Abstract
Elevated COX-2 activity is associated with the development of chronic lung diseases leading to bronchial obstruction, including sarcoidosis. The aim of the study was to examine expression pattern of COX-2 messenger RNA (mRNA). Expression was performed by q-PCR method in bronchoalveolar lavage (BAL) cells and peripheral blood (PB) lymphocytes in sarcoidosis patients (n = 61) and control group (n = 30). Analysis of COX-2 mRNA expression level in BAL fluid and PB revealed downregulation in sarcoidosis and control groups. In PB lymphocytes, the statistically significant difference between patients and controls was observed (P = 0.003, Mann–Whitney U test), with higher expression in patients. There were no statistically significant differences between patients without and with parenchymal involvement (stages I vs. II–IV), between patients with acute vs. insidious onset of disease and between patients with abnormal vs. normal spirometry (P > 0.05, Mann–Whitney U test). Results suggest that expression of COX-2 mRNA in patients with pulmonary sarcoidosis is not related to clinical classifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sarcoidosis is recognized as a multi-systematic granulomatous disease of not fully identified etiology. However, it is accepted that many factors, including environmental, inflammatory, and genetic predisposition, are involved in the pathomechanism of this diseases [1]. Sarcoidosis, diagnosed based on clinical, radiological, and pathological evaluations, may affect many organs, but predominately involves the intrathoracic lymph nodes and lungs [2]. It is documented that activated mononuclear phagocytes can release a variety of potent inflammatory mediators, such as interleukins which regulate granuloma formation and the fibrotic process in the lung during the advanced stage of the disease. Upregulation of inflammatory response in sarcoidosis may act via prostaglandin E2 (PGE2) with the participation of key enzyme—cyclooxygenase (COX) [3]. Two distinct isoenzymes of COX, COX-1 and COX-2, are distinguished. COX-2 expression may be stimulated by various pro-inflammatory cytokines, growth factors (such as TGF-β, HGF), hormones, oncogenes [4], and COX-2 itself is responsible for production of prostaglandins, especially PGE2 [5]. PGE2, a major product of lung fibroblasts, epithelial cells, and alveolar macrophages acts as a potent inhibitor of fibroblast proliferation and collagen production [6–10].
Among interstitial lung diseases (ILD), involving a wide group of diffuse lung disorders, downregulation of COX-2 expression—associated with COX-2 gene polymorphism—has been documented in idiopathic pulmonary fibrosis (IPF) [11–14] and also in sarcoidosis [9, 15, 16]. However, it should be pointed out that clinical significance of COX-2-altered gene expression level in the course of IPF or sarcoidosis has not yet been conclusively investigated. As so far, highly specific diagnostic markers for sarcoidosis monitoring have not been found; therefore, the search for new non-invasive markers, in particular molecular, seems to be necessary.
In our study, we examined the level of COX-2 expression in patients with pulmonary sarcoidosis, both in bronchoalveolar lavage fluids (BALFs) and in peripheral blood lymphocytes (PB) in order to determine the prognostic significance of potential gene expression changes in the course of the disease.
MATERIALS AND METHODS
The study was approved by the Ethics Committee at the Medical University of Lodz (RNN/141/10/KE). Written informed consent was obtained from each patient.
Study Group
A total of 61 patients with pulmonary sarcoidosis were recruited in the study. Patients were admitted to the Department of Pneumology and Allergy of Norbert Barlicki Memorial University Hospital No. 1 in Lodz, during the years 2010–2014. The diagnosis was made based on current standards [17, 18]. For each patient, consistent clinical and radiological picture of sarcoidosis, with the presence of non-caseating granuloma in tissue biopsy, was confirmed. The diagnosis was documented by EBUS-TBNA, bronchial mucosal biopsy, transbronchial peripheral lung biopsy, mediastinoscopy, or extrathoracic biopsy (skin, peripheral lymph nodes). Only in patients with bilateral hilar lymph nodes enlargement, acute symptoms of Löfgren syndrome, and typical bronchoalveolar lavage (BAL) results (increased percentage of lymphocytes with CD4/CD8 > 3.5) were not obligatory to the biopsy. Based on chest X-ray results, patients were divided into the following radiological subgroups: stage I (hilar lymph node enlargements without signs of parenchymal involvement), stage II (signs of parenchymal involvement in addition to hilar lymph node enlargements), stage III (parenchymal involvement without visible hilar lymph node enlargements), and stage IV (signs of irreversible extensive lung fibrosis). The independent comparison between patients with acute onset (Löfgren syndrome with arthritis, erythema nodosum, and elevated body temperature—with at least two symptoms present) and patients with insidious onset was done. Clinical and biological characteristics of the study group are presented Table 1.
Control group consisted of 30 non-smokers referred for bronchoscopy due to chronic cough or undefined changes on chest X-ray. After thorough examination, these patients were finally diagnosed either with idiopathic cough, or as healthy—when radiological signs were defined as clinically insignificant changes or artifacts.
Collection of Biological Material
Bronchoscopy was performed with a flexible bronchoscope (Pentax, Tokyo, Japan), according to the Polish Respiratory Society Guidelines [19]. BALF was collected from medial lobe, by instillation and subsequent withdrawal of 4 × 50 ml of 0.9 % NaCl. The fluid recovery was 52.1 ± 1.2 %. The crude BALF was filtered through a gazue, to clear the thick mucus and other contaminants, next centrifuged, and the pellet was suspended in a phosphate buffer. The total number of non-epithelial cells (total cell count—TCC) was presented as n × 106. Cytospin slides were prepared and stained by May-Grünwald-Giemsa stain. The number of macrophages, lymphocytes, neutrophils, and eosinophils was calculated under a light microscope and presented as percent of TCC. After the calculations, the fluid was centrifuged (10 min, 1200 rpm); supernatant of BALF was suspended in RNAlater RNA Stabilization Reagent (QIAGEN, Hilden, Germany) in a volume of about 350 μl of solution in eppendorf tubes, marked with an identification number, and frozen (−80 °C) until further RNA isolation procedures. Spirometry was performed according to the Polish Respiratory Society Guidelines (Polish Society of Respiratory Diseases, 2006) with a computer-based spirometer (Jaeger, Dortmund, Germany). Blood was collected into 5 ml EDTA containing tubes. For lymphocyte separation, a density gradient cell separation medium Histopaque was used.
Gene Expression Analysis
RNA isolation was performed using the mirVana™ miRNA Isolation Kit (Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s protocol. The quality and quantity of isolated RNA was spectrophotometrically assessed (Eppendorf BioPhotometrTM Plus, Eppendorf, Hamburg, Germany). The purity of total RNA (ratio of 16S to 18S fraction) was determined in the automated electrophoresis using the RNA Nano Chips LabChipplates in Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Complementary DNA (cDNA) was transcribed from 100 ng of total RNA, using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) in a total volume of 20 μl, according to manufacturer’s protocol. The relative expression analysis was performed in 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) using TaqMan probes for the genes COX-2 (Hs00153133_m1) and ACTB (Hs99999903_m1) used as an endogenous control. The PCR mixture contained cDNA (1 to 100 ng), 20× TaqManR Gene Expression Assay, 2× KAPA PROBE Master Mix (2×) ABI Prism Kit (Kapa Biosystems, Wilmington, MA, USA), and RNase-free water in a total volume of 20 μl. The expression levels (RQ values) of the studied gene were calculated using the delta delta CT method, with the adjustment to the β-actin expression level and in relation to the expression level of calibrator (Human Lung Total RNA Ambion®), for which RQ value was equal to 1.
Statistical Analysis
The Kruskal–Wallis test, Mann–Whitney test, Neuman–Keuls multiple comparison test, and Spearman rank correlation were used to assess the correlation between gene relative expression levels and sarcoidosis groups classified on the basis of chest X-ray results (stage I vs. II–IV), disease phenotype (acute vs. insidious onset), spirometric parameters, DLCOc, serum Ca2 + concentration, Ca2+ in 24 h urine collection, percentage of lymphocytes in BAL, phenotype of immune cells (CD4+/CD8+), age, and sex of patients. The P < 0.05 value was considered statistically significant.
RESULTS
Relative Expression Levels of the COX-2 mRNA in Sarcoidosis Patients Vs. Control Group
The relative expression levels of COX-2 in the studied groups (sarcoidosis patients and controls in relation to different biological material and disease classifications) were calculated based on delta delta CT method.
BALF Cells
In both studied groups, the expression of COX-2 messenger RNA (mRNA) was decreased (RQ <1). The expression level of COX-2 mRNA was lower in patients vs. controls, however without statistical significance (P > 0.05, Mann–Whitney U test) (data not shown).
PB Lymphocytes
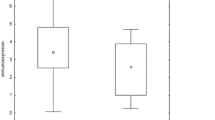
In both studied groups, COX-2 mRNA expression was decreased (RQ <1). Statistically significant difference between patients and controls was observed (P = 0.003, Mann–Whitney U test), with higher COX-2 mRNA expression level in sarcoidosis patients, see Fig. 1.
Reassuming analysis of COX-2 mRNA expression level in BALF and PB revealed downregulation of COX-2 in all studied groups. In sarcoidosis patients, the expression level of the study gene was lower in BALF and higher in PB compared to control group, as presented in Fig. 2.
Relative Expression Levels of COX-2 mRNA in Sarcoidosis Patients Classified According to Clinical and Radiological Assessment
BALF Cells
The results obtained in sarcoidosis patients are presented in Table 2.There were no statistically significant differences between patients without and with parenchymal involvement (stages I vs. II–IV), between patients with acute vs. insidious onset of disease and between patients with abnormal vs. normal spirometry (P > 0.05, Mann–Whitney U test).
PB Lymphocytes
The results obtained in sarcoidosis patients are presented in Table 2. There were no statistically significant differences between patients without and with parenchymal involvement (stages I vs. II–IV), between patients with acute vs. insidious onset of disease and between patients with abnormal vs. normal spirometry (P > 0.05, Mann–Whitney U test).
As it can be seen, downregulation of COX-2 in patient group was much more pronounced in BALF cells than in PB lymphocytes, and the difference was statistically significant (P = 0.0002, Mann–Whitney U test), as presented on Fig. 3.
Additionally, in BALF cells, several statistically significant correlations (Spearman’s rank correlation.) between gene expression levels and lung function test parameters, as well as selected laboratory markers in sarcoidosis patients were found (see Table 3). No similar correlations were found in patients’ PB lymphocytes (P > 0.05, Mann–Whitney U test).
DISCUSION
It has been well established that important inflammatory mediators, prostaglandins (PGE2), and COXs (especially COX-2) are implicated in the pathogenesis of several interstitial lung diseases (ILD) including sarcoidosis [9, 20–24]. In bronchiolar epithelial cells and macrophages, endogenous COX-2 and PGE2 are important regulators of inflammatory responses in the lung. It has been confirmed that immoderate COX-2 activity is associated with the development of chronic obstructive lung diseases, airway reactivity, and bronchial inflammation, while restricted activation of COX-2 protein following pro-inflammatory stimulation promotes lung fibrosis [9, 22, 23]. This biological role of COX-2 in pathology of the lung was confirmed also in vitro in cultured lung fibrosis isolated from idiopathic pulmonary fibrosis (IPF) patients [20] and in experimental models of asthma in rodent [21].
To the best of our knowledge, only two studies have reported COX-2 expression in sarcoidosis patients so far, however, on different biological material: lung tissue biopsies (nine) and BALF [24], and only one of them analyzed COX-2 on a mRNA level [24]. Petkova et al. [9] assessed immunoexpression of COX-2 and showed its significant reduction in bronchiolar epithelial cells and in alveolar macrophages. The results of our study, focused on mRNA COX-2 expression level in sarcoidosis patients, confirmed downregulation of COX-2 on transcription level. We conclude that reduced COX-2 mRNA levels may be associated with defect in PGE biosynthesis in the lung and also with increasing fibroblast activity and collagen deposition during sarcoidosis development. Similar effect was observed in IPF [9, 20]. Lack of statistically significant differences in COX-2 mRNA expression in BALF between our patients and controls may be related to small number of patients with advanced (III–IV) stage of the disease, where the lung fibrosis takes place. The dominance of early stage of sarcoidosis patients in our study group could result in limited reduction of COX-2 expression, covered by the presence of persistent inflammatory response (chronic lymphocytosis). This effect was demonstrated on bleomycin model of mice lung fibrosis [25, 26]. We suppose that decreased expression of COX-2 observed by us is associated primarily with inflammatory induction and EMT (epithelial–mesenchymal transition) in the lung and may precede the upregulation of COX-2 expression in the later stages of the disease, which protects against fibrosis [14]. Unfortunately, in our study, we did not document statistically significant differences in the decreasing expression of COX-2 in various clinical stages of sarcoidosis (II–IV vs. I), although we observed higher gene expression in radiological stage I vs II–IV and higher in acute form of sarcoidosis. However, we observed negative correlation in BALF between COX-2 expression and BALF% and 24 h Ca2+ loss with urine as well as with forced expiratory volume in the first second (FEV1), which were considered as factors with negative prognostic value in sarcoidosis. Our results give hope for further studies focused on COX-2 mRNA level in BALF, searching for prognostic markers of sarcoidosis progression and fibrosis.
Our results appear to be inconsistent with the observations of Chistophi et al. [24], who examined COX-2 expression on mRNA level in lung tissue biopsies and observed its significant upregulation in sarcoid granulomas. Surprisingly, so different results could be expected. During sarcoid formation, the accumulation of activated monocytes and alveolar macrophages as a source of secretion of COX-2 takes place. Also, granulomatous and bronchial epithelium inflammation influences COX-2 upregulation via accumulation of recruited monocytes and macrophages on the border of sarcoid granuloma [27, 28]. Therefore, higher expression level of COX-2 in lung sarcoid biopsy as compared to BALF may be expected.
In our study, we evaluated COX-2 mRNA expression also in PB lymphocytes of sarcoidosis patients to compare it with BALF and to answer the question whether the expression of COX-2 gene in the blood of patients can be a specific marker for sarcoidosis. There are no other published studies regarding this aspect, although sarcoidosis is known as a systemic disease marked by the upregulation of inflammatory factors in serum that are secreted from peripheral leukocytes [29]. Microarray analysis from peripheral blood leukocytes of sarcoidosis patients revealed a unique gene expression signature involving genes implicated in T cell differentiation and activation and cytokine signaling [29]. Interestingly, in our study, we found significantly higher COX-2 expression in PB lymphocytes in patients as compared to controls. We considered that this effect may be linked to systemic inflammatory process developing in sarcoidosis patients, where COX-2 is activated by pro-inflammatory cytokines derived from monocytes and lymphocytes as a T cell-mediated disease. Many published results suggest that the improved biosynthesis of COX-2 which takes place in both acutely and chronically inflamed tissues results from selective, local upregulation of COX-2 biosynthesis in PB, mainly in monocytes and activated T cell lymphocytes [30, 31]. Moreover, endogenous PGE2 may influence the balance of COX-2 expression in cells as a main autocrine regulator of inflammatory and immunological events, which was confirmed in vitro [32, 33]. Significantly increased expression level of COX-2 in PB of sarcoidosis patients is also associated with uncontrolled inflammatory responses in the course of disease, where immunoregulatory processes might be reduced and the activity of T-reg lymphocytes might be disturbed. Sarcoidosis is therefore associated with a global T-reg cell subset amplification whose activity would be insufficient to control local inflammation. At the same time, peripheral T-reg cells exert powerful antiproliferative activity that may account for the state of anergy. Decreased T-reg activity in blood of sarcoidosis patients was documented [34], while COX-2 was recognized as a potentially inhibiting factor of T-reg cells [35].
Reassuming, it is possible that COX-2 mRNA expression levels are interesting in the aspect of searching for new prognostic factors in sarcoidosis, but it does not fully reflect the functionality of this enzyme in cells due to the possible differences in mRNA stability on translational level, and the influence of different regulatory factors involved in immunological response.
References
Iannuzzi, M.C., B.A. Rybicki, and A.S. Teirstein. 2007. Sarcoidosis. The New England Journal of Medicine 357: 2153–2165.
Baughman, R.P., A.S. Teirstein, M.A. Judson, M.D. Rossman, H. Yeager Jr., E.A. Bresnitz, L. DePalo, G. Hunninghake, M.C. Iannuzzi, C.J. Johns, et al. 2001. Clinical characteristics of patients in a case control study of sarcoidosis. American Journal of Respiratory and Critical Care Medicine 164: 1885–1889.
Pueringer, R.J., D.A. Schwartz, C.S. Dayton, S.R. Gilbert, and G.W. Hunninghake. 1993. The relationship between alveolar macrophage TNF, IL-1, and PGE2 release, alveolitis, and disease severity in sarcoidosis. Chest 3: 832–838.
Coward, W.R., K. Watts, C.A. Feghali-Bostwick, A. Knox, and L. Pang. 2009. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Molecular and Cellular Biology 15: 4325–39.
Bauman, K.A., S.H. Wettlaufer, K. Okunishi, K.M. Vannella, J.S. Stoolman, S.K. Huang, A.J. Courey, E.S. White, C.M. Hogaboam CM, R.H. Simon, et al. 2010. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. Journal of Clinical Investigation 6: 1950–60.
Crofford, L.J. 1997. COX-1 and COX-2 tissue expression: Implications and predictions. Journal of Rheumatology. Supplement 49: 15–9.
Korn, J.H. 1983. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short-term exposure to mononuclear cell products. Journal of Clinical Investigation 5: 1240–1246.
Goldstein, R.H., and P. Polgar. 1982. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. Journal of Biological Chemistry 15: 8630–8633.
Petkova, D.K., C.A. Clelland, J.E. Ronan, S. Lewis, and A.J. Knox. 2003. Reduced expression of cyclooxygenase (COX) in idiopathic pulmonary fibrosis and sarcoidosis. Histopathology 4: 381–386.
O’Neill, G.P., and A.W. Ford-Hutchinson. 1993. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Letters 13: 156–160.
Asano, K., C.M. Lilly, and J.M. Drazen. 1996. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. American Journal of Physiology 271: 126–131.
Xaubet, A., W.J. Fu, M. Li, A. Serrano-Mollar, J. Ancochea, M. Molina-Molina, E. Rodriguez-Becerra, F. Morell, J.M. Rodríguez-Arias, J. Pereda, et al. 2010. A haplotype of cyclooxygenase-2 gene is associated with idiopathic pulmonary fibrosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases 27: 121–130.
Bonner, J.C., A.B. Rice, J.L. Ingram, C.R. Moomaw, A. Nyska, A. Bradbury, A.R. Sessoms, P.C. Chulada, D.L. Morgan, D.C. Zeldin, et al. 2002. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. American Journal of Pathology 161: 459–470.
Hodges, R.J., R.G. Jenkins, C.P. Wheeler-Jones, D.M. Copeman, S.E. Bottoms, G.J. Bellingan, C.B. Nanthakumar, G.J. Laurent, S.L. Hart, M.L. Foster, et al. 2004. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. American Journal of Pathology 165: 1663–1676.
Lopez-Campos, J.L., D. Rodriguez-Rodriguez, E. Rodriguez-Becerra, I. Alfageme Michavila, J.F. Guerra, F.J. Hernandez, A. Casanova, J. de Córdoba Gamero Fernández, A. Romero-Ortiz, et al. 2009. Cyclooxygenase-2 polymorphisms confer susceptibility to sarcoidosis but are not related to prognosis. Respiratory Medicine 103: 427–433.
Hill, M.R., A. Papafili, H. Booth, P. Lawson, M. Hubner, H. Beynon, C. Read, G. Lindahl, R.P. Marshall, R.J. McAnulty, et al. 2006. Functional prostaglandin-endoperoxide synthase 2 polymorphism predicts poor outcome in sarcoidosis. American Journal of Respiratory and Critical Care Medicine 15: 915–922.
Ianuzzi, M.C., B.A. Rybicki, and A.S. Teirstein. 2007. Sarcoidosis. NEJM 357: 2153–2165.
American Thoracic Society, European Respiratory Society, and World Association of Sarcoidosis and Other Granulomatous Disorders. 1999. Statement on sarcoidosis. American Journal Respiratory Critical Care Medicine 160: 736–755.
Chciałowski, A., J. Chorostowska-Wynimko, A. Fal, R. Pawłowicz, and J. Domagał-Kulawik. 2011. Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling processing and analysis methods. Pneumonologia i Alergologia Polska 79: 75–89.
Wilborn, J., L.J. Crofford, M.D. Burdick, S.L. Kunkel, R.M. Strieter, and M. Peters-Golden. 1995. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. Journal of Clinical Investigation 95: 1861–1868.
Peebles, R.S.J., K. Hashimoto, J.D. Morrow, R. Dworski, R.D. Collins, Y. Hashimoto, J.W. Christman, K.H. Kang, K. Jarzecka, J. Furlong, et al. 2002. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. American Journal of Respiratory and Critical Care Medicine 165: 1154–1160.
Xaubet, A., J. Roca-Ferrer, L. Pujols, J. Ramírez, J. Mullol, A. Marin-Arguedas, A. Torrego, J.M. Gimferrer, and C. Picado. 2004. Cyclooxygenase-2 is up-regulated in lung parenchyma of chronic obstructive pulmonary disease and down-regulated in idiopathic pulmonary fibrosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases 21: 35–42.
Lappi-Blanco, E., R. Kaarteenaho-Wiik, P.K. Maasilta, S. Anttila, P. Pääkkö, and H.J. Wolff. 2006. COX-2 is widely expressed in metaplastic epithelium in pulmonary fibrous disorders. American journal of Clinical Pathology 126: 717–24.
Christophi, G.P., T. Caza, C. Curtiss, D. Gumber, P.T. Massa, and S.K. Landas. 2014. Gene expression profiles in granuloma tissue reveal novel diagnostic markers in sarcoidosis. Experimental and Molecular Pathology 96: 393–399.
Hodges, R.J., R.G. Jenkins, C.P. Wheeler-Jones, D.M. Copeman, S.E. Bottoms, G.J. Bellingan, C.B. Nanthakumar, G.J. Laurent, S.L. Hart, and M.L. Foster. 2004. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. American Journal of Pathology 165: 1663–76.
Brown, J.R., and R.N. DuBois. 2004. Cyclooxygenase as a target in lung cancer. Clinical Cancer Research 15: 4266–4269.
Noor, A., and K.S. Knox. 2007. Immunopathogenesis of sarcoidosis. Clinics in Dermatology 25: 250–258.
Hastürk, S., B. Kemp, S.K. Kalapurakal, J.M. Kurie, W.K. Hong, and J.S. Lee. 2002. Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung carcinoma. Cancer 15: 1023–1031.
Zhou, T., W. Zhang, N.J. Sweiss, E.S. Chen, D.R. Moller, K.S. Knox, S.F. Ma, M.S. Wade, I. Noth, R.F. Machado, et al. 2012. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS ONE 7(9), e44818.
Davies, P., and D.E. MacIntyre: Prostaglandins and inflammation. Inflammation: Basic Principles and Clinical Correlates. J.I. Gallin, I.M.Goldstein, and R. Snyderman, editors. Raven Press, Ltd., New York. 123–137, 1992
Pablos, J.L., B. Santiago, P.E. Carreira, M. Galindo, and J.J. Gomez-Reino. 1999. Cyclooxygenase-1 and -2 are expressed by human T cells. Clinical and Experimental Immunology 115: 86–90.
Hinz, B., K. Brune, and A. Pahl. 2000. Prostaglandin E(2) upregulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochemical and Biophysical Research Communications 272: 744–748.
Bonazzi, A., M. Bolla, C. Buccellati, A. Hernandez, S. Zarini, T. Viganò, F. Fumagalli, S. Viappiani, S. Ravasi, P. Zannini, et al. 2000. Effect of endogenous and exogenous prostaglandin E(2) on interleukin-1 beta-induced cyclooxygenase-2 expression in human airway smooth-muscle cells. American Journal of Respiratory and Critical Care Medicine 162: 2272–2277.
Miyara, M., Z. Amoura, C. Parizot, C. Badoual, K. Dorgham, S. Trad, M. Kambouchner, D. Valeyre, C. Chapelon-Abric, P. Debré, et al. 2006. The immune paradox of sarcoidosis and regulatory T cells. Journal of Experimental Medicine 20: 359–370.
Akasaki, Y., G. Liu, N.H. Chung, M. Ehtesham, K.L. Black, and J.S. Yu. 2004. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. Journal of Immunology 173: 4352–4359.
Acknowledgments
This work was financed by the Medical Univeristy of Lodz, number of subsidy: 503/1-151/-4/503-11-002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee at the Medical University of Lodz (RNN/141/10/KE). Written informed consent was obtained from each patient.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kiszałkiewicz, J., Piotrowski, W.J., Pastuszak-Lewandoska, D. et al. Altered Cyclooxygenase-2 Expression in Pulmonary Sarcoidosis is not Related to Clinical Classifications. Inflammation 39, 1302–1309 (2016). https://doi.org/10.1007/s10753-016-0362-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0362-y