in Activity Budgets
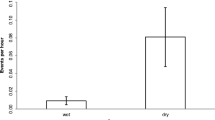
Based on a total of 3646 scan records (3004 for females and 642 for males) in the 1492 subgroup scans, we calculated that the study group spent 24.2 % of time feeding, 36.2 % inactive, 25.3 % traveling, 13.3 % in social activities, and 1.1 % engaged in other activities such as place sniffing, chest rubbing, and other scent marking behaviors. Overall, males and females differed significantly in their activity budgets (chi-square test: χ2 = 92.56, df = 4, P < 0.001; Fig. 3). Males spent significantly more time traveling (χ2 = 23.278, df = 1, P < 0.001) and engaging in other activities (χ2 = 29.336, df = 1, P < 0.001), although this was a very small percentage of their overall activity budget. In contrast, females spent more time being inactive (χ2 = 22.081, df = 1, P < 0.001). There was no sex differences in the time spent feeding (χ2 = 3.215, df = 1, P = 0.073), or engaged in social activities (χ2 = 0.14, df = 1, P = 0.708).
Using biweekly values, the mean κ between segregated and nonsegregated periods was not significant (Cohen’s κ coefficient: t = –1.922, P = 0.062).Mean κ values were < 0 for both segregated and nonsegregated periods, indicating that individuals within both periods were generally not synchronous in their activities (Fig. 4).However, consistent with the predictions of the activity budget hypothesis, the mean κ was lowest during periods of sexual segregation, which suggests that asynchronous behavior between individuals in subgroups correlates with sexual segregation.
Cohen’s κ coefficient for biweekly intervals during periods of sexual segregation and nonsegregation. Open circles are the κ coefficient values computed for all subgroups of a given time period. Black circles are the mean κ values, i.e., the mean of all open circles. The gray line highlights the difference in mean κ values between sexually segregated and nonsegregated periods.
Sex Differences in Diet
During years 2008–2009 the spider monkeys fed on approximately 100 species of trees, vines, and other epiphytic plants with fruit constituting 71 % of their diet, followed by 22 % immature leaves, 6 % flowers, and < 1 % other (i.e. insects, algae/moss, soil, limestone, and undetermined food items). Overall, adult males and females differed in diet (chi-square test: χ2 = 21.5, df = 3, P < 0.001). Both sexes fed primarily on ripe fruit, but females included significantly more leaves in their diet than did males (χ2 = 7.648, df = 1, P = 0.006), whereas males fed significantly more on ripe fruit (χ2 = 13.174, df = 1, P < 0.001).
Overall, there was a significant difference in the proportion of food items in the diet of males and females between segregated and nonsegregation periods (chi-square test: χ2 = 59.215, df = 12, P < 0.001). We found that females ate significantly more leaves than males during periods of no sexual segregation (χ2 = 9.67, df = 4, P = 0.04), but that males consumed more ripe fruits than females during periods of sexual segregation (χ2 = 12.88, df = 4, P = 0.01; Fig. 5).
The proportion of food items consumed by females and males during periods of sexual segregation and nonsegregation. F/no SS refers to females, nonsegregation; M/no SS to males, nonsegregation; F/SS to females, segregation; and M/SS to males, sexual segregation. Asterisk denotes food items that differed significantly in the diet of males and females during periods of sexual segregation and nonsegregation.
Female Fertility and Sexual Segregation
Twelve infants were born over the course of the data collection period. In the 2 yr combined, six infants were born in December and January (2008 and 2009, respectively), four in April–May (2009), and two in September–October. Based on a gestation period of ca. 7 mo, we estimated that in 2008–2009 a total of six infants were conceived in May–June (2008), four in October–September (2008), and two in February–March (2009).Contrary to our prediction, we found no significant relationship between either the timing of births (logistic regression: Wald χ2 = 0.077, df = 1, P = 0.781) or conceptions (logistic regression: Wald χ2 = 0.000, df = 1, P = 0.999) and periods of nonsegregation between adult males and females.
Discussion
We found that the spider monkeys at RCNR live primarily in sexually segregated societies, but patterns of sexual segregation varied slightly within and between the 2 study years. Sexual segregation appeared to follow a general pattern: males and females segregated for the majority of the year, except during the very beginning and end of the rainy season. Significant aggregation between the sexes never occurred in this group of spider monkeys.
We examined whether variation in one ecological variable —food availability— predicted the biweekly time periods in which the monkeys were sexually segregated over the course of 1 yr. We found a higher probability that males and females would segregate when overall food availability was relatively high, and that they were less likely to be segregated during the months when food availability was low. One possible explanation for this observation is that during periods of lower overall food availability, there may be fewer feeding locations from which to choose, which might lead to larger subgroups and an increasing likelihood of males and females coinciding at feeding sites. Conversely, when more food is available, there may be fewer constraints on sexual segregation as individuals have more feeding patches to choose from. However, we found no significant correlation between overall food availability and mean adult subgroup size. Although this does not necessarily mean that males and females were not coinciding at limited feeding sites, it suggests that, if they were, it was unlikely to be a chance artifact of larger subgroup sizes.
We then addressed the question as to whether there were any other factors that might drive male and female spider monkeys to associate with one another aside from the possible constraints imposed by limited feeding sites. Although spider monkeys do not display a distinct mating and birth season, one possibility is that the sporadic presence of sexually receptive females, or the birth of a new infant, could entice males into association with females. However, we found no evidence that males and females spend more time in mixed-sex associations either during periods surrounding estimated maximum female fertility or those surrounding infant births. Of course, one possible explanation for this observation is that, as asynchronous breeders, sexually receptive and/or new mother spider monkey females are distributed sparsely over time and space within a large home range, and they might therefore not have been captured during subgroup scan samples. This potential mechanism for sexual aggregation could be more directly evaluated with a longer-term dataset containing a larger sample of birth records, as well as more accurate physiological data on female reproductive status.
Why Do Spider Monkeys Segregate by Sex?
We tested two hypotheses concerning why the sexes are likely to segregate when the constraints of relatively lower food availability are relaxed. Sexual segregation as a result of differing energy budgets between males and females is hypothesized to occur when sexual dimorphism in body size and/or behavior requires each sex to optimize energetic intake and expenditure in relation to their respective fitness demands (Conradt 1998; Main et al. 1996; Ruckstuhl 1998).Despite low levels of sexual dimorphism in body size, we predicted that male philopatry and the cooperative defense of territorial boundaries containing females would place different energetic demands on males and females that would be evident in their respective activity budgets. As a consequence of satisfying sex-specific energy demands, we further predicted that behavioral synchrony within subgroups would be lower during periods of sexual segregation, as subgroup cohesiveness would become unstable and result in fission along sexual lines. Consistent with both predictions, we show that male and female spider monkeys differed significantly in the time they allocate to specific activities. Males were observed traveling more often than females, and females spent more time feeding and resting. We also demonstrate that activity synchronization in subgroups was lower during biweekly periods in which males and females were “segregated” vs. “nonsegregated.” This suggests that one possible mechanism of sexual segregation is the breakdown of subgroup stability due to differences in behavior between males and females (Conradt 1998; Conradt and Roper 2000; Ruckstuhl 1998).
Although both sexes of spider monkeys feed primarily on ripe fruit, we found that females included significantly more leaves in their diet than do males during periods of no segregation, but that males consumed more ripe fruit during periods of segregation. Like females in other sexually segregated species, e.g., Cervus elaphus (Clutton-Brock et al. 1982), Loxodonta africana (Stokke 1999), Diomedea spp. (Xavier et al. 2003), Delphinus delphis (Young and Cockcroft 1994), a more varied diet may provide a greater diversity of nutrients necessary for pregnancy and lactation. The preponderance of ripe fruit in the male diet may reflect the high energy demands of their daily activities when compared with the activity demands of females (males travel faster, have longer day ranges, and defend the territory: Chapman 1990; Shimooka 2005; Spehar et al. 2010). Moreover, because males range within a much larger area on a daily basis (Wallace 2008), they may come across more fruiting trees than are available in the smaller female ranges. In functional terms, these differences in activity and diet could result sex-specific strategies for fitness optimization, and from which emerge the unique social dynamics that are particular to a high fission–fusion dynamics system (Aureli et al. 2008). In spider monkeys, for instance, a relatively small number of strongly bonded, philopatric males, with low intra- but high intergroup competition for mates (Link et al. 2009; Shimooka et al. 2008) face the challenge of maintaining exclusive access to a much larger group of unrelated females with relatively weaker social bonds who are dispersed over a large home range.
Another factor that may contribute to social segregation, and that we did not test, is the active avoidance of males by females. Female directed aggression by males has been reported extensively in spider monkeys and at rates much higher than aggression directed by other females (Campbell 2003; Fedigan and Baxter 1984; Link et al. 2009). Although the function of female-directed aggression is unclear, it may represent an indirect form of sexual coercion (Link et al. 2009), and may even limit the ability of females to form social bonds in some animal species (Darden et al.2009). At RCNR, females also receive more aggression from males than from other females (unpubl. data), and they are often chased out of feeding trees when males join a subgroup of females. In a study by Slater et al. (2009), female spider monkeys spent significantly less time feeding in subgroups in which males were present than when males were absent and they fed at lower rates than males in mixed-sex subgroups. A male’s ability to displace females from higher quality ripe fruit patches may also explain the larger percentage of ripe fruit in the male diet, and the greater quantities of leaves that we observed in the female diet, specifically during periods of nonsegregation.
Another, nonmutually exclusive, possible mechanism of sexual segregation is the development of social preferences for same-sex associations. Social segregation attributed to affinities for same-sex group mates has been reported in a number of ungulate species (Bon 1991; Ruckstuhl and Neuhaus 2000), as well as in humans (Pellegrini and Long 2003), especially during juvenescence and adolescence. As the philopatric sex, spider monkey males are expected to invest more in social relationships with each other than are females, and indeed observations from other sites bear out this prediction (Fedigan and Baxter 1984).Although we did not observe a significant difference in rates of socializing between males and females, the adult males in the study group were more often observed with each other in cohesive subgroups than were females. Adult males at RCNR have also been reported to exhibit a preference for handling male infants over female infants, which has been interpreted as an attempt to foster relationships with future potential allies in cooperative territorial defense (Evans et al. 2012).Future research into the mechanisms of sexual segregation needs to address whether male and female spider monkeys experience different forms of socialization, and whether and when they develop and express preferences for socializing with same-sex group mates.
Sexual Segregation and Fission–Fusion Dynamics in Primates
In a recent reconceptualization of the term “fission–fusion societies,”Aureli et al. (2008) propose a three-dimensional framework within which species exhibiting particular variants of spatiotemporal group cohesion are represented. Specifically, the authors propose that mammalian social systems tend to vary along three fundamental dimensions, represented by subgroup size, composition, and spatial cohesion. Species that show high variability in all three dimensions are considered “high fission–fusion dynamics” species, in reference to the hypothesized complexity and dynamic nature of interactions between individual group members —including the inherent uncertainty of social relationships— that are thought inevitably to arise from spatiotemporal fluidity. Thus, spider monkeys, chimpanzees, and spotted hyenas (Crocuta crocuta), for example, are considered species with high fission–fusion dynamics because all three exhibit flexibility in the formation of subgroups, such that their composition, spatial cohesion, and size show consistent temporal variation (Aureli et al. 2008). The question, then, is where sexual segregation —a phenomenon that has also been described for all three of these species exemplars— might fit into this framework, if at all.
To date, no empirical studies have specifically addressed the relationship between sexual segregation and fission–fusion dynamics. Conradt and Roper (2000) developed a model to test the prediction that activity synchrony between the sexes leads to social segregation in red deer (Cervus elaphus). In their model, high fission-fusion dynamics are assumed a priori and, when combined with asynchrony in behavior between males and females, the predicted outcome is social segregation between the sexes. However, it remains unclear whether the causal factors implicated in leading to high fission–fusion dynamics in the first place, e.g., food availability and competitive regimes, might also underlie the likelihood of sexes segregating, or whether sexual segregation is a “first step” resulting from other causal mechanism(s), which then lead to the particular fission–fusion patterns exhibited by sexually segregated species. Although we do not purport to have adequately addressed these empirical or conceptual gaps in this study, we do propose that a starting point for future studies might be to consider sexual segregation as a “dimension-within-a-dimension,” namely that of subgroup composition. By its very definition, a subgroup’s composition incorporates the socially (and biologically) relevant characteristics of the individuals of which it is comprised, including sex. If the assortment of males and females in a given subgroup is not random, as is likely the case for a sexually segregated species, then there is a constraint on the potential variation in subgroup composition. This constraint then limits the degrees of freedom for different subgroup compositions within a given social group or community. In theory, then, sexually segregated species such as spider monkeys should be placed lower down the axis of variation in subgroup composition than has been proposed in the new fusion–fusion dynamics framework (Aureli et al. 2008). Although we recognize there are many possible constraints on subgroup compositions, e.g., ecological conditions and preferential associations between specific individuals, and that subgroup composition in mammals is likely not a random assortment of individuals in time and space, we also suggest that temporal variation in sexual segregation —as well as its underlying causal mechanisms— may be an additional and relevant underlying dimension of subgroup composition to consider when testing a null model within a fission–fusion framework.
References
Aureli, F., Schaffner, C. M., Boesch, C., Bearder, S. K., Call, J., Chapman, C. A., et al. (2008). Fission-fusion dynamics. Current Anthropology, 49, 627–654.
Boesch, C. (1996). Social grouping in Taï chimpanzees. In W. C. McGrew, L. F. Marchant, & T. Nishida (Eds.), Great ape societies (pp. 101–113). Cambridge, U.K: Cambridge University Press.
Bon, R. (1991). Social and spatial segregation of males and females in polygamous ungulates: Proximate factors. In F. Spitz, G. Janeau, G. Gonzalez, & S. Aulagnier (Eds.), Ongules/ungulates (pp. 195–198). Paris/Toulouse: SFEPM-IRGM.
Bonenfant, C., Gaillard, J., Dray, S., Loison, A., Royer, M., & Chessel, D. (2007). Testing sexual segregation: old ways are best. Ecology, 88, 3202–3208.
Bowyer, R. T. (2004). Sexual segregation in ruminants: definitions, hypotheses, and implications for conservation and management. Journal of Mammalogy, 85, 1039–1052.
Bowyer, T. R., Kie, J. G., & Van Ballenberghe, V. (1996). Sexual segregation in black-tailed deer: effect of scale. Journal of Wildlife Management, 60, 10–17.
Campbell, C. J. (2003). Female-directed aggression in free-ranging Ateles geoffroyi. International Journal of Primatology, 24, 223–237.
Campbell, C. J., & Gibson, K. N. (2008). Spider monkey reproduction and sexual behavior. In J. C. Campbell (Ed.), Spider monkeys: Behavior,ecology,and evolution of the genus Ateles (pp. 266–287). New York: Cambridge University Press.
Chapman, C. A. (1990). Association patterns of spider monkeys: the influence of ecology and sex on social organization. Behavioural Ecology and Sociobiology, 26, 409–414.
Clutton-Brock, T. H. (1983). Selection in relation to sex. In D. S. Bendall (Ed.), Evolution from molecules to men (pp. 457–481). Cambridge, U.K: Cambridge University Press.
Clutton-Brock, T. H., Iason, G. R., Albon, S. D., & Guiness, F. E. (1982). Effects of lactation on feeding behaviour and habitat use in wild red deer hinds. Journal of Zoology, 198, 227–236.
Conradt, L. (1998). Could asynchrony in activity between the sexes cause intersexual social segregation in ruminant? Proceedings of the Royal Society of London B: Biological Sciences, 265, 1359–1363.
Conradt, L. (1999). Social segregation is not a consequence of habitat segregation in red deer and feral soay sheep. Animal Behaviour, 57, 1151–1157.
Conradt, L., Clutton-Brock, T. H., & Guinness, F. E. (2000). Sex differences in weather sensitivity can cause habitat segregation: Red deer as an example. Animal Behaviour, 59, 1049–1060.
Conradt, L., & Roper, T. J. (2000). Activity synchrony and social cohesion: a fission-fusion model. Proceedings of the Royal Society of London B: Biological Sciences, 267, 2213–2218.
Cook, T. R., Cherel, Y., Bost, C. A., & Tremblayz, M. (2007). Chick-rearing Crozet shags (Phalacrocorax melanogenis) display sex-specific foraging behaviour. Antarctic Science, 19, 55–63.
Darden, S. K., James, R., Ramnarine, I. W., & Croft, D. P. (2009). Social implications of the battle of the sexes: sexual harassment disrupts female sociality and social recognition. Proceedings of the Royal Society of London B: Biological Sciences, 276, 2651–2656.
Darwin, C. (1871). The descent of man, and selection in relation to sex (1st ed.). London: John Murray.
Di Fiore, A., & Campbell, C. J. (2007). The Atelines: Variation in ecology, behavior, and social organization. In C. J. Campbell, S. Bearder, A. Fuentes, K. C. MacKinnon, & M. Panger (Eds.), Primates in perspective (pp. 155–185). New York: Oxford University Press.
Di Fiore, A., Link, A., Spehar, S. N., & Schmitt, C. A. (2009). Dispersal patterns in sympatric woolly and spider monkeys: integrating molecular and observational data. Behaviour, 146, 437–470.
Eisenberg, J. F. (1973). Reproduction in two species of spider monkeys, Ateles fusciceps and Ateles geoffroyi. Journal of Mammalogy, 54, 955–957.
Evans, K. J., Pavelka, M. S., Hartwell, K. S., & Notman, H. (2012). Do adult male spider monkeys (Atelesgeoffroyi) preferentially handle male infants? International Journal of Primatology, 33, 799–808.
Fedigan, L. M. (1992). Primate paradigms: Sex roles and social bonds. Chicago: University of Chicago Press.
Fedigan, L. M., & Baxter, M. J. (1984). Sex differences and social organization in free-ranging spider monkeys (Ateles geoffroyi). Primates, 25, 279–294.
Goodall, J. (1986). The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press.
Key, C., & Ross, C. (1999). Sex differences in energy expenditure in non-human primates. Proceedings of the Royal Society of London B: Biological Sciences, 266, 2479–2485.
Knott, C. D. (1999). Reproductive,physiological and behavioral responses of orangutans in Borneo to fluctuations in food availability. PhD dissertation, Harvard University, Cambridge, MA.
Link, A., Di Fiore, A., & Spehar, S. N. (2009). Female directed aggression and social control in spider monkeys. In M. N. Muller & R. W. Wrangham (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 157–187). Cambridge, MA: Harvard University Press.
Loe, L. E., Irvine, R. J., Bonenfant, C., Stien, A., Langvatn, R., Albon, S. D., et al. (2006). Testing five hypotheses of sexual segregation in an arctic ungulate. Journal of Animal Ecology, 75, 485–496.
Main, M. B., Weckerly, F. W., & Bleich, V. C. (1996). Sexual segregation in ungulates: new directions for research. Journal of Mammalogy, 77, 449–461.
Matsumoto-Oda, A., Hosaka, K., Huffman, M. A., & Kawanaka, K. (1998). Factors affecting party size in chimpanzees of the Mahale mountains. International Journal of Primatology, 19, 999–1011.
McCullough, D., Hirth, D., & Newhouse, S. (1989). Resource partitioning between the sexes in white-tailed deer. Journal of Wildlife Management, 53, 277–283.
Meerman, J. C. (1999). Rapid ecological assessment of Runaway Creek. Belize: Zoological Society of Milwaukee.
Milton, K. (1981). Estimates of reproductive parameters for free-ranging Ateles geoffroyi. Primates, 22, 574–579.
Newton-Fisher, N. E., Reynolds, V., & Plumptre, A. J. (2000). Food supply and chimpanzee (Pan troglodytes schweinfurthii) party size in the Budongo Forest Reserve, Uganda. International Journal of Primatology, 21, 613–628.
Pellegrini, A. D., & Long, J. D. (2003). A sexual selection theory longitudinal analysis of sexual segregation and integration in early adolescence. Journal of Experimental Child Psychology, 85, 257–278.
Ramos-Fernandez, G. (2005). Vocal communication in a fission-fusion society: Do spider monkeys stay in touch with close associates? International Journal of Primatology, 26, 1077–1092.
Ramos-Fernandez, G., Boyer, D., Aureli, F., & Vick, L. G. (2009). Association networks in spider monkeys (Ateles geoffroyi). Behavioural Ecology and Sociobiology, 63, 999–1013.
Ren, B., Li, D., Garber, P. A., & Li, M. (2012). Fission–fusion behavior in Yunnan snub-nosed monkeys (Rhinopithecus bieti) in Yunnan, China. International Journal of Primatology, 33, 1–14.
Ruckstuhl, K. E. (1998). Foraging behaviour and sexual segregation in bighorn sheep. Animal Behaviour, 55, 99–106.
Ruckstuhl, K. E. (2007). Sexual segregation in vertebrates: proximate and ultimate causes. Integrative and Comparative Biology, 47, 245–257.
Ruckstuhl, K. E., & Neuhaus, P. (2000). Sexual segregation in ungulates: a new approach. Behaviour, 137, 361–377.
Ruckstuhl, K. E., & Neuhaus, P. (2002). Sexual segregation in ungulates: a comparative test of three hypotheses. Biological Reviews of the Cambridge Philosophical Society, 77, 77–96.
Ruckstuhl, K. E., & Neuhaus, P. (2005). Sexual segregation in vertebrates: Ecology of the two sexes. New York: Cambridge University Press.
Sakura, O. (1994). Factors affecting party size and composition of chimpanzees (Pan troglodytes verus) Bossou, Guinea. International Journal of Primatology, 15, 167–183.
Shimooka, Y. (2005). Sexual differences in ranging of Ateles belzebuth belzebuth at La Macarena, Colombia. International Journal of Primatology, 26, 385–406.
Shimooka, Y., Campbell, C., Di Fiore, A., Felton, A. M., Izawa, K., Link, A., & Wallace, R. (2008). Demography and group composition of Ateles. In J. C. Campbell (Ed.), Spider monkeys: Behavior,ecology,and evolution of the genus Ateles (pp. 329–348). New York: Cambridge University Press.
Silk, J. B. (1999). Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Animal Behaviour, 57, 1021–1032.
Singh, N. J., Bonenfant, C., Yoccoz, N. G., & Cote, S. D. (2010). Sexual segregation in Eurasian wild sheep. Behavioral Ecology, 21, 410–418.
Slater, K. Y., Schaffner, C. M., & Aureli, F. (2007). Embraces for infant handling in spider monkeys: evidence for a biological market? Animal Behaviour, 74, 455–461.
Slater, K. Y., Schaffner, C. M., & Aureli, F. (2009). Sex differences in the social behavior of wild spider monkeys (Ateles geoffroyi yucatanensis). American Journal of Primatology, 71, 21–29.
Spehar, S. N., Link, A., & Di Fiore, A. (2010). Male and female range use in a group of white-bellied spider monkeys (Ateles belzebuth) in Yasuni National Park, Ecuador. American Journal of Primatology, 72, 129–141.
Stanford, C. B., Wallis, J., Mpongo, E., & Goodall, J. (1994). Hunting decisions in wild chimpanzees. Behaviour, 131, 1–18.
Stokke, S. (1999). Sex differences in feeding-patch choice in a megaherbivore: elephants in Chobe National Park, Botswana. Canadian Journal of Zoology, 77, 1723–1732.
Stumpf, R. M. (2007). Chimpanzees and bonobos. In C. J. Campbell, S. Bearder, A. Fuentes, K. C. MacKinnon, & M. Panger (Eds.), Primates in perspective (pp. 321–344). Oxford: Oxford University Press.
Sueur, C., Jacobs, A., Amblard, F., Petit, O., & King, A. J. (2011). How can social network analysis improve the study of primate behavior? American Journal of Primatology, 73, 703–719.
Symington, M. M. (1988). Demography, ranging patterns, and activity budgets of black spider monkeys (Ateles paniscus chamek) in the Manu National Park, Peru. American Journal of Primatology, 15, 45–67.
Trivers, R. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man: 1871–1971 (pp. 136–179). Chicago: Aldine.
Villaret, J. C., & Bon, R. (1995). Social and spatial segregation in Alpine ibex (Capra ibex) in Bargy, French Alps. Ethology, 101, 291–300.
Wallace, R. B. (2008). Factors influencing spider monkey habitat use and ranging patterns. In J. C. Campbell (Ed.), Spider monkeys: Behavior, ecology, and evolution of the genus Ateles (pp. 138–154). New York: Cambridge University Press.
Watts, D. P. (2005). Sexual segregation in non-human primates. In K. E. Ruckstuh & P. Neuhaus (Eds.), Sexual segregation in vertebrates: Ecology of the two sexes (pp. 327–347). New York: Cambridge University Press.
Whitehead, H., Waters, S., & Lyrholm, T. (1991). Social organization of female sperm whales and their offspring: constant companions and casual acquaintances. Behavioral Ecology and Sociobiology, 29, 385–389.
Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology, 3, 385–397.
Wrangham, R. W. (1979). Sex differences in chimpanzee dispersion. In D. A. Hamburg & E. R. McCown (Eds.), The great apes (pp. 481–489). Menlo Park, CA: Benjamin/Cummings.
Wrangham, R. W., & Smuts, B. B. (1980). Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility, 28(Supplement), 13–31.
Xavier, J. C., Croxall, J. P., & Reid, K. (2003). Interannual variation in the diets of two albatross species breeding in South Georgia: implications for breeding performance. Ibis, 14, 593–610.
Young, D. D., & Cockcroft, V. G. (1994). Diet of common dolphins (Delphinus delphis) off the south-east coast of southern Africa – opportunism or specialization. Journal of Zoology, 234, 41–53.
Acknowledgments
Thanks to Dr. Gil and Lillian Boese from the Zoological Society of Milwaukee and the Foundation for Wildlife Conservation for permission to work in RCNR and Birds without Borders/Aves sin Fronteras for their ongoing support and assistance with this project. Stevan Reneau in particular spent hundreds of hours assisting in data collection. Sharon Matola of the Belize Zoo is responsible for initiating the discussions that led to the establishment of this project in 2007, and we thank the staff at the Belize Zoo and Tropical Education Center for ongoing assistance of all kinds.We thank Kathreen Ruckstuhl for putting us in touch with Christophe Bonenfant and launching what is proving to be a very productive collaboration. We also thank Brittany Dean for assisting in data collection. This research was supported by the Natural Sciences and Engineering Research Council of Canada, the National Geographic Society, the University of Calgary, and Athabasca University. We also thank two anonymous reviewers and the editor for their careful and thoughtful reviews and constructive feedback.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartwell, K.S., Notman, H., Bonenfant, C. et al. Assessing the Occurrence of Sexual Segregation in Spider Monkeys (Ateles geoffroyi yucatanensis), Its Mechanisms and Function. Int J Primatol 35, 425–444 (2014). https://doi.org/10.1007/s10764-013-9746-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-013-9746-0