Abstract
Collagen triple helix repeat containing 1 (CTHRC1), a secreted glycoprotein, is widely expressed in many tissues. It has been recently defined as a novel marker for rheumatoid arthritis (RA), a systemic inflammatory disorder. However, the precise role of CTHRC1 in other chronic inflammatory diseases, like periodontal disease, remains unclear. This research aimed to explore the presence of CTHRC1 in periodontal inflammation, determine the precise role in inflammatory response modulation in periodontal ligament cells (PDLCs), and explore its underlying mechanisms. In vivo gingival crevicular fluid (GCF) and gingivae were obtained from healthy people and chronic periodontitis patients. Maxillary tissues of mice with or without ligature-induced periodontitis were immunostained for CTHRC1. In vitro human PDLCs were treated with tumor necrosis factor alpha (TNF-α) to mimic the inflammatory environment. Small interfering RNA (siRNA) was used to silence CTHRC1. SB203580 was used to inhibit the p38 mitogen-activated protein kinase (MAPK) pathway. CTHRC1 was highly expressed in GCF and gingival tissues of periodontitis patients. Animal models also revealed the same tendency. CTHRC1 knockdown promoted inflammatory cytokine production and activated the p38 MAPK signaling pathway in PDLCs. Inhibiting the p38 MAPK signaling pathway partially attenuated the inflammatory responses. This study revealed that CTHRC1 was highly expressed in periodontitis and suggested that CTHRC1 might play an important role in modulating periodontal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Periodontitis is a bacteria-induced inflammatory condition that destroys the supporting structures of the teeth. It induces alveolar bone resorption and finally causes tooth loss [1, 2]. The supporting structures consist of four parts: gingiva, cementum, periodontal ligament (PDL), and alveolar bone. The PDL is a connective tissue rich in blood vessels and fibroblasts which helps to connect the cementum and the alveolar bone [3]. PDL is anatomically close to the bacteria in dental plaque and periodontal pockets, suggesting PDL may play an essential role in defense against bacteria [4]. As the dominant cells in the periodontal membrane, periodontal ligament cells (PDLCs) isolated from the diseased site present more proinflammatory cytokine secretions [5]. Moreover, inflammation stimulation promotes PDLCs to secret more proinflammatory cytokines and chemokines, such as interleukin (IL)-1β, IL-6, IL-8, TNF-α, and monocyte chemoattractant protein-1 [6,7,8]. All of these results suggest that PDLCs participate in the onset and development of periodontitis.
Collagen triple helix repeat containing 1 (CTHRC1) is a 28-kDa secreted glycoprotein containing a short collagen motif with 12 Gly-X-Y repeats, and it is highly conserved from lower chordates to mammals [9]. CTHRC1 works in many aspects, such as vascular remodeling, adipogenic differentiation, bone metabolism, wound healing, and malignant tumor progression [9,10,11,12,13,14]. In the tooth movement rat model, PDLCs can express CTHRC1 physiologically, and CTHRC1 has a higher expression level during alveolar bone remodeling [15]. Besides, CTHRC1 can improve the mineralization ability of human periodontal ligament stem cells (PDLSCs) in vitro [15].
Meanwhile, increasing evidences have displayed that CTHRC1 has a close relationship with inflammatory diseases, and it is a suppressor of inflammation [11, 16,17,18,19,20]. Serum CTHRC1 level is higher in people suffering from an inflammatory disease such as fever, yeast and urinary tract infections, thrush, diabetes, systemic lupus erythematosus, and RA [16,17,18]. The CTHRC1 plasma level is also closely related to the severity of hepatic inflammation in primary biliary cholangitis [19]. The CTHRC1 expression level is also greatly up-regulated when the injured medial collateral ligament is at the early stages of healing, which is characterized as the inflammation stage [20]. Furthermore, CTHRC1 knockout mice have more severe symptoms after inducing arthritis with a collagen antibody, suggesting that CTHRC1 can suppress inflammation [11]. However, the specific effect of CTHRC1 on periodontitis and its underlying mechanisms remains unclear.
The P38 MAPK signaling pathway is activated in many inflammatory dental diseases such as temporomandibular joint disorder, oral mucous membrane diseases, and periodontitis [21]. Previous researches have verified that it is the common pathway involved in inflammation regulation. In addition, TNF-α is found to promote IL-8 production via phosphor-p38 (p-p38) MAPK accumulation in human PDLCs [22]. So, we wondered whether p38 MAPK participated in our study. Thus, the purpose of our study was to specify the presence of CTHRC1 in periodontal inflammation, determine its precise role in inflammatory response modulation in PDLCs, and explore the signaling pathway.
MATERIALS AND METHODS
Collection of Clinical Samples
The clinical study was approved by the Medical Ethics Committee of the Wuhan University School of Stomatology (authorized c-14/2014). The informed consent to participate in the study was obtained from patients. Human gingival tissues and GCF were collected in accordance with our previously reported protocols [23]. Briefly, human inflammatory gingivae were obtained from patients in need of surgical therapy, and healthy samples were taken from healthy individuals receiving crown-lengthening surgery. Then, these samples were fixed in 4% paraformaldehyde.
For GCF collection, 30 healthy individuals and 34 periodontitis patients were included through our inclusion and exclusion criteria described in our previous study [23]. The first molar that suffered the severest inflammation was isolated with cotton rolls and dried with air. An 8-mm-long and 2-mm-wide chromatography paper (Whatman, Little Chalfont, Buckinghamshire, UK) was inserted at the gingival crevice and maintained for 30 s. Each tooth was sampled in proximal and distal sites twice. Then, these four paper strips were collected together and eluted with 500-μL sterile phosphate-buffered saline (PBS) buffer (HyClone, Marlborough, MA, USA).
Induction of Experimental Periodontitis and Sample Collection
Ten male C57BL/6 mice (weighing 25–30 g) were obtained from the Provincial Center of Disease Control (Wuhan, China). They were shut in a specific-pathogen-free condition and separated into two groups: healthy and periodontitis groups. Briefly, general anesthesia was performed through an intraperitoneal injection of pentobarbital (1.3%, 5 mL/kg). Mice in the periodontitis group suffered a 5-0 silk suture around the cervixes of the bilateral maxillary second molar. Animals took food and water freely in pre-ligation and post-ligation periods. Seven days later, these mice were sacrificed, and maxillary samples were collected. To obtain gingival RNA, the buccal gingival tissues of the right maxillary second molar were separated. They were then placed in Eppendorf (EP) tubes filled with RNAlater Stabilization Solution (Invitrogen, Santa Clara, CA, USA) and stored at − 80 °C. After being fixed in 4% neutral buffered paraformaldehyde for 24 h, these right segments were preserved in neutral buffered paraformaldehyde, and these left segments were decalcified in 10% ethylenediaminetetraacetic acid for 6 weeks.
Hematoxylin and Eosin (H&E) Staining and Immunochemistry (IHC)
After demineralization, these left segments of mice and human gingival tissues were washed in PBS for 24 h, dehydrated by using graded ethanol, embedded in paraffin, and sliced into 4-μm thickness. For histological assessment, paraffin-embedded blocks of mice periodontal tissues were in the mesiodistal plane. Sections were dewaxed and rehydrated before stained with H&E. For IHC, the sections underwent an antigen retrieval step by Pepsin (MXB Biotechnologies, Fuzhou, China) and incubated with reagents of the UltraSensitive™ SP IHC Kit (MXB Biotechnologies) as instructed and with anti-CTHRC1 primary antibodies (1:600, Abcam, Cambridge, UK) overnight at 4 °C. The morphologies of the mice periodontal tissues and human gingivae were observed and photographed by using a microscope.
Enzyme-Linked Immunosorbent Assay (ELISA)
An ELISA kit was used to quantify CTHRC1 in human GCF, and experiments were performed as the manufacturer’s protocol (Reddot Biotech, Kelowna, British Columbia, Canada). The concentrations of IL-6 and IL-8 were also determined by using an ELISA kit (NeoBioscience, Shenzhen, China).
Isolation and Culture of PDLCs and Stimulation
Human wisdom teeth and premolars were extracted from healthy individuals (12–22 years of age) for orthodontic purposes from the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Wuhan University. All patients gave informed consent. We washed the teeth with PBS and scraped off the PDL from the central third of the roots. Then, the tissues were digested with 3 mg/mL collagenase I and cultured in alpha MEM supplemented with 10% fetal calf serum (ScienCell, San Diego, CA, USA). The medium was changed every 3 days, and cells of passages 2 through 5 were used for all experiments. Human PDLCs were treated with human TNF-α (20 ng/mL, Life Technologies, Carlsbad, CA, USA) to mimic the inflammatory environment.
Cellular Immunofluorescence
After being fixed with 4% paraformaldehyde for 15 min, PDLCs were permeabilized by 0.5% Triton X-100 for 15 min. Then, samples were incubated with normal goat serum at 37 °C for 60 min following rabbit anti-CTHRC1 antibodies (1:200, Abcam) until the next day at 4 °C. Next, secondary antibodies conjugated to Cy3 (1:500, Beyotime, Shanghai, China) were added for 1 h. DAPI (ZSGB-BIO, Beijing, China) was used to dye nuclei. A fluorescence microscope was used to observe and photograph the cells.
Small Interfering RNA (siRNA) Interference and Transient Transduction
PDLCs were inoculated in six-well plates. When the cell density reached 70–80% confluency, the cells were transiently transfected with si-CTHRC1 (5′-CCC AUU GAA GCU AUA AUU UTT-3′ and 5′-AAA UUA UAG CUU CAA UGG GTT-3′) or si-NC bought from GenePharma (Shanghai, China). The transfection reagent PupMute (SignaGen, MD, USA) was used as its instructions. Forty-eight hours later, we added human TNF-α to insult PDLCs.
RNA Extraction from Gingival Tissues and PDLCs
After dropping RNAlater Stabilization Solution, five samples in the same group were combined in an EP tube with TRIzol reagent (Invitrogen, Santa Clara, CA, USA). The tissues were placed on ice and ground until invisible to the human eyes. Then, we extracted RNA with chloroform–isopropanol and used ethanol to purify RNA. Total RNA of PDLCs was lysed with TRIzol reagent and then extracted in the same way without grinding.
Quantitative Real-Time PCR (qRT-PCR)
With the help of the PrimeScript RT Reagent Kit (Takara, Kusatsu, Shiga, Japan), first-strand cDNA was generated via reverse transcription. The primer sequences are listed in Table 1, and the related genes were quantified by using ChamQ™ SYBR® qPCR Master Mix (Vazyme, Nanjing, China) in Applied Biosystems QuantStudio 6. The relative fold change of gene expression was computed according to the 2−ΔΔCt method after being normalized to GAPDH [24].
Western Blot Assay
Cells were lysed in cell lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with phosphatase and protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Total proteins were extracted by sonication. After centrifugation at 15,000 ×g at 4 °C for 15 min, we collected the supernatant and assessed protein by a bicinchoninic acid assay kit (Beyotime). Then, we loaded 25 μg of total proteins per lane in 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred proteins to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Five percent nonfat dry milk was utilized to block unclear staining. Next, the blocked membranes were incubated with primary antibodies, anti-CTHRC1 (1:600, Abcam), p-p38 (1:1000, CST, Boston, USA), total-p38 (1:1000, CST), and β-actin (1:15,000, Proteintech, Wuhan, China). Target bands were detected using a horseradish peroxidase-conjugated secondary antibody, anti-mouse (1:10,000, Proteintech), or anti-rabbit (1:8000, Proteintech). The membranes were visualized by using an Odyssey LI-COR scanner with the addition of enhanced chemiluminescence detection reagents (Advansta, CA, USA).
Statistical Analysis
All experiments were repeated thrice separately. We analyzed data by GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) via t test and one-way analysis of variance (ANOVA). Quantitative data from experiments were presented as the mean ± SD. P < 0.05 meant statistically significant.
RESULTS
CTHRC1 Was Highly Expressed in Periodontitis Patients and Animal Models
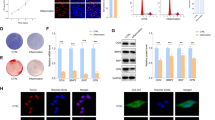
Clinical samples of GCF and gingival tissue were collected from healthy people and chronic periodontitis patients. Numerous inflammatory cells are distributed in the connective tissue (Fig. 1a). IHC of gingival tissue indicated more CTHRC1 expression in the chronic periodontitis gingival tissues, especially in inflammatory cells of the connective tissue (Fig. 1b). Detection of proinflammatory cytokine IL-1β, IL-6, and TNF-α was performed, and the concentration was reported in our previous study [25]. ELISA revealed that the production of CTHRC1 was significantly higher in chronic periodontitis patients with P = 0.0138 (Fig. 1c).
CTHRC1 had a higher expression level in periodontitis animal models and patients. a Images of H&E staining showed human gingivae. Large amounts of inflammatory cells were distributed in the connective tissue. b IHC staining for CTHRC1 in gingival tissues of human samples. Increased CTHRC1 protein can be found in inflammatory gingiva. c Measurement of CTHRC1 in the GCF with an ELISA kit. 30 healthy people and 34 patients with diagnosed chronic periodontitis were contained. d Images of H&E staining showed periodontal tissues of mice. Destroyed epithelium with obvious inflammatory cell infiltration could be observed in the ligature-induced periodontitis group. e IHC staining for CTHRC1 in periodontal tissues of mice. Increased CTHRC1 protein could be found in PDLCs. f The CTHRC1 mRNA level in mouse gingiva showed more CTHRC1 mRNA in the periodontitis group. *P < 0.05, ***P < 0.001 versus the healthy group.
H&E staining of mouse periodontal tissues showed the structural integrity of the epithelium in the healthy group. Destroyed epithelium could be observed in the ligature-induced periodontitis group (Fig. 1d). In the IHC section, we also found that CTHRC1 was positive in healthy ligament cells in mice, and PDLCs in the periodontitis group exhibited more brown positive staining (Fig. 1e). As expected, Cthrc1 mRNA expression was significantly up-regulated in the periodontitis group (Fig. 1f). To summarize, evidences from clinical samples and animal models demonstrated that CTHRC1 had a higher expression level in periodontitis.
CTHRC1 Was Increased in PDLCs when Stimulated by TNF-α In Vitro
Cellular immunofluorescence of CTHRC1 signals revealed that CTHRC1 was located in the cytoplasm of PDLCs (Fig. 2a). Increased mRNA levels of proinflammatory cytokines IL-6, IL-8, and IL-1β were observed in PDLCs when stimulated with TNF-α for 3, 6, and 12 h (Fig. 2b–d). Meanwhile, mRNA and protein levels of CTHRC1 were also obviously increased 3 h after stimulation. Results of in vitro experiments were also in favor of the notion that PDLCs expressed more CTHRC1 in an inflammatory environment.
CTHRC1 expression increased in PDLCs when stimulated by TNF-α in vitro. a Images of immunofluorescence assay. The blank group was incubated with PBS instead of primary antibody. Pictures illustrated that CTHRC1 was located in the cytoplasm of PDLCs. b–d PDLCs were stimulated with or without TNF-α for different hours. Proinflammatory cytokines IL-6, IL-8, and IL-1β were examined by qRT-PCR. e and f The mRNA level of CTHRC1 was examined by qRT-PCR, and the protein level was examined by western blotting assay after incubation with TNF-α for 0, 3, 6, and 12 h. *P < 0.05; **P < 0.01; ***P < 0.001 versus the 0-h group.
CTHRC1 Knockdown Promoted PDLC Inflammation
To further identify the function of CTHRC1 in the inflammatory environment, we used siRNA to silence CTHRC1 and Si-NC as the negative nontarget control. CTHRC1 can be effectively curb checked by qRT-PCR and western blot analysis (Fig. 3a, b). After PDLCs were incubated with or without TNF-α for 3 h, qRT-PCR was executed to measure proinflammatory cytokine gene transcription. The Si-C1 (Si-CTHRC1) group expressed more IL-6, IL-8, and IL-1β than the Si-NC group, although they were in the same inflammatory environment (Fig. 3c–e). Moreover, we used the ELISA kit to assess proinflammatory cytokine secretion in culture supernates. The data revealed that TNF-α significantly promoted the production of IL-6 and IL-8 in PDLCs. The blocking of the synthesis of CTHRC1 also promoted the stimulatory effects of TNF-α on cytokine expression (Fig. 3f, g). Based on the results, we concluded that CTHRC1 knockdown promoted PDLC inflammation.
CTHRC1 silencing promoted inflammation in PDLCs. a CTHRC1 was silenced by using siRNA for 48 h. The mRNA expression level was downregulated to approximately 20% according to qRT-PCR. b Western blot analysis showed hardly any bands in the siRNA treatment groups. c–e Proinflammatory cytokines IL-6, IL-8, and IL-1β were examined by qRT-PCR. CTHRC1 knockdown plus TNF-α group had higher levels of IL-6, IL-8, and IL-1β mRNA compared with the TNF-α alone group. f and g Culture supernates were collected to determine proinflammatory cytokine secretion with an ELISA kit. Si-C1 means siRNA of CTHRC1. ***P < 0.001 versus the Si-NC alone group. #P < 0.05, ###P < 0.001 versus the Si-NC with TNF-α group.
CTHRC1 Knockdown Promoted PDLC Inflammation Through the p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
To further elucidate the molecular mechanisms of CTHRC1 in inflammation modulation, we conducted Western blot analysis to analyze signaling pathway changes. Western blot analysis indicated that p-p38 was significantly enhanced after TNF-α treatment. The Si-C1 group had more p-p38 expression compared with the Si-NC group (Fig. 4a). We further validated the inhibitory effect of SB203580 on PDLCs. Western blot analysis showed p-p38 was dramatically decreased after treatment with 10 μM of SB203580 in dimethyl sulfoxide for 2 h (Fig. 4c). Meanwhile, SB203580 employment could suppress the mRNA levels of IL-6 and IL-8 in the Si-C1 plus TNF-α group and Si-NC plus TNF-α group although IL-1β seemed to show no difference with or without the inhibitor (Fig. 4c–e). Moreover, the results were further supported by ELISA. p38 MAPK inhibitor treatment reduced the secretion of proinflammatory cytokines IL-6 and IL-8 in the TNF-α groups (Fig. 4f, g). Collectively, inhibiting the p38 MAPK signaling pathway could partially restrain proinflammatory cytokine expression in the CTHRC1 knockdown plus TNF-α group.
CTHRC1 knockdown promoted PDLC inflammation through the p38 MAPK signaling pathway. a The protein level of p-p38 was measured by western blot analysis. P-p38 was dramatically enhanced in the CTHRC1 knockdown plus TNF-α group. b Histograms represented the quantification of p-p38/total p38. c Protein level of p-p38 after different treatments, and p-p38 could be suppressed by SB203580 (10 μM). d–f mRNA expression of IL-6, IL-8, and IL-1β was examined by qRT-PCR after different treatments. g and h The levels of secreted extracellular IL-6 and IL-8 were measured by ELISA. SB203580 could partially restrain proinflammatory cytokine expression in the CTHRC1 knockdown plus TNF-α group. Si-C1 means siRNA of CTHRC1. ***P < 0.001 versus the Si-NC alone group. ###P < 0.001 versus the Si-NC plus TNF-α group. †††P < 0.001 versus the Si-C1 plus TNF-α group.
DISCUSSION
In our study, we observed that as a suppressor of inflammation, CTHRC1 was highly expressed in periodontitis in vivo and in vitro, and it might play an important role in the process of periodontal inflammation. Knockdown CTHRC1 led to significant upregulation of IL-6, IL-8, and IL-1β at the TNF-α-stimulating level. The P38 MAPK inhibitor could partially rescue the activation of IL-6, IL-8, and IL-1β expression, suggesting that CTHRC1 suppressed the production of proinflammatory cytokines in human PDLCs partially by the p38 MAPK pathway (Fig. 5).
Upregulation of CTHRC1 has been reported in many inflammatory diseases like RA and primary biliary cholangitis [16,17,18,19]. CTHRC1 is also increased in the healing gingiva [12]. Meanwhile, TNF deficiency also has been reported to decrease the expression of CTHRC1 in brain tissues of mice [25]. These results indicate that CTHRC1 is indispensable in inflammatory reactions.
Previous studies have reported that CTHRC1 expression is closely related to IL-6, IL-8, and IL-1β production [26, 27]. To further explore the function of CTHRC1 in inflammatory response, we used siRNA to silence CTHRC1. The results showed that the PDLCs produced more proinflammatory cytokines (IL-6, IL-8, and IL-1β) in the inflammatory environment. IL-6, IL-8, and IL-1β are widely known as proinflammatory cytokines in periodontitis [28, 29]. They regulate the progression of periodontitis in different ways. IL-6 has an inhibitory effect on PDLC cementogenic differentiation, and it also contributes to alveolar bone resorption in periodontitis [30, 31]. IL-8 is a chemoattractant of polymorphonuclear leukocytes that can induce neutrophil recruitment to lesions, resulting in neutrophil-related periodontal tissue destruction [32]. IL-1β modulates the response of the host to infection and performs dual effects on the osteogenesis of PDLSCs; a low dose of IL-1β promotes while a high dose inhibits the osteogenesis of PDLSCs [33, 34].
Next, we investigated the underlying mechanism of how CTHRC1 modulated TNF-α-induced inflammatory responses. We found that increased production of IL-6, IL-8, and IL-1β involved the p38 MAPK signaling pathway. Previous studies have shown CTHRC1 regulates kinds of signaling pathway such as TGF-β, Wnt, and integrin β/FAK signaling pathway but not p38 MAPK signaling pathway [35]. P38 MAPK is essential in accelerating proinflammatory cytokine production via promoting gene transcription and mRNA translation [36]. The P38 MAPK pathway participates in IL-6, IL-8, and IL-1β production in human PDLCs [37,38,39]. Interestingly, in our study, when PDLCs were in a normal situation, knockdown of CTHRC1 increased phosphorylation of p38, but productions of inflammatory cytokines had no change. This could be due to the fact that besides MAPK signaling pathways, periodontal ligament cell cytokines can be mediated by many other intracellular signaling pathways [40, 41]. In the healthy condition, modulators may work together to keep the cytokine balance. SB203580, an inhibitor of p38 MAPK, could suppress the increased production of IL-6 and IL-8 in PDLCs due to CTHRC1 knockdown, whereas IL-1β had no change. This could be because CTHRC1 regulated IL-1β production through other ways or TNF-α facilitated IL-1β expression via p38-independent manner [35, 42]. These results supported the hypothesis that CTHRC1 knockdown promoted inflammatory responses partially by p38 MAPK activation in human PDLCs.
Surprisingly, the expression of CTHRC1 in PDLCs increased dramatically in the inflammatory environment. CTHRC1 acted as a suppressor of periodontal inflammation. This negative feedback might help to keep the host in balance. The result was consistent with the previous study, in which joints of Cthrc1 knockout mice suffered more severe inflammation than those of the wildtype after inducing arthritis with a collagen antibody [11]. Originally discovered in injured arteries, CTHRC1 can contribute to vascular remodeling and cell migratory ability [9]. CTHRC1 improves the mineralization ability of PDLSCs through upregulation of a transcriptional coactivator with PDZ-binding motif (TAZ) [15]. Additionally, it helps to inhibit osteoclast differentiation, which leads to reduced bone formation in Cthrc1 null mice [11]. These features can help periodontal regeneration. Along with our study, CTHRC1 could be an attractive therapeutic target in patients with periodontitis, which could not only suppress proinflammatory cytokine production but also promote periodontal regeneration. However, further research is required to investigate the functions of CTHRC1 in periodontitis progression and treatments.
In conclusion, this study illustrated the expression pattern of CTHRC1 in the periodontal tissue of periodontitis patients and animal models. CTHRC1 was highly expressed in periodontitis in vivo and in vitro. Knockdown of CTHRC1 promoted inflammation of PDLCs partially by activating the p38 MAPK signaling pathway. CTHRC1 might play an important role in the process of periodontal inflammation and implied that CTHRC1 could be a potential therapeutic regimen to relieve the inflammatory state in periodontitis.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kinane, D.F., P.G. Stathopoulou, and P.N. Papapanou. 2017. Periodontal diseases. Nature Reviews. Disease Primers 3: 17038.
Hajishengallis, G. 2015. Periodontitis: from microbial immune subversion to systemic inflammation. Nature Reviews. Immunology 15: 30–44.
Lekic, P., and C.A. McCulloch. 1996. Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. The Anatomical Record 245 (2): 327–341.
Jönsson, D., D. Nebel, G. Bratthall, and B.O. Nilsson. 2011. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. Journal of Periodontal Research 46 (2): 153–157.
El-Awady, A.R., R.L. Messer, A.Y. Gamal, M.M. Sharawy, K.H. Wenger, and C.A. Lapp. 2010. Periodontal ligament fibroblasts sustain destructive immune modulators of chronic periodontitis. Journal of Periodontology 81 (9): 1324–1335.
Liu, J., X. Tang, C. Li, C. Pan, Q. Li, F. Geng, and Y. Pan. 2015. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Archives of Oral Biology 60 (8): 1153–1161.
Abidi, A.H., C.S. Presley, M. Dabbous, D.A. Tipton, S.M. Mustafa, and B.M. Moore 2nd. 2018. Anti-inflammatory activity of cannabinoid receptor 2 ligands in primary hPDL fibroblasts. Archives of Oral Biology 87: 79–85.
Zhang, Y., and X. Li. 2015. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: the role of toll-like receptors 2 and 4 and the MAPK pathway. Journal of Periodontal Research 50 (2): 141–151.
Pyagay, P., M. Heroult, Q. Wang, W. Lehnert, J. Belden, L. Liaw, R.E. Friesel, and V. Lindner. 2005. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circulation Research 96 (2): 261–268.
Stohn, J.P., Q. Wang, M.E. Siviski, K. Kennedy, Y.R. Jin, D. Kacer, V. DeMambro, L. Liaw, C.P. Vary, C.J. Rosen, I. Prudovsky, and V. Lindner. 2015. Cthrc1 controls adipose tissue formation, body composition, and physical activity. Obesity (Silver Spring) 23 (8): 1633–1642.
Jin, Y.R., J.P. Stohn, Q. Wang, K. Nagano, R. Baron, M.L. Bouxsein, C.J. Rosen, V.A. Adarichev, and V. Lindner. 2017. Inhibition of osteoclast differentiation and collagen antibody-induced arthritis by CTHRC1. Bone. 97: 153–167.
Wang, Y., and D.N. Tatakis. 2017. Human gingiva transcriptome during wound healing. Journal of Clinical Periodontology 44 (4): 394–402.
Qin, S., J.H. Zheng, Z.H. Xia, J. Qian, C.L. Deng, and S.L. Yang. 2019. CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF-β and notch pathways. Biomedicine & Pharmacotherapy 113: 108594.
Wang, C., Z. Li, F. Shao, X. Yang, X. Feng, S. Shi, Y. Gao, and J. He. 2017. High expression of Collagen Triple Helix Repeat Containing 1 (CTHRC1) facilitates progression of oesophageal squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. Journal of Experimental & Clinical Cancer Research 36 (1): 84.
Wang, C., W. Gu, B. Sun, Y. Zhang, Y. Ji, X. Xu, and Y. Wen. 2017. CTHRC1 promotes osteogenic differentiation of periodontal ligament stem cells by regulating TAZ. Journal of Molecular Histology 48 (4): 311–319.
Wu, Q., Q. Yang, and H. Sun. 2018. Collagen triple helix repeat containing-1: a novel biomarker associated with disease activity in Systemic lupus erythematosus. Lupus. 27 (13): 2076–2085.
Duarte CW, Stohn JP, Wang Q, Emery IF, Prueser A, and Lindner V. 2014. Elevated plasma levels of the pituitary hormone Cthrc1 in individuals with red hair but not in patients with solid tumors. PLoS One. 19;9(6): e100449.
Shekhani, M.T., T.S. Forde, A. Adilbayeva, M. Ramez, A. Myngbay, Y. Bexeitov, V. Lindner, and V.A. Adarichev. 2016. Collagen triple helix repeat containing 1 is a new promigratory marker of arthritic pannus. Arthritis Research & Therapy 18: 171.
Li, Y.K., Y.M. Li, Y. Li, Y.R. Wei, J. Zhang, B. Li, Z.R. You, Y. Chen, B.Y. Huang, Q. Miao, Q.X. Wang, Y.S. Peng, M.E. Gershwin, R.Q. Tang, Z.L. Bian, and X. Ma. 2019. CTHRC1 expression in primary biliary cholangitis. Journal of Digestive Diseases 20 (7): 371–376.
Chamberlain, C.S., S.H. Brounts, D.G. Sterken, K.I. Rolnick, G.S. Baer, and R. Vanderby. 2011. Gene profiling of the rat medial collateral ligament during early healing using microarray analysis. Journal of Applied Physiology (Bethesda, MD: 1985) 111 (2): 552–565.
Patil, C.S., and K.L. Kirkwood. 2007. p38 MAPK signaling in oral-related diseases. Journal of Dental Research 86 (9): 812–825.
Lee, H.J., J.W. Cho, S.C. Kim, K.H. Kang, S.K. Lee, S.H. Pi, S.K. Lee, and E.C. Kim. 2006. Roles of p38 and ERK MAP kinases in IL-8 expression in TNF-alpha- and dexamethasone-stimulated human periodontal ligament cells. Cytokine. 35 (1-2): 67–76.
Xu, S., C. Jiang, H. Liu, H. Zhang, H. Liao, X. Wang, S. Yao, L. Ma, Y. Guo, and Z. Cao. 2020. Integrin-α9 and its corresponding ligands play regulatory roles in chronic periodontitis. Inflammation. 43 (4): 1488–1497.
Schmittgen, T.D., and K.J. Livak. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3 (6): 1101–1108.
Yli-Karjanmaa, M., K.S. Larsen, C.D. Fenger, L.K. Kristensen, N.A. Martin, P.T. Jensen, A. Breton, L. Nathanson, P.V. Nielsen, M.C. Lund, S.L. Carlsen, J.B. Gramsbergen, B. Finsen, J. Stubbe, L.H. Frich, H. Stolp, R. Brambilla, D.C. Anthony, M. Meyer, and K.L. Lambertsen. 2019. TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex. Brain, Behavior, and Immunity 82: 279–297.
Kudryavtseva, E., T.S. Forde, A.D. Pucker, and V.A. Adarichev. 2012. Wnt signaling genes of murine chromosome 15 are involved in sex-affected pathways of inflammatory arthritis. Arthritis and Rheumatism 64 (4): 1057–1068.
Myngbay, A., Y. Bexeitov, A. Adilbayeva, Z. Assylbekov, B.P. Yevstratenko, R.M. Aitzhanova, B. Matkarimov, V.A. Adarichev, and J. Kunz. 2019. CTHRC1: a new candidate biomarker for improved rheumatoid arthritis diagnosis. Frontiers in Immunology 10: 1353.
Buduneli, N., and D.F. Kinane. 2011. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. Journal of Clinical Periodontology 8 (Suppl 11): 85–105.
Stadler, A.F., P.D. Angst, R.M. Arce, S.C. Gomes, R.V. Oppermann, and C. Susin. 2016. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. Journal of Clinical Periodontology 43 (9): 727–745.
Han, P., T. Lloyd, Z. Chen, and Y. Xiao. 2016. Proinflammatory cytokines regulate cementogenic differentiation of periodontal ligament cells by Wnt/Ca (2+) signaling pathway. Journal of Interferon & Cytokine Research 36 (5): 328–337.
Baker, P.J., M. Dixon, R.T. Evans, L. Dufour, E. Johnson, and D.C. Roopenian. 1999. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infection and Immunity 67 (6): 2804–2809.
Silva, T.A., G.P. Garlet, S.Y. Fukada, J.S. Silva, and F.Q. Cunha. 2007. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. Journal of Dental Research 86 (4): 306–319.
Pan, W., Q. Wang, and Q. Chen. 2019. The cytokine network involved in the host immune response to periodontitis. International Journal of Oral Science 11 (3): 30.
Mao, C.Y., Y.G. Wang, X. Zhang, X.Y. Zheng, T.T. Tang, and E.Y. Lu. 2016. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death & Disease 7 (7): e2296.
Mei, D., Y. Zhu, L. Zhang, and W. Wei. 2020. The role of CTHRC1 in regulation of multiple signaling and tumor progression and metastasis. Mediators of Inflammation 2020: 9578701.
Kumar, S., J. Boehm, and J.C. Lee. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Reviews. Drug Discovery 2 (9): 717–726.
Guan, S.M., M. Zhang, J.J. He, and J.Z. Wu. 2009. Mitogen-activated protein kinases and phosphatidylinositol 3-kinase are involved in Prevotella intermedia-induced proinflammatory cytokines expression in human periodontal ligament cells. Biochemical and Biophysical Research Communications 386 (3): 471–476.
Huang, W., Y. Zhan, Y. Zheng, Y. Han, W. Hu, and J. Hou. Up-regulated ferritin in periodontitis promotes inflammatory cytokine expression in human periodontal ligament cells through transferrin receptor via ERK/P38 MAPK pathways. Clinical Science (London, England) 133 (1): 135–148.
Tang, L., X. Li, Y. Bai, P. Wang, and Y. Zhao. 2019. MicroRNA-146a negatively regulates the inflammatory response to Porphyromonas gingivalis in human periodontal ligament fibroblasts via TRAF6/p38 pathway. Journal of Periodontology 90 (4): 391–399.
Francis, M., G. Gopinathan, A. Salapatas, S. Nares, M. Gonzalez, T.G.H. Diekwisch, and X. Luan. 2020. SETD1 and NF-κB regulate periodontal inflammation through H3K4 trimethylation. Journal of Dental Research 99 (13): 1486–1493.
Du, L., Y. Li, and W. Liu. 2018. Maresin 1 regulates autophagy and inflammation in human periodontal ligament cells through glycogen synthase kinase-3β/β-catenin pathway under inflammatory conditions. Archives of Oral Biology 87: 242–247.
Song, H.K., E.M. Noh, J.M. Kim, Y.O. You, K.B. Kwon, and Y.R. Lee. 2019. Reversine inhibits MMP-3, IL-6 and IL-8 expression through suppression of ROS and JNK/AP-1 activation in interleukin-1β-stimulated human gingival fibroblasts. Archives of Oral Biology 108: 104530.
Funding
The study was supported by grants from the National Natural Science Foundation of China to Zhengguo Cao (81870776 and 81570946).
Author information
Authors and Affiliations
Contributions
YG contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; CJ, SY, LM contributed to design, data acquisition, and analysis, critically revised the manuscript; HZ, XW, SX contributed to data acquisition and analysis, critically revised the manuscript; ZC contributed to conception, design, and interpretation, drafted and critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal study was performed in accordance with the care and use of the laboratory animal manual by the US National Institutes of Health. This clinical study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Wuhan University School of Stomatology (authorized c-14/2014). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Y., Jiang, C., Yao, S. et al. CTHRC1 Knockdown Promotes Inflammatory Responses Partially by p38 MAPK Activation in Human Periodontal Ligament Cells. Inflammation 44, 1831–1842 (2021). https://doi.org/10.1007/s10753-021-01461-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01461-8