Abstract
Aquatic hyphomycetes are key organisms that perform the decomposition of plant organic matter in streams. They are strongly influenced by nitrogen availability and temperature. Both environmental parameters stimulate aquatic hyphomycete activity (litter decomposition and conidia production) while high temperature tends to lower their diversity. However, the past research mostly focused on temperate streams, and tropical studies remain comparatively too scarce to determine if current knowledge applies to tropical aquatic hyphomycetes. Here, we provide the first assessment of aquatic hyphomycete communities and microbial litter decomposition in streams of Guadeloupe, which exhibit naturally warm and oligotrophic conditions. Local gradients of temperature and nitrate concentration allowed to test the hypothesis that microbial litter decomposition is stimulated by nitrate and temperature. To the contrary, we expected the warmer downstream sites to exhibit lower aquatic hyphomycete diversity due to high temperature. Contrary to our expectations, diversity increased along both nitrate and temperature gradients, while decomposition process was stimulated by nitrate but not by temperature. One implication of these findings is that warm water in the tropics is not necessarily associated with low aquatic hyphomycete diversity. Future studies should investigate the effect of temperature using broad and independent gradients of temperature (latitude, altitude and season).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic hyphomycetes are a polyphyletic group of fungi that share several ecological and morphological features (Belliveau & Bärlocher, 2005). They often occur on dead leaves and produce hyaline, branched, tetraradiate or sigmoid conidia that are spread by stream flow and handle dispersal and asexual reproduction (Bärlocher, 2009). They are able to decompose lignin and cellulose, and are thereby considered to be the main microbial decomposers of plant litter in stream ecosystems, at least under temperate latitudes (Gessner & Chauvet, 1994). However, their diversity, ecological role and functional importance in streams located in the tropics remain unclear (Graça et al., 2016).
Several environmental parameters are known to influence aquatic hyphomycete activity and diversity. Among them, temperature and nitrogen (N) availability received considerable attention, since their current increases are major threats to freshwater ecosystem quality and biodiversity (Millenium Ecosystem Assessment, 2005). The influence of nitrate availability on microbial litter decomposition was extensively studied (see Ferreira et al., 2015 for a meta-analysis). It generally follows a Michaelis–Menten fit (Ferreira et al., 2006; Jabiol et al., 2019), which means that litter decomposition is stimulated by nitrate at low (limiting) concentration. At higher nitrate concentration, microbial decomposers become limited by the availability of other nutrients or by carbon (C) quality (Jabiol et al., 2019), and adding more nitrate does not further increase decomposition rates. However, the vast majority of the studies published so far focused on temperate regions, and it is not clear if the patterns described above hold true in tropical streams (Ferreira et al., 2015; Camelo et al., 2022). Positive effects of nitrate availability on aquatic hyphomycete species richness were also described in microcosm (Jabiol et al., 2018), stream enrichment (Gulis & Suberkropp, 2004) and correlative studies (Pérez et al., 2013). However, the reasons for this pattern remain unclear. It could reflect a better detection of rare species (Gulis & Suberkropp, 2004) or a faster succession of species on leaf litter (Jabiol et al., 2018) rather than an enlargement of the species pool.
Temperature also influences processes and communities. It stimulates the metabolism of poïkilotherm organisms (Brown et al., 2004) and accelerates aquatic hyphomycete activity (e.g., microbial litter decomposition, respiration and conidia production) accordingly. Indeed, positive effects of temperature on microbial litter decomposition were shown from microcosm studies (e.g., Geraldes et al., 2012; Martinez et al., 2014; Jabiol et al., 2020), field warming experiments (e.g., Ferreira & Canhoto, 2015) or correlative studies along altitudinal (e.g., Taylor & Chauvet, 2014) or latitudinal gradients (Irons et al., 1994; Boyero et al., 2011) (see Amani et al., 2019 for a meta-analysis).
Finally, temperature drives aquatic hyphomycete community structure and composition as well. Several experiments concluded that the diversity of aquatic hyphomycetes could be lower at high temperature (Bärlocher et al., 2008; Geraldes et al., 2012). This result matches the decrease in conidia production above 15–25 °C (depending on the species) observed in several temperate strains (Chauvet & Suberkropp, 1998; Dang et al., 2009), as well as the abrupt decline of the number of sporulating species above 25 °C reported in an experiment carried out on fungi from Indian streams (Rajashekhar & Kaveriappa, 2000). Accordingly, several cross-latitudes comparisons concluded that aquatic hyphomycete diversity decreases from intermediate (i.e., temperate) to low (i.e., tropical) latitude (e.g., Ferreira et al., 2012; Jabiol et al., 2013; Seena et al., 2019; Barreto et al., 2023). Again, data on tropical streams are still too patchy, and drawing general patterns remains a matter of conjecture (Duarte et al., 2016; Graça et al., 2016).
In this study, we tested the effect of temperature and nitrate availability on aquatic hyphomycete communities and activity in tropical streams of Guadeloupe. Twelve study sites were selected based on temperature and nitrate availability gradients provided by altitude and anthropogenic disturbance, respectively. According to existing knowledge (see above), we expected that both temperature and nitrate availability would influence aquatic hyphomycete community composition and stimulate aquatic hyphomycete activity. To the contrary, a decline of aquatic hyphomycete species richness was expected at high temperature.
Methods
Study sites
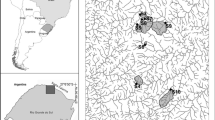
Litter decomposition and fungal communities were described in three streams distributed along a gradient of anthropogenic disturbance (Table 1) in Guadeloupe island (French West Indies). All streams are located in the Basse-Terre Island (Fig. 1), where most of the streams of Guadeloupe are located. Basse-Terre is a ca. 850 km2 volcanic island that culminates at 1468 masl (La Soufrière volcano). Streams in Basse-Terre take their source in forested areas belonging to the Core zone of the Guadeloupe National Park. Because of the topography, they are relatively short (39.5 km for the longest), torrential and well-oxygenated even in their lower parts, where they are impacted by agriculture and urbanization to various extents before flowing into the sea (Fig. 1). Three streams were selected to encompass different anthropogenic contexts and be representative of Basse-Terre streams (Fig. 1 and Table 1). On each stream, four sites (numbered from 1 to 4) were selected along the altitudinal gradient. Site 1 was located in a preserved (Core zone of the national park) forested upstream part (altitude > 200 m), while the three others belong to the buffer zone of the National Park. Site 2 was located at an intermediate altitude (ca. 50–100 m), and sites 3 and 4 were located in lowlands (altitude < 20 m) right upstream (site 3) and downstream (site 4) of domestic (wastewater treatment plant) or industrial (industrial distillery) effluents. In each site, pH, conductivity and oxygen saturation were analyzed in situ using a HQ40d probe (HACH, Loveland, CO, USA). Moreover, 50-mL samples of water were frozen at − 20 °C before analyzing for ion concentration using ion chromatography. Chemical analyses and in situ measurements were performed on three occasions for each site. Finally, water temperature was continuously monitored (each 1/2 h) with HOBO data loggers (HOBO UA-001–64, Bourne, MA, USA).
Litter decomposition
Leaf litter decomposition rates were determined using litter bags made of 0.5-mm mesh (ca. 15 × 20 cm) to quantify microbial decomposition rates (Bärlocher, 2020). Each litter bag contained 3 g of naturally abscised bamboo litter (Bambusa vulgaris Schrad.) that was collected close to the Grande Rivière à Goyaves (close to sites 3 and 4) and air-dried before the experiment. Four 3-g batches of dried litter were grinded and analyzed for elemental composition. C and N concentrations were determined using a CHN analyzer (Flash 2000 Thermo Scientific) and phosphorus (P) by spectrometry after oxidation by persulfate in acidic conditions. A fiber analysis following Goering & Van Soest (1970) protocols was also performed on three batches of litter. Litter composition was expressed as % dry mass.
From April 5 to 7, 2022, four litter bags were deployed in each of the 12 study sites and fastened on iron bars to anchor them on the streambed. A total of eight out of 48 litter bags were lost during the experiment, either due to a moderate flood that occurred on April 19 or to vandalism. On two sites (site 2 of the Grande Rivière à Goyaves and site 3 of the Petite Rivière à Goyave), only two out of four replicates remained. Either three or four replicates could be retrieved on the 10 other sites. After ca. 3 weeks (± 1 d) in the streams, litter bags were transported to the laboratory. Litter was rinsed using demineralized water, and 10 leaf disks were cut from each litter bag using a cork borer (diameter 10 mm) for fungal sporulation analysis. Litter was then dried at 60 °C for 48 h and weighed to the nearest 0.01 g. Litter decomposition rates (dry mass) were calculated using the inverse exponential relationship described in Bärlocher (2020) and expressed in day−1.
Fungal communities
Aquatic hyphomycete communities were studied following two protocols: sporulation from the bamboo leaf litter (leaf baits) and foam collection (Descals, 2020). Sporulation was induced using the 10 disks cut from the litter. They were immersed in glass Petri dishes containing 20 mL of demineralized water and put under constant agitation (ca. 100 rpm) in a room with air conditioning set at 25 °C. After 48 h, conidia suspension was fixed with formalin (2% final concentration) in Falcon tubes, and leaf disks were dried at 60 °C for 48 h and weighed to the nearest 0.01 mg. A 5-mL aliquot of each conidia suspension was filtered over a membrane filter (5-µm porosity), stained with Trypan blue (5% Trypan blue in 60% lactic acid) and set on a microscopic slide. Additionally, two foam samples were collected in each site when possible (i.e., in all sites but the site 4 on Grande Rivière de Vieux-Habitants). Foam was collected using a piece of 0.5-mm mesh net, transferred in a Petri dish and fixed with a few drops of FAA (formalin, ethanol and acetic acid). The resulting suspension was then poured in 2-mL tubes. Foam samples were filtered following the same procedure than above with 1-mL aliquots to get a semi-quantitative data. The two samples were pooled together to reach a sufficient amount of conidia in each sample. Conidia were counted and identified under the microscope at × 200 using identification keys from temperate and tropical areas (Santos-Flores & Betancourt López, 1997; Chan et al., 2000; Fuiza et al., 2017; Gulis et al., 2020). Sporulation rate was expressed as the number of conidia per mg of litter (dry mass) per day.
Data analysis
As a first step, we compared microbial litter decomposition rates, fungal diversity and species evenness in foam samples as well as sporulation rates between sites using ANOVAs with site location nested in stream identity. For this comparison, we used rarefied species richness for 90 identified conidia to account for a different number of conidia from one site to another. The 90 conidia threshold allowed us to calculate a rarefied species richness in all the study sites but 1, where only 35 conidia were found and that were excluded from the analyses. Finally, the Pielou’s index was calculated as a measure of evenness. Conidia densities in sporulation samples were extremely low (half of the samples contained less than 10 conidia and no more than 2 species), which makes the calculation of rarefied richness and ecological distances irrelevant. For this reason, the analyses of community structure and composition were performed on foam samples only.
As a second step, we assessed the variations of the same parameters along the gradients of temperature and nitrate concentrations using ANCOVAs with stream identity as factor. A two-level factor also accounted for the presence/absence of the wastewater effluents. Sporulation rates were ln-transformed for these analyses. Model simplification was performed by deleting non-significant variables and eventually grouping levels of factors, and the final model was selected using the AIC criteria.
Fungal community composition was analyzed from foam samples and compared between streams and sites using an analysis of similarity (ANOSIM) with either site or stream identity as factors. ANOSIMs were coupled with a Non-Metric Multidimensional Scaling (NMDS) for illustrative purpose. Moreover, similarities between communities were assessed using a hierarchical clustering analysis using Ward’s method. NMDS, ANOSIM and hierarchical clustering were performed using a Bray–Curtis distance after Hellinger’s transformation of the community matrix. All analyses were performed using R.4.0.3 (R core team, 2020) with packages vegan and MASS.
Results
Stream water and litter physical and chemical properties
Stream physical and chemical properties are summarized in Table 2. Overall, they were similar between streams, except for Ca2+ concentration which was higher in the Petite Rivière à Goyave. Nutrient concentrations were very low, with N-NO3− concentration ranging from 1.1 to 45.3 µg L−1 but exhibited variations across streams and along the longitudinal gradient. They were the highest at the downstream sites, in particular in the site 4 impacted by wastewater. For instance, N-NH4+ concentration in the Petite Rivière à Goyave was ca. 10 × higher below than above the wastewater treatment plant. Water was saturated with oxygen (Table 2) except downstream of the industrial effluents on the Grande Rivière à Goyaves. Temperature increased with decreasing altitude, with a 4.5–5 °C gradient from sites 1 to 4. Some data loggers were lost during the experiment, and temperature data are missing for sites 1 and 4 on the Grande Rivière de Vieux-Habitants. Initial bamboo litter CNP composition (N = 4) was 38.76% C (± 3.30 SD), 1.21% N (± 0.10 SD) and 0.042% P (± 0.011 SD). It contained 18.52% of lignin (± 0.27 SD) and 28.29% of cellulose (± 0.49 SD) based on fiber analysis (N = 3).
Aquatic hyphomycete community composition
A total of 44 species (or morphospecies) were found in foam samples (Online Resource 1; Table 3), with species richness ranging from 4 to 25 depending on the site. In comparison, sporulation samples were much less diverse, with a total of 17 species and species richness between 1 and 11 depending on the site. According to ANOSIM, aquatic hyphomycete communities were not significantly different between streams (R = − 0.014; P = 0.482), but varied between sites along the altitudinal (i.e., temperature) gradient (R = 0.362; P = 0.007). According to Fig. 2, communities in upstream sites (site 1 and eventually site 2) were similar among streams. They were dominated by a few species such as Triscelophorus acuminatus Nawawi, Triscelophorus monosporus Ingold and an unidentified Campylospora species, which accounted together for 77–92% of total abundances depending on the stream. In the downstream sites of the three streams, Tricladium angulatum Ingold was among the dominant species (35–72% of total abundance) while it was almost absent from upstream sites. One downstream site appeared dissimilar from others (site 4 in Petite Rivière à Goyave), but exhibited low conidia density (35 counted conidia) and species richness (four species).
Non-Metric Multidimensional Scaling A and hierarchical cluster analysis B based on aquatic hyphomycete communities from foam samples. In A, arrows link together the sites along each stream, with the arrow indicating the downstream direction. It is dotted on the Grande Rivière de Vieux-Habitants because site 3 is missing. Abbreviations of species names are provided in Table 3. In B, the dotted line indicates the threshold used to determine the clusters shown in A. Black: Grande Rivière à Goyaves, gray: Petite Rivière à Goyave and white: Grande Rivière de Vieux-Habitants. Dashed ellipses indicate the clusters identified by hierarchical clustering
Nitrate and temperature effects on aquatic hyphomycetes
Average litter decomposition rates ranged from 0.0097 d−1 (± 0.0007 SD) to 0.0271 d−1 (± 0.0027 SD) (Fig. 3A). As shown in Fig. 4A and B, decomposition rates were significantly stimulated by nitrate availability but only weakly by temperature. It was inhibited by the wastewater in two out of the three streams with a 33 and 51% decrease in Petite Rivière à Goyaves and Grande Rivière à Goyave, respectively.
Mean decomposition, sporulation rates and rarefied species richness and evenness from foam samples in the 12 study sites. Error bars are standard errors. In A, stars indicate sites where decomposition is statistically different from the other sites within the same stream (Tukey test P < 0.05). A bar in C and D is lighter to indicate a conidia density lower than the 90 conidia threshold used to calculate rarefied species richness
Relationships between biological variables (litter decomposition, sporulation rates, fungal rarefied species richness and evenness) and stream nitrate concentration and temperature. A regression curve is provided when significant. The statistical results are provided for the variables included in the final model after model simplification. Black: Grande Rivière à Goyaves, gray: Petite Rivière à Goyave and white: Grande Rivière de Vieux-Habitants. Stars indicate the sites impacted by wastewater when its effect was significant
Fungal sporulation rates were low and ranged from 0.05 conidia d−1 mg−1 litter dry mass (± 0.07 SD) (site 1 of the Petite Rivière à Goyave) to 2.64 conidia d−1 mg−1 litter dry mass (± 0.99 SD) (site 3 of the Grande Rivière à Goyaves) (Fig. 3B). Contrary to decomposition rates, no effect of temperature or nitrate availability was found (Fig. 4C and D), which was probably due to the high variability of sporulation rates between replicates. Yet, a marginally significant effect of wastewater was found despite this variability and suggests that conidia production was inhibited by wastewater.
Finally, rarefied species richness and evenness based on foam samples were the lowest in the most upstream sites within each stream (Fig. 3C and 4D). Rarefied species richness but not evenness increased significantly along both nitrate availability and temperature gradients (Fig. 4E and F). The nitrate effect, though, was significant only when including a stream effect. This accounts to a significantly lower aquatic hyphomycete species richness in the Petite Rivière à Goyave irrespective of its nitrate concentration. We did not find any wastewater effect on species richness, but this result might be non-representative since foam sample was excluded from rarefied richness data (less than 35 conidia) in one of the wastewater sites.
Discussion
A first finding of this study is that the effect of nitrate on microbial litter decomposition and aquatic hyphomycete communities was broadly consistent with the previous knowledge—mostly based on temperate experiments. The ca. 1.8 × increase in litter decomposition along our moderate nitrate concentration gradient is largely in accordance with the previous results, which predict a limitation of microbial leaf litter decomposition below ca. 100 µgN L−1 (Ferreira et al., 2006; Jabiol et al., 2019). This concentration is more than 2 × higher than the highest N-NO3− concentration we reported across our study sites (i.e., 45.3 µg L−1). Nitrate availability also correlated positively with aquatic hyphomycete species richness. However, as pointed out in the previous studies, it is impossible to determine if the limiting nutrient availability actually narrowed the species pool, or if it inhibited the conidia production for some species that thus remained unnoticed (Gulis & Suberkropp, 2004). The previous studies suggested that low litter decomposition rates occur in—at least some—tropical streams due to low nutrient availability (Gonçalves et al., 2007; Medeiros et al., 2015), which is a common feature of tropical streams due to intensive weathering (Boulton et al., 2008). To the contrary, in a recent microcosm experiment (Camelo et al., 2022), providing nutrients failed to stimulate microbial decomposition rates. The reason for this discrepancy between our results and this microcosm study is unclear. It is possible that decomposition in the study by Camelo et al. (2022) was constrained by the availability of one or several other nutrients that were not limiting along our nitrate gradient.
Temperature effects were more surprising and contrary to our hypotheses. First, litter decomposition was not clearly stimulated along our 5 °C temperature gradient. Though, positive effects of temperature on litter decomposition are well-documented (Amani et al., 2019), including along altitudinal gradients (e.g., Fabre & Chauvet, 1998). For instance, a positive effect of temperature (ca. 6 °C) along an altitudinal gradient was reported by Taylor & Chauvet (2014), but was removed when expressing decomposition rates per degree-days. Results by Follstad Shah et al. (2017) even suggest that temperature effect on decomposition rates could be stronger in tropical than in temperate streams, and predict a 10% increase in litter decomposition for each 1 °C rise. This was clearly not the case in our study, maybe because nutrient limitation lowered the apparent effect of temperature on the decomposition process (Cross et al., 2015). This could occur, for instance, if aquatic hyphomycete lacks the necessary amounts of nutrients to increase the synthesis of litter degrading enzymes.
It is also possible that the variations of community composition masked the effect of temperature. Species found in upstream (i.e., colder) sections of the stream could be more efficient decomposers than the species found downstream, because leaf litter is a more significant resource for these headwater food webs. Species composition of communities actually varied along our altitudinal gradient, following common patterns between streams. However, several studies suggest that temperature could be largely involved in aquatic hyphomycete species distribution along latitudinal (Seena et al., 2019) or seasonal (Suberkropp, 1984) gradients, and reflect species thermal preferences. This is supported by the presence in our upstream sites of several species that are also common in temperate streams, such as Alatospora acuminata Ingold, Anguillospora crassa Ingold or Tetracladium marchalianum de Wild. Together with species composition, community structure also varied along the longitudinal gradient. Foam samples contained higher amounts of conidia in the most upstream sites, which increased the probability of observing rare species. However, they were dominated by a few species, and both evenness and rarefied species richness were consequently low. At lower altitude, conidia densities were lower, but higher rarefied species richness suggests that the species pool could be wider than upstream.
This result is contrary to our expectations and to the hypothesis that high temperature limits aquatic hyphomycete diversity within the tropics (Barreto et al., 2023). In fact, the species richness we observed does not support the general expectation that aquatic hyphomycete communities are less diverse in tropical than in temperate streams. Rather, it is broadly comparable to temperate richness levels according to several surveys using similar methodologies (i.e., foam collection) and sampling effort (e.g., Wood-Eggenschwiler & Bärlocher, 1983; Chauvet, 1990)—though a higher number of species were also reported in Portugal (Pascoal et al., 2005). Most of the primary research that concluded on the lower aquatic hyphomycete richness at low latitude was based on leaf baits (i.e., conidia produced from leaf litter) (Ferreira et al., 2012; Jabiol et al., 2013; Barreto et al., 2023). It is possible that relying on this methodology in tropical streams leads to an underestimation of aquatic hyphomycete species richness, since the species richness in our samples was 3 × higher in foam than in bamboo leaf baits. Similar discrepancies between methods were found by Maddodi et al. (2008) and Iqbal (1994) in tropical streams of India and Pakistan (respectively), while Wood-Eggenschwiler & Bärlocher (1983) reported more comparable levels of diversity between methods (similar to a 1.75 × higher richness in water and foam than in leaf baits).
Graça et al. (2016) suggested that tropical aquatic hyphomycetes could invest less energy into spore production compared with temperate species. Low sporulation rates could result in low aquatic hyphomycete diversity when assessed at the leaf scale at a single time (leaf baits), even if the species pool at the stream scale (as assessed from foam) is high.
Huge spore production can provide a competitive advantage when resource availability is pulsed (litterfall) and short-lived, as in temperate streams. In tropical streams, litterfall is less seasonal, and litter usually decomposes more slowly because of high lignin and tannins concentrations (Boyero et al., 2017). The selection pressure could be lower on dispersal and colonization efficiency (i.e., sporulation), but stronger on enzymatic capabilities and resistance to litter secondary metabolites.
Finally, though it was not the primary goal of our study, we could assess the effect of different effluents on leaf decomposition and aquatic hyphomycete diversity. Two effluents in particular strongly inhibited leaf decomposition (by 33 and 51%). They, respectively, originate from a food-processing factory (distillery) and a high capacity (8560 population equivalent) domestic wastewater treatment plant, which was qualified as non-compliant with legal standards by the French Ministry of Ecological Transition. By contrast, the 3rd wastewater effluent, that had no discernible impact on microbial litter decomposition, originated from a smaller domestic wastewater treatment plant (1800 population equivalent) that was compliant with legal standards. Together with the stimulation of litter decomposition by nitrate availability, these findings support that the use of litter decomposition for assessing stream ecological status (Ferreira et al., 2020; Frainer et al., 2021; Brosed et al., 2022) is also useful under tropical climates (Pérez et al., 2013).
Conclusions
The main conclusions of our study are that the effects of nitrate availability on aquatic hyphomycete activity and communities in streams of Guadeloupe are largely consistent with existing knowledge based on temperate experiments. However, the positive effect of temperature on aquatic hyphomycete diversity is more surprising and contradictory with previous cross-latitudes comparisons. We suggest that the conclusions of broad-scale comparisons are strongly dependent on the methodology used to assess aquatic hyphomycete diversity (most often conidia production from leaf baits), and encourage upcoming surveys to use several complementary techniques simultaneously. Moreover, future studies should be dedicated to disentangling between the confounding influence of temperature and nutrient limitation (both high under the tropics) on global aquatic hyphomycete diversity patterns. This can be achieved using broad nutrient availability gradients as well as different sources of temperature variations (e.g., seasonal, altitudinal and geothermal).
Data availability
All the data are available from the authors upon request. Biodiversity data and metadata were transferred to national and international open-access databases (SINP and GBIF).
Code availability
Not applicable.
References
Amani, M., M. A. S. Graça & V. Ferreira, 2019. Effects of elevated atmospheric CO2 concentration and temperature on litter decomposition in streams: a meta-analysis. International Review of Hydrobiology 104: 14–25. https://doi.org/10.1002/iroh.201801965.
Bärlocher, F., 2009. Reproduction and dispersal in aquatic hyphomycetes. Mycoscience 50: 3–8. https://doi.org/10.1007/s10267-008-0449-x.
Bärlocher, F., 2020. Leaf Mass Loss Estimated Using The Litter Bag Technique. In Bärlocher, F., M. O. Gessner & M. A. S. Graça (eds), Methods to Study Litter Decomposition: A Practical Guide, Switzerland Springer, Cham: 43–52. https://doi.org/10.1007/978-3-030-30515-4_6.
Bärlocher, F., J. E. Helson & D. D. Williams, 2010. Aquatic hyphomycete communities across a land-use gradient of Panamanian streams. Fundamental and Applied Limnology 3: 209–221. https://doi.org/10.1127/1863-9135/2010/0177-0209.
Bärlocher, F., S. Seena, K. P. Wilson & D. D. Williams, 2008. Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshwater Biology 53: 368–379. https://doi.org/10.1111/j.1365-2427.2007.01899.x.
Barreto, G. G., L. U. Hepp, R. de Souza Rezende, J. F. Gonçalves Jr., M. Moretti, Y. Moretto, R. Chaves Loureiro, R. M. Restello & A. O. Medeiros, 2023. The cooler the better: Increased aquatic hyphomycete diversity in subtropical streams along a neotropical latitudinal gradient. Fungal Ecology 62: 101223. https://doi.org/10.1016/j.funeco.2022.101223.
Belliveau, M. J. R. & F. Bärlocher, 2005. Molecular evidence confirms multiple origins of aquatic hyphomycetes. Mycological Research 109: 1407–1417. https://doi.org/10.1017/S0953756205004119.
Boulton, A. J., L. Boyero, A. P. Covich, M. Dobson, S. Lake & R. Pearson, 2008. Are Tropical Streams Ecologically Different From Temperate Streams? In Dudgeon, D. (ed), Tropical Stream Ecology Academic Press, Cambridge: 257–284. https://doi.org/10.1016/B978-012088449-0.50011-X.
Boyero, L., M. A. S. Graça, A. M. Tonin, J. Pérez, A. J. Swafford, V. Ferreira, A. Landeira-Dabarca, M. A. Alexandrou, M. O. Gessner, B. G. McKie, R. J. Albariño, L. A. Barmuta, M. Callisto, J. Chará, E. Chauvet, C. Colón-Gaud, D. Dudgeon, A. C. Encalada, R. Figueroa, A. S. Flecker, T. Fleituch, A. Frainer, J. F. Gonçalves Jr., J. E. Helson, T. Iwata, J. Mathooko, C. M’Erimba, C. M. Pringle, A. Ramírez, C. M. Swan, C. M. Yule & R. G. Pearson, 2017. Riparian plant litter quality increases with latitude. Scientific Reports 7: 10562. https://doi.org/10.1038/s41598-017-10640-3.
Boyero, L., R. G. Pearson, M. O. Gessner, L. A. Barmuta, V. Ferreira, M. A. S. Graça, D. Dudgeon, A. J. Boulton, M. Callisto, E. Chauvet, J. E. Helson, A. Bruder, R. J. Albariño, C. M. Yule, M. Arunachalam, J. N. Davies, R. Figueroa, A. S. Flecker, A. Ramírez, R. G. Death, T. Iwata, J. M. Mathooko, C. Mathuriau, J. F. Gonçalves Jr., M. S. Moretti, T. Jinggut, S. Lamothe, C. M’Erimba, L. Ratnarajah, M. Schindler, J. Castela, L. M. Buria, A. Cornejo, V. D. Villanueva & D. West, 2011. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecology Letters 14: 289–294. https://doi.org/10.1111/j.1461-0248.2010.01578.x.
Brosed, M., J. Jabiol & E. Chauvet, 2022. Towards a functional assessment of stream integrity: a first large-scale application using leaf litter decomposition. Ecological Indicators 143: 109403. https://doi.org/10.1016/j.ecolind.2022.109403.
Brown, J. H., J. F. Gillooly, A. P. Allen, V. M. Savage & G. B. West, 2004. Toward a metabolic theory of ecology. Ecology 85: 1771–1789. https://doi.org/10.1890/03-9000.
Camelo, F. R. B., A. M. Tonin, L. Salgueiro, G. Sena, I. Braga, A. O. Medeiros & J. F. Gonçalves Jr., 2022. Tropical stream microcosms of isolated fungal species suggest nutrient enrichment does not accelerate decomposition but might inhibit fungal biomass production. FEMS Microbiol Lett 369: fnac113. https://doi.org/10.1093/femsle/fnac113.
Chan, S. Y., T. K. Goh & K. D. Hyde, 2000. Ingoldian fungi in Hong Kong. Fungal Diversity 5: 89–107.
Chauvet, E. & K. Suberkropp, 1998. Temperature and sporulation of aquatic hyphomycetes. Applied and Environmental Microbiology 64: 1522–1525. https://doi.org/10.1128/AEM.64.4.1522-1525.1998.
Chauvet, E., 1990. Hyphomycètes aquatiques du sud-ouest de la France. Gaussenia 6: 3–31.
Cross, W. F., J. M. Hood, J. P. Benstead, A. D. Huryn & D. Nelson, 2015. Interactions between temperature and nutrients across levels of ecological organization. Global Change Biology 21: 1025–1040. https://doi.org/10.1111/gcb.12809.
Dang, C. K., M. Schindler, E. Chauvet & M. O. Gessner, 2009. Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90: 122–131. https://doi.org/10.1890/07-1974.1.
Descals, E., 2020. Techniques for Handling Ingoldian Fungi. In Bärlocher, F., M. O. Gessner & M. A. S. Graça (eds), Methods to Study Litter Decomposition: A Practical Guide Springer, Cham: 197–210. https://doi.org/10.1007/978-3-030-30515-4_23.
Do Nascimiento, E. G. R., M. A. Barbosa, W. L. Tavares & E. Malosso, 2021. Diversity of hyphomycetes on submerged leaf litter in two Atlantic Forest areas in the Northeast of Brazil with comments on the water quality. Acta Limnologica Brasiliensa 33: e30. https://doi.org/10.1590/S2179-975X0921.
Duarte, S., F. Bärlocher, C. Pascoal & F. Cássio, 2016. Biogeography of aquatic hyphomycetes: current knowledge and future perspectives. Fungal Ecology 19: 169–181. https://doi.org/10.1016/j.funeco.2015.06.002.
Fabre, E. & E. Chauvet, 1998. Leaf breakdown along an altitudinal stream gradient. Archiv Für Hydrobiologie 141: 167–179.
Ferreira, V. & C. Canhoto, 2015. Future increase in temperature may stimulate litter decomposition in temperate mountain streams: evidence from a stream manipulation experiment. Freshwater Biology 60: 881–892. https://doi.org/10.1111/fwb.12539.
Ferreira, V., A. Elosegi, S. D. Tiegs, D. von Schiller & R. Young, 2020. Organic matter decomposition and ecosystem metabolism as tools to assess the functional integrity of streams and rivers–A systematic review. Water 12: 3523. https://doi.org/10.3390/w12123523.
Ferreira, V., A. C. Encalada & M. A. S. Graça, 2012. Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshwater Science 31: 945–962. https://doi.org/10.1899/11-062.1.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet & M. A. S. Graça, 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688. https://doi.org/10.1111/brv.12125.
Ferreira, V., V. Gulis & M. A. S. Graça, 2006. Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149: 718–729. https://doi.org/10.1007/s00442-006-0478-0.
Fiuza, P. O., T. Cantillo-Pérez, V. Gulis & L. F. P. Gusmão, 2017. Ingoldian fungi of Brazil: some new records and a review including a checklist and a key. Phytotaxa 306: 171–200. https://doi.org/10.11646/phytotaxa.306.3.1.
Follstad Shah, J., J. S. Kominoski, M. Ardón, W. K. Dodds, M. O. Gessner, N. A. Griffiths, C. P. Hawkins, S. L. Johnson, A. Lecerf, C. J. LeRoy, D. W. P. Manning, A. D. Rosemond, R. L. Sinsabaugh, C. M. Swan, J. R. Webster & L. H. Zeglin, 2017. Global synthesis of the temperature sensitivity of leaf litter breakdown in streams and rivers. Global Change Biology 23: 3064–3075. https://doi.org/10.1111/gcb.13609.
Frainer, A., A. Bruder, F. Colas, V. Ferreira & B. G. McKie, 2021. Plant Litter Decomposition as a Tool for Stream Ecosystem Assessment. In Swan, C. M., L. Boyero & C. Canhoto (eds), The Ecology of Plant Litter Decomposition in Stream Ecosystems Springer, Cham: 483–509. https://doi.org/10.1007/978-3-030-72854-0_21.
Geraldes, P., C. Pascoal & F. Cássio, 2012. Effects of increased temperature and aquatic fungal diversity on litter decomposition. Fungal Ecology 5: 734–740. https://doi.org/10.1016/j.funeco.2012.05.007.
Gessner, M. O. & E. Chauvet, 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75: 1807–1817. https://doi.org/10.2307/1939639.
Goering, H.K. & P.J. Van Soest, 1970. Forage fiber analysis (apparatus, reagents, procedures, and some applications). Agriculture Handbook 379, Department of Agriculture: 1–20.
Gonçalves, J. F., Jr., M. A. S. Graça & M. Callisto, 2007. Litter decomposition in a Cerrado savannah stream is retarded by leaf toughness, low dissolved nutrients and a low density of shredders. Freshwater Biology 52: 1440–1451. https://doi.org/10.1111/j.1365-2427.2007.01769.x.
Graça, M. A. S., K. Hyde & E. Chauvet, 2016. Aquatic hyphomycetes and litter decomposition in tropical—subtropical low order streams. Fungal Ecology 19: 182–189. https://doi.org/10.1016/j.funeco.2015.08.001.
Gulis, V. & K. Suberkropp, 2004. Effects of whole-stream nutrient enrichment on the concentration and abundance of aquatic hyphomycete conidia in transport. Mycologia 96: 57–65. https://doi.org/10.1080/15572536.2005.11832997.
Gulis, V., L. Marvanová & E. Descals, 2020. An Illustrated Key to the Common Temperate Species of Aquatic Hyphomycetes. In Bärlocher, F., M. O. Gessner & M. A. S. Graça (eds), Methods to Study Litter Decomposition: A Practical Guide Switzerland, Springer, Cham: 223–240. https://doi.org/10.1007/978-3-030-30515-4_25.
Iqbal, S. H., 1994. Species diversity of freshwater hyphomycetes in some streams of Pakistan Comparison of Sampling Techniques. Mycoscience 35: 331–343. https://doi.org/10.1007/BF02268503.
Irons, J. G., M. W. Oswood, R. J. Stout & C. M. Pringle, 1994. Latitudinal patterns in leaf litter breakdown: is temperature really important? Freshwater Biology 32: 401–411. https://doi.org/10.1111/j.1365-2427.1994.tb01135.x.
Jabiol, J., A. Bruder, M. O. Gessner, M. Makkonen, B. G. McKie, E. T. H. M. Peeters, V. C. A. Vos & E. Chauvet, 2013. Diversity patterns of leaf-associated aquatic hyphomycetes along a broad latitudinal gradient. Fungal Ecology 6: 439–448. https://doi.org/10.1016/j.funeco.2013.04.002.
Jabiol, J., A. Gossiaux, A. Lecerf, T. Rota, F. Guérold, M. Danger, P. Poupin, F. Gilbert & E. Chauvet, 2020. Variable temperature effects between heterotrophic stream processes and organisms. Freshwater Biology 65: 1543–1554. https://doi.org/10.1111/fwb.13520.
Jabiol, J., A. Lecerf, S. Lamothe, M. O. Gessner & E. Chauvet, 2019. Litter quality modulates effects of dissolved nitrogen on leaf decomposition by stream microbial communities. Microbial Ecology 77: 959–966. https://doi.org/10.1007/s00248-019-01353-3.
Jabiol, J., J. Cornut, A. Tlili & M. O. Gessner, 2018. Interactive effects of dissolved nitrogen, phosphorus and litter chemistry on stream fungal decomposers. FEMS Microbiology Ecology 93: fiy151. https://doi.org/10.1093/femsec/fiy151.
Maddodi, N. D., N. S. Raviraja & M. Rajashekhar, 2008. Diversity of Aquatic Hyphomycetes of the Western Ghat Rivers. In Sridhar, K. R. (ed), Frontiers in Fungal Ecology Diversity and Metabolites I.K International Publishing House, New Delhi: 17–23.
Martínez, A., A. Larrañaga, J. Pérez, E. Descals & J. Pozo, 2014. Temperature affects leaf litter decomposition in low-order forest streams: field and microcosm approaches. FEMS Microbiology Ecology 87: 257–267. https://doi.org/10.1111/1574-6941.12221.
Mathuriau, C. & E. Chauvet, 2002. Breakdown of leaf litter in a neotropical stream. Journal of the North American Benthological Society 21: 384–396. https://doi.org/10.2307/1468477.
Medeiros, A. O., M. Callisto, M. A. S. Graça, V. Ferreira, C. A. Rosa, J. França, A. Eller, R. S. Rezende & J. F. Gonçalves Jr., 2015. Microbial colonisation and litter decomposition in a Cerrado stream are limited by low dissolved nutrient concentrations. Limnetica 34: 283–292. https://doi.org/10.23818/limn.34.22.
Millenium Ecosystem Assessment, 2005. Ecosystems and Human Well-Being: Wetlands and Water, World Resources Institue, Washington:, 68.
Pascoal, C., L. Marvanová & F. Cássio, 2005. Aquatic hyphomycete diversity in streams of Northwest Portugal. Fungal Diversity 19: 109–128.
Pérez, J., A. Basaguren, E. Descals, A. Larrañaga & J. Pozo, 2013. Leaf-litter processing in headwater streams of northern Iberian Peninsula: moderate levels of eutrophication do not explain breakdown rates. Hydrobiologia 718: 41–57. https://doi.org/10.1007/s10750-013-1610-x.
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rajashekhar, M. & K. Kaveriappa, 2000. Effects of temperature and light on growth and sporulation of aquatic hyphomycetes. Hydrobiologia 441: 149–153. https://doi.org/10.1023/A:1017591109362.
Santos-Flores, C. J. & C. Betancourt-López, 1997. Aquatic and water-borne hyphomycetes (Deuteromycotina) in streams of Puerto Rico (including records from other neotropical locations). Caribbean Journal of Science 2: 1–116.
Schoenlein-Crusius, I. H. & R. A. P. Grandi, 2003. The diversity of aquatic hyphomycetes in South America. Brazilian Journal of Microbiology 34: 183–193. https://doi.org/10.1590/S1517-83822003000300001.
Seena, S., F. Bärlocher, O. Sobral, M. O. Gessner, D. Dudgeon, B. G. McKie, E. Chauvet, L. Boyero, V. Ferreira, A. Frainer, A. Bruder, C. D. Matthaei, S. Fenoglio, K. R. Sridhar, R. J. Albariño, M. M. Douglas, A. C. Encalada, E. Garcia, S. D. Ghate, D. P. Giling, V. Gonçalves, T. Iwata, A. Landeira-Dabarca, D. McMaster, A. O. Medeiros, J. Naggea, J. Pozo, P. M. Raposeiro, C. M. Swan, N. S. D. Tenkiano, C. M. Yule & M. A. S. Graça, 2019. Biodiversity of leaf litter fungi in streams along a latitudinal gradient. Science of the Total Environment 661: 306–315. https://doi.org/10.1016/j.scitotenv.2019.01.122.
Smits, G., R. Fernández & C. Cressa, 2007. Preliminary study of aquatic hyphomycetes from Venezuelan streams. Acta Botanica Venezuelica 30: 345–355.
Suberkropp, K., 1984. Effect of temperature on seasonal occurrence of aquatic hyphomycetes. Transactions of the British Mycological Society 82: 53–62. https://doi.org/10.1016/S0007-1536(84)80211-9.
Taylor, B. R. & E. Chauvet, 2014. Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia 721: 239–250. https://doi.org/10.1007/s10750-013-1666-7.
Wood-Eggenschwiler, S. & F. Bärlocher, 1983. Aquatic hyphomycetes in sixteen streams in France, Germany and Switzerland. Transactions of the British Mycological Society 81: 371–379. https://doi.org/10.1016/S0007-1536(83)80089-8.
Acknowledgements
We thank the members of the ASSET Research Unit (INRAE) for their help and access to their laboratory and equipment. We are grateful to Andreas Bruder who provided small equipment as well as suggestions about fungal identification and manuscript writing. We also thank Eric Chauvet for giving clues on fungal identification, as well as Marie Robert and two anonymous referees for their helpful and constructive comments on a previous version of the manuscript.
Funding
The project was supported by the Office de l’Eau de la Guadeloupe (Grant n 286) and the Parc National de la Guadeloupe (Grant n 2022–039) with the support of the France Relance Programme. The biodiversity inventory was conducted under the National Inventory of the Natural Heritage (inpn.mnhn.fr). It received support from PatriNat (OFB-MNHN-CNRS-IRD).
Author information
Authors and Affiliations
Contributions
The study was coordinated by ML. JJ and ML designed the study with inputs from FJ. All authors performed the research. JJ carried out the statistical analyses and drafted the manuscript. All authors contributed to the revision of the manuscript and gave their final approval before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: V. Ferreira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabiol, J., Julien, F. & Labeille, M. Aquatic hyphomycetes and litter decomposition in tropical streams: insights from the first study in Guadeloupe. Hydrobiologia 851, 4487–4501 (2024). https://doi.org/10.1007/s10750-024-05602-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05602-6