Abstract
Lung fibrosis is a complication of sarcoidosis, in which TGF-β/Smad pathway may play an important role. We evaluated gene expression of TGF-β1, SMAD2, 3 and 7 in bronchoalveolar lavage (BAL) cells and peripheral blood (PB) lymphocytes of sarcoidosis patients (n = 94) to better understand the mechanisms of sarcoid inflammation. The relative gene expression was analyzed by qPCR method. Selected clinical/radiological features and biochemical markers were taken into account in the analysis. We found that TGF-β1 and SMAD3 expressions in PB lymphocytes were significantly higher in sarcoidosis patients. Up-regulation of SMAD7 (inhibitory Smad) and down-regulation of SMAD3 in BAL cells in all subgroups were found. The expression of TGF-β1 in PB lymphocytes was the highest in patients with lung parenchymal involvement and in the insidious onset phenotype. The expression of TGF-β1 in BAL cells was higher in patients with abnormal spirometry (p = 0.012), and TGF-β1 and SMAD3 in patients with restrictive pattern (p = 0.034 and 0.031, respectively). Several statistically significant negative correlations were found between the expression levels of SMAD2 and 3 in BAL cells and various LFT parameters. We conclude that TGF-β/Smad pathway is involved in the pathogenesis of pulmonary sarcoidosis. These biomarkers (especially TGF-β1, SMAD2 and 3) are of a negative prognostic value.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Sarcoidosis is a chronic inflammatory disease, presenting – in the majority of patients – as intrathoracic lymph nodes enlargement and parenchymal lung disease, with the possible involvement of many extrapulmonary organs. The formation of non-caseating granulomas and lymphocytic inflammation with the predominance of CD4+ Th1 lymphocytes are the most characteristic histologic features. The overall prognosis is good, as about 60 % of patients presenting various phenotypes may recover without treatment. Lung fibrosis is the most unfavorable outcome, and may occur in 5–25 % of patients. It is difficult to anticipate at the beginning which patients will develop this severe complication (Ianuzzi et al. 2007; ATS&ERS&WASOG 1999).
Transforming growth factor beta (TGF-β) is a key cytokine responsible for tissue regeneration and scarring. Its role in the pathogenesis of experimental lung fibrosis (Shi et al. 2014) and idiopathic pulmonary fibrosis (IPF) (Khalil et al. 1996) is well documented. Biological effects of TGF-β include fibroblast recruitment, activation, proliferation and transformation to myofibroblast phenotype, epithelial-mesenchymal transition (EMT), and epithelial cells apoptosis (Patterson et al. 2012). It is believed to play a role in the late phase of sarcoid inflammation, being responsible either for tissue repair or for pathologic fibrosis. Moreover, it may play an important immunoregulatory role in early stages of sarcoid inflammation. An ambiguous role of TGF-β makes its use as a disease marker difficult.
Activation of TGF-β receptors (TβRI and TβRII) by TGF-β and phosphorylation of the receptor complex activates the Smad intracellular signaling proteins through the Smads (Smad2 and 3) and co-Smad 4 receptors. Activation of Smads 2–4 is inhibited by Smad7 (inhibitory Smad) (Flanders et al. 2002). Therefore, the activity of TGF-β should always be considered in relation to the whole TGF-β/Smad signaling pathway. To our best knowledge, the entire pathway has not been studied in the context of sarcoidosis so far.
Taking into account the information above outlined, the aim of this study was to verify the hypothesis, that TGF-β/Smad intracellular pathway signaling elements (TGF-β1, Smad2, 3 and 7) are important in the pathogenesis of sarcoidosis. In order to assess their significance as negative prognostic markers we evaluated the expression of TGF-β1, SMAD2, 3, 7 genes in bronchoalveolar lavage fluid (BALF) cells and peripheral blood (PB) lymphocytes in sarcoidosis patients in relation to signs of lung parenchymal involvement, lung function results, clinical phenotypes, and several biochemical markers.
2 Methods
The study was approved by the Ethics Committee of the Medical University of Lodz (RNN/141/10/KE). Written informed consent was obtained from each patient.
2.1 Study Group
A total of 94 patients with pulmonary sarcoidosis were recruited for the study. Patients were admitted to the Department of Pneumology and Allergy of the Norbert Barlicki memorial University Hospital No. 1 in Lodz (Poland) during the years 2010–2014. The diagnosis was based on the current standards (Ianuzzi et al. 2007; ATS&ERS&WASOG 1999). Consistent clinical and radiological picture of sarcoidosis, with the presence of non-caseating granuloma in tissue biopsy, was confirmed for each patient. The diagnosis was documented by EBUS-TBNA, bronchial mucosal biopsy, transbronchial peripheral lung biopsy, mediastinoscopy, or extrathoracic biopsy (skin, peripheral lymph nodes). Only in patients with typical clinico-radiological picture (bilateral hilar lymph nodes enlargement) and typical BAL results (increased percentage of lymphocytes with CD4/CD8 > 3.5) the biopsy was not obligatory. The patients were divided based on chest X-ray results into the following radiological subgroups: stage I (hilar lymph nodes enlargement without signs of parenchymal involvement), stage II (signs of parenchymal involvement in addition to hilar lymph nodes enlargement), stage III (parenchymal involvement without visible hilar lymph nodes enlargement), and IV (signs of irreversible extensive lung fibrosis). An independent comparison between the patients with acute onset (Löfgren syndrome with arthritis, erythema nodosum, elevated body temperature – with at least two symptoms present) and patients with insidious onset was done. Clinical and biological characteristics of the study group is presented Table 1.
Control group consisted of 50 non-smokers referred for bronchoscopy due to chronic cough or undefined changes on chest X-ray. These patients after thorough examination were finally diagnosed either with idiopathic cough, or as healthy – when radiological signs were defined as clinically insignificant changes or artifacts.
2.2 Bronchoscopy and Bronchoalveolar Lavage Fluid (BALF) Collection
Bronchoscopy was performed with a flexible bronchoscope (Pentax, Tokyo, Japan) according to the Polish Respiratory Society Guidelines (Chciałowski et al. 2011). Patients optionally received midanium and atropine before the examination, 2 % lidocaine was used as a topical anaesthetic. BAL fluid (BALF) was collected from medial lobe, by instillation and subsequent withdrawal of 4 × 50 mL of 0.9 % NaCl. The fluid recovery was 52.1 ± 1.2 %. The crude BALF was filtered through a gauze, to clear the thick mucus and other contaminants, next centrifuged, and the pellet was suspended in a phosphate buffer. The total number of non-epithelial cells (total cell count – TCC) was presented as n × 106. Cytospin slides were prepared and stained by May-Grünwald-Giemsa stain. The number of macrophages, lymphocytes, neutrophils, and eosinophils was calculated under a light microscope and presented as % of TCC. After the calculations, all fluid was centrifuged (10 min 1,200 rpm), supernatant of BALF was suspended in RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) in a volume of about 350 μl of solution in Eppendorf tubes, marked with an identification number, and was frozen (−80 °C) until further RNA isolation procedures.
2.3 Lung Function
Spirometry was performed according to the Polish Respiratory Society Guidelines (2006) with a computer-based spirometer (Jaeger, Dortmund, Germany). Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were measured, and the Tiffenau index (FEV1/FVC) was calculated. Lung diffusion capacity for carbon monoxide was measured in patients with lung parenchymal disease only (stage II–IV) with a single breath method, with a Lungtest 1000 SB (MES, Cracow, Poland) according to ATS/ERS standards (European Respiratory Society 1993). The values were corrected for the hemoglobin concentration (DLCOc). All data (except the Tiffeneau index) were presented as % of predicted value.
2.4 Peripheral Blood Samples Collection
Blood was collected into 2 mL EDTA containing tubes (labeled with the identification number). For lymphocyte separation, a density gradient cell separation medium Histopaque-1077 (Sigma-Aldrich, Poznan, Poland) was used. Blood (3 mL) was carefully layered onto 3 mL of Histopaque-1077 in a 15-mL conical centrifuge tube. Next, samples were centrifuged at 400 × g for 30 min at room temperature. After centrifugation, mononuclear cells were transferred into a clean conical centrifuge tube. Cells were washed by adding 10 mL of isotonic phosphate buffer and mixed gently, then centrifuged at 250 × g for 10 min. The supernatant was discarded. Cells were resuspended in 5 mL of isotonic phosphate buffered saline solution and centrifuged at 250 × g for 10 min. The supernatant was discarded and cells were resuspended in 350 μl RNAlater RNA Stabilization Reagent and frozen (−80 °C).
2.5 Gene Expression Analysis
RNA isolation was performed using mirVana™ miRNA Isolation Kit (Life Technologies, Carlsbad, CA), according to the manufacturer’s protocol. The quality and quantity of isolated RNA was spectrophotometrically assessed (Eppendorf BioPhotometrTM Plus, Eppendorf, Hamburg, Germany). The purity of total RNA (ratio of 16S to 18S fraction) was determined in the automated electrophoresis using RNA 6000 Pico LabChipplates on Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
cDNA was transcribed from 100 ng of total RNA, using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) in a total volume of 20 μl. Reverse transcription (RT) master mix contained the following: 10 x RT buffer, 259 dNTP Mix (100 mM), 10 x RT Random Primers, MultiScribeTM Reverse Transcriptase, RNase Inhibitor, and nuclease-free water. RT reaction was performed in a personal thermocycler (Eppendorf, Hamburg, Germany) in the following conditions: 10 min at 25 °C, followed by 120 min at 37 °C; then the samples were heated to 85 °C for 5 s, and held at 4 °C. The relative expression analysis was performed in 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) using TaqMan probes for the studied genes: TGF-β1 (Hs00998133_m1), SMAD2 (Hs00183425_m1), SMAD3 (Hs00969210_m1), SMAD7 (Hs00998193_m1). The PCR mixture contained: cDNA (1–100 ng), 20 × TaqManR Gene Expression Assay, 2 × KAPA PROBE Master Mix (2x) ABI Prism Kit (Kapa Biosystems, Wilmington, MA) and RNase-free water in a total volume of 20 μl. The expression levels (RQ values) of the studied genes were calculated using the delta delta CT method, with the adjustment to the β-actin expression level and in relation to the expression level of calibrator (Human Lung Total RNA Ambion®), for which RQ value was equal to 1.
2.6 Statistical Analysis
The Kruskal–Wallis test, the Mann–Whitney U test, the Neuman–Keuls’ multiple comparison test, and the Spearman rank correlation were used to assess the correlation between the relative gene expression levels and sarcoidosis groups classified on the basis of chest X-ray results (stage I vs. II–IV) and disease phenotype (acute vs. insidious onset), spirometric parameters, DLCOc, serum Ca2+ concentration, Ca2+ in 24 h urine collection, the percentage of lymphocytes in BAL, the phenotype of immune cells (CD4+/CD8+), age, and sex of patients. P < 0.05 was considered statistically significant.
3 Results
3.1 Relative Expression of Genes in BALF Cells
There were significant differences between the expression level of TGF-β1 and SMAD3, TGF-β1 and SMAD7, and between all SMAD genes: SMAD2, 3 and 7 in BALF cells (P < 0.05, Neuman-Keuls multiple comparison test) (data not shown).
In the patients with radiological stage I, the highest expression level of SMAD7 (mean RQ = 3.41) and the lowest of SMAD3 (mean RQ = 0.26) was found in BALF cells. Likewise, the patients with radiological stages II–IV revealed the highest mean expression level of SMAD7 (mean RQ = 3.54) and the lowest of SMAD3 (mean RQ = 0.33). In both acute and insidious disease onset groups, the highest mean expression level was observed for SMAD7 (mean RQ: 3.49 and 3.40, respectively), and the lowest for SMAD3 (0.36 and 0.23, respectively).
Summarizing, the expression of SMAD3 gene was decreased in the majority of samples (90–94 %), and the highest expression level was observed for SMAD7 gene (82–85 %), regardless of the disease classification (Table 2).
3.2 Relative Expression of Genes in Peripheral Blood Lymphocytes of Patients with Sarcoidosis
There were significant differences between the expression level of TGF-β1 and SMAD2 in PB cells (P < 0.05, Neuman-Keuls multiple comparison test) (data not shown). In the patients with radiological stage I, the highest expression of SMAD3 (mean RQ = 1.060) and the lowest of SMAD2 (mean RQ = 0.494) was found. In the patients with radiological stages II–IV, the highest mean expression of TGF-β1 (0.92) and the lowest of SMAD2 (0.53) was observed.
In the acute onset group, the highest mean expression of SMAD3 (1.11) and the lowest of SMAD2 (0.47) was revealed. In the insidious onset group, the highest mean expression level for TGF-β1 (1.08) and the lowest for SMAD2 (0.55) was found.
The expression of the TGF-β1 gene increased in 23–38 % of the samples, regardless of the clinical disease classification groups. Decreased expression levels were observed for SMAD2 gene in the majority of patients (86–94 %) (Table 3).
3.3 Expression Levels of Genes (TGF-β1, SMAD 2,3,7) in Sarcoidosis Patients vs. Controls
In BAL cells of the control group, the mean RQ values of the genes were as follows: 0.470 for TGF-β1, 0.468 for SMAD2, 0.161 for SMAD3, and 2.670 for SMAD7. The mean RQ values of these genes for patients with sarcoidosis are shown in Table 2. No statistically significant differences were found for any of the studied genes between sarcoidosis and control subjects (P > 0.05; Mann–Whitney U test).
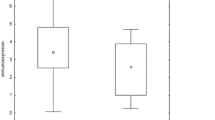
In PB lymphocytes of the control group, the mean RQ values were as follows: 0.51 for TGF-β1, 0.49 for SMAD2, 0.41 or SMAD3 and 0.81 for SMAD7. The mean RQ values of these genes for patients with sarcoidosis are shown in Table 3. Significant differences between the patients and controls were observed for the TGF-β1 gene (P = 0.004) and for the SMAD3 gene (P = 0.002, Mann–Whitney U test), with a higher gene expression in sarcoidosis patients (Figs. 1 and 2).
3.4 BAL Cells vs. Lymphocytes
Significant differences between the BAL cells and lymphocytes for the TGF-β1 gene and for the SMAD3 gene (P = 0.001, Mann–Whitney U test) were observed, with a higher expression level of those genes in lymphocytes. In contrast, The SMAD7 gene was significantly higher (P = 0.001) in the BAL cells.
3.5 Expression Levels of Genes in Different Sarcoidosis Subgroups
There were no significant differences regarding the expression of the genes between the patients without and those with parenchymal involvement (stages I vs. II–IV) or between the clinical phenotypes (acute vs. insidious onset), either in BAL cells or in PB lymphocytes.
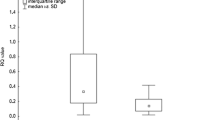
Significantly increased expression of TGF-β1 was observed in BAL cells in the patients with abnormal compared with those having normal spirometry (P = 0.012). Likewise, expression of both TGF-β1 and SMAD3 in BAL cells was higher in a subgroup of patients with restrictive spirometric pattern (P = 0.003 both, Mann–Whitney U test) (Figs. 3 and 4).
3.6 Gene Expression Levels in BAL Cells in Relation to Patients’ Gender, and Biochemical and Immunological Markers
There were significant inter-gender differences for the TGF-β1 gene in a total patient group (P = 0.032), with a higher gene expression level in men. Likewise, in men with radiological stage I, a significantly higher TGF-β1 gene expression level was observed (P = 0.029). In females, a significantly higher SMAD2 gene expression level was found in the insidious onset phenotype group (P = 0.042, Mann–Whitney U test). We found several negative correlations between the lung function parameters and RQ values of the genes studied. Those and other correlations are presented in Table 4.
3.7 Gene Expression in Peripheral Blood Lymphocytes in Relation to Patients’ Gender, and Biochemical and Immunological Markers
Significant inter-gender differences were observed for the SMAD7 gene in a total patient group (P = 0.021; U Mann–Whitney U test), with a higher gene expression level in females.
Correlations between RQ values of the genes studied in peripheral blood lymphocytes and lung function and laboratory results are presented in Table 4.
4 Discussion
Although we found inappreciable differences between the sarcoidosis and control groups regarding the expression of genes in BAL cells, we did find upregulated TGF-β1 and SMAD3 genes in peripheral blood lymphocytes in sarcoidosis patients. The TGF-β1 gene was overexpressed exclusively in peripheral blood lymphocytes in the patients with lung parenchymal involvement and insidious disease onset (both known to be related to increased risk of chronic and progressive disease). Interestingly, BAL fluid cells obtained from the sarcoidosis patients showed signs of overexpression of inhibitory SMAD7, with presumably a subsequent downregulation of SMAD3. The most informative parameters of bronchial and parenchymal distortion and damage – lung function results and diffusion capacity – correlated negatively with SMAD3, SMAD2, and TGF-β1. These findings were confirmed in the whole sarcoidosis group and in the subgroups of patients with radiological signs of lung parenchymal involvement and insidious disease onset, both features related to chronicity and progression. In addition, increased expression of SMAD3 and TGF-β1 genes were also confirmed in patients with abnormal spirometry, especially those with restrictive pattern.
Our results are in concordance with other authors (Salez et al. 1998; Ishioka et al. 1996) who reported the lack of significant differences in TGF-β1 protein/mRNA expression in BALF of sarcoidosis patients in comparison with healthy controls. The possible explanation of this phenomenon is a complicated system of adjustment of the TGF- β1 activity in lung cells by a set of receptors which reveal serine-threonine kinase activity and collaborate with different cytokines via TGF/Smad signaling and other downstream pathways (e.g., Ras/MEK/ERK signaling) (Massague 1998). Moreover, according to the earlier published data, a class of intracellular signaling proteins such as Smads has been confirmed as the pivotal mediators of TGF-β1 activity (Derynck et al. 1998).
These signaling proteins may strongly modulate the TGF-β1 expression. Especially, Smad3, which interacts with many transcription factors, oncogenes, and with glucocorticoid receptors, may influence the TGF-β1 synthesis in the inflammatory process. In the present study we confirmed a reduced expression of TGF-β1 followed by a reduced SMAD3 gene expression in BAL cells of sarcoidosis patients. Consequently, we did not observe any difference in TGF-β1 expression between the patient and control groups. Additionally, we observed an overexpression of inhibitory SMAD7 in the patients’ BAL cells, which possibly is also responsible for the downregulation of TGF-β1 gene.
It seems interesting that, according to other published results, TGF-β1 immunoexpression in BAL cells is increased in sarcoidosis patients with altered lung function (Salez et al. 1998). Likewise, in the present study in BAL cells of patients with lung function impairment, the increased expression of TGF-β1 on the transcriptional level and a negative correlation between TGF-β1 mRNA level and lung function results were found. The overwhelming source of information on the role of Smads in TGF-β-mediated responses comes from the studies on wound healing. We can learn from these studies that SMAD3 gene and its protein Smad3 may play a key role in the regulation of this process (Ashcroft and Roberts 2000). For instance, the rate of wound healing in Smad3ex8/ex8 (null) mice is substantially accelerated when compared to wild-type animals (Ashcroft et al. 1999). Also, in a skin radiation fibrosis model, Smad3 seems to play a modulatory role, as Smad3ex8/ex8 mice are protected against cutaneous injury induced by ionizing radiation, and show reduced epidermal acanthosis, reduced influx of neutrophils and mast cells, and, most importantly, reduced accumulation of myofibroblasts and extracellular matrix components (Flanders et al. 2002). Recent studies also point out a critical role of TGFβ/Smad3 signaling in bleomycin-induced lung fibrosis in mice. Tang et al. (2014) have reported that TGF-β1wt transgenic mice, showing increased levels of latent TGF-β1 in plasma and lung tissue, are protected from bleomycin-induced lung inflammation and collagen matrix accumulation. This effect was accompanied by inactivation of TGFβ/Smad3 pathway and increased levels of an inhibitory Smad7. Such studies on the role of the TGFβ/Smad signaling pathway in sarcoidosis have not yet been published. Therefore, we presume that the present data showing the role of SMAD genes in sarcoidosis are the first ones published.
Many studies dedicated to the significance of TGF-β in interstitial lung diseases provide evidence for a negative prognostic value of this cytokine. For instance, the correlation between the concentration of TGF-β1 in BAL fluid and the high resolution computed tomography (HRCT) score of the lavaged segment has been found (Szlubowski et al. 2010). The authors conclude that this cytokine may be a good, but not specific, marker of fibrosis in the plethora of interstitial lung diseases.
Interestingly, glucocorticosteroids, which are the mainstay of treatment in severe sarcoidosis and other interstitial lung diseases potentially leading to fibrosis, downregulate the TGF-β receptor 1 (tgfbr1)/Smad2/3 pathway in lung fibroblasts in vitro (Schwartze et al. 2014). Also, inhibition of extracellular signal-regulated kinase (ERK-5), a MAP-kinase known to play a role in an experimental model of bleomycin-induced lung fibrosis, ameliorates fibrosis via inhibition of Smad3 acetylation (Kim et al. 2013). Therefore, Smad3 and SMAD3 gene may be a target for pharmacological modulation. It may be presumed that this pathway will be considered in the development of new antifibrotic drugs.
In conclusion, we provide data showing upregulation of TGF-β1 and SMAD3 in peripheral blood lymphocytes in sarcoidosis patients, while in BAL cells these genes may be downregulated by inhibitory SMAD7. The increased activity of these genes in patients presenting signs known to be related to worse prognosis (insidious disease onset, parenchymal involvement, lung function impairment) as well as several negative correlations between lung function results and mRNA levels of these molecules, point to the TGFβ1/Smad pathway as being critical for the development of such unfavorable outcome in sarcoidosis as lung fibrosis.
References
American Thoracic Society: European Respiratory Society: World Association of Sarcoidosis and Other Granulomatous Disorders (1999) Statement on sarcoidosis. Am J Respir Crit Care Med 160:736–755
Ashcroft GS, Roberts AB (2000) Loss of Smad3 modulates wound healing. Cytokine Growth Factor Rev 11:125–131
Ashcroft GS, Yang X, Glick A, Weinstein M, Letterio JJ, Mizel DE (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1:260–266
Chciałowski A, Chorostowska-Wynimko J, Fal A, Pawłowicz R, Domagał-Kulawik J (2011) Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling processing and analysis methods. Pneumonol Alergol Pol 79:75–89
Derynck R, Zhang Y, Feng XH (1998) Smads: transcriptional activators of TGF-beta response. Cell 95:737–740
European Respiratory Society (1993) Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J 16(Suppl):1–100
Flanders KC, Sullivan CD, Fuji M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB (2002) Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 160:1057–1068
Ianuzzi MC, Rybicki BA, Teirstein AS (2007) Medical progress. Sarcoidosis. N Engl J Med 357:2153–2165
Ishioka S, Saito T, Hiyama K, Haruta Y, Maeda A, Hozawa S, Inamizu T, Yamakido M (1996) Increased expression of tumor necrosis factor-alpha, interleukin-6, platelet-derived growth factor-B and granulocyte-macrophage colony-stimulating factor mRNA in cells of bronchoalveolar lavage fluids from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 13:139–145
Khalil N, O’Connor RN, Flanders KC, Unruh H (1996) TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol 14:131–138
Kim S, Lim JH, Woo CH (2013) ERK5 inhibition ameliorates pulmonary fibrosis via regulating Smad3 acetylation. Am J Pathol 183:1758–1768
Massague J (1998) TGF-beta signaling transduction. Annu Rev Biochem 67:753–791
Patterson KC, Hogarth K, Husain AN, Sperling AI, Niewold TB (2012) The clinical and immunologic features of pulmonary fibrosis in sarcoidosis. Transl Res 160:321–331
Polish Respiratory Society Guidelines for Spirometry (2006) Zalecenia Polskiego Towarzystwa Chorób Płuc dotyczce wykonywania badań spirometrycznych. Pneumonologia i Alergologia Polska 74(Suppl 1) (in Polish)
Salez F, Gosset P, Copin MC, Lamblin Degros C, Tonnel AB, Wallaert B (1998) Transforming growth factor-β1 in sarcoidosis. Eur Respir J 12:913–919
Schwartze JT, Becker S, Sakkas E, Wujak ŁA, Niess G, Usemann J, Reichenberger F, Herold S, Vadász I, Mayer K, Seeger W, Morty RE (2014) Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. J Biol Chem 289:3262–3275
Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, Song P, Yu Z, Liu C, Zhu Q, Zheng J (2014) Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir Physiol Neurobiol 190:113–117
Szlubowski A, Soja J, Grzanka P, Tomaszewska R, Papla B, Kużdżał J, Ćmiel A, Sładek K (2010) TGF-b1 in bronchoalveolar lavage fluid in diffuse parenchymal lung diseases and high-resolution computed tomography score. Pol Arch Med Wewn 120:270–275
Tang YJ, Xiao J, Huang XR, Zhang Y, Yang C, Meng XM, Feng YL, Wang XJ, Hui DS, Yu CM, Lan HY (2014) Latent TGF-β1 protects against bleomycin-induced lung injury in mice. Am J Respir Cell Mol Biol (Epub ahead of print), 51(6):761–771
Acknowledgements
The study was funded by grant 2011/01/B/NZ5/04239 from the National Science Center.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Piotrowski, W.J. et al. (2014). TGF-β and SMADs mRNA Expression in Pulmonary Sarcoidosis. In: Pokorski, M. (eds) Respiratory Carcinogenesis. Advances in Experimental Medicine and Biology(), vol 852. Springer, Cham. https://doi.org/10.1007/5584_2014_106
Download citation
DOI: https://doi.org/10.1007/5584_2014_106
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16921-7
Online ISBN: 978-3-319-16922-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)