Abstract
Among species of Corbicula, only the hermaphroditic androgenetic lineages are invasive while sexual species are restricted to the native range (mainly Asia). Four androgenetic lineages of Corbicula spp. have been identified in American and European freshwater systems, with the Corbicula sp. form A/R lineage being abundant on both continents. This lineage is considered an important invasive species because of its impact on aquatic ecosystems and industrial cooling systems. The present study identified the invasive lineages of Corbicula spp. in freshwater environments of South America based on morphometric data and used genetic marker (mtDNA COI gene) to investigate their genetic relatedness to the invasive lineages from Europe and North America. Four important results arise: (1) there are two invasive lineages of Corbicula in South America, Corbicula sp. form C/S and Corbicula sp. form A/R, and they are clonally expanding their distribution in continental aquatic systems of South America; (2) populations with intermediate morphotype were detected in three distinct sites of Brazil; (3) one population with individuals presenting a new COI haplotype was recovered with an intermediate morphotype; (4) the morphotype of the invasive lineages of Corbicula spp. in South America presents high correlation with their COI haplotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Corbicula clams are native to Asia, Australia, Africa, and the Middle East, inhabiting both estuarine and freshwater environments (Araújo et al., 1993; Park & Kim, 2003). The genus includes dioecious sexual species, which seem mainly restricted to the native region in Asia, and the hermaphroditic androgenetic lineages that were genetically recognized invasive lineages were detected in America, Europe (Pigneur et al., 2014a), and Russia (Bespalaya et al., 2018).
Androgenetic reproduction is a peculiar asexual mode of reproduction in which the offspring are paternal clones, inheriting only the nuclear chromosomes from the sperm, while all the maternal nuclear chromosomes from the oocyte are extruded as polar bodies after fertilization (Komaru et al., 2006; Houki et al., 2011; Pigneur et al., 2012; Vastrade et al., 2022). In the species of Corbicula, this reproductive mode is characterized by the presence of biflagellate and unreduced sperm (Komaru et al., 1997; Konishi et al., 1998). Moreover, the sperm from an androgenetic lineage of a species of Corbicula can fertilize the egg of a different lineage, combining its nuclear genome with the mitochondrial genome of the other, resulting in a cytonuclear mismatch (Komaru et al., 2006; Hedtke et al., 2008; Vastrade et al., 2022). When the maternal nuclear genome is incompletely extruded, an admixture with the paternal genome can occur—event known as androgenetic parasitism—and a hybrid lineage is formed (e.g., Komaru et al., 2006; Hedtke et al., 2008; Bespalaya et al., 2018).
The invasive Corbicula spp. presently identified in America and Europe were divided into four lineages (Pigneur et al., 2014a; Tiemann et al., 2017; Vastrade et al., 2022) and appear to reproduce through androgenesis. First, Corbicula sp. form A/R is abundant and widely distributed in Europe and America (Lee et al., 2005; Hedtke et al., 2008; Pigneur et al., 2014a). Second, Corbicula sp. form C/S is present in South America and Europe (Pigneur et al., 2014a; Santos et al., 2016). Third, Corbicula sp. form B is known only in North and South America (Lee et al., 2005; Hedtke et al., 2008). Four, Corbicula sp. form Rlc is reported only in Europe (Pigneur et al., 2011b; 2014a). Recently, Corbicula sp. form D was recovered in Illinois, USA (Tiemann et al., 2017). However, form D appears to be a hybrid at the nuclear and morphological level, with a COI haplotype identical to that of Corbicula sp. form A/R, as observed in several Corbicula sp. populations of form A/R (Hedtke et al., 2008; Pigneur et al., 2014a; Tiemann et al., 2017; Vastrade et al., 2022).
These invasive Corbicula spp., especially the form A/R lineage, are considered one of the most invasive mollusks worldwide (Darrigran, 2002; Sousa et al., 2008; Crespo et al., 2015; Darrigran et al., 2020) due to their large geographic distribution in freshwater ecosystems, the high densities they can reach (5000 individuals/m2) (Mansur et al., 2012; Ferreira-Rodriguez et al., 2018), their high fecundity (e.g., 90.000 descendants in one reproductive period) (McMahon, 1999; Paschoal et al., 2013), their impact on phytoplankton abundance (Pigneur et al., 2014b), and their invasive behavior (Darrigran, 2002). As is common for most invasive species, Corbicula sp. form A/R has a high reproductive capacity (Cao et al., 2017). Cao et al. (2017) demonstrated, in populations of Corbicula sp. from Argentina, that neither the oocyte incubation nor the gamete spawning was affected by low temperatures (8.2–9 °C) and low salinities (0–3‰). Corbicula spp., therefore, appear capable of locally adapting during temporal water level fluctuations, surviving low temperatures (2 °C) (Castañeda et al., 2018), and promoting morphological changes in Neotropical regions (Paschoal et al., 2015) with distinct incubation pattern (Ludwig et al., 2014). In temperate climates, immature individuals are released from gill chambers being almost juvenile, while in subtropical climates, the release occurs at a pediveliger stage (Cao et al., 2017). These characteristics likely maximize their success of invasion in a wide range of environments. Additionally, a single hermaphroditic androgen is enough to initiate a new population owing to its ability to self-reproduce (Pigneur et al., 2014a).
Compared to the native species, the invasive Corbicula spp. present a much lower genetic diversity in their introduced range (Pigneur et al., 2014a; Gomes et al., 2016). This is probably the consequence of their androgenetic mode of reproduction (Siripattrawan et al., 2000; Lee et al., 2005; Hedtke et al., 2008; Pigneur et al., 2011b; 2012). Indeed, according to Pigneur et al. (2014a), a few individuals recently invaded America and Europe and, through their clonal mode of reproduction, the established clonal diversity was maintained. Within the native range, however, where sexual and androgenetic species co-occur, high genetic diversity is observed (Pigneur et al., 2014a; Vastrade et al., 2022).
In South America, the first report of Corbicula spp. was in Argentina around 1980 (Ituarte, 1981). Since then, the number of published records of Corbicula spp. has increased (Santos et al., 2012) and their current distribution encompasses the northern (39°5'S, 68°34'W) and southern (43°21′18.28"S 65°39′34.93"W) Argentine region of Patagonia (Semenas & Flores, 2005; Labaut et al., 2021a, b; Perez et al., 2022), the Negro River in Northern Brazil (Pimpão & Martins, 2008), the Orinoco basin in Venezuela (Lasso et al., 2009), and the island of Salamanca in Colombia (Aristizábal, 2008). According to Castañeda et al. (2018), their distribution is usually limited to temperatures between 2 °C and 34 °C.
Four species of Corbicula were reported from South America and identified solely on basis of morphological characteristics, are Corbicula fluminea (O.F. Müller, 1774), Corbicula largillierti (R.A. Philippi, 1844), Corbicula cf. fluminalis (O.F. Müller, 1774), and Corbicula sp. (detected only by Mansur et al., 2004) (Mansur et al., 2004; Santos et al., 2016). In 2005, three distinct morphotypes of Corbicula were genetically identified, each one corresponding to a distinct mitochondrial COI haplotype and nuclear 28S genotype with no genetic variability observed within each form (Lee et al., 2005) and, years later, were assigned to the invasive lineages Corbicula sp. forms A/R, Corbicula sp. forms B, and Corbicula sp. forms C/S (Pigneur et al., 2014a). Lee et al. (2005) also detected hybrid specimens in the Iguazu Falls (Iguaçu River, Paraná state in Brazil), which presented ‘mixed’ genotypes between form C/S lineage and form B lineage associated with C/S morphotype. Besides, based on ISSR markers, Bagatini et al. (2005) demonstrated that there are three distinct morphotypes of Corbicula in the Rosana reservoir (Paraná state, Brazil) which shared a single multilocus genotype (MLG) and were assigned to Corbicula sp. form A/R lineage. In 2014, Pigneur et al. (2014a) performed a world phylogeographic survey on specimens of Corbicula spp. and identified in two localities in South America the presence of the invasive lineages Corbicula sp. forms A/R and C/S, with no genetic variability observed within each form using both COI haplotype and 10 microsatellite markers. These two invasive lineages are widely distributed in South American rivers (Santos et al., 2012; Santana, 2013) that were linked to C. fluminea which corresponds to Corbicula sp. form A/R, and C. largillierti corresponding to Corbicula sp. form C/S (Hedtke et al., 2008; Pigneur et al., 2014a). On the other hand, C. cf. fluminalis is apparently restricted to Patos Lagoon and Guaiba Lake (Southern Brazil) (Mansur et al., 2012; Santos et al., 2012), which is genetically recognized as Corbicula sp. form B lineage (Lee et al., 2005; Hedtke et al., 2008). However, there are projection studies attesting suitable areas to Corbicula cf. fluminalis be present in South America (Reyna et al., 2018).
In the present study, a geographically wide sampling effort across South American basins was performed to determine which invasive lineages of Corbicula are present in South America using geometric morphometrics and genetic analyses of the mtDNA COI gene. Besides, we evaluated if there are individuals with cytonuclear mismatches as suggested by Pigneur et al. (2012).
Materials and methods
Sample collection
From 2011 to 2014, 507 specimens of Corbicula spp. of 22 putative populations were collected from rivers of Argentina, Brazil, and Colombia (Table 1). All specimens were preserved in 96% ethanol. In the Laboratory of Molecular Ecology and Evolutionary Parasitology at the Universidade Federal do Paraná, Brazil (LEMPE), the shells were separated from the soft tissue and the right shell of each specimen was photographed with a Canon Rebel EOS T3 digital camera for morphological analysis. The preserved tissues (in 96% ethanol) were used for molecular analyses.
COI gene amplification
A fragment of 650 bp of the mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified for each sampled specimen of Corbicula clams by Polymerase Chain Reaction (PCR) using the primers LCO1490 and HCO2198 (Folmer et al., 1994). Amplifications were performed in 25 µl total volume including 0.5 µl of gDNA, 1 × Reaction buffer, 200 µM of dNTPs, 0.5 µM of both primers, and 0.1 µl of Taq DNA polymerase (Life Technologies). PCR conditions were 5 min at 95 °C followed by 35 cycles of the 30 s at 94 °C, 30 s at 44 °C, and 40 s at 72 °C, and then a final extension of 5 min at 72 °C. Amplified fragments were sequenced in both directions in an Applied Biosystems 3130 automatic sequencer. Sequences were assembled and edited, and a consensus was generated using Geneious® 6.1.2 (Biomatters). We compiled 40 COI haplotypes that were available on GenBank (Table S1) and added those sequences into our COI alignment to further be analyzed.
COI haplotypes identification and phylogenetic analysis
The COI haplotypes (H) of each specimen from South America were compared to reference sequences from GenBank (Table S1), and frequencies (f) were individually counted. The evolutionary relationships among the COI haplotypes for all Corbicula spp. were investigated by reconstructing a Bayesian phylogeny with MrBayes 3.1.2 (Ronquist & Huelsenbeck, 2003) implemented by the CIPRES Science Gateway (available at http://www.phylo.org/news/mrbayes). The best evolutionary model was assessed by jModelTest, and based on Akaike information criteria (AIC) (Posada, 2008), the TrN + I + Γ model for all codon positions was used. In each analysis, we ran four independent Markov Chain Monte Carlo (MCMC) for 10 million generations. We evaluated burn-in by plotting the log-likelihood scores for each sampling point using TRACER v1.5 (Rambaut & Drummond, 2007), all ESS values in the final runs were > 900, and the first 25% of the trees were discarded as burn-in for each run. The residual trees were used to build a consensus tree using LogCombiner v.1.5.4 (part of the BEAST package, Drummond & Rambaut, 2007) and estimate Bayesian posterior probabilities (PP). For all analyses, a published COI sequence of Neocorbicula limosa (Maton, 1811) was used as an outgroup. Phylogenetic trees were visualized and edited using FigTree v1.3.1 (Rambaut, 2009).
Morphological data analysis
Using our dataset, we tested the hypothesis of Pigneur et al. (2012) suggesting a correlation between the COI lineage with the morphotype of invasive Corbicula lineages. The presence of intermediate morphotypes—i.e., hybrids between lineages—was also investigated as suggested by Lee et al. (2005) and Pigneur et al. (2014a).
For the morphological analysis, we obtained the length (L), width (W), and height (H) ratios (L/W, L/H, and H/W) for each right shell of the 507 individuals, corresponding to quantitative characteristics. In addition, geometric morphometric analyses were performed using two-dimensional anatomical landmarks from 11 internal homologous points (Fig. S1) using tpsDig2 software (Rohlf, 2006), following the protocol of Sousa et al. (2007). The ratios and each landmark were taken three times for each individual and a mean was calculated for each individual. The shape variables (quantitative characteristics and landmarks compiled) generated by the x and y coordinates of each landmark were adjusted to minimize any effect in translation, rotation, and scale, as suggested by Sousa et al. (2007), and used to construct a matrix for subsequent analysis. The morphological variation (ratios and landmarks compiled) among the COI haplotypes, within and between populations, was assessed by PCA. For both analyses, lineages of Corbicula were assigned according to their respective COI haplotypes. Furthermore, the generalized Procrustes ANOVA analysis (GPA) algorithm (Dryden & Mardia, 1998) was performed to test the morphological differences by Wilk’s λ, in which significant P values were accessed for 1000 replications between the lineages assigned by COI. All morphological analyses were performed in the software MorphoJ (Klingenberg, 2011).
Results
COI haplotypes and phylogenetic relationships
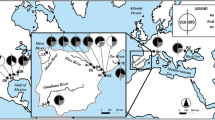
We identified three distinct COI haplotypes in our entire dataset (N = 507) (Table 1): haplotypes FW5 and FW17, and a third one named herein as FWBra (GenBank accession number: MH460421; Fig. S2), which are distinctly distributed within the South American hydrographic basins (Table 1; Fig. 1). The haplotype FW5 was predominant in our sampling, being detected in 19 out of the 22 South American sites (Table 1; Fig. 1). The haplotype FW17 was identified in six South American sites (ARG1, ARG2, PP, SOB, BRA, and COL) (Table 1; Fig. 1). The FWBra haplotype was identified only in the PU site (Table 1; Fig. 1).
Distribution of the invasive Corbicula lineages detected in the South America hydrographic basins and its respective COI haplotype, and morphotypes. The pie charts correspond to the distribution of each lineage and the colors (as indicated in the legend) and their respective frequency proportions for each site (details are in Table 1). The Corbicula mismatches are also shown
Based on our phylogeny, the FW5 haplotype matches with the invasive lineage Corbicula sp. form A/R and the FW17 haplotype matches with the invasive lineage Corbicula sp. form C/S (Fig. 2). The new FWBra haplotype was separately clustered from the others within the freshwater clade (Fig. 2) and, comparatively, it presents 94,67%, and 98,54% of identity with FW17 and FW5, respectively (Table S1; Fig. S2); therefore, we assign it as a putative new lineage called Corbicula sp. FWBra. The other two invasive lineages, European Corbicula sp. form Rlc (haplotype FW4) and Corbicula sp. form B (haplotype FW1), were not identified in our sampling.
Bayesian phylogenetic relationship of the COI haplotypes for estuarine and freshwater Corbicula spp. with their respective occurrence. Names in bold indicate the four invasive Corbicula lineages with androgenetic reproduction. The three lineages detected in this study are highlighted following red for Corbicula sp. form A/R, green for Corbicula sp. form C/S, and gray for Corbicula sp. FWBra. The posterior probabilities are indicated above each branch
Shells morphotype
We identified 327 individuals belonging to Corbicula sp. form A/R lineage, 115 individuals of Corbicula sp. form C/S lineage, and 65 individuals with an intermediate morphotype based on the morphological analyses (Table 1). Based on quantitative characteristics, Corbicula sp. form A/R presents high morphological variability but is distinguished from the remaining morphotypes by presenting a robust shell with a high umbo with spaced concentric rings (Fig. 1; Table S2). The Corbicula sp. form C/S presents a triangular, ovate shell with juxtaposed concentric rings and a low and round umbo; the internal color can be dark purple and the pallial line is evident (Fig. 1). The specimens with intermediate morphotypes were characterized by ovoid shells and flat often eroded umbo, closely juxtaposed concentric lines, light-purple internal color, and pallial lines scarcely evident. This intermediate morphotype was found in PU and JAC populations and some individuals in BRA (Fig. 1; Table 1).
The PCA described 99,7% of the total variability between the lineages, which PC1 axis described 77,8% retrieving the L/H and L/W ratios and PC2 described 21,9% retrieving the H/W ratio (Fig. 3), and demonstrated more morphological variations within Corbicula sp. form A/R than between Corbicula sp. form A/R and form C/S. The PCA with COI haplotypes assigned showed that the morphotype of C/S, A/R, and FWBra strongly correlated with their lineages (Wilk’s λ = 0.082, F= 33.93, P ≤ 0.001), except for few specimens of Corbicula sp. form C/S and Corbicula sp. form A/R that overlapped with Corbicula sp. FWBra (Fig. 3).
Principal components analysis of morphological variation of three lineages of Corbicula sp. detected in this study in South American hydrographic basins, with their respective COI haplotypes. The colors are following red for Corbicula sp. form A/R, green for Corbicula sp. form C/S, and gray for Corbicula sp. FWBra
Discussion
Clonal invasion of Corbicula clams in South America
The present study confirms the existence of two invasive lineages of Corbicula well-established and widespread in South American hydrographic systems: Corbicula sp. form C/S and Corbicula sp. form A/R. Moreover, an additional lineage with a distinct COI haplotype (FWBra) and intermediate morphotype was retrieved in one population in Brazil. This genetic homogeneity within each population suggests clonal propagation, as previously observed in Europe and North America (Hedtke et al., 2008; Pigneur et al., 2011a, b; 2014a; Vastrade et al., 2022).
The Corbicula sp. form C/S was detected in populations ARG1, and ARG2 from Argentina, in populations PP, SOB, and BRA from Brazil, and in populations COL in Colombia. All South American individuals that shared the COI FW17 haplotype, also shared the morphotype C/S with a similar description to Corbicula sp. form C/S lineage from Pigneur et al. (2014a). The Corbicula sp. form C/S was apparently introduced around 1980 in Europe (Haeslopp, 1992) and Argentina/Brazil because it was then first reported on both continents (Ituarte, 1994); its propagules were likely accidentally introduced in both continents (Pigneur et al., 2014a). A recent study by Vastrade et al. (2022) showed a biogeographic origin of Corbicula sp. form C/S in South Africa, clustering with C. fluminalis africana (Krauss,1848), which ancestor could therefore have colonized both continents independently or sequentially around the same time, from the same pool of South Africa. The morphotype of Corbicula sp. form C/S found in South America diverges morphologically from clams from other regions (Table S2) and suggests to us phenotypic plasticity within the Corbicula whose shell shape likely depends on habitat patterns, despite low genetic differences (for the marker studied, Pigneur et al., 2014a; López-Soriano et al., 2018; Bespalaya et al., 2018).
The individuals of Corbicula sp. form A/R detected in this study share the same COI FW5 haplotypes with the Corbicula sp. form A/R from other parts of the world (Hedteke et al., 2008; Pigneur et al., 2014a; Bespalaya et al., 2021), but they presented a wide morphological variability across South America. The first report of Corbicula sp. form A/R in North America was around 1930, in Europe in 1983 (McMahon, 1983), and in South America around 1980 (Ituarte, 1981). The origin of Corbicula sp. form A/R points to Japan, lake Biwa where the sexual C. sandai is found (Vastrade et al., 2022). Since identical clones of Corbicula sp. form A/R are widely distributed across Europe and America, one clone must have started the colonization in North America and has been maintained through androgenesis (Pigneur et al., 2014a), and based on our results, they depict the same pattern of geographic expansion in South America. Moreover, since there was no genetic distinction among specimens within the studied populations, we advocate that Corbicula sp. form A/R and Corbicula sp. form C/S from South America are composed of clones, each lineage is strictly correlated with a single COI haplotype but both lineages presented high phenotypic variability even in other regions of the world (Table S2).
Gradual changes in shell morphology have been observed in other invasive mollusks such as Melanoides tuberculatus (Müller, 1774) (e.g., Peso et al., 2011), and Dreissena polymorpha (Pallas, 1771) (Lajtner et al., 2004), but also to another Corbicula spp. worldwide (Table S2). We presume that the wide phenotypic variability detected herein is likely influenced by spatial and local abiotic characteristics of the distinct Brazilian hydrographic basins (Mansur et al., 2012)—the same is likely true also for the European populations of Corbicula (Pfenninger et al., 2002; Sousa et al., 2007; Pigneur et al. 2011b) (Table S2). Information about the influence of how local abiotic characteristics of the newly invaded environment influence the phenotype of the shells of invasive Corbicula spp. is still scarce, but according to Crespo et al. (2015), Corbicula sp. form A/R populations presents a broad environmental tolerance especially influenced by changing temperatures. Hence, this lineage appears more plastic and generalized, reaching a wider range in geographical and ecological distribution (Pigneur et al., 2014a; Crespo et al., 2015; this study). In addition, the recognition that Corbicula spp. in South American rivers do not always incubate early larval stages in their gills—but instead, release the first D-larvae stages in the environment or are released from gill chambers being almost juvenile,—favors new pools of clones in a short period, resulting in high dispersion rates during the reproductive seasons (Ludwig et al., 2014). These novel discoveries indicate the invasiveness of clonal Corbicula spp. is not affected by the reduction in genetic variability and natural barriers do not appear to be a limiting factor in the new environment. Nevertheless, further studies should attempt to correlate the clonal fitness and invasiveness in South American Corbicula sp. form A/R to evaluate if they are associated with the phenotypic plasticity of this lineage influenced by local environmental conditions.
Populations with intermediate morphotypes
We observed discrepancies between the shell morphotypes and COI haplotypes in two populations: JAC and BRA from Brazil. The JAC and some BRA individuals shared the same FW5 haplotype with Corbicula sp. form A/R but exhibited an intermediate morphotype, which might indicate local adaptation and phenotype plasticity. The intermediate morphotype found in this study is like those found in Iguazu Falls by Lee et al. (2005), which described as “the shell resembles Corbicula. sp. form C/S but visibly less triangular and lightly coarse and with spaced external co-marginal rings” (Table S2). But also resemble the morphotypes found in Indonesia for Corbicula tobae Martens, 1900 (Fig. 3a of Bespalaya et al., 2021).
Based on our results, 23 individuals from the population PU site might represent a distinct lineage since they shared the FWBra haplotype and their shells have flat umbos, contrasting to the traditional description of any other Corbicula spp. (e.g., Ituarte, 1981; Lee et al., 2005; Sousa et al., 2007; Lee et al., 2005; Bespalaya et al. 2018; López-Soriano et al., 2018). The PCA demonstrated that the PU individuals exhibit an intermediate morphotype that fits between the Corbicula. sp. C/S and A/R forms. Interestingly, Lee et al. (2005) detected ‘mixed’ populations of Corbicula at Iguassu Falls—at the mouth of Iguaçu River—which is the same river where the PU site is located and reported genetic similarity between Corbicula. sp. form B (mtDNA) and mixed 28S (nDNA) probably between Corbicula. sp. form B and form C/S lineages. The detection of a single maternally inherited COI haplotype (FWBra) across 23 specimens from the PU site and presently restricted to this population suggests that these individuals could have resulted from cytonuclear mismatches (Hedtke et al., 2008; Pigneur et al., 2012). On the other hand, the fact that the FWBra haplotype is a sister clade of the subclade C. leana/C. sp. form A/R—FW5/C. sp. fluminea—FW7/C. sp.—FW8/C. javanica—FW9 reinforces our assumption that it is a distinct lineage. Another restricted invasive lineage is observed in Europe, the Corbicula sp. form Rlc, which also presents a unique COI haplotype (FW4) and distinct multilocus genotype pattern from the other invasive Corbicula lineages (Pigneur et al., 2012). We do not know for sure if it has an invasive behavior or if it is a lineage originating in South America such as Corbicula. sp. form C/S (Vastrade et al., 2022). Hence, we tentatively propose that these PU individuals should be treated as a distinct lineage as Corbicula sp. FWBra with a cryptic status, since we do not identify it in other sampling did not cover all South American hydrographic basins.
The intermediate morphotypes could represent hybrid specimens between distinct invasive lineages Corbicula sp. form C/S and form A/R that live in sympatry in some sites as we detected, following androgenetic parasitism with incomplete maternal nuclear expulsion (Hedtke et al., 2008; Pigneur et al., 2012), which was also detected in Russia and Indonesia (Bespalaya et al., 2018). Moreover, according to Pigneur et al. (2011b), a Corbicula individual exhibiting a mitochondrial/nuclear mismatch should have the morphotype of the nuclear genotype combined with the mitochondrial haplotype of another lineage. According to the “hybridization-invasion” hypothesis of Ellstrand & Schierenbeck (2000), interspecific hybridization may promote invasiveness because nuclear/mitochondrial hybrids and mismatches can create intermediate phenotypes relative to the parental taxa, which may increase the likelihood of survival and the success of establishment in novel habitats (Facon et al., 2005; Rius & Darling, 2014). Besides, these populations may be the result of a cytonuclear mismatch between the other two invasive lineages (Komaru et al., 2006; Hedtke et al., 2008; Pigneur et al., 2012), due to the maternal nuclear genome extruded, and an admixture with the paternal genome with intermediate morphotype (e.g., Komaru et al., 2006; Hedtke et al., 2008; Bespalaya et al., 2018). Thus, we know that tracing back the origin of clonal individuals is challenging due to the low population resolution level, as detected in this study for Corbicula spp., but further genomic studies may circumvent such limitation.
Invasion process in South America
The introduction process of an exotic population into a new environment can give us a unique opportunity to evaluate invasion dynamics in expanding populations (e.g., Darrigran, 1992; Borges et al., 2017; Ludwig et al., 2021; Carranza et al., 2023). The dispersal of lineages of Corbicula in South America, as it happens in general with other non-native species in the world (Seebens et al., 2017), is characterized by the increase in the rate of first geographical records over time but does not show any sign of saturation. Herein, we postulate that, based on our results, the invasion of the Corbicula sp. forms C/S and A/R lineages occurred through multiple introductions (and/or multiple propagules) which could explain their wide geographic distribution in South America, as also observed for Limnoperna fortunei (Dunker, 1857) (Ludwig et al., 2021). Based on the first records, the first introduction in South America likely occurred in the Río de la Plata River in Argentina (Ituarte, 1981); a second in the Patos Lagoon, in Southern Brazil (Mansur et al., 2004); the third in the Amazon estuary, in Northern Brazil (Beasley et al., 2003; Pimpão & Martins, 2008); and a fourth through the river systems surrounding of the Caribbean Sea (Martínez, 1987). The Río de la Plata River in Argentina is an international commercial port and one of the largest rivers in the world, into which flows Argentinian, Brazilian, and Uruguayan rivers (Ituarte, 1994). The specific geographic position of this basin in South America and the connectivity with tributaries of neighborhood countries favor the dissemination of new propagules of Corbicula into other continental waters in South America (Ghabooli et al., 2013; Darrigran et al., 2020). This hypothesis is supported by the detection of Corbicula sp. form A/R in ARG1 and of Corbicula sp. form C/S detected in ARG2. Thus, based on our data, the Rio del Plata could represent the genetic pool source for IGU, GUA, CLM, RJ, GO, MAT, IMI, ROS, and ITA populations, like the proposal for L. fortunei (Ludwig et al., 2021).
In the Patos Lagoon (city of Porto Alegre, Southern Brazil), a distinct morphotype was detected in the JAC population. There are four Corbicula spp. living sympatrically in the Patos Lagoon: Corbicula sp. form A/R, form C/S, form B, and Corbicula sp. (Mansur et al., 2012; Santos et al., 2012). In addition, the JAC population is situated in the city of Agudo on the Jacuí River, which flows directly into the Patos Lagoon, which is less than 19 km from it, respectively. Based on the proximity of those sites to the Patos Lagoon, it seems probable that the propagules Corbicula originated from the Patos Lagoon.
The Amazon estuary (Belém city, Pará state, Northern Brazil), which is a port region, is connected by water with ITU and PP sites in the Tocantins River. These two locations are located 572,6 km far from each other. The Tocantins River is a very important river located in Northern Brazil, it crosses four Brazilian states (Goiás, Tocantins, Pará, and Maranhão), and there are six hydroelectric power plants installed in its waters. According to Belz et al. (2012), a vector of dissemination of Corbicula in South American basins is sand transport, which is dredged from hydropower reservoirs. Thus, we postulate that there is an exchange of propagules of those rivers and between the PP and ITU sites, in the Praia da Prata site we identified the Corbicula sp. form C/S and form A/R lineages living in sympatry. The rapid spread of C. fluminea through North American freshwater has been the result of human activities (e.g., as fish bait) (Darrigran, 2002). Although no data are available on the dispersal of C. fluminea in South America, similar spread mechanisms to those observed in North America could occur (e.g., C. fluminea for live bait fishing) (Labaut et al., 2021a, b).
Our ability to identify the dispersion mechanisms during the invasion process remains limited by the clonal genetic pattern of Corbicula lineages detected herein, thus, we presume that human activities have indirectly aided the propagation of these invasive clams in South America via sand transportation in Brazilian hydrographic basins, as also detected by L. fortunei (Belz et al., 2012; Ludwig et al., 2021; Carranza et al., 2023). Nevertheless, we cannot rule out the fact that free-swimming Corbicula larvae can also disperse naturally (at least 1.2 km/year upstream) without human aid as suggested by Voelz et al. (1998).
Data availability
The data of this study are included as an electronic supplementary file.
References
Arano, B., G. Llorent, M. Gardia-Paris & P. Herrero, 1995. Species translocation menaces iberian waterfrogs. Conservation Biology 9: 196–198.
Araújo, R., D. Moreno & R. A. Ramos, 1993. The asiatic clam Corbicula fluminea (Muller 1774) (Bivalvia, Corbiculidae) in Europe. American Malacological Bulletin 10: 39–49.
Aristizábal, M. V. D. L. H., 2008. Primer registro en Colombia de Corbicula fluminea (Mollusca: Bivalvia: Corbiculidae), una espécie invasora. Boletín de Investtigaciones Marinas y Costeras 37: 197–202.
Bagatini, Y. M., R. S. Panarari, J. Higuti, E. Benedito-Cecilio, A. J. Prioli & S. M. A. P. Prioli, 2005. Morphological and molecular characterization of Corbicula (Mollusca: Bivalvia) at Rosana Resevoir, Brazil. Acta Scientiarum Biological Science 27: 397–404.
Beasley, C. R., C. H. Tagliaro & W. B. Figueiredo, 2003. The Occurrence Of The Asian Clam Corbicula Fluminea In The Lowe Amazon Basin. Acta Amazonica 33: 317–324.
Belz, C. E., G. Darrigran, O. S. M. Netto, W. A. Boeger & P. J. R. Junior, 2012. Analysis of four dispersion vectors in Inland Waters: The case of the invading bivalves in South America. Journal of Shellfish Research 31: 777–784. https://doi.org/10.2983/035.031.0322.
Bespalaya, Y. V., I. N. Bolotov, O. V. Aksenova, A. V. Kondakov, M. Y. Gofarov, T. M. Laenko, S. E. Sokolova, A. R. Shevchenko & O. V. Travina, 2018. Aliens are moving to the Arctic frontiers: an integrative approach reveals selective expansion of androgenic hybrid Corbicula lineages towards the North of Russia. Biological Invasions. https://doi.org/10.1007/s10530-018-1698-z.
Bespalaya, Y. V., O. V. Aksenova, M. Y. Gofarov, A. V. Kondakov, A. V. Kropotin, O. D. Kononov & I. N. Bolotov, 2021. Who inhabits the world’s deepest crater lake? A taxonomic review of Corbicula (Bivalvia: Cyrenidae) clams from Lake Toba, North Sumatra, Indonesia. Journal of Zoological Systematics and Evolutionary Research 59: 400–410. https://doi.org/10.1111/jzs.12428.
Borges, P. D., S. Ludwig & W. A. Boeger, 2017. Testing hypotheses on the origin and dispersion of Limnoperna fortunei (Bivalvia, Mytilidae) in the Iguassu River (Paraná, Brazil): molecular markers in larvae and adults. Limnology 18: 31–39. https://doi.org/10.1007/s10201-016-0485-8.
Cao, L., C. Damborenea, P. E. Penchaszadeh & G. Darrigran, 2017. Gonadal cycle of Corbicula fluminea (Bivalvia: Corbiculidae) in Pampean streams (Southern Neotropical Region). PLoS ONE 12: 1–16. https://doi.org/10.1371/journal.pone.0186850.
Castañeda, R. A., E. Cvetanovska, K. M. Hamelin, M. A. Simard & A. Ricciardi, 2018. Distribution, abundance, and condition of an invasive bivalve (Corbicula fluminea) along an artificial thermal gradient in the St. Lawrence River. Aquatic Invasions 13: 379–392.
Carranza, A., Agudo-Padrón, I., Collado, G.A. et al. 2023. Socio-environmental impacts of non-native and transplanted aquatic mollusc species in South America: What do we really know?. https://doi.org/10.1007/s10750-023-05164-z
Crespo, D., M. Dolbeth, S. Leston, R. Sousa & M. A. Pardal, 2015. Distribution of Corbicula fluminea (Müller, 1774) in the invaded range: a geographic approach with notes on species traits variability. Biological Invasions 17: 2087–2101.
Darrigran, G., 1992. Variación temporal y espacial de la distribución de las especies de Corbicula Megerle, 1811 (Bivalvia, Corbiculidae), en el estuario del Río de la Plata, República Argentina. Neotropica 38.
Darrigran, G., 2002. Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biological Invasions 4: 145–156.
Darrigran, G., I. Agudo-Padrón, P. Baez, C. Belz, F. Cardoso, A. Carranza, G. Collado, M. Correoso, M. G. Cuezzo, A. Fabres, D. E. Gutiérrez-Gregoric, S. Letelier, S. Ludwig, M. C. Mansur, G. Pastorino, P. Penchaszadeh, C. Peralta, A. Rebolledo, A. Rumi, S. Santos, S. Thiengo, T. Vidigal & C. Damborenea, 2020. Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biological Invasions 22: 853–871. https://doi.org/10.1007/s10530-019-02178-4.
Darrigran, G., I. Agudo-Padrón, P. Baez, et al., 2022. Species movements within biogeographic regions: exploring the distribution of transplanted mollusc species in South America. Biological Invasions. https://doi.org/10.1007/s10530-022-02942-z.
Drummond, A. J. & A. Drummond, 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7: 214. https://doi.org/10.1186/1471-2148-7-214.
DrydenDrydenMardia, I. L. K. V., 1992. Size and shape analysis of landmark data. Biometrika 79: 57–68. https://doi.org/10.1093/biomet/79.1.57.
Ellstrand, N. C. & K. A. Schierenbeck, 2000. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences 97: 7043–7050.
Facon, B., P. Jame, J. P. Pointier & P. David, 2005. Hybridization and invasiveness in the freshwater snail Melanoides tuberculata: hybrid vigour is more important than increase in genetic variance. Journal of Evolutionary Biology 18: 524–535. https://doi.org/10.1111/j.1420-9101.2005.00887.x.
Ferreira-Rodríguez, N., R. Sousa & I. Pardo, 2018. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 810: 85–95.
Folmer, O., M. Black, W. Hoeh, et al., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Ghabooli, S., A. Zhan, P. Sardiña, E. Paolucci, F. Sylvester, P. V. Perepelizin, et al., 2013. Genetic diversity in introduced golden mussel populations corresponds to vector activity. PLoS ONE 8: e59328. https://doi.org/10.1371/journal.pone.0059328.
Gomes, C., R. Sousa, T. Mendes, R. Borges, P. Vilares, V. Vasconcelos & A. Antunes, 2016. Low genetic diversity and high invasion success of Corbicula fluminea (Bivalvia, Corbiculidae)(Müller, 1774) in Portugal. PLoS ONE 11: e0158108. https://doi.org/10.1371/journal.pone.0158108.
Hedtke, S. M., K. Stanger-Hall, R. J. Baker & D. M. Hillis, 2008. All-male asexuality: origin and maintenance of androgenesis in the Asian clam Corbicula. Evolution 62: 1119–1136. https://doi.org/10.1111/j.1558-5646.2008.00344.x.
Hedtke, S. M., M. Glaubrecht & D. M. Hillis, 2011. Rare gene capture in predominantly androgenic species. PNAS 108: 9520–9524. https://doi.org/10.1073/pnas.1106742108.
Houki, S., M. Yamada, T. Honda & A. Komaru, 2011. Origin and Possible role of males in hermaphroditic androgenic Corbicula clams. Zoological Science 28: 526–531. https://doi.org/10.2108/zsj.28.526.
Ituarte, C. F., 1981. Primera noticia acerca de la introduccion de pelecipodos asiaticos en el area rioplatense. Neotropica 27: 79–82.
Ituarte, C. F., 1994. Corbicula and Neocorbicula (Bivalvia: Corbiculidae) in the Paraná, Uruguay, and Río de La Plata Basins. The Nautilus 107: 129–135.
Jombart, T., 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405. https://doi.org/10.1093/bioinformatics/btn129.
Jombart, T., S. Devillard & F. Balloux, 2010. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics 11: 1–15. https://doi.org/10.1186/1471-2156-11-94.
Keenan, K., P. McGinnity, T. F. Cross & P. A. Prodöhl, 2013. diveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods in Ecology and Evolution 4: 782–788. https://doi.org/10.1111/2041-210X.12067.
Klingenberg, C. P., 2011. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources 11: 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x.
Komaru, A., K. Konishi, I. Nakayama, T. Kobayashi, H. Sakai & K. Kawamura, 1997. Hermaphroditic freshwater clams in the genus Corbicula produce non-reductional spermatozoa with somatic DNA content. Biological Bulletin 193: 320–323.
Komaru, A., A. Kumamoto, T. Kato, R. Ishibashi, M. Obata & N. Nemoto, 2006. A hypothesis of ploidy elevation by formation of a female pronucleus in the androgenic clam Corbicula fluminea in the Tone River estuary, Japan. Zoological Science 23: 529–532. https://doi.org/10.2108/zsj.23.529.
Konishi, K., K. Kawamura, H. Furuita & A. Komaru, 1998. Spermatogenesis of the freshwater Clam Corbicula aff. fluminea Müller (Bivalvia: Corbiculidae). Journal of Shellfish Research 17: 185–189.
Labaut, Y., P. A. Macchi, F. M. Archuby & G. Darrigran, 2021a. Homogenization of macroinvertebrate assemblages and Asiatic Clam Corbicula fluminea invasion in a river of the Arid Patagonian Plateau. Argentina. Frontiers in Environmental Science 9: 728620. https://doi.org/10.3389/fenvs.2021.728620.
Labaut Y., P. A. Macchi, F. M. Archuby, & G. Darrigran, 2021. Pesca con carnada viva como vector dispersante de la invasión de la almeja asiática Corbicula fluminea en Patagonia, Argentina. Memoria del III Simposio Internacional de Aguas Continentales de las Américas Restauración y Conservación de los Ecosistemas con Enfoque Participativo. 5, 6 y 7 de julio, 2021, Panajachel, Sololá, Guatemala. pp. 35. http://rid.unrn.edu.ar/handle/20.500.12049/8092
Lajtner, J., Z. Marusic, G. I. V. Klobucar, I. Maguire & R. Erben, 2004. Comparative shell morphology of the zebra mussel, Dreissena polymorpha in the Drava river (Croatia). Biologia-Bratislava 59: 595–600.
Lasso, C. A., R. Martínez-Escarbassiere, J. C. Capelo, M. A. Morales-Betancourt & A. Sánchez-Maya, 2009. Lista de los moluscos (Gastropoda-Bivalvia) dulceacuícolas y estuarinos de La cuenca del Orinoco (Venezuela). Biota Colombiana 10: 63–74.
Lee, T., S. Siripattrawan, C. Ituarte & D. Ó Foighil, 2005. Invasion of the clonal clams: Corbicula lineages in the New World. American Malacological Bulletin 20: 113–122.
Librado, P. & J., Rozas, 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. https://doi.org/10.1093/bioinformatics/btp187.
López-Soriano, J., S. Quiñonero-Salgado, C. Cappelletti, F. Faccenda & F. Ciutti, 2018. Unraveling the complexity of Corbicula clams invasion in Lake Garda (Italy). Advances in Oceanography and Limnology. https://doi.org/10.4081/aiol.2018.7857.
Ludwig, S., M. K. Tschá, R. P. Patella, A. J. Oliveira & W. Boeger, 2014. Looking for a needle in a haystack: molecular detection of larvae of invasive Corbicula clams. Management of Biological Invasions 5: 143–149. https://doi.org/10.3391/mbi.2014.5.2.07.
Ludwig, S., E. H. Sari, H. Paixão, L. C. Montresor, J. Araújo, C. F. Brito & C. B. Martinez, 2021. High connectivity and migration potentiate the invasion of Limnoperna fortunei (Mollusca: Mytilidae) in South America. Hydrobiologia 848: 499–513. https://doi.org/10.1007/s10750-020-04458-w
Mansur, M.C.D., Callil, C.T., Cardoso, F.R., Ibarra, J.A.A., Silva, J.S.V., & R.C.C.L. Souza, 2004. Uma retrospectiva e mapeamento da invasão de espécies de Corbicula (Mollusca, Bivalvia, Veneroida, Corbiculidae) oriundas do sudeste asiático, na América do Sul. In: Silva JSV and Souza RCCL, Água de lastro e bioinvasão. Interciência, Rio de Janeiro, pp. 39.
Mansur, M.C.D., Santos, C.P., M.V., Nehrke, 2012. Corbiculidae na América do Sul: Espécies nativas e invasoras, dispersão e a situação das pesquisas no Brasil (Mollusca: Bivalvia). In: Ecos do XIX Encontro Brasileiro de Malacologia, Rio de Janeiro, Technical Books, pp 467.
Martínez, R., 1987. Corbicula manilensis molusco introducido en Venezuela. Acta Científica Venezeolana 38: 384–385.
McMahon, R.F., 1983. Ecology of an Invasive Pest Bivalve, Corbicula. In: The Mollusca, Vol. 6, W. D. Russell-Hunter, ed. Academic Press, New York. pp.505.
McMahon, R.F., 1999. Invasive Characteristics of the Freshwater Bivalve, Corbicula fluminea. Lewis Publisher: 315–343.
Park, J. K., J. S. Lee & W. Kim, 2002. A single mitochondrial lineage is shared by morphologically and allozymatically distinct freshwater Corbicula clams. Molecular and Cells 14: 318–322.
Park, J. & W. Kim, 2003. Two Corbicula (Corbiculidae: Bivalvia) mitochondrial lineages are widely distributed in Asian freshwater environment. Molecular Phylogenetics and Evolution 29: 529–539. https://doi.org/10.1016/S1055-7903(03)00138-6.
Paschoal, L. R. P., D. D. P. Andrade & G. Darrigran, 2015. How the fluctuations of water levels affect populations of invasive bivalve Corbicula fluminea (Müller, 1774) in a Neotropical reservoir? Brazilian Journal of Biology 75: 135–143. https://doi.org/10.1590/1519-6984.09113.
Pérez, C. H. F., C. S. Cárdenas & P. L. Simon, 2022. Patagonia Invadida: Primer Registro de Corbicula Fluminea (Müller, 1774) en la Cuenca del Río Chubut, Chubut, Argentina. Historia Natural 121: 165–172.
Peso, J. G., D. C. Pérez & R. E. Vogler, 2011. The invasive snail Melanoides tuberculata in Argentina and Paraguay. Limnologica 41: 281–284. https://doi.org/10.1016/j.limno.2010.12.001.
Peñarrubia, L., R. M. Araguas, O. Vidal, C. Pla, J. Viñas & N. Sanz, 2017. Genetic characterization of the Asian clam species complex (Corbicula) invasion in the Iberian Peninsula. Hydrobiologia 784: 349–365. https://doi.org/10.1007/s10750-016-2888-2.
Pfenninger, M., F. Reinhardt & B. Streit, 2002. Evidence for cryptic hybridization between different evolutionary lineages of the invasive clam genus Corbicula (Veneroida, Bivalvia). Journal of Evolutionary Biology 15: 818–829.
Pigneur, L. M., A. M. Risterucci, N. Dauchot, X. Li & K. Van Doninck, 2011. Development of novel microsatellite markers to identify the different invasive lineages in the Corbicula complex and to assess androgenesis. Molecular Ecology Resources 11: 573–577. https://doi.org/10.1111/j.1755-0998.2010.02963.x.
Pigneur, L. M., J. Marescaux, K. Roland, E. Etoundi, J. P. Descy & K. Van Doninck, 2011b. Phylogeny and androgenesis in the invasive Corbicula clams (Bivalvia, Corbiculidae) in Western Europe. BMC Evolutionary Biology 11: 147. https://doi.org/10.1186/1471-2148-11-147.
Pigneur, L. M., S. M. Hedtke, E. Etoundi & K. Van Doninck, 2012. Androgenesis: a review through the study of the shellfish Corbicula spp. Heredity 108: 581–591. https://doi.org/10.1038/hdy.2012.3.
Pigneur, L. M., E. Etoundi, D. C. Aldridge, J. Marescaux, N. Yasuda & K. Van Doninck, 2014a. Genetic uniformity and long-distance clonal dispersal in the invasive androgenic Corbicula clams. Molecular Ecology 23: 5102–5116. https://doi.org/10.1111/mec.12912.
Pigneur, L. M., E. Etoundi, D. C. Aldridge, J. Marescaux, N. Yasuda & K. Van Doninck, 2014. Genetic uniformity and long-distance clonal dispersal in the invasive androgenic Corbicula clams. Molecular Ecology 23: 5102–5116. https://doi.org/10.1111/mec.12912.
Pimpão, D. M. & D. S. Martins, 2008. Occurrence of the Asian mollusk Corbicula fluminea (Müller 1774) (Bivalvia: Corbiculidae) in the lower Rio Negro, Central Amazoan Region, Brazil. Acta Amazonica 38: 589–592.
Posada, D., 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. https://doi.org/10.1093/mollbev/msn083.
Rambaut, A. & A. J. Drummond, 2007. Tracer v1.4: MCMC tracer analyses tool. See http://tree.bio.ed.ac.uk/software/tracer/]. PubMed.
Rambaut, A., 2009. FigTree v1.3.1: Tree figure drawing tool. Website: http://tree.bio.ed.ac.uk/software/figtree.
Reyna, P., J. Nori, M. Ballesteros, A. Hued & M. Tatián, 2018. Targeting clams: insights into the invasive potential and current and future distribution of Asian clams. Environmental Conservation 45: 387–395. https://doi.org/10.1017/S0376892918000139.
Rius, M. & J. A. Darling, 2014. How important is intraspecific genetic admixture to the success of colonizing populations? Trends in Ecology & Evolution 29: 233–242. https://doi.org/10.1016/j.tree.2014.02.003.
Robertson, A. & W. G. Hill, 1984. Deviation from Hardy-Weinberg proportions: sampling variances and use in estimation of inbreeding coefficient. Genetics 107: 703–718.
Rohlf, F. J., 2006. TpsDig2 Software, ver.2.10, State University New York, Stony Brook:
Ronquist, F. & J. P. Huelsenbeck, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180.
Santana, D. O., M. J. M. Silva, A. Bocchiglieri, S. M. Pantaleão, R. G. Faria, B. B. Souza, S. M. Rocha & L. F. O. Lima, 2013. Mollusca, Bilvavia, Corbiculidae, Corbicula flumínea (Müller, 1774): first record for the Caatinga biome, northeastern Brazil. Check List 9: 1072–1074.
Santos, S. B., S. C. Thiengo, M. A. Fernandez, et al., 2012. Espécies de moluscos límnicos invasores no Brasil. In: Moluscos límnicos invasores do Brasil: biologia, prevenção e controle, Porto Alegre, Redes Editora, pp 412.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, et al., 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 14435. https://doi.org/10.1038/ncomms14435.
Semenas, L. & V. Flores, 2005. Presence of Corbicula fluminea in the Upper Negro River Basin (Patagonia, Argentina). Journal of Freshwater Ecology 20: 615–616.
Siripattrawan, S., J. K. Park & Ó. Foighil, 2000. Two lineages of the introduced Asian freshwater clam Corbicula occur in North America. Journal of Molluscan Studies 66: 423–429. https://doi.org/10.1093/mollus/66.3.423.
Sousa, R., R. Freire, M. Rufino, J. Méndez, M. Gaspar, et al., 2007. Genetic and shell morphological variability of the invasive bivalve Corbicula fluminea (Müller, 1774) in two Portuguese estuaries. Estuarine, Coastal and Shelf Science 74: 166–174. https://doi.org/10.1016/j.ecss.2007.04.011.
Sousa, R., A. J. A. Nogueira, M. B. Gaspar, C. Antunes & L. Guilhermino, 2008. Growth and extremely high production of the non-indigenous invasive species Corbicula fluminea (Müller, 1774): Possible implications for ecosystem functioning. Estuarine, Coastal and Shelf Science 80: 289–295. https://doi.org/10.1016/j.ecss.2008.08.006.
Tiemann, J. S., A. E. Haponski, S. A. Douglass, T. Lee, K. S. Cummings, M. A. Davis & Ó. Foighil, 2017. First record of a putative novel invasive Corbicula lineage discovered in the Illinois River, Illinois, USA. BioInvasions Records 6: 159–166. https://doi.org/10.3391/bir.2017.6.2.12.
Thompson, J. D., 1991. The biology of an invasive plant. BioScience 41: 393–401.
Van Oosterhout, C., W. F. Hutchinson, D. P. M. Wills & P. Shipley, 2004. MICRO-CHECKER: software for identifying and correcting genotyping erros in microsatellite data. Molecular Ecology 4: 535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x.
Vastrade, M., E. Etoundi, T. Bournonville, M. Colinet, N. Debortoli, S. M. Hedteke, E. Nicolas, L. M. Pigneur, L. Virgo, J. F. Flot, J. Marescaux & K. Van Doninck, 2022. Substantial genetic mixing among sexual and androgenetic lineages within the clam genus Corbicula. Peer Community Journal 2: 73. https://doi.org/10.24072/pcjournal.180.
Voelz, N. J., J. V. McArthur & R. B. Rader, 1998. Upstream mobility of the Asiatic clam Corbicula fluminea: identifying potential dispersal agents. Journal of Freshwater Ecology 13: 39–45.
Zhan, A., P. V. Perepelizin, S. Ghabooli, E. Paolucci, F. Sylvester, P. Sardina, et al., 2012. Scale-dependent post-establishment spread and genetic diversity in an invading mollusk in South America. Diversity and Distributions 18: 1042–1055. https://doi.org/10.1111/j.1472-4642.2012.00894.x.
Acknowledgements
Special thanks to Damminsky PB (Institutos Lactec), S.B. dos Santos (UERJ), H. Tagliaro (UFPE), M.C.D. Mansur (UFRS), K. Kotzian (UFSM), T.D.H.A. Vidigal (UFMG), O. M. Netto (MAxClean), and D. Pimpão (INPA), for providing samples for this study. We also thank the Instituto Lactec, UNLP (11/N927), CONICET (PIP 1017), PICT-2019-01417 and Conselho de Desenvolvimento Científico e Tecnológico (CNPq) for supporting this study, and for Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarship assistance of SL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ludwig, S., Darrigran, G. & Boeger, W.A. Opening the black box of the invasion of Corbicula clams (Bivalvia, Corbiculidae) in South America: a genetic and morphological evaluation. Hydrobiologia 851, 1203–1217 (2024). https://doi.org/10.1007/s10750-023-05378-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05378-1