Abstract
Asthma is a chronic inflammatory disease characterized by airway remodeling and lung inflammation. Collagen triple helix repeat containing 1 (CTHRC1), a glycoprotein, is involved in multiple pathological processes, including inflammation and fibrosis. However, the function of CTHRC1 in asthma remains unclear. In the present study, the mouse asthma model was successfully generated by sensitizing and challenging mice with ovalbumin (OVA). CTHRC1 expression at both RNA and protein levels was significantly upregulated in lung tissues of asthmatic mice. Asthmatic mice exhibited significant airway remodeling as evidenced by increased bronchial wall and smooth muscle cell layer thickness, goblet cell hyperplasia and collagen deposition, and epithelial-mesenchymal transition (EMT), but those characteristics were reversed by CTHRC1 silencing. The cell model with transforming growth factor-β1 (TGF-β1) induction in bronchial epithelial cells (BEAS-2B) was conducted to verify the effects of CTHRC1 on EMT, a classic mechanism that mediates airway remodeling. The results showed that TGF-β1 stimulation increased CTHRC1 expression, and CTHRC1 knockdown inhibited TGF-β1-induced EMT. OVA-treated mice also showed increased inflammatory cell infiltration and the production of OVA-specific immunoglobulin E (IgE), interleukin (IL)-4, IL-5, and IL-13, which were decreased by CTHRC1 downregulation. The effects of CTHRC1 on OVA-induced airway inflammation were further determined by treating BEAS-2B cells with IL-13, in which CTHRC1 knockdown reduced the IL-13-induced secretion of pro-inflammatory factors, including IL-4 and IL-5. In conclusion, these results indicate that CTHRC1 silencing attenuates asthmatic airway remodeling and inflammation in vivo and in vitro, suggesting that CTHRC1 may be a potential target for asthma treatment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Asthma is a chronic allergic disease of the airways characterized by airway hyper-responsiveness, and airway inflammation and remodeling [1, 2]. Currently, the available therapeutic interventions are based on resolution of chronic airway inflammation and dilating narrowed airways [3, 4]. Asthma airway remodeling is the key pathologic feature of asthma and is involved in multiple pathological alterations, including aberrant repair of the epithelium and vascular or fibrotic changes [5, 6]. The degree of airway remodeling in asthma correlates with the severity of the disease [7]. Epithelial-mesenchymal transition (EMT) is considered to be a hallmark pathological change and a classic mechanism of airway remodeling [8, 9]. Together, these studies suggest that targeting EMT may contribute to the development of airway remodeling in asthma.
Collagen triple helix repeat containing-1 (CTHRC1), a 28 kDa secreted glycoprotein, plays extensive roles in various pathological processes, including malignant tumors, tissue repair, fibrosis, and inflammatory diseases [10, 11]. CTHRC1 is considered a cancer-related protein and is implicated in crucial pathological processes of cancer, such as metastasis and EMT [12, 13]. The effect of CTHRC1 on tumorigenesis and tissue fibrosis has been well-documented in several published reports [14,15,16]. In addition, CTHRC1 is associated with the transforming growth factor-β (TGF-β) signaling pathway and subsequent reaction [17, 18]. The activation of the TGF-β pathway results in the upregulation of CTHRC1 [19]. It has been investigated that TGF-β-activated EMT is a key pathophysiological process in asthma-related airway remodeling [8, 20]. Although the regulation of EMT by CTHRC1 has been extensively reported in studies of tumor [12, 21], the functional role of CTHRC1 in asthma EMT and airway remodeling has not been explored. In addition, CTHRC1 is implicated in many inflammatory diseases, such as periodontitis and rheumatoid arthritis [10, 22, 23]. Results of previous studies indicated that CTHRC1 is associated with the severity of inflammatory diseases and correlated with the pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and interferon gamma (IFN-γ) expression [24]. Although previous studies have demonstrated the critical role of CTHRC1 in inflammatory diseases, the regulatory role of CTHRC1 in chronic asthmatic inflammation has not been yet been determined.
The present study aimed to investigate the functionality of CTHRC1 in the pathogenesis of asthma. A well-established ovalbumin (OVA)-induced asthma model was conducted and administrated with lentivirus containing the specific small hairpin RNA (shRNA) that targeted CTHRC1 to verify the functional role of CTHRC1 deficiency in OVA-stimulated-asthma. We here provided experimental evidence that CTHRC1 was essential for EMT in OVA-induced asthmatic mice. TGF-β1-induced EMT was performed to verify the impact of CTHRC1 on the EMT process in human bronchial epithelial cells BEAS-2B. In addition, the effects of CTHRC1 on inflammatory response were further confirmed in IL-13-stimulated human bronchial epithelial cells by focusing on the level of CTHRC1 expression and production of cytokines. Taken together, results of the present study demonstrated that CTHRC1 knockdown inhibited asthma airway remodeling and airway inflammation, which shed light upon the essential effects of CTHRC1 in OVA-induced asthma airway remodeling and provided a novel target for the treatment of asthma.
MATERIALS AND METHODS
Lentivirus Construction
Lentiviral particles contain target-specific constructs that encode short hairpin RNA (shRNA) designed to knock down CTHRC1 expression. In brief, target sites for mouse CTHRC1 mRNA (GenBank accession number: NM_026778) were selected using RNAi design sites. The formed shuttle plasmid (pLVX-shRNA1) was selected for silencing the CTHRC1 gene. The recombinant lentiviruses, shRNA-CTHRC1 and shRNA-control, were constructed and synthesized by YouBio Technology (VT1456, Changsha, China). The target sequence against CTHRC1 is as follows: 5′-GAGTTAAATTCAACTATTAAT-3′. The shRNA was cloned into a pLVX vector at the BamH1 and EcoRI sites. When 293 T packaging cells (ZQ0033, Zhong Qiao Xin Zhou Biotechnology, Shanghai, China) reached 70% confluence, all the constructs were transfected with the lentiviral packaging plasmids (pSPAX2, pMD2G, and CTHRC1 shRNA) using Lipofectamine 3000 (L3000015, Invitrogen, CA, USA). After 48 h of transfection, the viral particles were collected for titer determination.
Animal Experiments
All procedures involving animals were performed following the Ethics Committee of the Shengjing Hospital of China Medical University (No. 2019PS536K). Specific pathogen-free (SPF) female BALB/c mice (Certificate No. SCXK (Liao) 2020–0001), weighing 18–22 g, were purchased from ChangSheng Biotech (Liaoning, China, License No. SYXK (Liao) 2017–0004), and acclimatized for 1 week prior to the experiments under a 12-h light/dark cycle condition, and received food and water. Six animals per study group were utilized for all in vivo assays. Mice were randomly assigned to four groups including the control (CTL) group, OVA group, OVA + shCTL group, and OVA + shCTHRC1 group. To establish the OVA model, mice were intraperitoneal injection of 10 µg of ovalbumin (A107820, Aladdin, Shanghai, China) and 1 mg of aluminum hydroxide gel in a total volume of 200 µL on days 1, 7, and 14. Subsequently, the mice were received with aerosolized 5% OVA in saline 3 days/week from day 21 to day 77 for 30 min per day, while the control mice were injected with normal saline and challenged with saline in the same manner. Lentivirus containing shCTHRC1 (3 × 106 TU) or control shRNA (3 × 106 TU) was intratracheally delivered into the OVA animals at 18 days and supplemented at 5 weeks, while the control mice were treated with normal saline. Mice were euthanized at 48 h after the final challenge and the lung tissues were collected and fixed with 4% paraformaldehyde solution, and the rest was stored at −80 °C for further analysis. Moreover, bronchoalveolar lavage fluid (BALF) and blood samples were also collected. BALF was centrifuged to separate the supernatant and sediment and stored at −80 °C for further analysis.
Cell Culture and Transfection
Human bronchial epithelial cells, BEAS-2B, were obtained from iCell Bioscience Inc. (iCell-h023, Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (09231, Zhong Qiao Xin Zhou Biotechnology, Shanghai, China) supplemented with 10% fetal bovine serum (ZQ500, Zhong Qiao Xin Zhou Biotechnology, Shanghai, China). All cells were incubated at 37 °C with 5% CO2. For IL-13 stimulation, BEAS-2B cells were treated with 20 ng/mL recombinant human IL-13 (200–13, Peprotech, MN, USA) and incubated for 48 h. The culture supernatant or cells were collected for further analysis. For transfection, cells were cultured to final confluency at around 90%, and cells were transfected with small interfering RNA (siRNA) to form the CTHRC1-silencing group (siCTHRC1) or negative control group (siCTL). siCTHRC1 (5′-GGATGGATTCAAAGGAGAA-3′) was synthesized by General Biosystems (China). For CTHRC1 overexpression, the pcDNA3.1 vector (YouBio Technology, G103616, Changsha, China) was constructed by inserting the CDS of CTHRC1 (GenBank accession number: NM_138455). pcDNA3.1 empty vector served as a control. BEAS-2B cells were transfected with the siRNA specific for CTHRC1 or CTHRC1 overexpression plasmid and the corresponding empty vector by using Lipofectamine 3000 (L3000015, Invitrogen, CA, USA) according to the manufacturer’s instructions. After 24-h incubation, BEAS-2B cells were treated with TGF‐β1 (5 ng/mL, 10804-HNAC, Sino Biological Inc., Beijing, China) for 48 h, and TGF‐β1-treated cells were harvested and then divided into the following groups: CTL group, TGF‐β1 group, TGF‐β1 + siCTL group, and TGF‐β1 + siCTHRC1 group; CTL group, vector group, CTHRC1OE group, TGF‐β1 group, TGF‐β1 + vector group, and TGF‐β1 + CTHRC1OE group.
Immunofluorescence Assay
For immunofluorescence staining of lung tissues, the sections were fixed in 4% paraformaldehyde and embedded in paraffin. Slides were heated for antigen retrieval and incubated in blocking solution for 15 min. The slides were then incubated with CTHRC1 antibody (16534–1-AP, Proteintech, IL, USA), E-cadherin antibody (A20798, ABclonal Biotechnology, Wuhan, China), N-cadherin antibody (A19083, ABclonal Biotechnology, Wuhan, China), and α-SMA antibody (14395–1-AP, Proteintech, IL, USA) overnight at 4 °C. Subsequently, the sections were incubated with the Cy3-conjugated goat anti-rabbit IgG (A27039, Invitrogen, CA, USA) for 1 h. The slides were fixed using mounting media with DAPI (D106471-5 mg, Aladdin, Shanghai, China). The immunofluorescent images were obtained using a fluorescence microscope (DP73, Olympus, Tokyo, Japan) and analyzed using Image-Pro Plus 6.0 software according to the mean optical density of positively stained areas in a standard protocol.
For immunofluorescence staining in vitro, cells were grown on glass slides and fixed with 4% paraformaldehyde. After permeabilization with 0.1% Triton X-100 (ST795, Beyotime, Shanghai, China), cells were incubated with primary antibodies against CTHRC1 (16534–1-AP, Proteintech, IL, USA), E-cadherin (A20798, ABclonal Biotechnology, Wuhan, China), and N-cadherin (A19083, ABclonal Biotechnology, Wuhan, China) overnight at 4 °C. Cy3-labeled goat anti-rabbit IgG (A27039, Invitrogen, CA, USA) were used as secondary antibodies. Cell nuclei were stained with DAPI (D106471-5 mg, Aladdin, Shanghai, China). The immunofluorescent images were obtained using a fluorescence microscope (DP73, Olympus, Tokyo, Japan) and quantified using Image-Pro Plus 6.0 software according to the mean optical density of positively stained areas in a standard protocol.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from lung tissues or BEAS-2B cells by using TRIpure Reagent (RP1001, BioTeke, Beijing, China). Reverse transcription was performed using the qRT-PCR system (Exicycler™ 96, Bioneer, Daejeon, Korea). Quantitative RT-PCR was performed with the SYBR Green PCR system (Exicycler™ 96, Bioneer, Daejeon, Korea). Gene expression was normalized with the β-actin mRNA signals and performed by the relative quantification method of 2–ΔΔCT. The following primer sequences were used: Homo CTHRC1 forward 5′-CATTTACAAAGATGCGTTCA-3′ and reverse 5′-CCACTAATCCAGCACCAA-3′; for Mus CTHRC1 forward 5′-AGTGTTCGTGGAGTTCG-3′ and reverse 5′-CCAATCCCTTCACAGAGT-3′.
Western Blot Analysis
Total protein of tissues and BEAS-2B cells was extracted using the RIPA buffer (P0013B, Beyotime, Shanghai, China) containing PMSF (ST506, Beyotime, Shanghai, China) and quantified using the BCA protein assay kit (P0009, Beyotime, Shanghai, China). Protein samples were electrophoresed on SDS-PAGE (P0015, Beyotime, Shanghai, China) and blotted onto PVDF membranes (LC2005, Thermo Fisher Scientific, PA, USA). The membranes were then blocked in 5% bovine serum albumin (BS043, Biosharp, Hefei, China) for 1 h, followed by incubation with primary antibodies overnight at 4 °C. Horseradish peroxidase-labeled goat anti-rabbit IgG (SA00001-2, Proteintech, IL, USA) and goat anti-mouse IgG (SA00001-1, Proteintech, IL, USA) were used as the secondary antibodies. The blots were developed by incubation with ECL substrate reagents (E003, Seven Sea biotech, Shanghai, China). The proteins were visualized using the Gel-Pro-Analyzer (WD-9413B, Beijing Liuyi Biotechnology, Beijing, China). The primary antibodies were as follows: CTHRC1 (16534–1-AP, Proteintech, IL, USA), E-cadherin (A3044, ABclonal Biotechnology, Wuhan, China), N-cadherin (A19083, ABclonal Biotechnology, Wuhan, China), α-SMA (14395–1-AP, Proteintech, IL, USA), fibronectin (A12932, ABclonal Biotechnology, Wuhan, China), and Snail (13099–1-AP, Proteintech, IL, USA).
Lung Tissue Histological Analysis
The total wall area (Wat), smooth muscle wall area (Wam), and basement membrane perimeter (Pbm) were further measured. The Wat/Pbm and Wam/Pbm ratio was used to indicate airway remodeling. Pathological changes were evaluated by hematoxylin–eosin (H&E), periodic acid-Schiff (PAS), and Masson staining analysis to assess the inflammation, epithelial injury, and degree of collagen deposition, respectively. Fixed lung tissues were dehydrated and processed routinely for paraffin embedding, and sliced. H&E, PAS, and Masson reaction staining were performed on the tissue sections following the manufacturing instructions. The images were visualized under light microscopy (DP73, Olympus, Tokyo, Japan). The inflammatory cell infiltration degree was determined according to the formula: 0, no inflammatory cell infiltration; 1, a small number of infiltrated inflammatory cells; 2, an inflammatory cell monolayer around the airway; 3, 2–4 layers of inflammatory cells around the airway; 4, > 4 layers of inflammatory cells around the airway. Goblet cell hyperplasia were performed on PAS-stained tissues, and goblet cells were further quantified and scored (0, no goblet cells; 1, < 25%; 2, 25 to 50%; 3, 51 to 75%; 4, > 75%) [25]. The degree of fibrosis was graded from 0 to 3 using Masson-stained sections (0, no fibrosis; 1, mild fibrosis, lesions range < 20%; 2, moderate fibrosis, lesions ranged from 20 to 50%; 3, severe fibrosis, lesions range > 50%) [26].
Differential Counts of Inflammatory Cells in BALF
The degree of inflammatory response was estimated by the total inflammatory cell counts, and eosinophils, neutrophils, lymphocytes, and monocyte macrophages recruited into the mouse lungs and recovered from the BALF. In brief, the harvested BALF sediment was re-suspended in 0.5 mL PBS, and the 10 µL suspension was used to conduct smear staining and to count and classify inflammatory cells. Inflammatory cells in the BALF were stained via Wright-Giemsa Stain Kit (D010, Jiancheng Bioengineering Institute, Nanjing, China). The number of different inflammatory cells in BALF was counted with a hemocytometer according to morphological characteristics. The experiment was repeated three times for each group.
ELISA Assay
Cytokine levels including IL-4 (EK204, MultiSciences, Hangzhou, China), IL-5 (EK205, MultiSciences, Hangzhou, China), IL-13 (EK213, MultiSciences, Hangzhou, China), and IFN-γ (EK280, MultiSciences, Hangzhou, China) in BALF supernatants were measured with the Mouse ELISA kits according to the manufacturer’s instructions. IL-4 levels (EK104, MultiSciences, Hangzhou, China), IL-5 (EK105, MultiSciences, Hangzhou, China), and IFN-γ (EK180, MultiSciences, Hangzhou, China) in IL-13-induced cell culture supernatant were evaluated using Human ELISA kits. Mouse blood was collected and centrifuged to extract the serum. Serum OVA-specific IgE content was assayed by the mouse OVA sIgE ELISA kit (EM1254, Wuhan Fine Biotech, Wuhan, China) according to the manufacturer’s protocol.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software Inc., CA, USA). Comparisons of two groups were calculated with Student’s t-test and comparison of more than three groups was calculated with one-way analysis of variance (ANOVA). A post hoc power analysis was performed for power analysis. Data were expressed as the mean ± SD. Differences with p-values below 0.05 were considered significant.
RESULTS
CTHRC1 Deficiency Attenuates Airway Remodeling in OVA-Induced Asthmatic Mouse Models
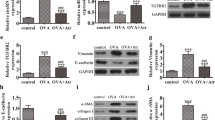
The CTHRC1 expression pattern was determined in a mouse model of asthma induced by the OVA challenge, a well-established asthma model. Lentiviruses carrying CTHRC1 shRNA particles were intratracheally delivered into mice with an OVA challenge. The expression of CTHRC1 in lung tissues was explored by immunofluorescent staining and further quantified. As indicated in Fig. 1a, CTHRC1 was predominantly detected in the pulmonary bronchus of lung tissues and CTRHC1 expression was significantly increased in lung tissues of OVA-induced asthmatic mice compared with the control group, while the treatment of shCTHRC1 caused a reduction of CTHRC1 expression in OVA-challenged mice, indicating that the shRNA lentiviral vectors were delivered into the pulmonary bronchus. The results of Western blot and qRT-PCR assays further indicated that OVA treatment markedly increased the expression levels of CTHRC1, and asthmatic mouse administrated with CTHRC1 shRNA reversed OVA-induced elevation of CTHRC1 (Fig. 1b, c). These data suggested that lentivirus treatment successfully interfered with CTHRC1 gene expression. The bronchial wall and smooth muscle layer airway wall area, smooth muscle layer area, and basement membrane perimeter were commonly employed to indicate airway remodeling [27]. The thickness of the bronchial wall and smooth muscle cell layer was obviously increased in the OVA-challenged mice, and CTHRC1 knockdown indicated a significant reduction of these alterations (Fig. 1d). To explore the potential functional role of CTHRC1 in the processes of airway remodeling, pathological morphology changes were detected. Morphologically, no significant abnormalities were observed in the control group. OVA mouse showed severe bronchial tissue injury marked by thickened bronchial mucosa, lumen stenosis, and severe inflammatory cell infiltration. Notably, these pathological changes and inflammatory responses were improved in the CTHRC1 knockdown asthma model (Fig. 1e), which was further confirmed by the inflammation score. Both PAS staining and quantification results demonstrated obvious goblet cell hyperplasia in the tissues of OVA-challenged mice and the goblet cell hyperplasia response was reduced following CTHRC1 knockdown (Fig. 1f).
CTHRC1 affects airway remodeling in the OVA-induced asthmatic model. a The expression of CTHRC1 in mouse tissues was detected by immunofluorescence staining and quantified. (n = 6, ***P < 0.001 compared with the CTL or OVA + shCTL group). b Representative blots of CTHRC1 assessed by Western blot analysis. β-actin was used as a normalization control (n = 6). c Results of CTHRC1 mRNA expression levels were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). (n = 6, ***P < 0.001 compared with the CTL or OVA + shCTL group). d Quantified results of the airway wall area thickness (Wat/Pbm) and smooth muscle layer thickness (Wam/Pbm). (n = 6, *P < 0.05, ***P < 0.001 compared with the CTL or OVA + shCTL group). e Mouse pathological morphology changes in OVA-sensitized mouse tissues were detected by hematoxylin–eosin (H&E) staining assay and the degree of inflammatory cell infiltration was quantified. (n = 6, **P < 0.01, ***P < 0.001 compared with the CTL or OVA + shCTL group). f PAS staining and corresponding quantification assays were performed to measure goblet cell hyperplasia response in OVA-sensitized mouse tissues. (n = 6, *P < 0.05, ***P < 0.001 compared with the CTL or OVA + shCTL group). CTHRC1, collagen triple helix repeat containing 1; OVA, ovalbumin; Wat, total wall area; Wam, smooth muscle wall area; Pbm, basement membrane perimeter. Dot presents the single data result in bar graph. Data are presented as means ± SD for 6 mice in each group from a minimum of three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
CTHRC1 Deficiency Suppresses Fibrosis and EMT in OVA-Induced Asthmatic Mouse Models
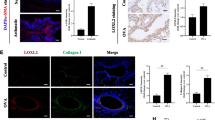
The impact of CTHRC1 deficiency on fibrosis was determined in OVA-induced asthmatic mice. Masson staining and quantification assays were performed to evaluate airway fibrosis in bronchial asthma. As shown in Fig. 2a, OVA challenge resulted in visible fibrosis, evidenced by increased collagen deposition. These symptoms were improved following CTHRC1 knockdown under asthmatic conditions. In addition, we further investigate whether EMT occurred in the OVA-induced asthmatic mice. Cadherin switch is a fundamental event in EMT [28]. As indicated by immunofluorescence and corresponding quantitative analysis in Fig. 2b–d, OVA-induction resulted in a significantly increased expression of N-cadherin and α-SMA, and decreased expression of E-cadherin. Interestingly, expression of epithelium marker E-cadherin was significantly upregulated, while expressions of the mesenchymal marker N-cadherin and α-SMA were downregulated in OVA-induced asthmatic mouse tissues following CTHRC1 knockdown (Fig. 2b–d). Western blot analysis consistently confirmed that CTHRC1 deficiency increased the expression of E-cadherin and decreased N-cadherin, Snail, α-SMA, and fibronectin expression (Fig. 2e, f). These results suggested that CTHRC1 might orchestrate the EMT process in the asthmatic model.
CTHRC1 affects airway epithelial-mesenchymal transition (EMT) in the OVA-induced asthmatic model. a Masson staining assay was performed to measure fibrosis in OVA-sensitized mouse tissues and the total fibrosis score was quantified. (n = 6, *P < 0.05, ***P < 0.001 compared with the CTL or OVA + shCTL group). b–d Representative immunofluorescent staining and relative quantitative data of epithelial marker E-cadherin, mesenchymal markers N-cadherin, and α-smooth muscle actin (α-SMA) in OVA-sensitized mouse tissues as indicated. (n = 6, ***P < 0.001 compared with the CTL or OVA + shCTL group). e Representative blots of E-cadherin, N-cadherin, and snail were observed by Western blot analysis in airway tissues. β-actin was used as a normalization control (n = 6). f Representative blots of α-SMA and fibronectin were observed by Western blot analysis in airway tissues. β-actin was used as a normalization control. Dot presents the single data result in bar graph (n = 6). Data are presented as means ± SD for 6 mice in each group from a minimum of three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
CTHRC1 Deficiency Attenuates OVA-Induced Airway Inflammation
The role of CTHRC1 deficiency in the inflammatory responses was further explored, and the cell populations including inflammatory cells, eosinophils, neutrophils, lymphocytes, and monocyte macrophage in BALF were detected. A robust accumulation of total protein expression and the number of inflammatory cells, eosinophils, neutrophils, lymphocytes, and monocyte macrophage in BALF, as expected, accompanied OVA sensitization and challenge in mice, while the treatment of shCTHRC1 decreased the number of these inflammatory cells (Fig. 3a), suggesting that CTHRC1 deficiency modified the inflammatory cell pattern in the OVA-induced asthmatic animals. In addition, total IgE levels in sera and T helper type 2 (Th2)-type cytokines IL-4, IL-5, IL-13, and Th1 cytokine IFN-γ levels in BALF were also detected using ELISA analysis. As indicated in Fig. 3b, OVA-specific IgE expression was significantly elevated in the OVA mice, and CTHRC1 knockdown reduced the IgE concentration. The expression of pulmonary cytokines, including IL-4, IL-5, and IL-13, was increased and IFN-γ was decreased in the OVA mice. CTHRC1 deficiency reduced IL-4, IL-5, and IL-13 levels and promoted IFN-γ expression in BALF (Fig. 3b). These results suggested that CTHRC1 deficiency might alleviate asthma disease severity.
CTHRC1 affects inflammatory response in OVA-induced asthmatic model. a Total protein expression, the number of inflammatory cells, eosinophils, neutrophils, lymphocytes, and monocyte macrophage in bronchoalveolar lavage fluid (BALF) were detected. (n = 6, *P < 0.05, **P < 0.01, ***P < 0.001 compared with the CTL or OVA + shCTL group). b The level of OVA-specific IgE in serum was measured by ELISA assay. The levels of Th2-type cytokines interleukin (IL)-4, IL-5, IL-13, and interferon-γ (IFN-γ) levels in the BALF supernatant were measured by ELISA analysis. (n = 6, *P < 0.05, **P < 0.01, ***P < 0.001 compared with the CTL or OVA + shCTL group). Dot presents the single data result in bar graph. Data are presented as means ± SD for 6 mice in each group from a minimum of three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
CTHRC1 Deficiency Represses EMT in TGF-β1-Stimulated Bronchial Epithelial Cells
TGF-β has been considered to be the most common EMT inducer in lung diseases including asthma [29]. Therefore, the role of CTHRC1 deficiency in TGF-β1-induced EMT was further investigated in vitro. CTHRC1 knockdown was carried out using siRNA in BEAS-2B cells and the transfection efficiency was evaluated via the Western bolt assay (Fig. 4a). The expression levels of CTHRC1 in TGF-β1-stimulated BEAS-2B cells were detected using immunofluorescent staining. As indicated in Fig. 4b, TGF-β1 treatment dramatically upregulated CTHRC1 expression, whereas CTHRC1 knockdown reversed the increased CTHRC1 expression of TGF-β1-stimulated BEAS-2B cells. Consistently, Western blot and qRT-PCR assays further confirmed that CTHRC1 levels were obviously increased in TGF-β1-stimulated BEAS-2B cells, and CTHRC1 knockdown significantly reversed the effect of TGF-β1 on the expression of CTHRC1 (Fig. 4c, d). As shown in Fig. 4e–g, TGF-β1 treatment caused a significant increase in EMT, evidenced by increased expression of N-cadherin, α-SMA, and fibronectin, and decreased expression of E-cadherin. CTHRC1 knockdown markedly attenuated the increased expression of N-cadherin, α-SMA, and fibronectin, and increased E-cadherin expression in TGF‐β1‐stimulated BEAS-2B cells. These results indicated that CTHRC1 regulated the EMT process in TGF-β1-stimulated bronchial epithelial cells.
CTHRC1 deficiency represses airway EMT in TGF-β1-treated BEAS-2B cells. BEAS-2B cells were treated with a CTHRC1 siRNA and stimulated with TGF-β1 for 48 h. a CTHRC1 protein expression was measured by Western blot analysis. β-actin was used as a normalization control. b Immunofluorescence analysis indicated the expression of CTHRC1 in different groups. c CTHRC1 mRNA in TGF-β1-treated BEAS-2B cells was measured by qRT-PCR. (n = 3, ***P < 0.001 compared with the CTL or TGF-β1 + siCTL group). d CTHRC1 protein expression in TGF-β1-treated BEAS-2B cells was measured by Western blot analysis. β-actin was used as a normalization control. e Representative blots of E-cadherin, N-cadherin, α-SMA, and fibronectin were observed by Western blot analysis in TGF-β1-stimulated BEAS-2B cells. β-actin was used as a normalization control. f Immunofluorescence analysis indicated the expression of epithelial marker E-cadherin. g Immunofluorescence analysis indicated the expression of mesenchymal markers N-cadherin. Dot presents the single data result in bar graph. Data are presented as means ± SD. All results are representative of or combined from at least three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
CTHRC1 Overexpression Promotes EMT in TGF-β1-Induced Bronchial Epithelial Cells
To further confirm the effect of CTHRC1 on TGF-β1-stimulated EMT, CTHRC1 was overexpressed via an expression plasmid in BEAS-2B cells. The transfection efficiency was evaluated via the Western bolt assay (Fig. 5a). Immunofluorescent staining showed that CTHRC1 overexpression significantly upregulated the expression of CTHRC1, and TGF-β1 treatment aggravated CTHRC1 expression in the presence of CTHRC1 (Fig. 5b). Accompanied by CTHRC1 overexpression, CTHRC1 mRNA and protein expression levels were remarkably increased (Fig. 5c, d). In addition, CTHRC1 overexpression dramatically increased the expression of CTHRC1 in TGF-β1-stimulated BEAS-2B cells. Notably, the introduction of CTHRC1 dramatically aggravated TGF-β1-stimulated N-cadherin, α-SMA, and fibronectin expression in BEAS-2B cells, whereas further suppressed E-cadherin expression, demonstrated by Western blot assay (Fig. 5e). Immunofluorescence analysis further confirmed that CTHRC1 overexpression promoted TGF-β1-stimulated EMT, evidenced by decreased expression of E-cadherin and increased expression of N-cadherin (Fig. 5f, g).
CTHRC1 overexpression promotes airway EMT in TGF-β1-treated BEAS-2B cells. BEAS-2B cells were treated with a CTHRC1 overexpression plasmid and stimulated with TGF-β1 for 48 h. a Protein levels of CTHRC1 were detected by western blotting. β-actin was used as a normalization control. b Immunofluorescence analysis indicated the expression of CTHRC1 in different groups. c CTHRC1 mRNA in TGF-β1-treated BEAS-2B cells was measured by qRT-PCR. (n = 3, ***P < 0.001 compared with the vector or TGF-β1 + vector group). d CTHRC1 protein expression in TGF-β1-treated BEAS-2B cells was measured by Western blot analysis. β-actin was used as a normalization control. e Representative blots of E-cadherin, N-cadherin, α-SMA, and fibronectin were observed by Western blot analysis in TGF-β1-stimulated BEAS-2B cells. β-actin was used as a normalization control. f Immunofluorescence analysis indicated the expression of epithelial marker E-cadherin. g Immunofluorescence analysis indicated the expression of mesenchymal markers N-cadherin. Dot presents the single data result in bar graph. Data are presented as means ± SD. All results are representative of or combined from at least three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
CTHRC1 Knockdown Attenuates Pulmonary Cytokines Production in IL-13-Stimulated Bronchial Epithelial Cells
To further validate the effect of CTHRC1 airway epithelial inflammation, we used IL-13 to stimulate BEAS-2B epithelial cells. CTHRC1 knockdown was carried out using siRNA in BEAS-2B cells. Western blot analysis indicated that the protein levels of CTHRC1 in IL-13-stimulated BEAS-2B cells were higher than those in control cells, consistent with in vivo results (Fig. 6a). In addition, Th2 cytokine levels of IL-4 and IL-5 were higher in IL-13-induced BEAS-2B cells (Fig. 6b), but there was no significant difference in IFN-γ production, indicating that inflammatory cytokines might be induced by IL-13 in epithelial cells. Notably, similar to the effects in the mouse model of asthma, the increased concentrations of IL-4 and IL-5 in IL-13-induced BEAS-2B cells were suppressed following CTHRC1 knockdown.
CTHRC1 affects inflammatory response in IL-13-induced BEAS-2B epithelial cells. a Western blot of CTHRC1 expression following IL-13 stimulation. β-actin was used as a normalization control. b The levels of IL-4, IL-5, and IFN-γ levels in the IL-13-induced BEAS-2B epithelial cell supernatant were measured by ELISA analysis. (n = 3, **P < 0.01, ***P < 0.001 compared with the CTL or IL-13 + siCTL group). Dot presents the single data result in bar graph. Data are presented as means ± SD. All results are representative of or combined from at least three independent experiments. One-way ANOVA followed by a post hoc test for multiple comparisons was used for three or more groups.
DISCUSSION
Asthma is a chronic respiratory disease with complicated pathogenesis, and airway remodeling is an active and highly complex process in the pathogenesis of asthma [30]. The present study focused on the role of CTHRC1 deficiency in asthma. Results of the present study demonstrated that CTHRC1 expression was increased both in the OVA-induced asthma model and TGF-β or IL13-induced bronchial epithelial cells compared with corresponding control group. We therefore sought to investigate the functional role of CTHRC1 knockdown in asthma both in vitro and in vivo. Results of the present study identified that CTHRC1 deficiency exerted a protective function in airway remodeling, airway inflammation, fibrotic changes, and EMT in the asthmatic model.
Several mouse models have been developed that closely reproduce the symptoms of the human asthmatic phenotype. OVA, house dust mite, and ragweed are the common allergens that have been used to induce asthma in murine models [31]. OVA-sensitized and challenged mice represent a very well-characterized model of allergic asthma and produce a robust Th2-mediated asthma-like disease in mice [32]. In the experiment, OVA and house dust mite trigger asthma models were commonly used, which exhibited a variety of similar asthma-related symptoms, including airway smooth muscle thickening, basement membrane thickening, subepithelial fibrosis, and goblet cell hyperplasia [33, 34]. In the present study, pathological morphology changes in OVA-sensitized mice were detected by H&E and PAS staining assay, indicating symptoms of asthma. Specifically, the OVA challenge resulted in inflammatory cell infiltration, airway wall thickening, airway smooth muscle hyperplasia, and goblet cell hyperplasia, consistent with the results of previous studies [35]. Notably, CTHRC1 inhibition in vivo mitigated the OVA-induced airway remodeling and airway inflammation. To our knowledge, this is the first study reported that CTHRC1 deficiency against airway remodeling in OVA-induced asthma. All of these data supported our hypothesis that CTHRC1 might act as a key regulator in the regulation of airway remodeling in chronic asthma.
Fibrosis is a major feature of chronic asthma [36]. CTHRC1 was considered to be involved in tissue fibrosis and highly expressed in various fibrotic tissues [11, 37]. However, the specific effect of CTHRC1 on fibrosis remains enigmatic. It has been demonstrated that CTHRC1 acted as a specific inhibitor of TGF-β that suppressed excessive fibroblast collagen deposition and exerted protective effects against fibrosis in the skin and liver [38, 39]. In the current work, the OVA challenge caused severe fibrotic response, confirmed by increased collagen deposition in the bronchial tissues. Results of the present study demonstrated that CTHRC1 knockdown reduced OVA-induced collagen deposition, indicating CTHRC1 might be involved in fibrosis in asthma. However, the underlying molecular mechanisms of CTHRC1 in fibrosis remain unclear, and more experiments are needed to validate.
EMT is complex progress and has been proposed as a vital mechanism in airway remodeling in asthma [40]. In the present study, we focused on EMT and provided insights into the effects of CTHRC1 on EMT. EMT is a process by which loss of function of epithelial cells and transformation to mesenchymal cells, and the development of EMT is commonly accompanied by a decreased expression of epithelial marker E-cadherin, and an increased expression of mesenchymal markers including N-cadherin, Snail, α-SMA [41]. Increasing evidence indicated that TGF-β1 was a profibrotic cytokine, which could induce EMT in asthma [42, 43]. Here, the role of CTHRC1 on EMT was investigated in vivo and in vitro. EMT was initiated in OVA-challenged asthmatic mice and triggered in human bronchial epithelial cells BEAS-2B by pro-fibrotic TGF-β1, demonstrated by altered expression of E-cadherin, N-cadherin, Snail, α-SMA, and fibronectin. CTHRC1 was associated with EMT progress, and CTHRC1 knockdown inhibited EMT in various malignant tumors [12, 44]. Results of the present study indicated that CTHRC1 deficiency suppressed the EMT process by reversing decreased expression of E-cadherin and increased expression of N-cadherin, Snail, and α-SMA. Therefore, we here expanded the role of CTHRC1 in the EMT process from cancer to airway remodeling in OVA-induced asthma.
In asthma, inflammatory response activation is one of the main reasons for the pathogenesis of asthma, accompanied by the influx of a large number of inflammatory cells such as eosinophils, neutrophils, lymphocytes, macrophages, and mast cells [45, 46]. The inflammatory cells are commonly infiltrating the lung tissue and further infiltrating into the BALF in the development of airway inflammation. Results of the present study demonstrated that increased numbers of these inflammatory cells in BALF samples in OVA-sensitized mice were significantly repressed following CTHRC1 knockdown, suggesting that CTHRC1 deficiency might be a potential strategy for asthma treatment partially by mitigating airway inflammation. However, the reciprocal regulatory mechanisms of CTHRC1 in immune cell production warrant further study. Increasing evidence has indicated that asthma was primarily driven by the overproduction of proinflammatory cytokines, IL-4, IL-5, and IL-13 and they were considered important molecules for management of allergic asthma [47, 48]. To confirm the undergoing molecular mechanism, we further conducted an IL-13-induced asthma model in bronchial epithelial cells, which is a central asthma model [49]. After stimulation with IL-13, epithelial cells produce large amounts of Th2-type cytokines IL-4 and IL-5, while inhibition of CTHRC1 significantly suppressed the generation of inflammatory factors, suggesting that CTHRC1 might directly regulate Th2 cytokines secretion in the airway epithelium. Mechanically, proinflammatory cytokines were involved in the recruitment of immune cells into the mucosal airway and could induce IgE production in OVA-induced asthma model, and the reduction of IgE levels could improve asthmatic reaction [50]. It has been demonstrated that the neutralization of IL-13 ameliorated eosinophilic inflammation in an OVA-induced asthma model [31]. The knowledge of the specific molecular process involved in CTHRC1 in airway inflammation will be addressed in future studies.
Our current study was mainly focused on the basic roles of CTHRC1 in the asthma model and bronchial epithelial cells; however, the association between CTHRC1 expression and asthma severity was not investigated, which may be an important area for future research. Additionally, the deeper exploration mechanisms of CTHRC1 in asthma might remove bottlenecks associated with the progress of patients with asthma in the clinical field, and future studies on airway remodeling in asthma require the development of more specific biomarkers. There was also no investigation of underlying mechanisms of CTHRC1 in inflammatory mediators and immune cell production in the pathogenesis of bronchial asthma. In the future, these limitations are to be addressed. Due to the limitation of our experimental conditions, our animal experimental and in vitro cell experimental sample amount is relatively small. In future work, we will make up these deficiencies.
To our knowledge, this is the first report providing evidence for a potentially important role of CTHRC1 in OVA-induced asthma. The present study demonstrated that CTHRC1 expression was increased in asthma and CTHRC1 deficiency reduced airway remodeling via suppression of EMT progression in the asthma model. Collectively, the results of the present study highlighted the important role of CTHRC1 in asthma, indicating that CTHRC1 may be considered an alternative candidate target for the treatment of asthma [21].
Author Contribution
Yong Feng and Yunxiao Shang conceived and directed research, prepared the figures, and wrote the manuscript; Yong Feng carried out most of the experiments and analyzed the data; Jiapeng Hu and Fen Liu provided technical assistance; all authors reviewed the manuscript and provided comments of the manuscript.
Data Availability
All data used or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Papi, A., C. Brightling, S.E. Pedersen, and H.K. Reddel. 2018. Asthma. Lancet (London, England) 391: 783–800.
Holgate, S.T., S. Wenzel, D.S. Postma, S.T. Weiss, H. Renz, and P.D. Sly. 2015. Asthma. Nature Reviews Disease Primers 1: 15025.
Wang, K.C.W., G.M. Donovan, A.L. James, and P.B. Noble. 2020. Asthma: Pharmacological degradation of the airway smooth muscle layer. The International Journal of Biochemistry & Cell Biology 126: 105818.
Finotto, S. 2019. Resolution of allergic asthma. Seminars In Immunopathology 41: 665–674.
Morimoto, Y., K. Hirahara, M. Kiuchi, T. Wada, T. Ichikawa, T. Kanno, M. Okano, K. Kokubo, A. Onodera, D. Sakurai, Y. Okamoto, and T. Nakayama. 2018. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity 49: 134-150.e6.
Hough, K.P., M.L. Curtiss, T.J. Blain, R.-M. Liu, J. Trevor, J.S. Deshane, and V.J. Thannickal. 2020. Airway remodeling in asthma. Frontiers In Medicine 7: 191.
Lambrecht, B.N., and H. Hammad. 2015. The immunology of asthma. Nature immunology 16: 45–56.
Sun, Z., N. Ji, Q. Ma, R. Zhu, Z. Chen, Z. Wang, Y. Qian, C. Wu, F. Hu, M. Huang, and M. Zhang. 2020. Epithelial-mesenchymal transition in asthma airway remodeling is regulated by the IL-33/CD146 axis. Frontiers In Immunology 11: 1598.
Riemma, M.A., I. Cerqua, B. Romano, E. Irollo, A. Bertolino, R. Camerlingo, E. Granato, G. Rea, S. Scala, M. Terlizzi, G. Spaziano, R. Sorrentino, B. D’Agostino, F. Roviezzo, and G. Cirino. 2022. Sphingosine-1-phosphate/TGF-β axis drives epithelial mesenchymal transition in asthma-like disease. British Journal of Pharmacology 179: 1753–1768.
Guo, Y., C. Jiang, S. Yao, L. Ma, H. Zhang, X. Wang, S. Xu, and Z. Cao. 2021. CTHRC1 knockdown promotes inflammatory responses partially by p38 MAPK activation in human periodontal ligament cells. Inflammation 44: 1831–1842.
Tsukui, T., K.H. Sun, J.B. Wetter, J.R. Wilson-Kanamori, L.A. Hazelwood, and N.C. Henderson. 2020. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nature Communications 11: 1920.
Jin, X.-F., H. Li, S. Zong, and H.-Y. Li. 2016. Knockdown of collagen triple helix repeat containing-1 inhibits the proliferation and epithelial-to-mesenchymal transition in renal cell carcinoma cells. Oncology Research 24: 477–485.
Wang, C., Z. Li, F. Shao, X. Yang, X. Feng, S. Shi, Y. Gao, and J. He. 2017. High expression of collagen triple helix repeat containing 1 (CTHRC1) facilitates progression of oesophageal squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. Journal of Experimental Clinical Cancer Research 36: 84.
Jiang, N., Y. Cui, J. Liu, X. Zhu, H. Wu, Z. Yang, and Z. Ke. 2016. Multidimensional roles of collagen triple helix repeat containing 1 (CTHRC1) in malignant cancers. Journal of Cancer 7: 2213–2220.
Mei, D., Y. Zhu, L. Zhang, and W. Wei. 2020. The role of CTHRC1 in regulation of multiple signaling and tumor progression and metastasis. Mediators of Inflammation 2020: 9578701.
Tsukui, T., K.-H. Sun, J.B. Wetter, J.R. Wilson-Kanamori, L.A. Hazelwood, N.C. Henderson, T.S. Adams, J.C. Schupp, S.D. Poli, I.O. Rosas, N. Kaminski, M.A. Matthay, P.J. Wolters, and D. Sheppard. 2020. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nature Communications 11: 1920.
LeClair, R.J., T. Durmus, Q. Wang, P. Pyagay, A. Terzic, and V. Lindner. 2007. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circulation research 100: 826–833.
Li, J., Y. Wang, M. Ma, S. Jiang, X. Zhang, Y. Zhang, X. Yang, C. Xu, G. Tian, Q. Li, Y. Wang, L. Zhu, H. Nie, M. Feng, Q. Xia, J. Gu, Q. Xu, and Z. Zhang. 2019. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. eBioMedicine 40: 43–55.
Myngbay, A., and L. Manarbek. 2021. The role of collagen triple helix repeat-containing 1 protein (CTHRC1) in rheumatoid arthritis. International Journal of Molecular Sciences 22: 2426.
Smith, L.C., S. Moreno, L. Robertson, S. Robinson, K. Gant, A.J. Bryant, and T. Sabo-Attwood. 2018. Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells. Respiratory Research 19: 160.
Ni, S., F. Ren, M. Xu, C. Tan, W. Weng, Z. Huang, W. Sheng, and D. Huang. 2018. CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Medicine 7: 5643–5654.
Tai, Y., Y. Zhu, D. Mei, H. Wang, Q. Yu, C. Hong, X. Cai, L. Xu, J. Ge, F. Liang, C. Jiang, Z. Xue, L. Hu, R. Liu, T. Zhang, P. Wang, X. Zhang, F. Zhang, W. Wei, and L. Zhang. 2021. IgD promotes pannus formation by activating Wnt5A-Fzd5-CTHRC1-NF-κB signaling pathway in FLS of CIA rats and the regulation of IgD-Fc-Ig fusion protein. International immunopharmacology 101: 108261.
Jin, Y.R., J.P. Stohn, Q. Wang, K. Nagano, R. Baron, M.L. Bouxsein, C.J. Rosen, V.A. Adarichev, and V. Lindner. 2017. Inhibition of osteoclast differentiation and collagen antibody-induced arthritis by CTHRC1. Bone 97: 153–167.
Myngbay, A., Y. Bexeitov, A. Adilbayeva, Z. Assylbekov, B.P. Yevstratenko, R.M. Aitzhanova, B. Matkarimov, V.A. Adarichev, and J. Kunz. 2019. CTHRC1: A new candidate biomarker for improved rheumatoid arthritis diagnosis. Frontiers In Immunology 10: 1353.
Myou, S., A.R. Leff, S. Myo, E. Boetticher, J. Tong, A.Y. Meliton, J. Liu, N.M. Munoz, and X. Zhu. 2003. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. The Journal of Experimental Medicine 198: 1573–1582.
Pu, Y., Y. Liu, S. Liao, S. Miao, L. Zhou, and L. Wan. 2018. Azithromycin ameliorates OVA-induced airway remodeling in Balb/c mice via suppression of epithelial-to-mesenchymal transition. International Immunopharmacology 58: 87–93.
Wu, L.Q., R.L. Wang, Y.R. Dai, F.Q. Li, H.Y. Wu, S.S. Yan, L.R. Wang, L.D. Jin, and X.D. Xia. 2015. Roxithromycin suppresses airway remodeling and modulates the expression of caveolin-1 and phospho-p42/p44MAPK in asthmatic rats. International immunopharmacology 24: 247–255.
Loh, C.-Y., J.Y. Chai, T.F. Tang, W.F. Wong, G. Sethi, M.K. Shanmugam, P.P. Chong, and C.Y. Looi. 2019. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 8: 1118.
Lee, H.-W., C.C. Jose, and S. Cuddapah. 2021. Epithelial-mesenchymal transition: Insights into nickel-induced lung diseases. Seminars In Cancer Biology 76: 99–109.
Banno, A., A.T. Reddy, S.P. Lakshmi, and R.C. Reddy. 2020. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clinical Science 134: 1063–1079.
Gubernatorova, E.O., O.A. Namakanova, A.V. Tumanov, M.S. Drutskaya, and S.A. Nedospasov. 2019. Mouse models of severe asthma for evaluation of therapeutic cytokine targeting. Immunology Letters 207: 73–83.
Bates, J.H.T., M. Rincon, and C.G. Irvin. 2009. Animal models of asthma. American Journal of Physiology. Lung Cellular and Molecular Physiology 297: L401–L410.
Komi, Elieh Ali, and D. and L. Bjermer. 2019. Mast cell-mediated orchestration of the immune responses in human allergic asthma: Current insights. Clinical Reviews in Allergy & Immunology 56: 234–247.
Jia, A., Y. Wang, W. Sun, B. Xiao, L. Mu, Y. Wei, L. Xu, C. Peng, D. Zhang, H. Shen, and X. Xiang. 2017. Comparison of the roles of house dust mite allergens, ovalbumin and lipopolysaccharides in the sensitization of mice to establish a model of severe neutrophilic asthma. Experimental and Therapeutic Medicine 14: 2126–2134.
Liu, Y., L. Wei, C. He, R. Chen, and L. Meng. 2021. Lipoxin A4 inhibits ovalbumin-induced airway inflammation and airway remodeling in a mouse model of asthma. Chemico-biological Interactions 349: 109660.
Al-Muhsen, S., J.R. Johnson, and Q. Hamid. 2011. Remodeling in asthma. The Journal of Allergy and Clinical Immunology 128: 451–462.
Jin, J., and S. Togo. 2019. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respiratory Research 20: 119.
Bian, Z., Q. Miao, W. Zhong, H. Zhang, Q. Wang, Y. Peng, X. Chen, C. Guo, L. Shen, F. Yang, J. Xu, D. Qiu, J. Fang, S. Friedman, R. Tang, M.E. Gershwin, and X. Ma. 2015. Treatment of cholestatic fibrosis by altering gene expression of Cthrc1: Implications for autoimmune and non-autoimmune liver disease. Journal of Autoimmunity 63: 76–87.
Shen, Z., T. Su, J. Chen, Z. Xie, and J. Li. 2021. Collagen triple helix repeat containing-1 exerts antifibrotic effects on human skin fibroblast and bleomycin-induced dermal fibrosis models. Annals of Translational Medicine 9: 801.
Pain, M., O. Bermudez, P. Lacoste, P.J. Royer, K. Botturi, A. Tissot, S. Brouard, O. Eickelberg, and A. Magnan. 2014. Tissue remodelling in chronic bronchial diseases: From the epithelial to mesenchymal phenotype. European Respiratory Review 23: 118–130.
Yang, Z.C., Z.H. Qu, M.J. Yi, Y.C. Shan, N. Ran, L. Xu, and X.J. Liu. 2019. MiR-448-5p inhibits TGF-β1-induced epithelial-mesenchymal transition and pulmonary fibrosis by targeting Six1 in asthma. Journal of cellular physiology 234: 8804–8814.
Fan, Q., and Y. Jian. 2020. MiR-203a-3p regulates TGF-β1-induced epithelial-mesenchymal transition (EMT) in asthma by regulating Smad3 pathway through SIX1. Bioscience reports 40: BSR20192645.
Serrano-Gomez, S.J., M. Maziveyi, and S.K. Alahari. 2016. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Molecular Cancer 15: 18.
Liu, J., W. Li, S. Liu, X. Zheng, L. Shi, W. Zhang, and H. Yang. 2017. Knockdown of collagen triple helix repeat containing 1 (CTHRC1) inhibits epithelial-mesenchymal transition and cellular migration in glioblastoma cells. Oncology Research 25: 225–232.
Mostaço-Guidolin, L.B., E.T. Osei, J. Ullah, S. Hajimohammadi, M. Fouadi, X. Li, V. Li, F. Shaheen, C.X. Yang, F. Chu, D.J. Cole, C.A. Brandsma, I.H. Heijink, G.N. Maksym, D. Walker, and T.L. Hackett. 2019. Defective fibrillar collagen organization by fibroblasts contributes to airway remodeling in asthma. American Journal of Respiratory and Critical Care Medicine 200: 431–443.
Boonpiyathad, T., Z.C. Sözener, P. Satitsuksanoa, and C.A. Akdis. 2019. Immunologic mechanisms in asthma. Seminars In Immunology 46: 101333.
Lambrecht, B.N., H. Hammad, and J.V. Fahy. 2019. The cytokines of asthma. Immunity 50: 975–991.
Hammad, H., and B.N. Lambrecht. 2021. The basic immunology of asthma. Cell 184: 1469–1485.
Seibold, M.A. 2018. Interleukin-13 stimulation reveals the cellular and functional plasticity of the airway epithelium. Annals of the American Thoracic Society 15: S98–S102.
Tay, H.L., and P.S. Foster. 2020. Biologics or immunotherapeutics for asthma? Pharmacological Research 158: 104782.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were approved by the Ethics Committee of the Shengjing Hospital of China Medical University (No. 2019PS536K).
Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Hu, J., Liu, F. et al. Collagen Triple Helix Repeat Containing 1 Deficiency Protects Against Airway Remodeling and Inflammation in Asthma Models In Vivo and In Vitro. Inflammation 46, 925–940 (2023). https://doi.org/10.1007/s10753-022-01781-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01781-3