Abstract
Contact calls, which function to coordinate group movement and maintain contact between conspecifics, are predicted to show high levels of acoustic variability and individual distinctiveness. We investigated interindividual variation in whinnies, a contact call, between two geographically distinct communities of wild Geoffroyi’s spider monkeys (Ateles geoffroyi), which were experiencing different degrees of stability in membership due to immigration. We recorded whinnies from 18 subjects, including 9 females ranging within the Otoch Ma’ax Yetal Kooh Reserve, Punta Laguna, Mexico, and 9 females ranging within the Santa Rosa Sector, Area de Conservación Guanacaste, Costa Rica. We examined 13 acoustic parameters of female whinnies using principal component analysis and discriminant function analysis. Individual acoustic variability was significantly different between the two communities. A higher percentage of the whinnies of females were assigned to the correct caller in the community with only 3 individuals immigrating within 36 mo before and during data collection than in the community with 15 immigrant individuals during the same period. We suggest that the variation in interindividual distinctiveness for each community was influenced by the stability of the vocal environment, which was quantitatively different between communities because of changes in membership.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individual recognition via vocalizations is important for effective communication, particularly when individuals are separated from visual contact (Spillmann et al. 2010), and it can occur in a variety of situations including parent–offspring interactions, kin recognition, mate-pair recognition, and intragroup interactions (Amorim and Vasconcelos 2008; Aubin and Jouventin 2002; Catchpole and Slater 2008; Mulard et al. 2008; Waser 1977). Individual recognition reflects a vocal identity or in-group membership when intraindividual variation of acoustic parameters is less than interindividual variation (Amorim and Vasconcelos 2008; Bee et al. 2001). The production of distinctive calls and their discrimination is expected whenever there is a positive tradeoff between the benefits and costs of being recognized (Wiley 1994). Individual distinctiveness in the acoustic features of calls has been documented in several species of nonhuman primates, including the pant-hoots of chimpanzees (Pan troglodytes schwienfurthii: Notman and Rendall 2005), long calls of male orangutans (Pongo spp.: Delgado 2007), loud calls of male baboons (Papio cynocephalus ursinus: Fischer et al. 2002), screams of juvenile vervets (Cercopithecus aethiops: Cheney and Seyfarth 1980), whinnies of spider monkeys (Ateles geoffroyi: Chapman and Weary 1990; Teixidor and Byrne 1997, 1999), lost calls and food calls of capuchins (Cebus capucinus: Digweed et al. 2007; Gros-Louis 2006), phee calls and long calls of Wied’s marmosets (Callithrix kuhlii: Jorgensen and French 1998; Rukstalis et al. 2003), and phee calls of common marmosets (C. jacchus: Jones et al. 1993).
Of the multiple calls in a species vocal repertoire, contact calls function to coordinate community movement or to instigate and maintain contact between conspecifics (Kondo and Watanabe 2009; Marler 2004). Given that contact calls operate in intragroup social interactions, selection for individual distinctiveness is more likely in contact calls than in call types for which caller identification is not the primary function, e.g., alarm or intergroup loud calls (Bouchet et al. 2012). For example, of the previously mentioned call types that contain individually distinct cues, several are classified as contact calls, including chimpanzee pant-hoots (Notman and Rendall 2005), male orangutan long calls (Delgado 2007), spider monkey whinnies (Chapman and Weary 1990; Teixidor and Byrne 1997, 1999), capuchin lost calls (Digweed et al. 2007), and marmoset phee calls (Jones et al. 1993; Rukstalis et al. 2003). Although the basic categories of calls are thought to be innate and fixed, modification of the structure of existing calls is possible and enables a degree of vocal plasticity (Egnor and Hauser 2005; Fedurek and Slocombe 2011). For example, adult female Campbell’s monkeys (Cercopithecus campbelli) produce a usually stable combined-harmonic contact call of which various acoustic features are individually specific. Some of these acoustic features change in response to changes in the community members’ social interactions and may function to advertise recently established affiliative bonds between individuals (Lemasson and Hausberger 2004). The acoustic similarities of these calls are also better explained by the amount of time females spend grooming one another, rather than genetic similarities between females (Lemasson et al. 2011).
Vocal plasticity can occur when individuals are exposed to social learning opportunities in their acoustic environment, including the calls of conspecifics (Snowdon and Elowson 1999). Changes in community membership, as well as changes in affiliation between individuals, create significant social variability (Rukstalis et al. 2003) and affect opportunities for social learning to occur. Aspects of individuals’ vocal communication that have a learned component are therefore likely affected by community membership changes as novel vocal elements are introduced into their acoustic environment and more familiar vocal elements cease to be practiced. Several species modify the acoustic structure of their calls as a result of changes to their social and therefore vocal environment, such as the arrival of immigrants, e.g., budgerigars (Melopsittacus undulates: Bartlett and Slater 1999), pygmy marmosets (Cebuella pygmaea: Snowdon et al. 1997), and acoustic contact with an unfamiliar group, e.g., budgerigars (Farabaugh et al. 1994), yellow-naped amazon parrots (Amazona auropalliata: Wright et al. 2008). Thus, a community experiencing greater stability in its membership, e.g., fewer immigrants, will exhibit a more stable vocal environment than a community experiencing lesser stability in its membership, e.g., more immigrants, as different vocal learning opportunities arise.
Individual vocal discrimination is particularly important for arboreal species as group members are often out of visual contact with one another (Ghazanfer and Santos 2004). For the same reason, it should be fundamental for species with a social organization characterized by a high degree of fission–fusion dynamics (Benson-Amram et al. 2011), where individuals from a stable community merge and separate over the course of hours, days, and weeks, resulting in subgroups of highly variable membership (Aureli et al. 2008). Spider monkeys (Ateles spp.) have such a social organization that requires consistent monitoring of subgroup membership for effective social interactions (Aureli and Schaffner 2008), and likely rely on individual vocal discrimination during intra- and intercommunity interactions (Ramos-Fernández 2005; cf. Crockford et al. 2004).
The vocal repertoire of Geoffroyi’s spider monkey contains ca. 14 call types, and the “whinny” is the most common call produced (Chapman et al. 1989; Eisenberg 1976; Ramos-Fernández 2008). Whinnies are contact calls (Eisenberg 1976) that can carry for up to 300 m (Ramos- Fernández 2005) and often elicit a “whinny” response from one or more subgroup members (Ramos-Fernández 2008). The main function of whinnies is the maintenance of contact between community members (Eisenberg 1976; Ramos-Fernández 2005). Over larger distances, loud calls may be given to establish contact between highly dispersed subgroups (Spehar and Di Fiore 2013). Individual distinctiveness in whinnies has been already identified in previous studies of the same species (Chapman and Weary 1990; Ramos-Fernandez 2005; Teixidor and Byrne 1997, 1999). We investigated whether individual distinctiveness differs with the degree of stability in community membership by examining the nature of variation in the assignment of calls to the correct individual in two geographically distinct communities. We predicted that the community experiencing greater stability in its membership, i.e., fewer immigrants, and therefore a more stable vocal environment, would have a higher level of correct call assignment to individuals than the community experiencing lesser stability in its membership.
Methods
Ethical Note
Research was conducted at all times in accordance with the laws of participating countries. Permission to conduct research was granted by the University of Chester Psychology Department Ethics Committee, the Costa Rica Ministry of Environment and Energy (MINAE) permit no. ACG-PL-030-2006, and the Mexican government permit no. SGPA/DGVS/ 00910/13.
Study Sites and Subjects
We collected calls from individuals in two communities of wild Geoffroyi’s spider monkeys (Ateles geoffroyi) that were identified using unique facial and body characteristics. One community ranged within the Otoch Ma’ax Yetel Kooh Reserve, Punta Laguna, Yucatan Peninsula, Mexico (hereafter the Punta Laguna community) (20°38′ N, 87°37′ W, 25 m elevation). The other community ranged within Santa Rosa Sector, Area de Conservación Guanacaste, Costa Rica (hereafter the Santa Rosa community) (10°50′ N, 85.37′ W, 25 m elevation). These two distinct geographic regions are phenologically similar, with seasonally dry tropical climates and a mosaic of primary and regenerating forests (Janzen 1986; Ramos-Fernández and Ayala-Orozco 2003). During the study period monthly rainfall totaled 2340 mm at Punta Laguna and 2648 mm at Santa Rosa (data courtesy of M. M. Chavarria Diaz, Santa Rosa and A. Alpuche Castillo, Comision National del Agua, Mexico). During the study period the Santa Rosa community consisted of 27–30 individuals, and the Punta Laguna community of 22–23 individuals (Table I). Before the study period the number of male and female adults and subadults that were resident for >36 mo was 10 (out of 25 adults and subadults = 40%) in the Santa Rosa community and 13 (out of 16 adults and subadults =81%) in the Punta Laguna community. During the 36 mo before the study period, 11 (44%) individuals immigrated into the Santa Rosa community and 2 (13%) immigrated into the Punta Laguna community. In addition, 4 (16%) individuals immigrated into Santa Rosa community and 1 (6%) immigrated into Punta Laguna community during the study period. Newly immigrant females were classified as those joining a community within the period of data collection. All other females from which calls were collected and analyzed had been resident in the community for ≥1 yr before recording calls commenced.
Data Collection
We collected data over an 18-mo study period, which incorporated wet and dry seasons at each site. In 2006 we collected data at Punta Laguna from January until mid-May and at Santa Rosa from mid-May through September. In 2007 we collected data at Santa Rosa from January until mid-May and at Punta Laguna from mid-May through September. Whinnies were recorded on an ad libitum basis from each community using a Sennheiser MKH shotgun microphone and a Marantz PMD 671 digital recorder. Visual identification of the caller and the context in which the monkey called had to be unambiguous for the call to be recorded. To reduce interference from background noise the distance between the caller and the microphone was kept to ≤15 m.
Acoustic Analysis
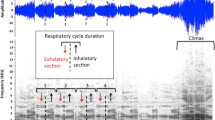
After sampling, we selected calls based on those with a high signal-to-noise ratio. Recordings were digitized using Avisoft SASLab Pro 4.40 (R. Sprecht, Berlin, Germany) with a sampling frequency of 44.1 kHz and 16 bits. We created spectrograms for each call using a 256-point fast-Fourier transform, flat top window function, bandwidth 648 Hz, and resolution 172 Hz. We measured 13 acoustic parameters from each spectrogram using the in-built program cursers (chosen based on Chapman and Weary 1990; Ramos-Fernández 2005; Teixidor and Byrne 1999; Catherine Crockford pers. comm., October 31, 2006). Nine of these parameters were temporal measurements and the remaining four were frequency measurements (Fig. 1; Table II).
Spectrogram of Geoffroyi’s spider monkey ‘whinny’ call showing examples of the 13 vocal parameters measured for analysis. Numbers in brackets refer to the corresponding numbers for each parameter in Table II.
Statistical Analysis
We used a stepwise discriminant function analysis (DFA) to classify calls into predetermined, discrete categories, i.e., individual identities, based on their vocal structure. Before DFA we applied principal component analysis (PCA) to reduce the 13 acoustic parameters to a smaller set of uncorrelated variables in order to minimize problems associated with multicollinearity and to ensure that the smallest sample group exceeded the number of predictor variables, and thereby protect from type I errors (Tabachnick and Fidell 2007). We used a varimax rotation and an eigenvalue >1.0 to determine the components that were extracted from the PCA (Tabachnick and Fidell 2007). All DFA tests were cross-validated using a leave-half-out procedure, creating the DFA model with half of the data set and then randomly testing it against the other half to test the classification power on a different set of calls than those on which the function was constructed. We set default levels used for entry criterion for the F value at 3.48 and removal was 2.71 (Tabachnick and Fidell 2007). We used χ2 goodness-of-fit tests to determine if classification of call assignment differed from chance classification following the DFA. We used a paired t-test to examine the difference in correct classification of community individuals’ calls when newly immigrant females were included and excluded. As the chance level differed owing to the inclusion and exclusion of newly immigrant females, we used the increase above chance level in correct cross-validated classification for each of the six remaining females to compare the two conditions. We used an unpaired t-test to determine whether correct call classification differed between communities. Statistical tests, except for the χ2 tests, were performed using SPSS version 17.0.
Call Sample Sizes
Obtaining multiple, good quality calls from a large number of male monkeys was difficult as they travel more frequently at the boundaries of their home ranges than females (Chapman 1990) and were not encountered as often or for as long as females. For these reasons we analyzed only calls from females for this study. We collected a variable number of calls from each female. We excluded females with fewer than 10 viable calls from analysis. As our preliminary analysis on call parameters confirmed that there was no significant structural difference in the whinnies of adult and subadult females, individuals from these two age categories were analyzed together. These criteria provided viable calls from nine females from each community. For each female, we chose 10 calls randomly for analysis.
Results
Santa Rosa
Five components were extracted using PCA that together explained 80% of call variance between individual females. The temporal call parameters loaded highly on the first three components, whereas frequency parameters loaded highly on the remaining two components (Table III).
The DFA procedure resulted in three functions that accounted for 100% of call variation between Santa Rosa females. The first function explained 60% of variance, the second 30%, and the third 10%. All three of these functions contributed significantly to explaining call variation (Table IV). Of the 90 calls analyzed, 41% were assigned to the correct caller, which was significantly better than classification by chance, which was 11% (χ2 test: χ2 = 96.816, df = 8, P < 0.001). With cross-validation 30% of calls were classified correctly, which was also significantly better than classification by chance (χ2 test: χ2 = 61.078, df = 8, P < 0.001). Correct classification varied between 0 and 60% across individuals (Table V).
When analyses for the Santa Rosa community were re-run excluding the three newly immigrant females, classifications improved considerably. Using PCA five components were extracted that explained 81% of call variance (Table VI). Temporal parameters loaded highly on components 1, 2, and 4, whereas frequency parameters loaded highly on components 3 and 5.
DFA resulted in four functions that accounted for all call variance, with the first function explaining 52.4%, the second 32.1%, the third 13%, and the fourth 2.4%. The first three functions contributed significantly to call variance (Table VII). Of the 60 calls classified, 60% were assigned to the correct caller, which was significantly better than classification by chance, which was 16.7% (χ2 test: χ2 = 69.757, df = 5, P< 0.001). These results held for the cross-validated procedure with 55% of calls correctly assigned (χ2 test: χ2 = 57.194, df = 5, P < 0.001). Correct classification varied between 40 and 70% for individuals (Table VIII). The increase above chance level in correct cross-validated classification of the six females was not significantly higher when newly immigrant females were excluded from the analysis procedure (55 ± 4.944) than when they were included (30 ± 7.993; paired t-test: t(5) = 1.999, P = 0.102).
Punta Laguna
Five components were extracted in PCA, which together explained 80% of call variance between individual females. Temporal variables contributed heavily to the first three components, and frequency variables (with the exception of duration of first modulation) loaded highly on the fourth and fifth components (Table IX).
The DFA procedure resulted in five functions that explained 100% of call variance between individual females in the Punta Laguna community. The first function explained 35.1% of this variation, the second 27.5%, the third 23.8%, the fourth 11.3%, and the fifth 2.3%. The first four of these functions were significant (Table X). Of the 90 calls analyzed, correct classification was considerably higher than for the Santa Rosa community, with 64.4% of calls assigned to the correct caller. This classification was significantly better than classification by chance, which was 11% (χ2 test: χ2 = 229.406, df = 8, P < 0.001). Cross-validated results classified 57.8% of calls correctly, which was also significantly better than classification by chance (χ2 test: χ2 = 180.75, df = 8, P < 0.001). Correct classification varied between 30 and 100% for individuals (20–80% cross-validated) (Table XI). Excluding the only newly immigrant from the analyses did not affect results for Punta Laguna females, with cross-validated classification 57.5%.
When we compared the cross-validated classification levels between the two communities we found a significant difference in individual classification between the nine Santa Rosa females and the nine Punta Laguna females (unpaired t-test: t(16) = 2.64, P = 0.018; Fig. 2), indicating that the individual calls of Punta Laguna females were assigned to the correct caller significantly more often than the individual calls of Santa Rosa females.
Mean (±SE) cross-validated correct call classification of the two Geoffroyi’s spider monkey study communities at Santa Rosa and Punta Laguna during 2006–2007. The hash line indicates a chance classification rate (11%). There is a significant difference in intragroup individual call classification between the two communities (unpaired t-test: t(16) = 2.64, P = 0.018).
Discussion
As in previous studies examining whinnies of Geoffroyi’s spider monkeys, we found evidence that calls contain information about the identity of the caller (Ramos-Fernández 2008). Our acoustic analysis provides evidence that variation in correct call assignment may be related to the stability in community membership. Although in each community females’ calls were assigned to the correct caller significantly better than chance, the two communities showed different levels of correct call assignment. Significantly more calls were assigned to the correct caller in the Punta Laguna community than in the Santa Rosa community, which experienced more changes in membership. Within the Santa Rosa community, classification to the correct caller was higher when immigrants were removed from the analysis than when they were included, but not significantly higher, a result that could have been due to small sample size (N = 6). Further, the PCA conducted on each community identified different underlying components to describe the variance between females’ calls (Tables III and IX). Our PCA results suggest that variance between females’ calls within the same community was not dominated by one specific call characteristic, e.g. differences in call duration or differences in frequency range, but was accounted for by individual variation in the use of several call parameters. This finding is consistent with many studies showing that significant variation in animals’ calls lies in multiple acoustic parameters (Ehret 1990; Fischer et al. 2001; Hammerschmidt and Todt 1995; Owren et al. 1992).
Various factors might account for the differences we observed across the two sites other than the degree of stability in community membership. It is possible that some variation in the acoustic structure of females’ calls from the two communities was due to habitat differences affecting call transmission of recordings (Brown and Gomez 1992). However, this is unlikely as all recordings took place in areas with little undergrowth, resulting in a clear visual path between the recorder and caller, and the distance between the recorder and caller was never >15 m. The possibility that body size differences in females between sites accounted for the variation (Fitch 1997; Fischer et al. 2002) is also unlikely, as no discernible size differences were observed and no such differences have been reported for Geoffroyi’s spider monkey (Ford and Davis 1992). The degree of genetic relatedness among the analyzed females in each community was low, except for a single mother–daughter dyad in the Punta Laguna community (F. Aureli, A. Di Fiore, and C. Schaffner unpubl. data), and there were no errors in the classification of whinnies between them. That the majority of females were not first-, second-, or even third-degree relatives suggests genetics do not play a large role in successful whinny classification in our study. Given the likely negligible impact of the above three factors, stability in community membership, i.e., the relative number of new immigrants in the community, remains an important factor that may account for the variation in successful call assignment across sites.
Residency duration was very different between the two communities: 81% of adult and subadult individuals resided in the Punta Laguna community for >3 yr before the study, whereas only 40% of individuals did so in the Santa Rosa community. In addition to the four females who joined the Santa Rosa community during the data collection period, in the 5 mo before data collection five males joined the community and the long-term resident males disappeared along with other adult and juvenile community members (Aureli et al. 2013). This is not typical for spider monkey communities (Shimooka et al. 2008). Thus, stability of membership was different between the two communities, a factor known to affect levels of affiliation among spider monkeys and overall community cohesion in captive settings (Pastor-Nieto 2001).
Changes in community membership likely affected the vocal environment of the Santa Rosa community, as the relatively high number of recent immigrant individuals, likely coming from different communities, probably introduced a number of novel call elements, resulting in an influx of unfamiliar acoustic cues. In previous studies, monkeys appear to share more vocal characteristics as a means to establish or strengthen new social relationships. For example, in female Campbell’s monkeys the sharing of vocal variants between individuals was suggested to occur more often in socially disturbed groups than in stable groups, as a way of advertising the formation of novel affiliative bonds to other group members (Lemasson and Hausberger 2004). Pygmy marmosets also modify characteristics of their trill calls after pairing with an unfamiliar individual, including duration, peak frequency, and modulation rate (Snowdon and Elowson 1999). Thus, the presence of more recent immigrants in the Santa Rosa community might have resulted in a greater intraindividual variation in call structure.
As there is evidence for community-specific parameters in Geoffroyi’s spider monkey whinnies (Santorelli, C., Aureli, F., Ramos-Fernández, G. & Schaffner, C. unpubl. data), the lower level of call assignment to the correct individual in the Santa Rosa community may capture a transitional acoustic phase as individuals adjust to changes in community membership. Call convergence, i.e., when novel acoustic features are incorporated into an existing repertoire, also results in individually distinct acoustic features being reduced (Candiotti et al. 2012), and may be the process through which this transitional acoustic phase occurs. Further, like unfamiliar male budgerigars forming new “cage”-specific calls when housed together (Farabaugh et al. 1994) and pygmy marmosets sharing elements of their trill vocalization when newly paired (Snowdon and Elowson 1999), the ability to adjust call structure in the face of changing community membership may not be restricted to new immigrants. Indeed, our study indicated that poor classification also included females that belonged to the community for several years at both sites. In Punta Laguna the lowest correct classification level belonged to a female, which was in the community as an adult in 1997, and a female that joined the community 17 mo before data collection. In Santa Rosa they were from a new immigrant, a female that had been an established adult in the community at least since 2003, and a female that was a juvenile in the community in 2003 and a subadult by the time data collection began. This pattern of findings has two potential implications: Successful individual call classification is not always associated with how long a female has resided in a community, as females that belonged to a community for several years did not necessarily have a more distinctive call structure than newer immigrants, and both new and longer term residents may also change elements of their call structure when exposed to new call variants.
There is also variation in the extent of correct call assignment across studies conducted on the same Santa Rosa community. In our study correct call classification was 30%, whereas it was 44% in Chapman and Weary (1990) and 72% in Teixidor and Byrne (1999). In addition to possible differences in membership stability, differences in methodological procedures between our study and the two previous studies may have contributed to the discrepancy between correct assignment levels. We collected calls from a closer distance (≤15m) than Chapman and Weary (1990), which may have increased the background noise they recorded, reducing the fine details of auditory signals. Additional temporal and frequency parameters were also measured in our study. Further, we applied PCA to the data before DFA, whereas neither earlier study did so. The earlier studies also used smaller sample sizes for analysis and included individuals from different age cohorts, and individual variation was likely heightened by variation due to age differences (cf. Fischer 2002). Finally, although the three studies focused on the same community, it is highly likely that monkey subjects were different in each study, as there was nearly a decade between the two closest data collection periods.
Our analyses confirm that individual distinctiveness is a feature of whinnies, and demonstrate its variability between communities, particularly when there is high turnover in community membership. Our findings suggest that vocal learning, when an individual modifies the acoustic structure of a vocalization as a result of experience (Janik and Slater 2000), is an ongoing process in Geoffroyi’s spider monkeys, as it occurs in adulthood and is strongly supported by the possibility that both new and longer term residents may change elements of their call structure when exposed to new call variants. Investigating the influence of call convergence as a possible mechanism underlying this learning processes would be an intriguing possibility for future research. Overall, our study provides an important and novel contribution to the understanding of how changes in the vocal environment, such as those due to immigrant individuals, can lead to novel acoustic features being incorporated into a current repertoire.
References
Amorim, M. C. P., & Vasconcelos, R. O. (2008). Variability in the mating calls of the Lusitanian toadfish Halobatrachus didactylus: cues for potential individual recognition. Journal of Fish Biology, 73, 1267–1283.
Aubin, T., & Jouventin, P. (2002). How to vocally identify kin in a crowd: the penguin model. Advances in the Study of Behaviour, 31, 243–277.
Aureli, F., Di Fiore, A., Murillo-Chacon, E., Kawamura, S., & Schaffner, C. M. (2013). Male philopatry in spider monkeys revisited. American Journal of Physical Anthropology. doi:10.1002/ajpa.22331.
Aureli, F., & Schaffner, C. M. (2008). Social interactions, social relationships and the social system of spider monkeys. In C. J. Campbell (Ed.), Spider monkeys: Behavior, ecology and evolution of the genus Ateles (pp. 236–265). Cambridge, U.K.: Cambridge University Press.
Aureli, F., Schaffner, C. M., Boesch, C., Beader, S. K., Call, J., Chapman, C .A., et al. (2008). Fission-fusion dynamics: New research frameworks. Current Anthropology, 49, 627–654.
Bartlett, P., & Slater, P. J. B. (1999). The effect of new recruits on the flock specific call of budgerigars (Melopsittacus undulatus). Ethology, Ecology and Evolution, 11, 139–147.
Bee, M. A., Kozich, C. E., Blackwell, K. J., & Gerhardt, H. C. (2001). Individual variation in advertisement calls of territorial male green frogs, Rana clamitans: implications for individual discrimination. Ethology, 107, 65–84.
Benson-Amram, S., Heinen, V. K., Dryer, S. L., & Holekamp, K. E. (2011). Numerical assessment and individual call discrimination by wild spotted hyaenas (Crocuta crocuta). Animal Behaviour, 82, 743–752.
Bouchet, H., Blois-Heulin, C., Pellier, A.-S., Zuberbühler, K., & Lemasson, A. (2012). Acoustic variability and individual distinctiveness in the vocal repertoire of red capped mangabeys (Cercocebus torquatus). Journal of Comparative Psychology, 126, 45–56.
Brown, C. H., & Gomez, R. (1992). Functional design features in primate vocal signals: The acoustic habitat and sound distortion. In T. Nishida, W. M. McGrew, P. Marler, M. Pickford, & F. B. M. de Waal (Eds.), Topics in primatology (Vol. 1). Tokyo: University of Tokyo Press.
Candiotti, A., Zuberbühler, K., & Lemasson, A. (2012). Convergence and divergence in Diana monkey vocalizations. Biology Letters, 8, 382–385.
Catchpole, C. K., & Slater, P. J. B. (2008). Bird song: Biological themes and variations (2nd ed.). Cambridge, U.K.: Cambridge University Press.
Chapman, C. A. (1990). Association patterns of spider monkeys: the influence of ecology and sex of social organization. Behavioral Ecology and Sociobiology, 26, 409–414.
Chapman, C. A., Chapman, L. J., & Lefebvre, L. (1989). Spider monkey alarm calls: honest advertisement or warning kin? Animal Behaviour, 39, 197–198.
Chapman, C. A., & Weary, D. M. (1990). Variability in spider monkeys’ vocalizations may provide basis for individual recognition. American Journal of Primatology, 22, 279–284.
Cheney, D. L., & Seyfarth, R. M. (1980). Vocal recognition in free-ranging vervet monkeys. Animal Behaviour, 28, 362–367.
Crockford, C., Herbinger, I., Vigilant, L., & Boesch, C. (2004). Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology, 110, 221–243.
Delgado, R. A. J. (2007). Geographic variation in the long calls of male orangutans (Pongo spp.). Ethology, 113, 487–498.
Digweed, S. M., Fedigan, L. M., & Rendall, D. (2007). Who cares who calls? Selective responses to the lost calls of socially dominant group members in the white-faced capuchin (Cebus capucinus). American Journal of Primatology, 69, 829–835.
Egnor, S. E. R., & Hauser, M. D. (2005). A paradox in the evolution of primate vocal learning. Trends in Neuroscience, 27, 649–654.
Ehret, G. (1990). Categorical perception of sound signals: Facts and hypotheses from animal studies. In S. Harnad (Ed.), Categorical perception: The groundwork of cognition (pp. 301–331). Cambridge, U.K.: Cambridge University Press.
Eisenberg, J. F. (1976). Communication mechanisms and social integration in the black spider monkey, Ateles fusciceps robustus, and related species. Smithsonion Contributions to Zoology, 213, 1–108.
Farabaugh, S. M., Linzenbold, A., & Dooling, R. J. (1994). Vocal plasticity in Budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. Journal of Comparative Psychology, 108, 81–92.
Fedurek, P., & Slocombe, K. E. (2011). Primate vocal communication: a useful tool for understanding human speech and language evolution? Human Biology, 83, 153–172.
Fischer, J. (2002). Developmental modifications in the vocal behavior of non-human primates. In A. A. Ghazanfar (Ed.), Primate audition: Ethology and neurobiology (pp. 109–125). London: CRC Press.
Fischer, J., Hammerschmidt, K., Cheney, D. L., & Seyfarth, R. M. (2001). Acoustic features of female chacma baboon barks. Ethology, 107, 33–54.
Fischer, J., Hammerschmidt, K., Cheney, D. L., & Seyfarth, R. M. (2002). Acoustic features of male baboon loud calls: influences of context, age, and individuality. Journal of the Acoustical Society of America, 111, 1465–1474.
Fitch, W. T. S. (1997). Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. Journal of the Acoustical Society of America, 102, 1213–1222.
Ford, S. M., & Davis, L. C. (1992). Systematics and body size: implications for feeding adaptations in New World Monkeys. American Journal of Physical Anthropology, 88, 415–468.
Ghazanfer, A. A., & Santos, L. (2004). Primate brains in the wild: the sensory bases for social interactions. Nature Reviews Neuroscience, 5, 603–616.
Gros-Louis, J. (2006). Acoustic analysis and contextual description of food-associated calls in white-faced capuchin monkeys (Cebus capucinus). International Journal of Primatology, 27, 273–294.
Hammerschmidt, K., & Todt, D. (1995). Individual differences in vocalisations of young Barbary macaques (Macaca sylvanus): a multi-parametric analysis of to identify critical cues in acoustic signalling. Behaviour, 132, 381–399.
Janik, V. M., & Slater, P. J. B. (2000). The different roles of social learning in vocal communication. Animal Behaviour, 60, 1–11.
Janzen, D. H. (1986). Guanacaste National Park: Tropical, ecological and cultural restoration. Editorial Universidad Estatal a Distancia, Costa Rica/Fundación Parque Nacionales. San Jose, Costa Rica.
Jones, B. S., Harris, D. H. R., & Catchpole, C. K. (1993). The stability of the vocal signature in phee calls of the common marmoset, Callithrix jacchus. American Journal of Primatology, 31, 67–75.
Jorgensen, D. D., & French, J. A. (1998). Individuality but not stability in marmoset long calls. Ethology, 104, 729–742.
Kondo, N., & Watanabe, S. (2009). Contact calls: information and social function. Japanese Psychological Research, 51, 197–208.
Lemasson, A., & Hausberger, M. (2004). Patterns of vocal sharing and social dynamics in a captive group of Campbell’s monkeys (Cercopithecus campbelli campbelli). Journal of Comparative Psychology, 118, 347–359.
Lemasson, A., Ouattara, K., Petit, E. J., & Zuberbühler, K. (2011). Social learning of vocal structure in a nonhuman primate? BMC Evolutionary Biology, 11, 362.
Marler, P. (2004). Bird calls: A cornucopia for communication. In P. Marler & H. Slabbekoom (Eds.), Nature’s music: The science of birdsong (pp. 132–176). San Diego: Elsevier Academic Press.
Mulard, H., Aubin, T., White, J. F., Hatch, S. A., & Danchin, É. (2008). Experimental evidence of vocal recognition in young and adult black-legged kittiwakes. Animal Behaviour, 76, 1855–1861.
Notman, H., & Rendall, D. (2005). Contextual variation in chimpanzee pant hoots and its implications for referential communication. Animal Behaviour, 70, 177–190.
Owren, M. M., Seyfarth, R. M., & Hopp, S. L. (1992). Categorical vocal signalling in nonhuman primates. In H. Papousek & U. Jürgens (Eds.), Nonverbal vocal communication: Comparative and developmental approaches (pp. 102–122). Cambridge, U.K.: Cambridge University Press.
Pastor-Nieto, R. (2001). Grooming, kinship, and co-feeding in captive spider monkeys (Ateles geoffroyi). Zoo Biology, 20, 293–303.
Ramos-Fernández, G. (2005). Vocal communication in a fission-fusion society: do spider monkeys stay in touch with close associates? International Journal of Primatology, 26, 1077–1092.
Ramos-Fernández, G. (2008). Communication in spider monkeys: The function and mechanisms underlying the use of whinny. In C. J. Campbell (Ed.), Spider monkeys: Behavior, ecology and evolution of the genus Ateles (pp. 220–235). Cambridge, U.K.: Cambridge University Press.
Ramos-Fernández, G., & Ayala-Orozco, B. (2003). Population size and habitat use of spider monkeys at Punta Laguna, Mexico. In L. K. Marsh (Ed.), Primates in fragments: Ecology and conservation (pp. 191–209). New York: Kluwer Academic/Plenum Press.
Rukstalis, M., Fite, J. E., & French, J. A. (2003). Social change affects vocal structure in a callitrichid primate (Callithrix kuhlii). Ethology, 109, 327–340.
Shimooka, Y., Campbell, C. J., Di Fiore, A., Felton, A. M., Izawa, K., Link, A., et al. (2008). Demography and group composition of Ateles. In C. J. Campbell (Ed.), Spider monkeys: Behavior, Ecology and Evolution of the Genus Ateles (pp. 329–348). Cambridge: Cambridge University Press.
Snowdon, C. T., & Elowson, A. M. (1999). Pygmy marmosets modify call structure when paired. Ethology, 105, 893–908.
Snowdon, C. T., Elowson, A. M., & Roush, R. S. (1997). Social influences on vocal development in new world primates. In C. T. Snowdon & M. Hausberger (Eds.), Social influences on vocal development (pp. 234–248). Cambridge, U.K.: Cambridge University Press.
Spehar, S. N., & Di Fiore, A. (2013). Loud calls as a mechanism of social coordination in a fission-fusion taxon, the white-bellied spider monkeys (Ateles belzebuth). Behavioral Ecology and Sociobiology, 67, 947–961.
Spillman, B., Dunkel, L. P., van Noordwijk, M. A., Amda, R. N. A., Lameira, A. R., Wich, S. A., et al. (2010). Acoustic properties of long calls given by flanged male orang-utans (Pongo pygmaeus wurmbii) reflect both individual identity and context. Ethology, 116, 385–395.
Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics (5th ed.). London: Pearson International.
Teixidor, P., & Byrne, R. W. (1997). Can spider monkeys (Ateles geoffroyi) discriminate vocalizations of familiar individuals and strangers? Folia Primatologica, 68, 254–264.
Teixidor, P., & Byrne, R. W. (1999). The ‘whinny’ of spider monkeys: individual recognition before situational meaning. Behaviour, 136, 279–308.
Waser, P. M. (1977). Individual recognition, intragroup cohesion and intergroup spacing: evidence from sounds playback to forest monkeys. Behaviour, 60, 28–74.
Wiley, R. H. (1994). Errors, exaggeration, and deception in animal communication. In L. Real (Ed.), Behavioral mechanisms in ecology (pp. 157–189). Chicago: University of Chicago Press.
Wright, T. F., Dahlin, C. R., & Salinas-Melgoza, A. (2008). Stability and change in vocal dialects of the yellow-napped amazon. Animal Behaviour, 76, 1017–1027.
Acknowledgments
We thank Elvin Murillo, Roger Blanco, and María Marta Chavarría at Area de Conservación Guanacaste (Santa Rosa) and Nicola Forshaw, Eulogio Canul-Aban, Macedonio Canul-Chan, Juan Canul-Chan, Augusto Canul-Aban, Laura Vick, and staff at Pronatura (Punta Laguna). In addition, we thank Eric Patel and Catherine Crockford for advice on vocalization analysis, and Roger Mundry for advice on the DFA procedure. Research at Santa Rosa and Punta Laguna was supported by The British Academy, the Wenner-Gren Foundation, the Leakey Foundation, and the North of England Zoological Society. C. J. Santorelli was supported by a University of Chester Gladstone bursary and the Santander University Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santorelli, C.J., Aureli, F., Ramos-Fernández, G. et al. Individual Variation of Whinnies Reflects Differences in Membership Between Spider Monkey (Ateles geoffroyi) Communities. Int J Primatol 34, 1172–1189 (2013). https://doi.org/10.1007/s10764-013-9736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-013-9736-2