Abstract
Angiogenesis/angiostasis regulated by hypoxia inducible factor-1A (HIF-1A)/vascular endothelial growth factor (VEGF)/inhibitor of growth protein 4 (ING-4) axis may be crucial for the course and outcome of sarcoidosis. Overexpression of angiogenic factors (activation of VEGF through HIF-1A) may predispose to chronic course and lung fibrosis, whereas immunoangiostasis (related to an overexpression of inhibitory ING-4) may be involved in granuloma formation in early sarcoid inflammation, or sustained or recurrent formation of granulomas. In this work we investigated gene expression of HIF-1A, VEGF and ING-4 in bronchoalveolar fluid (BALF) cells and in peripheral blood (PB) lymphocytes of sarcoidosis patients (n = 94), to better understand mechanisms of the disease and to search for its biomarkers. The relative gene expression level (RQ value) was analyzed by qPCR. The results were evaluated according to the presence of lung parenchymal involvement (radiological stage I vs. II–IV), acute vs. insidious onset, lung function tests, calcium metabolism parameters, percentage of lymphocytes (BALL %) and BAL CD4+/CD8+ in BALF, age, and gender. In BALF cells, the ING-4 and VEGF RQ values were increased, while HIF-1A expression was decreased. In PB lymphocytes all studied genes were overexpressed. Higher expression of HIF-1A in PB lymphocytes of patients with abnormal spirometry, and in BALF cells of patients with lung volume restriction was found. VEGF gene expression in BALF cells was also higher in patients with abnormal spirometry. These findings were in line with previous data on the role of HIF-1A/VEGF/ING-4 axis in the pathogenesis of sarcoidosis. Up-regulated HIF-1A and VEGF genes are linked to acknowledged negative prognostics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Sarcoidosis is an inflammatory granulomatous disorder of unknown etiology, affecting multiple organs, but mainly intrathoracic lymph nodes and the lungs (Baughman et al. 2011; Hunninghake et al. 1999). It has been reported in all racial and ethnic groups. In the majority of patients the disease disappears without treatment, but it may become chronic and progressive, leading to debilitating lung fibrosis, and in some cases also to death (Ianuzzi et al. 2007). Genetic susceptibility seems to be crucial for the development of the disease in subjects exposed to unknown environmental (infectious or non-infectious) factors (antigens) (Lazarus 2009; Ianuzzi et al. 2007; Thomas and Hunninghake 2003). Genetic factors strongly influence the disease course and long-term prognosis. However, it is very difficult to predict in the clinical setting, which patients are at increased risk of unfavorable outcome in the future.
A few studies have been published recently, showing that angiogenic and angiostatic factors may be involved in the pathogenesis of sarcoidosis (Cui et al. 2010; Lazarus 2009; Ianuzzi et al. 2007; Antoniou et al. 2006). It is claimed that angiogenesis-angiostasis balance is implicated in the formation of granuloma (angiostasis), and in hypoxia-induced lung remodeling and fibrosis (angiogenesis) (Cui et al. 2010). Hypoxia-inducible factor-1A (HIF-1A, located in the chromosome 14q21-q24) is a key transcription factor in the cellular response to hypoxia, and is recognized as a major oxygen homeostasis regulator which controls the activation of genes essential to cellular adaptation to low oxygen conditions, such as vascular endothelial growth factor (VEGF). However, also under normoxic conditions, several dozen of genes implicated in different cellular functions have been found to be induced by growth factors and vascular hormones through the mediation of HIF-1A (Déry et al. 2005). HIF-1 is a key regulator of VEGF, which stimulates mobility and maturation of endothelial cells in hypoxic environment. In addition, the group of HIF-1A dependent genes (including MMP2) influence the extracellular matrix turnover. Under hypoxic conditions, HIF-1 upregulation may induce alveolar cell apoptosis and epithelial-mesenchymal transmission (EMT), thus potentially contributing to pulmonary fibrosis (Kim et al. 2006). VEGF, by controlling monocyte recruitment, may be involved in granuloma formation (Tzouvelekis et al. 2012; Tolnay et al. 1998). In chronic experimental hypoxia, leading to acute lung fibrosis, inflammation, fibrosis, and pulmonary hypertension, the increased HIF-1A protein and its DNA binding activity have been observed in pulmonary epithelial cells (Shimoda and Semenza 2011; Yu et al. 1998). Recently, it has been documented that HIF-1A dependent lung epithelial remodeling involves changes in the profile of chemokines and cytokines, properties of myofibroblasts, and a variety of proangiogenic factors, including VEGF (Stenmark et al. 2006). Inhibitor of growth protein 4 (ING-4) has been recognized as a potential tumor suppressor gene, and effective suppressor of HIF-1A. It is involved in the regulation of cell cycle arrest, apoptosis or senescence, inhibiting cell proliferation and angiogenesis (Guérillon et al. 2014).
Currently, no useful diagnostic or prognostic biomarkers are available to support the clinical judgment of patients suffering from sarcoidosis. Moreover, relatively little is known about the significance of HIF1-A/VEGF/ING-4 axis in sarcoidosis development and disease course. Therefore, in the present study we analyzed the expression levels of HIF-1A, VEGF, ING-4 genes and evaluated their potential diagnostic and prognostic value in sarcoidosis patients.
2 Methods
2.1 Study Group
The study was approved by Ethics Committee of the Medical University of Lodz, Poland (permission RNN/141/10/KE) and patients participating in the study signed written informed consent. This study is part of the project on research into the molecular mechanisms underlying the pathogenesis of sarcoidosis. The project encompassed 94 hospitalized patients with the diagnosis of pulmonary sarcoidosis and 50 non-smoking patients suffering from idiopathic cough or other unrelated to sarcoidosis conditions. All subject were recruited for the study in the years 2010–2014. The diagnosis was based on the current international guidelines (Ianuzzi et al. 2007; ATS & ERS & WASOG 1999). Clinical and radiological features of sarcoidosis, with the presence of non-caseating granuloma in tissue biopsy, were confirmed in each patient. Demographic characteristics of the sarcoidosis and control patients have been previously described in detail (Piotrowski et al. 2014a).
The current study on the role in sarcoid formation of angiogenesis/angiostasis balance controlled by HIF-1A/VEGF interplay expands on the previous investigation concerning the expression of the cytokine transforming growth factor beta (TGF-β)/SMAD receptor signaling cascade in the development of clinical features of sarcoidosis. The source of research material for the current study were the same samples of bronchoalveolar lavage fluid (BALF) and peripheral blood which were used in research on the TGF-β/SMAD cascade previously published (Piotrowski et al. 2014a). The angiogenesis/angiostasis status was considered an independent research ramification of the molecular pathogenesis of sarcoidosis, and, therefore, was herein described as a separate entity.
2.2 Collection of Biological Material
Bronchoscopy was performed with a flexible bronchoscope (Pentax, Tokyo, Japan) according to the Polish Respiratory Society Guidelines (Chciałowski et al. 2011). BALF was collected from the medial lung lobe, by instillation and subsequent withdrawal of 4 × 50 ml of 0.9 % NaCl. The fluid recovery was 52.1 ± 1.2 %. The crude BALF was filtered through a gauze to clear the thick mucus and other contaminants, centrifuged, and the pellet was suspended in a phosphate buffer. The total number of non-epithelial cells (total cell count – TCC) was presented as n × 106. Cytospin slides were prepared and stained by May-Grünwald-Giemsa stain. The number of macrophages, lymphocytes, neutrophils, and eosinophils was calculated under a light microscope and presented as percent of TCC. After the calculations, all fluid was centrifuged (10 min, 1,200 rpm), supernatant of BALF was suspended in RNAlater RNA Stabilization Reagent (QIAGEN, Hilden, Germany) in a volume of about 350 μl of solution in Eppendorf tubes, marked with an identification number, and was frozen (−80 °C) until further RNA isolation procedures.
Spirometry was performed according to the Polish Respiratory Society Guidelines (Polish Society of Respiratory Diseases 2006) with a computer-based spirometer (Jaeger, Dortmund, Germany).
Blood was collected into 5 ml EDTA containing tubes. For lymphocyte separation, a density gradient cell separation medium Histopaque-1077 (Sigma-Aldrich, Poznan, Poland) was used according to the manufacturer’s protocol.
2.3 RNA Extraction, Real-Time qPCR
RNA isolation was performed using mirVana™ miRNA Isolation Kit (Life Technologies, Carlsbad, CA), according to the manufacturer’s protocol. The quality and quantity of isolated RNA was assessed spectrophotometrically (BioPhotometerTM Plus, Eppendorf, Hamburg, Germany). The purity of total RNA (ratio of 16S to 18S fraction) was determined in the automated electrophoresis using RNA Nano Chips LabChipplates on Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
cDNA was transcribed from 100 ng of total RNA, using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) in a total volume of 20 μl according to manufacturer’s protocol. The relative expression analysis was performed in 7900HT fast real-time PCR System (Applied Biosystems, Carlsbad, CA) using TaqMan probes for the studied genes: HIF-1A (Hs00153153_m1), VEGF (Hs00900055_m1), ING-4 (Hs01088026_m1), ACTB (Hs99999903_m1). The PCR mixture contained: cDNA (1 to 100 ng), 20 × TaqManR Gene Expression Assay, 2 × KAPA PROBE Master Mix (2x) ABI Prism Kit (Kapa Biosystems, Wilmington, MA) and RNase-free water in a total volume of 20 μl. The expression levels (RQ values) of the studied genes were calculated using the delta CT method, with the adjustment to the β-actin expression level and in relation to the expression level of calibrator (Human Lung Total RNA Ambion®; Applied Biosystems, Carlsbad, CA), for which RQ value was equal to 1.
2.4 Data Elaboration
The Kruskal-Wallis test, the Mann-Whitney U test, the Neuman–Keuls multiple comparison test, and the Spearman rank correlation were used for statistical data elaboration (StatSoft, Cracow, Poland). Statistically significant differences were defined as P < 0.05.
3 Results
3.1 Relative Expression Analysis of Genes in Bronchoalveolar Lavage Fluid Cells
In BALF cells from patients with radiological stage I sarcoidosis, the highest expression level (mean RQ) of ING-4 (0.629), and the lowest of HIF-1A (0.037) were observed. Similarly, in patients with radiological stages II–IV, the highest RQ values were revealed for ING-4 (0.634), and the lowest for HIF-1A (0.031).
In the acute onset phenotype of sarcoidosis, the highest expression level (mean RQ) was observed for ING-4 (0.568) and the lowest for HIF-1A (0.033). Similarly, in the chronic insidious onset phenotype, the highest expression level (mean RQ) was found for ING-4 (0.686), and the lowest for HIF-1A (0.035). Decreased (RQ < 1) and increased (RQ > 1) expression values of studied genes are shown in Table 1.
3.2 Relative Expression Analysis of Genes in Peripheral Blood Lymphocytes
In peripheral blood (PB) lymphocytes in patients with radiological stage I sarcoidosis, the highest mean expression level (mean RQ) of ING-4 (0.540) and the lowest of HIF-1A (0.321) were observed. In patients with radiological stages II–IV, the highest mean expression level of ING-4 (0.586) and the lowest of VEGF (0.025) were found.
In the acute onset phenotype, the highest mean expression level of ING-4 (0.592) and the lowest of HIF-1A (0.246) were observed. Similarly, in the insidious onset phenotype, the highest mean expression level for ING-4 (0.526) and the lowest for VEGF (0.089) were found. Decreased (RQ < 1) and increased (RQ > 1) expression values of studies genes are shown in Table 2.
3.3 Expression of Genes in Sarcoidosis Patients vs. Controls
BALF Cells
In BALF cells, in the whole group of sarcoidosis patients, the mean RQ values of the genes were the following: 0.030 for HIF1-A, 0.172 for VEGF, and 0.703 for ING-4 gene (for specific clinical classification see Table 1). In the control group, the RQ values were the following: 0.050 for both HIF1-A and VEGF, and 0.331 for ING-4 gene. There were no statistically significant differences regarding the level of genes expression between the sarcoidosis and control groups (P > 0.05; Mann-Whitney U test).
PB Lymphocytes
In PB lymphocytes, in the whole group of sarcoidosis patients, the mean RQ values of the genes were the following: 0.233 for HIF1-A, 0.197 for VEGF, and 0.561 for ING-4 gene (for specific clinical classification see Table 2). In the control group, the RQ values were the following: 0.024 for HIF-1A, 0.013 for VEGF, and 0.468 for ING-4 gene. The level of expression of the HIF-1A gene was here significantly greater in sarcoidosis patients than that in the control subjects (P = 0.003, Mann-Whitney U test).
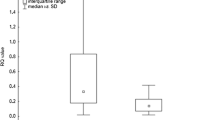
3.4 Expression of Genes in BALF Cells vs. PB Lymphocytes in Sarcoidosis Patients
There was significantly greater HIF-1A expression in PB lymphocytes than that in BALF cells in sarcoidosis patients (P <0.0001; Mann-Whitney U test) (Fig. 1).
3.5 Expression of Genes in Relation to Radiological and Clinical Classification and Lung Function
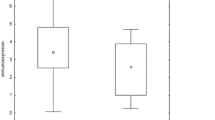
In both BALF cells and PB lymphocytes, there were no significant differences in the expression of the genes studied between the subgroups with and without parenchymal involvement (stage I vs. II–IV), and between the acute vs. insidious onset phenotypes. However, a significantly higher expression level of HIF-1A in BALF cells in patients with abnormal spirometry, especially those with lung volume restriction pattern was found (P = 0.033) (Fig. 2). We also observed a significantly higher expression level of VEGF gene in BALF cells in patients with abnormal spirometry (P = 0.028).
3.6 Relationship Between the Expression of Genes and Lung Function, Patients’ Features, and Laboratory Markers in Bronchoalveolar Lavage Fluid Cells and in Peripheral Blood Lymphocytes
Several negative correlations were found between the HIF-1A and VEGF gene expression in BALF, and VEGF expression in PB lymphocytes, on one side, and spirometric tests or DLCOc on the other side (Table 3).
4 Discussion
In the present study we documented down-regulated HIF-1A, and up-regulated VEGF and ING-4 gene expression in BALF cells, while up-regulated gene mRNAs of the entire axis was observed in peripheral blood lymphocytes. Our results are partly in accord with the observations of other authors (Tzouvelekis et al. 2012) who also found suppressed HIF-1A and abundant VEGF and ING-4 expression in tissue biopsies containing sarcoid granuloma. This unusual biological cascade of genes expression results, according to the cited authors, from the functional feedback between HIF-1A and ING-4 genes. Interestingly, we also observed a negative interaction between ING-4 and HIF-1A, as the overexpression of ING-4 frequently coexisted with decreased expression of HIF-1A in BALF cells in sarcoidosis patients. Other authors also confirmed that ING-4 is able to inhibit HIF-1A, or ING-4 may also be suppressed by other growth factors present in the cytokine milieu of sarcoid inflammatory site (Tzouvelekis et al. 2012; Antoniou et al. 2006; Déry et al. 2005).
The known mechanism of inhibitory properties of ING-4 is based on the interaction with HIF prolyl hydroxylases and NF-κB subunit – ReIA. The net effect is the repression of angiogenesis related genes, such as IL-6, IL-8, and COX-2. Immunoangiostatic environment in sarcoidosis may also be confirmed by the studies showing the role of CXCR3 ligands (interferon inducible cytokines, such as IP-10, ITAC, and MIG). These cytokines are related to more severe and chronic course of sarcoidosis (Piotrowski et al. 2014b). Apparently, the inconsistent rise in VEGF gene expression is the evidence of axis impairment and may be explained by a dual character of sarcoid inflammation – on the one side immunoangiostatic and on the other side inflammatory, in which VEGF may also be involved in inflammatory pathways different than angiogenesis. However, this dual immunoangiostatic and inflammatory nature of sarcoid granuloma formation involves stimuli unrelated to HIF-1/VEGF axis (Tzouvelekis et al. 2012). An elevated concentration of VEGF protein in the serum and BALF of sarcoidosis patients and increased VEGF protein expression in tissue biopsies has also been found by Yamashita et al. (2013). Additionally, VEGF concentration has been shown to be higher in patients with extrapulmonary involvement, and the level of VEGF is helpful in predicting which patients would deserve corticosteroid treatment (Sekiya et al. 2003). A comparison of VEGF concentration in BALF retrieved from lung segments with more and less intensive parenchymal changes revealed a higher concentration of VEGF in more involved lung areas. Moreover, the relation to the disease activity was confirmed (Ziora et al. 2000). This up-regulation of VEGF on the translational level has also been confirmed in the present study on the mRNA level, and a negative correlation between VEGF expression, and lung function results and parenchymal involvement in sarcoidosis patients was also recognized. Our observation concerning the up-regulation of VEGF is important for the recognition of this gene as a strong angiogenic stimulator, which activates mobility of endothelial cells toward the hypoxic environment and in consequence leads to lung epithelial remodeling. Therefore, VEGF expression and immunoexpression in the serum or BALF seem to have a prognostic value in sarcoidosis patients.
In idiopathic pulmonary fibrosis (IPF), a chronic progressive disease leading to extensive interstitial fibrosis, the pro-angiogenic environment has been documented. That is interesting in the context of possible mechanisms of lung fibrosis in the course of sarcoidosis. In a study comparing sarcoidosis and IPF, the increased VEGF mRNA expression in BALF of IPF patients, and also lower expression of CXCL12 and CXCR4 mRNA, constitute the pro-angiogenic microenvironment (Antoniou et al. 2009). The same group of authors reported a different profile of pro-angiogenic (GRO-a, ENA-78, IL-8) and anti-angiogenic (CXCR3 ligands) cytokines in BALF, with increased levels of pro-angiogenic cytokines in IPF (Antoniou et al. 2006). This is in line with the observation of other authors who found a negative correlation between angiogenic activity of sera of patients with interstitial lung disease and their lung diffusion capacity (Zielonka et al. 2010).
In the present study, we also documented the negative correlation in PB lymphocytes between expression of mRNA of another angiogenic factor, i.e., HIF-1A and lung function (FEV1/FVC) in sarcoidosis patients with parenchymal involvement. It seems an important observation due to the fact that up-regulation of HIF-1A on the translational level may inhibit the epithelial cell repair mechanisms, which facilitates the development of pulmonary fibrosis. The process of pulmonary fibrosis, as a consequence of granuloma healing in the lung sarcoidosis, may impair respiratory function and has to do with diseases progression. Therefore, the expression of HIF-1A mRNA may be useful as a progression marker in sarcoidosis. Reassuming, up-regulation of the pro-angiogenic HIF-1A and VEGF genes observed in the present study is linked to the acknowledged negative prognostic factors in sarcoidosis. However, the results cannot provide data confirming direct relationship between the shift to pro-angiogenic environment and progression of sarcoidosis toward extensive lung fibrosis. That would deserve a separate follow-up study, with the recruitment of a number of patients with disease progression.
References
American Thoracic Society: European Respiratory Society: World Association of Sarcoidosis and Other Granulomatous Disorders (1999) Statement on sarcoidosis. Am J Respir Crit Care Med 160:736–755
Antoniou KM, Tzouvelekis A, Alexandrakis MG, Sfiridaki K, Tsiligianni I, Rachiotis G, Tzanakis N, Bouros D, Milic-Emili J, Siafakas NM (2006) Different angiogenic activity in pulmonary sarcoidosis and idiopathic pulmonary fibrosis. Chest 130:982–988
Antoniou KM, Soufla G, Proklou A, Margaritopoulos G, Choulaki C, Lymbouridou R, Samara KD, Spandidos DA, Siafakas NM (2009) Different activity of the biological axis VEGF-Flt-1 (fms-like tyrosine kinase 1) and CXC chemokines between pulmonary sarcoidosis and idiopathic pulmonary fibrosis: a bronchoalveolar lavage study. Clin Dev Immunol 537929. doi:10.1155/2009/537929
Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R, Grutters JC, Judson MA, Lambiri I, Lower EE, Muller-Quernheim J, Prasse A, Rizzato G, Rottoli P, Spagnolo P, Teirstein A (2011) Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis 28:56–64
Chciałowski A, Chorostowska-Wynimko J, Fal A, Pawłowicz R, Domagała-Kulawik J (2011) Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling, processing and analysis methods. Pneumonol Alergol Pol 79(2):75–89 (Article in Polish)
Cui A, Anhenn O, Theegarten D, Ohshimo S, Bonella F, Sixt SU, Peters J, Sarria R, Guzman J, Costabel U (2010) Angiogenic and angiostatic chemokines in idiopathic pulmonary fibrosis and granulomatous lung disease. Respiration 80:372–378
Déry MA, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37:535–540
Guérillon C, Bigot N, Pedeux R (2014) The ING tumor suppressor genes: status in human tumors. Cancer Lett 345:1–16
Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP (1999) ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 16:149–173
Iannuzzi MC, Rybicki BA, Teirstein AS (2007) Sarcoidosis. N Engl J Med 357:2153–2165
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103:13180–13185
Lazarus A (2009) Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics. Dis Mon 55:649–660
Piotrowski WJ, Kiszałkiewicz J, Pastuszak-Lewandoska D, Antczak A, Górski P, Migdalska-Sęk M, Górski W, Czarnecka KH, Nawrot E, Domańska D, Brzeziańska-Lasota E (2014a) TGF-β and SMADs mRNA expression levels in pulmonary sarcoidosis. Adv Exp Med Biol. doi:10.1007/5584_2014_106
Piotrowski WJ, Młynarski W, Fendler W, Wyka K, Marczak J, Górski P, Antczak A (2014b) Associations between chemokine receptor cxcr3 ligands in bronchoalveolar lavage fluid and radiological pattern, clinical course and prognosis in sarcoidosis. Pol Arch Med Wewn 124:395–402
Sekiya M, Ohwada A, Miura K, Takahashi S, Fukuchi Y (2003) Serum vascular endothelial growth factor as a possible prognostic indicator in sarcoidosis. Lung 181:259–265
Shimoda LA, Semenza GL (2011) HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183:152–156
Stenmark KR, Fagan KA, Frid MG (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99:675–691
Thomas KW, Hunninghake GW (2003) Sarcoidosis. JAMA 289:3300–3303
Tolnay E, Kuhnen C, Voss B, Wiethege T, Muller KM (1998) Expression and localization of vascular endothelial growth factor and its receptor flt in pulmonary sarcoidosis. Virchows Arch 432:61–65
Tzouvelekis A, Ntolios P, Karameris A, Koutsopoulos A, Boglou P, Koulelidis A, Archontogeorgis K, Zacharis G, Drakopanagiotakis F, Steiropoulos P, Anevlavis S, Polychronopoulos V, Mikroulis D, Bouros D (2012) Expression of hypoxia-inducible factor (HIF)-1a-vascular endothelial growth factor (VEGF)-inhibitory growth factor (ING)-4- axis in sarcoidosis patients. BMC Res Notes 5:654
Yamashita M, Mouri T, Niisato M, Kowada K, Kobayashi H, Chiba R, Satoh T, Sugai T, Sawai T, Takahashi T, Yamauchi K (2013) Heterogeneous characteristics of lymphatic microvasculatures associated with pulmonary sarcoid granulomas. Ann Am Thorac Soc 10:90–97
Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL (1998) Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol 275:L818–L826
Zielonka TM, Demkow U, Radzikowska E, Bialas B, Filewska M, Życińska K, Obrowski MH, Kowalski J, Wardyn KA, Skopińska-Różewska E (2010) Angiogenic activity of sera from interstitial lung disease patients in relations to pulmonary function. Eur J Med Res 15(Suppl 2):229–234
Ziora D, Dworniczak S, Niepsuj G, Niepsuj K, Jarosz W, Sielska-Sytek E, Ciekalska K, Oklek K (2000) Proangiogenic cytokines (bFGF and VEGF) in BALF from two different lung segments examined by high resolution computed tomography (HRCT) in patients with sarcoidosis. Pneumonol Alergol Pol 68:120–130
Acknowledgements
This work was partially funded by the grant of the National Science Center (grant no. 2011/01/B/NZ5/04239).
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Piotrowski, W.J. et al. (2015). Expression of HIF-1A/VEGF/ING-4 Axis in Pulmonary Sarcoidosis. In: Pokorski, M. (eds) Noncommunicable Diseases. Advances in Experimental Medicine and Biology(), vol 866. Springer, Cham. https://doi.org/10.1007/5584_2015_144
Download citation
DOI: https://doi.org/10.1007/5584_2015_144
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19973-3
Online ISBN: 978-3-319-19974-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)