Abstract
This study explores the intraspecific trait variation and ecological strategies of three macrophyte species in the Amazon region, focusing on leaf traits and CSR strategies (competitors, stress tolerators, and ruderals). Individuals of Eleocharis interstincta, Fuirena umbellata, and Nymphaea rudgeana were examined across 22 sampling sites. Traits including leaf area, specific leaf area, and leaf dry matter content were measured, along with environmental variables. Results demonstrated significant differences in leaf traits among the species, highlighting their distinct strategies. Individuals of F. umbellata exhibited the lowest leaf dry matter content values, indicating a conservative and stress-tolerator strategy. N. rudgeana had the highest leaf area values, reflecting an acquisitive strategy and varied from a S to S/CS strategy, while E. interstincta showed intermediate trait values and a stress-tolerator strategy. Furthermore, intraspecific variation was observed within each species, influenced by environmental factors (nutrient availability, water conductivity, dissolved oxygen, and sediment composition). Our findings contribute to understanding the intraspecific trait variations and ecological strategies of macrophytes in the Amazon region, providing insights into plant adaptation and response to environmental changes. Future research should incorporate additional traits and encompass different macrophyte life forms, further enhancing our understanding of their strategies and responses to ongoing environmental change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Community structure is shaped by various processes, including dispersal, biotic interactions, and the abiotic environment (Cadotte & Tucker, 2017). Environmental filters, tied to resource availability, are pivotal in this process by influencing organisms’ distribution, survival, and competition patterns (Cadotte & Tucker, 2017). Additionally, individual variation within populations impacts community structure (Violle et al., 2012; Dalla Vecchia & Bolpagni, 2022), with intraspecific variation playing a significant role alongside interspecific variation (Siefert et al., 2015; Des Roches et al., 2018).
Plants can adapt to change in response to environmental conditions and biotic factors, which is reflected in their high phenotypic variability (Sultan, 2000; Nicotra et al., 2010). This involves resource allocation (trade-offs) (Weiner, 2004) and trait variations among genotypes, individuals, populations, and species (Violle et al., 2007). In resource-limited situations, these strategies are crucial for plant survival (Grime, 1977). Thus, intraspecific variation manifests in multiple plant functional traits. Among these, three key traits are commonly used to define ecological strategies (Wright et al., 2004; Pierce et al., 2017): leaf area (LA), specific leaf area (SLA), and leaf dry matter content (LDMC). Plant size and leaf economics, along with stem and root traits, work together to shape a plant’s survival strategy in the environment (Díaz et al., 2016). These traits influence and may be indicative of each other, shaping the plant economics spectrum (Reich, 2014).
For the plant economics spectrum, environmental conditions may trigger a growth vs. survival trade-off, which is associated with the acquisitive and conservative plant strategies (Reich, 2014). Plants with acquisitive strategies invest in growing fast by producing short-lived leaves with little tissue density (less carbon and more nitrogen) and a high photosynthetic rate (presenting high leaf area and specific leaf area and low leaf dry matter content) (Wright et al., 2004; Donovan et al., 2011; Reich, 2014). In contrast, plants exhibiting a conservative strategy grow at slower rates, investing in leaves with a long lifespan, that are structurally expensive (more carbon) and a low photosynthetic rate (presenting low leaf area and specific leaf area and high leaf dry matter content) (Wright et al., 2004; Donovan et al., 2011; Reich, 2014).
Through a comprehensive analysis of multiple traits, plant ecological strategies can be identified, offering insights into the phenotypic outcomes of natural selection within specific environments (Pierce et al., 2013, 2017; Estarague et al., 2022). Grime’s CSR scheme further classifies plant strategies into three main categories: competitors (C), stress tolerators (S), and ruderals (R) (Grime, 1977, 2002; Grime & Pierce, 2012; Pierce et al., 2017). Competitors invest in rapid growth to outcompete for resources, stress tolerators focus on resource retention for survival in harsh environments, and ruderals prioritize the production of propagules for regeneration after disturbance events (Grime & Pierce, 2012; Pierce et al., 2017). However, this scheme has been thoroughly applied to classify species as a whole (interspecific variation), while overlooking that such variation may also occur between individuals in a given population or among populations across contrasting environments (May et al., 2017; Vasseur et al., 2018; Estarague et al., 2022).
Aquatic plants are characterized by their highly acquisitive strategies compared to their terrestrial counterparts, reflected in investment in higher SLA and nitrogen content and lower LDMC (Poorter et al., 2009; Pierce et al., 2012; Pan et al., 2020). Macrophytes exhibit a diverse range of leaf sizes and morphologies (Barrett et al., 1993; Poorter et al., 2009; Pierce et al., 2012). These traits reflect their adaptation to water-saturated environments, with a reduced investment in structural tissues and an emphasis on maximizing photosynthetic surface area, either by producing large leaves (as seen in Nymphaeaceae) or numerous small ones (as observed in Myriophyllum spp.) (Díaz et al., 2016; Pan et al., 2020). However, it is important to consider that environmental variations, such as different physical structures of aquatic systems (e.g., lotic or lentic, perennial or temporary ecosystems, and saline environments) and the biomes associated with them, can influence the strategies employed by aquatic plants (Lacoul & Freedman, 2006; Fu et al., 2023; Gao et al., 2023).
Macrophytes also exhibit similar patterns in their ecological strategies. They are normally classified closer to the ruderal side of the ecological strategy scheme due to the frequent disturbances encountered in freshwater environments, such as flooding, trampling, and seasonal drought (Pierce et al., 2012; Albuquerque et al., 2020). Consequently, many species invest heavily in producing propagules and have a rapid regeneration capacity after biomass loss (Grime & Pierce, 2012; Pierce et al., 2017). However, certain macrophytes display a more competitive strategy by allocating resources toward faster growth (e.g., Nymphaeiden) (Pierce et al., 2012; Albuquerque et al., 2020). While it is argued that no macrophyte species could exhibit a strong conservative strategy to be classified as a true stress tolerator (Pierce et al., 2012), there are exceptions (Lacoul & Freedman, 2006), especially among sedges (Cyperaceae). Some sedges (e.g., Eleocharis spp.) are considered true macrophytes, and the global patterns for the Cyperaceae family indicate strong stress-tolerant characteristics (Pierce et al., 2017).
Therefore, further research on the variation in ecological strategies of macrophytes is necessary, since macrophyte communities have been understudied when it comes to assessing functional traits and ecological strategies and because the evidence of intraspecific variation of ecological strategies (especially CSR) are at the beginning stages of research. In particular, investigation of these strategies in tropical forests like the Amazon is of interest, as tropical forests have a very heterogeneous environments, and could show clear patterns. These regions exhibit high nutrient cycling but severe soil and sediment nutrient deficiency (Yu et al., 2015; Figueiredo et al., 2018), in addition to more acidic waterbodies (Ríos-Villamizar et al., 2013). Species in these environments tend to exhibit more competitive and stress-tolerant strategies (Pierce et al., 2017; Araujo da Costa et al., 2020). Furthermore, these environmental conditions may lead to macrophyte strategies differing from the known global pattern, potentially shifting dominance from ruderal species to competitors and stress-tolerant species.
In this study, we aimed to investigate the variation of leaf traits (LA, SLA, and LDMC) and ecological strategies in individuals of three macrophyte species: Eleocharis interstincta (Vahl) Roem. & Schult. (Cyperaceae), Fuirena umbellata Rottb. (Cyperaceae), and Nymphaea rudgeana G.Mey. (Nymphaeaceae), across an environmental gradient in Eastern Amazon. Our hypotheses are that (1) in resource-rich environments (with high water temperature and conductivity and high concentrations of sediment phosphorus, potassium, and magnesium), individuals of all species have a more acquisitive strategy (high SLA and LA and low LDMC). In contrast, individuals in resource-poor environments exhibit a conservative strategy (low SLA and LA and high LDMC). However, we expect individuals of N. rudgeana to have higher LA and SLA than E. interstincta and F. umbellata, which have a more conservative strategy, due their tolerance to environmental stress. (2) There is a contrasting intraspecific variation in ecological strategies of macrophyte species. More specifically, a shift in N. rudgeana from ruderal to competitors as resource availability increases in response to environmental change. In contrast, we expect individuals of E. interstincta and F. umbellata to be more conservative and vary little in their ecological strategies, remaining stress tolerators, as it is a tendency of the Cyperaceae family worldwide (Pierce et al., 2017). This study is, so far, the first one that investigates the intraspecific variation of CSR strategies in aquatic plant communities.
Material and methods
Study area
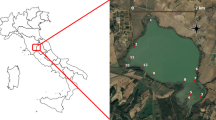
Data sampling was performed in September 2022, across 22 sites, at the Capim River Basin, located in the municipalities of Paragominas (Lat: 02º 59′ 45ʺ S; Long: 47º 21′ 10ʺ W) and its surroundings, in the northeastern portion of the State of Pará, Brazil (Fig. 1). The sites comprise mostly lentic ecosystems, such as lakes and ponds. The climate is wet and hot (mean annual temperature of 26º mean air humidity of 81%, and mean annual precipitation of 1.800 mm (Pinto et al., 2009). The vegetation of the area consists of tropical rainforest.
Sampling design
Environmental variables
At each site, we sampled environmental variables, which comprise water and sediment parameters, and physical aspects of the sites. For the water variables, we measured temperature (°C), conductivity (µs/cm), pH, and dissolved oxygen (mg/L), using a multiparameter probe (Model Akso AK48). We measured water depth using a meter (cm), and the inclination of the shoreline using a digital clinometer (°). These measurements were taken twice in each site.
For the measurement of sediment variables, we collected composed sediment samples. This consists of 15 to 20 simple samples collected randomly along each site. These samples were taken using a shovel at a 20 cm depth. Then, the simple samples are placed in a tray, homogenized, and stored in plastic bags. We collected 400 g of sediment for each site. Sediment samples were then taken to the Laboratory of Soils of the EMBRAPA Amazônia Oriental, located in the city of Belém, Pará (Brazil), where the chemical (amount of nutrients and elements present in the sediment) and physical (distribution of particles—granulometry) parameters of the sediment were assessed. We obtained the following measures: Chemical parameters: amount of Phosphorous (P), Potassium (K), and Sodium (Na) (mg/dm3) and amount of Aluminum (Al), Calcium (Ca), and Calcium + Magnesium (Ca + Mg) (cmolc/dm3) and physical parameters: amount of coarse and fine sand, silt, and total clay (g/kg). The procedure was performed following the protocol proposed by (Teixeira et al., 2017).
Biological sampling
For the collection of macrophyte species, we selected three species that were representative of the macrophyte community in the region:
-
(1)
Eleocharis interstincta (Cyperaceae): emergent, perennial herb, with rhizomes, leaves are underdeveloped, and the culms perform photosynthesis (Lorenzi, 2008; Pott & Pott, 2000), is native from tropical America, occurs in all regions of Brazil (Nunes et al., 2023), and normally inhabits wetlands and other stillwater habitats, forming stands closer to the shorelines (Lorenzi, 2008).
-
(2)
Fuirena umbellata (Cyperaceae): amphibious, perennial herb, rhizomatous, reaching from 40 to 100 cm (Lorenzi, 2008), inhabits waterlogged and flooded environments, at the shorelines (Lorenzi, 2008). Species native from America, it is present in all regions of Brazil (Alves et al., 2023), and reproduces by rhizome and seeds (Pott & Pott, 2000).
-
(3)
Nymphaea rudgeana (Nymphaeaceae): Floating-leaved herb, rooted in the sediment with flexible petioles below the water column (Moreira & Bove, 2017) and inhabits more lentic freshwaters (personal observation). In Brazil, it is present in all regions except for the central-western region (Pellegrini, 2023)
The selection followed two criteria: these species were the most frequent ones in the field (E. intersticta—16 occurrences; F. umbellata—12; and N. rudgeana—10 occurrences) and, when found, they were the most abundant ones. However, they rarely occurred at the same site together, normally there was a co-occurrence of two species at a time. Because of that, we performed the analyses separately for each species, to truly assess their intraspecific variation.
For the sampling, we selected three individuals of each species in the sites. From those individuals, we selected three middle leaves (for E. interstincta, we considered as ‘leaves’ the unfertile stems, following the recommendation of Pérez-Harguindeguy et al. (2013) for special cases) that were nor too young or too old and that had no signs of herbivory or any other damage. We took photographs of these leaves and then hydrated them in trays filled with water to measure the water-saturated fresh mass, using a digital scale (0.001 g). The leaves were then oven-dried at 65ºC for 72 h and weighted again to obtain the dry mass. With these measurements, we were able to calculate the following functional traits, as described by the protocol proposed by Pérez-Harguindeguy et al. (2013):
-
(a)
Leaf area (LA) measures leaf size and is the one-sided area of a leaf (mm2). It is associated with the investment of plants in the photosynthetic surface. We used the photos of the leaves to measure LA, using the package BiocManager (Morgan, 2022) in the R program (R Core Team, 2022).
-
(b)
Specific leaf area (SLA) is the one-sided area of a fresh leaf divided by its oven-dried mass (SLA = leaf area / dry mass). We used the area calculated in the same leaves used to measure LA. It expresses the amount of carbon invested in the photosynthetic area of a leaf.
-
(c)
Leaf dry matter content (LDMC) is the dry mass of a leaf divided by its water-saturated mass (mg/g). LDMC measures the average density of leaf tissues.
We considered these leaf traits because they are more accessible to measure in field conditions and they are suitable to test our hypotheses, as these traits vary with environmental change and are efficient in expressing plant strategies (i.e., more conservative or acquisitive). Additionally, these traits are required to calculate the CSR functional strategies as proposed by Pierce et al. (2017).
Statistical analysis
Prior to the hypothesis testing analyses, we assessed multicollinearity among predictors, using a Pearson Correlation Matrix. We considered the correlation coefficient value of r ≥ ± 0.65 as high (Table S1). Whenever two variables were correlated, we selected one to be retained based on the literature concerning the macrophyte community, where we chose the variables to be retained based on what elements or components would be important or limiting to plant establishment and growth in freshwaters (Lacoul & Freedman, 2006; Akasaka et al., 2010; Bornette & Puijalon, 2011; Aoki et al., 2017).
For the hypothesis testing analyses, we considered the individuals as the sample unit and used the mean values of the three leaves of each individual. To test the difference in trait variation among species, we performed a Permutational multivariate analysis of variance (PERMANOVA), using the species as predictors and the trait values as response variables (Anderson et al., 2008). As PERMANOVA tests only if there is a variation among the groups but does not tell which groups are significantly different from one another, we tested the pairwise difference between species using the Pairwise Adonis analysis (Martinez Arbizu, 2017). For this analysis, we included the trait values of all species in one matrix, and the trait matrix was standardized prior the analysis. In addition, to better visualize the distribution of the traits of individuals of each species, we performed a Principal Component Analysis (PCA).
To test the effects of the environmental variables on the trait variation of individuals in each species, we performed a Redundancy analysis (RDA (Gotelli & Ellison, 2012), using the variables selected after the Pearson correlation analysis as predictors and the functional traits (LA, SLA and LDMC) as response variables. We performed one model for each species, as they did not occur in all the sampling sites. Before running the RDA models, we performed a parametric forward selection of explanatory variables based on the functional traits and environmental variables. Environmental variables were standardized prior to the analysis, and the functional trait matrix was Hellinger-transformed to ensure linearity among predictors and response variables (Gotelli & Ellison, 2012). We validated the models using a permutation test at 10,000 permutations, and model adjustment was assessed using the Adjusted R2.
Finally, to assess the intraspecific variation of CSR strategies (Competitor, Stress tolerator, and Ruderal), we calculated the relative proportions of each component of CSR strategies of each individual, based on their trait (LA, SLA, LDMC) values using the StrateFy tool (Pierce et al., 2017). Then, to better represent and visualize this variation, we produced a ternary plot using the proportions of each strategy for each individual.
All the analyses were performed in RStudio version 4.2.0 (R Core Team, 2022). Pearson correlation was performed using the ‘rcorr’ function of the ‘Hmisc’ package (Harrell Jr, 2023). PERMANOVA was performed using the ‘adonis2’ function, and PCA was performed using the ‘prcomp’ function, also from the ‘vegan’ package (Oksanen et al., 2022). Pairwise Adonis comparison between groups was performed using the ‘pairwise.adonis’ function from the ‘pairwiseAdonis’ package (Martinez Arbizu, 2017). RDA was performed using the ‘rda’ function, model validation was performed using the ‘anova.cca’ function, and the adjusted R2 was calculated using the ‘RsquareAdj’ function, all from the ‘vegan’ package. Model selection for functional traits of each species was performed using the ‘forward.sel.par’ function from the adespatial package (Dray et al., 2022). Finally, all graphs were plotted using the ‘ggplot2’ package (Wickham, 2016), except from the ternary plot of the CSR strategies which was built using the ‘ggtern’ package (Hamilton & Ferry, 2018).
Results
Environmental variables
The environmental variables that were retained to be used in the models to test our hypothesis were water temperature, pH, conductivity, dissolved oxygen and depth, the amount of coarse sand and total clay of the sediment, and the amount of phosphorus, potassium, and calcium + magnesium in the sediment. Water temperature varied from 24.80 to 32.10 °C. pH varied from 3.47 (acidic) to 9.96 (basic). Conductivity varied from 15.90 to 87.60 µs/cm. Dissolved oxygen varied from 1.10 to 4.80 mg/L. Water depth varied from 5 to 44 cm. For the sediment variables, the amount of coarse sand varied from 34 to 554 g/kg, while total clay varied from 60 to 480 g/mg. Phosphorus concentration varied from 0.45 to 9.35 mg/dm3. Potassium concentrations varied from 4.61 to 64.89 mg/dm3, while sodium varied from 0.75 to 48.58 mg/dm3. Finally, the amount of Calcium + Magnesium concentrations varied from 0.10 to 2.87 cmolc/dm3 (Table 1).
Variation in functional traits and differences among species
We analyzed three leaves of 117 individuals (a total of 351 leaves) of three macrophyte species. Eleocharis interstincta had the highest number of individuals (48), followed by Fuirena umbellata (39), and Nymphaea rudgeana (30). Overall, LA varied from 54.06 to 5462.52 mm2, LDMC varied from 3.95 to 92.02%, and SLA varied from 0.57 to 14.75 mm2mg−1 (for more information, see table S1).
The PERMANOVA results showed that there is a difference in the trait values (Pseudo-F = 40.72; p = 0.001) among all species (Table 2). The first axis of the PCA represented 55.95% of total data variance, while the second axis represented 27.30% (Fig. 2). On average, E. individuals had the lowest SLA values, while F. umbellata individuals presented the highest LDMC values and the lowest LA values and individuals of N. rudgeana showed the opposite pattern (highest LA values and lowest LDMC values) (Fig. 2).
Effect of environmental variables on individuals’ functional traits
The result of the RDA models showed that different variables drove the intraspecific trait variation of the studied species. More specifically, the model for E. interstincta showed water conductivity, dissolved oxygen and Calcium + Magnesium explained 62.01% of variance (F = 26.57, p = 0.001, df = 3, Adjusted R2 = 0.62). The first RDA axis explained 99.50% (p > 0.001) of the fitted variance in E. interstincta and was positively related with Ca + Mg concentrations (0.51; p > 0.001) and negatively correlated with water conductivity (− 0.57; p > 0.001) and dissolved oxygen (− 0.51; p > 0.001). The second axis explained 0.04% (p = 0.85) of the fitted variation and was negatively associated with Ca + Mg (− 0.70) and dissolved oxygen (− 0.69). LDMC values increase with increased conductivity and dissolved oxygen concentrations and decrease with high sediment Ca + Mg concentrations, while LA values increase with low water conductivity and increased with an increase in Ca + Mg concentrations (Fig. 3A).
Redundancy Analysis performed between the traits (LA, SLA, and LDMC) of individuals of three macrophyte species (Eleocharis interstincta, Fuirena umbellata, and Nymphaea rudgeana) and environmental variables: Calcium + Magnesium (Ca + Mg), sediment Phosphorus (P), Coarse sand (coarse_s), Total clay (t_clay), water conductivity (Cond), depth, and dissolved oxygen (Oxy_mg)
The model for F. umbellata indicates water conductivity, dissolved oxygen, and sediment Phosphorus explained 39.91% of total trait variance (F = 9.41; p = 0.001, df = 3; Adjusted R2 = 0.40). The first axis explained 99.324% (p > 0.001) of the fitted variance and was positively correlated with water conductivity (0.49; p = 0.002) and dissolved oxygen (0.60; p > 0.001) and negatively related with sediment phosphorus concentrations (− 0.48; p = 0.01). The second axis explained 0.030% (p = 0.92) of the fitted variation and was negatively associated with sediment phosphorus (− 0.53) and dissolved oxygen (− 0.41). LDMC values of individuals increase with high water conductivity and dissolved oxygen and decrease with high concentrations of sediment P, while LA values decrease in those conditions (Fig. 3B).
Furthermore, the model for N. rudgeana explained 50.73% of trait variance and was explained by the amount of total clay, coarse sand, and water depth (F = 10.61, p = 0.001, df = 3, Adjusted R2 = 0.51). The first axis explained 99.75% (p > 0.001) of the fitted variance and was positively correlated with coarse sand (0.34; p = 0.04) and negatively related with water depth (− 0.84; p > 0.001). The second axis explained 0.24% (p = 0.98) of the fitted variation and was positively associated with total clay (0.93), and negatively correlated with water depth (− 0.43). SLA decrease with increased water depth and total clay, while LDMC decrease in sites with decreased water depth and amount of clay (Fig. 3C).
CSR strategies
We did not find large variation in individuals CSR strategies of the macrophyte species along the resource availability gradient (Fig. 3). All individuals of E. interstincta and F. umbellata remained stress tolerators (S > 80% of this strategy) in all sites. While individuals of N. rudgeana varied from stress tolerators to stress tolerators/competitors (S/CS) across the sites. Additionally, only one individual of N. rudgeana exhibited a small percentage of ruderal strategy (Fig. 4).
Discussion
According to the results, our first hypothesis that in resource-rich environments individuals of all species have a more acquisitive strategy was partially corroborated, because there was a variation in traits between all species. The intraspecific variation in E. interstincta and F. umbellata was driven by a combination of variables associated with nutrient availability (water conductivity and concentrations of phosphorus and calcium + magnesium). However, N. rudgeana was associated with variables that are not directly related with resource availability: amount of coarse sand, total clay, and water depth. In addition, our second hypothesis that there is contrasting intraspecific variation in ecological strategies of macrophyte species was also partially corroborated, as individuals of E. interstincta and F. umbellata were all stress tolerators; however, individuals of N. rudgeana presented a slight variation of strategies from competitors to stress tolerators, contrary with what we expected (a variation from ruderals to competitors).
Trait differences among species
There was a variation in traits among all species (Fig. 2; Table 2). E. interstincta had the lowest SLA, while F. umbellata had the highest LDMC and N. rudgeana had the highest LA and lowest LDMC. This is indicative of the contrasting strategies of the species if compared with one another. N. rudgeana is the most acquisitive of them, this means that this species invests more in growing fast, by producing larger leaves that are richer in nitrogen than carbon (less structural tissue) (Díaz et al., 2016) and their size vary greatly with environmental conditions. F. umbellata was the most conservative, this species invests in leaves that are more resistant (more carbon), with long lifespan, meaning they are more resistant to desiccation and can resist drought periods (Rodríguez-Alarcón et al., 2022). E. interstincta exhibited an intermediate strategy, tending to be more conservative than N. rudgeana, with the lowest SLA. This species also invests in having photosynthetic parts that are more resilient, being also resistant to desiccation (Albuquerque et al., 2020).
The variation found in this study agrees with what is expected for aquatic plants, especially the differences in strategies according with life form (Lacoul & Freedman, 2006; Pierce et al., 2012). However, the pattern found is slightly different from the global one: SLA was not inversely proportional to LDMC (Pierce et al. 2017; Fig. 2). This may be due to the overall low investment in tough tissue structure of aquatic plants (Poorter et al., 2009; Pierce et al., 2013; Pan et al., 2020), that, despite showing a variation among E. interstincta and F. umbellata (that invested in more resistant leaves with lower leaf area) and N. rudgeana (that invested more in high leaf area and less in tissue density), were still low in proportion if compared, for example, with terrestrial plants (Poorter et al., 2009; Pan et al., 2020). Additionally, it is suggested that macrophytes have distinct functional strategies to surpass the stressors present in freshwater ecosystems and inhabit them (Pan et al., 2020). Furthermore, another way to better understand this variation would be to assess other traits, such as leaf thickness and N and P content, as they would give insights in the plant’s investments to survive in a water-saturated environment (Pan et al., 2020). Also, there are few studies assessing the variation in traits of aquatic macrophytes and we emphasize that these are the initial results of environmental effects on the foliar traits of macrophytes in the Amazon, so in order to consolidate the comparison with a global pattern, it is necessary to investigate more species and more traits.
Intraspecific variation in response to environmental conditions
The intraspecific variation in E. interstincta is influenced by water conductivity, dissolved oxygen, and the amount of sediment Calcium + Magnesium. Similarly, in F. umbellata, conductivity and oxygen levels affect leaf traits. The positive relationship between dissolved oxygen and LDMC in plants can be attributed to the essential role of oxygen in plant metabolism and growth (Sousa & Sodek, 2002). When sediment lacks sufficient oxygen, microbial activity shifts, and certain substances become scarce (like nitrite, sulfide, iron, and manganese), potentially acting as phytotoxins and imposing stress on plants (Armstrong et al., 2006). This can reach a point where plant survival becomes compromised. Aquatic plants have evolved morpho-anatomical adaptations to overcome anoxic conditions prevalent in their habitats (Lemoine et al., 2012). For instance, E. interstincta, a rooted macrophyte, features long culms with segmented air cavities (Gil & Bove, 2007), while F. umbellata possesses aerenchyma in its roots and stems (Pott & Pott, 2000). These adaptations facilitate the transference of oxygen from well-oxygenated parts to hypoxic regions (e.g., from leaves to roots), enabling plants to endure anoxic conditions. In sites with higher oxygen availability, individuals can maintain metabolic processes, promote growth, and allocate more carbon toward building denser leaf tissues, resulting in higher LDMC. Conversely, conditions with limited oxygen pose challenges for plant survival, as they hinder vital physiological functions.
The changes observed in E. interstincta individuals, with an increase in LA and a decrease in LDMC corresponding to higher levels of sediment calcium and magnesium, and in F. umbellata individuals, with an increase in LA and a decrease in LDMC associated with sediment phosphorus concentrations, indicate a strategic shift in response to nutrient availability (Vance et al., 2003; Wright et al., 2004; Grassein et al., 2010). Magnesium is an element present in the chlorophyll molecule, being important to the photosynthetic activity of a plant, while calcium is important for plant growth (especially at cell levels) and phosphorus is a macronutrient required many fundamental processes of a plant’s metabolism (Grusak et al., 2016). Thus, when those nutrients are scarce, the individuals tend to exhibit a more conservative strategy, investing in producing long-lived leaves (higher LDMC) so they can retain these nutrients as much as possible and avoid losing them to the environment (Wright et al., 2004; Reich, 2014). In contrast, when these elements are abundant in the system, the individuals invest in growing faster to be more competitive (higher LA and lower LDMC), exhibiting a more acquisitive strategy (Wright et al., 2004; Reich, 2014).
Water conductivity is related with the concentrations of ions in water, which is also related with nutrient availability, like nitrogen (Lacoul & Freedman, 2006). However, some of those ions (such as ammonia and ammonium) can be harmful for plants (Kinsman-Costello et al., 2015; Esteban et al., 2016). For instance, the diversity of macrophyte can either increase (Rolon & Maltchik, 2006) or decrease with water conductivity (Murphy et al., 2003). We believe the positive relationship between E. interstincta and F. umbellata’s individual LDMC and the negative relationship with LA may be indicative of resistance to a minor ammonia stress. A possible cause for this increase in nitrogen-based compounds may be the presence of agricultural and livestock activities in the surrounding areas, which leads us to believe that this phenomenon occurs due to anthropogenic disturbance on the sites (Obi et al., 2016).
Moreover, contrary to our hypothesis, the variation in the functional traits of individuals of N. rudgeana were affected by variables more related with the structure of freshwater ecosystems: water depth and the amount of clay. Nympheids have an intrinsic relationship with water depth and sediment due to their life form (they are rooted but their leaves float in the water surface) and are very sensitive to water-level fluctuation, as they can quickly elongate their petioles to keep their leaves above water (Richards et al., 2012; Dalla Vecchia & Bolpagni, 2022).
Dalla Vecchia and Bolpagni (2022) found that the leaf area and petiole area of Nuphar lutea (L.) Sm. (Nymphaeaceae) increases with water depth, which they associated with the cost in investing in building the petiole: the plant is rewarded with a higher photosynthetic surface. Our findings, of a negative relationship between SLA and water depth and a positive relationship with LDMC, may indicate a trade-off in investment on petiole mass vs leaf area (Li et al., 2008). In sites with increased water depth, the individuals tend to invest in petiole length, to increase height, in order to reach the water surface (and then be able to photosynthesize), in detriment of having a high photosynthetic surface (Titus & Sullivan, 2001; Richards et al., 2012). An alternative hypothesis is that the individuals in deeper sites invest in having a higher number of leaves that are smaller in area, to increase overall photosynthetic surface while avoiding losing them completely in case the plant gets fragmented or cut (Richards et al., 2012). In addition, individuals in deeper sites invest more in structural tissue (both in leaves and petioles), so the leaves can support the stress caused by the increased water level (e.g., trampling and tidals) and not perish easily under such conditions (Titus & Sullivan, 2001; Dalla Vecchia & Bolpagni, 2022).
Furthermore, the negative relationship between N. rudgeana SLA and the amount of clay and the positive relationship between LDMC and clay are indicative of the importance of the sediment type to the establishment and growth of individuals of this species. Since the aquatic environment is prone to disturbance caused by currents (flooding, trampling, tidals), sediments that are good for root anchorage, such as the ones rich in clay, are advantageous to the establishment of some macrophyte life forms (such as submersed, emergent, and floating leaved species) (Schwarz et al., 2015; De Wilde et al., 2017). Additionally, sediments rich in clay retain more water and elements, including nutrients for plants, which can be good for the growth of rooted macrophytes (De Wilde et al., 2017). Thus, in sites with more clay, the individuals invest in more tissue density, while in sites with less clay, the plants invested in increasing their SLA.
Intraspecific variation in CSR strategies
Our analysis regarding the variation in CSR strategies among individuals showed that there was no variation in the ecological strategies of E. interstincta and F. umbellata, who remained stress tolerators, but N. rudgeana varied slightly from stress tolerator to competitive strategy. This result partially corroborated our hypothesis, as F. umbellata and E. interstincta followed the global pattern for the Cyperaceae family (closer to the stress tolerator axis) (Pierce et al., 2017; Albuquerque et al., 2020). However, we expected some individuals of N. rudgeana to exhibit a more ruderal strategy, as most macrophytes are expected to be ruderals (Pierce et al., 2012; Albuquerque et al., 2020), due to the characteristics of the aquatic environment to be more prone to constant disturbance (e.g., flooding, trampling, and drought periods). Indeed, Albuquerque et al. (2020), in a study performed in temporary pools in the Brazilian semi-arid region (Caatinga), found that E. interstincta exhibited a ruderal strategy, while several species from the Nymphaea genus exhibited a R/CR strategy, which they concluded that the communities’ strategies were structured by regional disturbance (e.g., changes in evapotranspiration and precipitation) which is quite contrasting from our results. Furthermore, the variation in individuals of N. rudgeana from stress tolerators to competitors (S to S/CS strategy) may be to the high diversity of macrophytes found in some sites. In those conditions, where resource availability is not a limiting factor, these individuals invest in increasing their biomass to acquire more resources and outgrow other plants. Species from the Nymphaeaceae family are known to be more competitive for resources in the environments they inhabit, which reflects in their traits: investment in high leaf area, moderate relative growth rate, limited vegetative dispersal, and seeds that sink immediately (Pott et al., 2011; Pierce et al., 2012). Moreover, some individuals may exhibit a more stress-tolerant strategy in an environment where some resources are limited, but they are also excellent competitors where resources are more abundant and species diversity is high. Therefore, the pattern observed in this study aligns more closely with the patterns found in tropical forests than with other macrophyte communities worldwide. However, it is important to note that Lacoul & Freedman (2006) classified macrophyte species inhabiting infertile, acidic, alkaline, and saline habitats as stress tolerators, suggesting that they exhibit a different ecological strategy. Our results indicate that individuals of the same species can modify their ecological strategies to thrive in diverse environments, highlighting the phenotypic plasticity observed in macrophytes.
Conclusion
This study is the first one assessing the intraspecific variation of aquatic plant CSR strategies, revealing intraspecific variation in functional traits and ecological strategies among the studied species, despite the local scale. Nutrient availability influenced the functional traits of E. interstincta and F. umbellata, while their ecological strategies remained relatively conservative as stress tolerators. However, it was observed that the strategies exhibited by these species in the study area differed from those found in another biome (ruderal), suggesting potential intraspecific variation at a regional or global scale. Those species in our study exhibited a strategy that align more with the niche requirements of tropical forests, such as the Amazon.
In contrast, N. rudgeana demonstrated intraspecific variation along the resource availability gradient, transitioning from stress tolerators to competitors. This species exhibited high sensitivity to environmental changes and a highly acquisitive strategy, even at a local level, but certain individuals adopted a more conservative strategy when resources were limited. The findings highlight the adaptability and phenotypic plasticity of aquatic plants at both the species and individual levels. Future research should incorporate other macrophyte life forms (such as submerged and free-floating) and additional traits (e.g., petiole length, mass and area, leaf thickness, leaf phosphorus and nitrogen content, and root traits) to further comprehend their strategies, life history, and responses to environmental changes driven by climate and land-use shifts, which pose ongoing challenges to biological communities.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
References
Akasaka, M., N. Takamura, H. Mitsuhashi & Y. Kadono, 2010. Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshwater Biology 55: 909–922. https://doi.org/10.1111/j.1365-2427.2009.02334.x.
Albuquerque, A. C., C. A. D. S. Rodrigues-Filho & L. Q. Matias, 2020. Influence of climatic variables on CSR strategies of aquatic plants in a semiarid region. Hydrobiologia 847: 61–74. https://doi.org/10.1007/s10750-019-04072-5.
Alves, K. N. L., C. F. Hall, L. J. C. Schneider, C. S. Nunes, J. F. Maciel-Silva, A. S. B. Gil, & A. J. Fernandes-Júnior, 2023. Fuirena. Flora e Funga do Brasil, Jardim Botânico do Rio de Janeiro. , https://floradobrasil.jbrj.gov.br/FB7210.
Anderson, M. J., R. N. Gorley, & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E Ltd, Plymouth.
Aoki, C., M. C. Teixeira-Gamarra, R. M. Gamarra, S. C. H. de Medeiros, V. J. Pott, G. A. Damasceno-Junior, A. Pott & E. Scremin-Dias, 2017. Abiotic factors drive the structure of aquatic plant assemblages in riverine habitats of the Brazilian “Pantanal.” Revista Brasileira De Botanica 40: 405–415. https://doi.org/10.1002/ecs2.1930.
Armstrong, J., R. E. Jones & W. Armstrong, 2006. Rhizome phyllosphere oxygenation in Phragmites and other species in relation to redox potential, convective gas flow, submergence and aeration pathways. New Phytologist 172: 719–731. https://doi.org/10.1111/j.1469-8137.2006.01878.x.
Barrett, S. C. H., C. G. Eckert & B. C. Husband, 1993. Evolutionary processes in aquatic plant populations. Aquatic Botany 44: 105–145. https://doi.org/10.1016/0304-3770(93)90068-8.
Bornette, G. & S. Puijalon, 2011. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences 73: 1–14. https://doi.org/10.1007/s00027-010-0162-7.
Cadotte, M. W. & C. M. Tucker, 2017. Should environmental filtering be abandoned? Trends in Ecology and Evolution 32: 429–437. https://doi.org/10.1016/j.tree.2017.03.004.
da Costa, H. D. J. A., E. S. C. Gurgel, D. D. do Amaral, L. V. Vasconcelos, L. G. B. Rebelo & G. S. Teodoro, 2020. CSR ecological strategies, functional traits and trade-offs of woody species in Amazon sandplain forest. Flora 273: 151710. https://doi.org/10.1016/j.flora.2020.151710.
Dalla Vecchia, A. & R. Bolpagni, 2022. The importance of being petioled: leaf traits and resource-use strategies in Nuphar lutea. Hydrobiologia 849: 3801–3812. https://doi.org/10.1007/s10750-022-04803-1.
de Sousa, C. A. F. & L. Sodek, 2002. The metabolic response of plants to oxygen deficiency. Brazilian Journal of Plant Physiology 14: 83–94. https://doi.org/10.1590/S1677-04202002000200002.
De Wilde, M., S. Puijalon & G. Bornette, 2017. Sediment type rules the response of aquatic plant communities to dewatering in wetlands. Journal of Vegetation Science 28: 172–183. https://doi.org/10.1111/jvs.12473.
Des Roches, S., D. M. Post, N. E. Turley, J. K. Bailey, A. P. Hendry, M. T. Kinnison, J. A. Schweitzer & E. P. Palkovacs, 2018. The ecological importance of intraspecific variation. Nature Ecology & Evolution Springer, US 2: 57–64. https://doi.org/10.1038/s41559-017-0402-5.
Díaz, S., J. Kattge, J. H. C. Cornelissen, I. J. Wright, S. Lavorel, S. Dray, et al., 2016. The global spectrum of plant form and function. Nature Nature Publishing Group 529: 167–171. https://doi.org/10.1038/nature16489.
Donovan, L. A., H. Maherali, C. M. Caruso, H. Huber & H. de Kroon, 2011. The evolution of the worldwide leaf economics spectrum. Trends in Ecology & Evolution 26: 88–95. https://doi.org/10.1016/j.tree.2010.11.011.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, & H. H. Wagner, 2022. adespatial: Multivariate multiscale spatial analysis. R package version 0.3–16. https://cran.r-project.org/package=adespatial.
Estarague, A., F. Vasseur, K. Sartori, C. C. Bastias, D. Cornet, L. Rouan, G. Beurier, M. Exposito-Alonso, S. Herbette, J. Bresson, D. Vile & C. Violle, 2022. Into the range: a latitudinal gradient or a center-margins differentiation of ecological strategies in Arabidopsis thaliana. Annals of Botany 129: 343–356. https://doi.org/10.1093/aob/mcab149.
Esteban, R., I. Ariz, C. Cruz & J. F. Moran, 2016. Review: mechanisms of ammonium toxicity and the quest for tolerance. Plant Science 248: 92–101. https://doi.org/10.1016/j.plantsci.2016.04.008.
Figueiredo, F. O. G., G. Zuquim, H. Tuomisto, G. M. Moulatlet, H. Balslev & F. R. C. Costa, 2018. Beyond climate control on species range: The importance of soil data to predict distribution of Amazonian plant species. Journal of Biogeography 45: 190–200. https://doi.org/10.1111/jbi.13104.
Fu, H., J. Guo, X. He, Y. Chen, Z. Wu, Y. Ge & G. Cai, 2023. Individual traits modify environmental effects on interaction, connectivity, and productivity of macrophyte community. Hydrobiologia. https://doi.org/10.1007/s10750-023-05185-8.
Gao, X., H. Liu, G. Liu, W. Huang & W. Xing, 2023. How functional traits of submerged macrophytes response to underwater light quality? Hydrobiologia. https://doi.org/10.1007/s10750-023-05142-5.
Gil, A. D. S. B. & C. P. Bove, 2007. Eleocharis R.Br. (Cyperaceae) no Estado do Rio de Janeiro, Brasil. Biota Neotropica 7: 163–193. https://doi.org/10.1590/S1676-06032007000100020.
Gotelli, N. J. & A. M. Ellison, 2012. A Primer of ecological statistics, Oxford University Press, Oxford:
Grassein, F., I. Till-Bottraud & S. Lavorel, 2010. Plant resource-use strategies: The importance of phenotypic plasticity in response to a productivity gradient for two subalpine species. Annals of Botany 106: 637–645. https://doi.org/10.1093/aob/mcq154.
Grime, J. P., 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist 111: 1169–1194. https://doi.org/10.1086/283244.
Grime, J. P., 2002. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. Journal of Ecology 86: 5. https://doi.org/10.1046/j.1365-2745.1998.00306.x.
Grime, J. P. & S. Pierce, 2012. The evolutionary strategies that shape ecosystems, Wiley, New Jersey:
Grusak, M. A., M. R. Broadley & P. J. White, 2016. Plant macro- and micronutrient minerals eLS, Wiley, Newyork:, 1–6. https://doi.org/10.1093/aob/mcq154.
Hamilton, N. E. & M. Ferry, 2018. ggtern: ternary diagrams using ggplot2. Journal of Statistical Software, Code Snippets 87: 1–17. https://doi.org/10.1002/etc.2801.
Harrell Jr, F. E., 2023. Hmisc: Harrell Miscellaneous. https://CRAN.R-project.org/package=Hmisc.
Kinsman-Costello, L. E., J. M. O’Brien & S. K. Hamilton, 2015. Natural stressors in uncontaminated sediments of shallow freshwaters: The prevalence of sulfide, ammonia, and reduced iron. Environmental Toxicology and Chemistry 34: 467–479. https://doi.org/10.1002/etc.2801.
Lacoul, P. & B. Freedman, 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews 14: 89–136. https://doi.org/10.1139/a06-001.
Lemoine, D. G., F. Mermillod-Blondin, M. H. Barrat-Segretain, C. Massé & E. Malet, 2012. The ability of aquatic macrophytes to increase root porosity and radial oxygen loss determines their resistance to sediment anoxia. Aquatic Ecology 46: 191–200. https://doi.org/10.1007/s10452-012-9391-2.
Li, G., D. Yang & S. Sun, 2008. Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude. Functional Ecology 22: 557–564. https://doi.org/10.1111/j.1365-2435.2008.01407.x.
Lorenzi, H., 2008. Plantas daninhas do Brasil: terrestres, aquática, parasitas e toxicas. Instituto Plantarum, Nova Odessa.
May, R. L., S. Warner & A. Wingler, 2017. Classification of intra-specific variation in plant functional strategies reveals adaptation to climate. Annals of Botany 119: 1343–1352. https://doi.org/10.1093/aob/mcx031.
Martinez Arbizu, P., 2017. pairwiseAdonis: Pairwise Multilevel Comparison using Adonis.
Moreira, A. D. R. & C. P. Bove, 2017. Flora do Rio de Janeiro: Nymphaeaceae. Rodriguesia Instituto De Pesquisas Jardim Botanico Do Rio De Janeiro 68: 91–97.
Morgan, M., 2022. BiocManager: Access the Bioconductor Project Package Repository. https://CRAN.R-project.org/package=BiocManager.
Murphy, K. J., G. Dickinson, S. M. Thomaz, L. M. Bini, K. Dick, K. Greaves, M. P. Kennedy, S. Livingstone, H. McFerran, J. M. Milne, J. Oldroyd & R. A. Wingfield, 2003. Aquatic plant communities and predictors of diversity in a sub-tropical river floodplain: The upper Rio Paraná, Brazil. Aquatic Botany 77: 257–276. https://doi.org/10.1016/S0304-3770(03)00108-6.
Nicotra, A. B., O. K. Atkin, S. P. Bonser, A. M. Davidson, E. J. Finnegan, U. Mathesius, P. Poot, M. D. Purugganan, C. L. Richards, F. Valladares & M. van Kleunen, 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. https://doi.org/10.1016/j.tplants.2010.09.008.
Nunes, C. S., J. F. Maciel-Silva, R. Trevisan, & A. S. B. Gil, 2023. Eleocharis. Flora e Funga do Brasil, Jardim Botânico do Rio de Janeiro, https://floradobrasil.jbrj.gov.br/FB17192.
Obi, F., B. Ugwuishiwu & J. Nwakaire, 2016. Agricultural waste concept, generation, utilization and management. Nigerian Journal of Technology African 35: 957. https://doi.org/10.4314/njt.v35i4.34.
Oksanen, J., G. L. Simpson, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, et al., 2022. vegan: community ecology package. https://CRAN.R-project.org/package=vegan.
Pan, Y., E. Cieraad, J. Armstrong, W. Armstrong, B. R. Clarkson, T. D. Colmer, O. Pedersen, E. J. W. Visser, L. A. C. J. Voesenek & P. M. van Bodegom, 2020. Global patterns of the leaf economics spectrum in wetlands. Nature Communications 11: 4519. https://doi.org/10.1038/s41467-020-18354-3.
Pellegrini, M. O. O., 2023. Nymphaeaceae. Flora e Funga do Brasil, Jardim Botânico do Rio de Janeiro. https://floradobrasil.jbrj.gov.br/FB10941.
Pérez-Harguindeguy, N., S. Díaz, E. Garnier, S. Lavorel, H. Poorter, P. Jaureguiberry, et al., 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. https://doi.org/10.1071/BT12225.
Pierce, S., G. Brusa, M. Sartori & B. E. L. Cerabolini, 2012. Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Annals of Botany 109: 1047–1053. https://doi.org/10.1093/aob/mcs021.
Pierce, S., G. Brusa, I. Vagge & B. E. L. Cerabolini, 2013. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Functional Ecology 27: 1002–1010. https://doi.org/10.1111/1365-2435.12095.
Pierce, S., D. Negreiros, B. E. L. Cerabolini, J. Kattge, S. Díaz, M. Kleyer, et al., 2017. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31: 444–457. https://doi.org/10.1111/1365-2435.12722.
Pinto, A., P. Amaral, C. Souza-Jr, A. Veríssimo, R. Salomão, G. Gomes, & C. Balieiro, 2009. Diagnóstico Socioeconômico e Florestal do Município de Paragominas. Instituto do Homem e Meio Ambiente da Amazônia - Imazon. Belém.
Poorter, H., Ü. Niinemets, L. Poorter, I. J. Wright & R. Villar, 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x.
Pott, V. J., A. Pott, L. C. P. Lima, S. N. Moreira & A. K. M. Oliveira, 2011. Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Brazilian Journal of Biology 71: 255–263. https://doi.org/10.1590/s1519-69842011000200004.
Pott, V. J., & A. Pott, 2000. Plantas Aquáticas do Pantanal. Embrapa Comunicação para Transferência de Tecnologia, Brasília.
R Core Team, 2022. R: a language and environment for statistical computing. Vienna, Austria, https://www.R-project.org/.
Reich, P. B., 2014. The world-wide “fast-slow” plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. https://doi.org/10.1111/1365-2745.12211.
Richards, J. H., D. N. Kuhn & K. Bishop, 2012. Interrelationships of petiolar air canal architecture, water depth, and convective air flow in Nymphaea odorata (Nymphaeaceae). American Journal of Botany 99: 1903–1909. https://doi.org/10.3732/ajb.1200269.
Ríos-Villamizar, E. A., M. T. F. Piedade, J. G. Da Costa, J. M. Adeney & W. J. Junk, 2013. Chemistry of different Amazonian water types for river classification: a preliminary review. WIT Transactions on Ecology and the Environment 178: 17–28. https://doi.org/10.2495/WS130021.
Rodríguez-Alarcón, S., R. Tamme & C. P. Carmona, 2022. Intraspecific trait changes in response to drought lead to trait convergence between—but not within—species. Functional Ecology 36: 1900–1911. https://doi.org/10.1111/1365-2435.14099.
Rolon, A. S. & L. Maltchik, 2006. Environmental factors as predictors of aquatic macrophyte richness and composition in wetlands of southern Brazil. Hydrobiologia 556: 221–231. https://doi.org/10.1007/s10750-005-1364-1.
Schwarz, C., T. J. Bouma, L. Q. Zhang, S. Temmerman, T. Ysebaert & P. M. J. Herman, 2015. Interactions between plant traits and sediment characteristics influencing species establishment and scale-dependent feedbacks in salt marsh ecosystems. Geomorphology 250: 298–307. https://doi.org/10.1016/j.geomorph.2015.09.013.
Siefert, A., C. Violle, L. Chalmandrier, C. H. Albert, A. Taudiere, A. Fajardo, et al., 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18: 1406–1419. https://doi.org/10.1111/ele.12508.
Sultan, S. E., 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. https://doi.org/10.1016/S1360-1385(00)01797-0.
Teixeira, P. C., G. K. Donagemma, A. Fontana, & W. G. Teixeira, 2017. Manual de métodos de análise de solo. Embrapa, Brasília.
Titus, J. E. & P. G. Sullivan, 2001. Heterophylly in the yellow waterlily, Nuphar variegata (Nymphaeaceae): effects of [CO2], natural sediment type, and water depth. American Journal of Botany 88: 1469–1478. https://doi.org/10.2307/3558455.
Vance, C. P., C. Uhde-Stone & D. L. Allan, 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x.
Vasseur, F., K. Sartori, E. Baron, F. Fort, E. Kazakou, J. Segrestin, E. Garnier, D. Vile & C. Violle, 2018. Climate as a driver of adaptive variations in ecological strategies in Arabidopsis thaliana. Annals of Botany 122: 935–945. https://doi.org/10.1093/aob/mcy165.
Violle, C., M.-L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional! Oikos 116: 882–892. https://doi.org/10.1111/j.2007.0030-1299.15559.x.
Violle, C., B. J. Enquist, B. J. McGill, L. Jiang, C. H. Albert, C. Hulshof, V. Jung & J. Messier, 2012. The return of the variance: Intraspecific variability in community ecology. Trends in Ecology and Evolution. 27: 244–252. https://doi.org/10.1016/j.tree.2011.11.014.
Weiner, J., 2004. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics 6: 207–215. https://doi.org/10.1078/1433-8319-00083.
Wickham, H., 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag New York, http://ggplot2.org.
Wright, I. J., P. B. Reich, M. Westoby, D. D. Ackerly, Z. Baruch, F. Bongers, et al., 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. https://doi.org/10.1038/nature02403.
Yu, H., M. Chin, T. Yuan, H. Bian, L. A. Remer, J. M. Prospero, A. Omar, D. Winker, Y. Yang, Y. Zhang, Z. Zhang & C. Zhao, 2015. The fertilizing role of African dust in the Amazon rainforest: a first multiyear assessment based on data from Cloud-Aerosol Lidar and infrared pathfinder satellite observations. Geophysical Research Letters 42: 1984–1991. https://doi.org/10.1002/2015GL063040.
Acknowledgements
We are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Bolsas FUNBIO conservando o Futuro (Project number 0092020), Hydro Paragominas Company, Brazilian Research Consortium and L’Oréal Brasil, UNESCO and ABC for scholarship and all financial support. We thank Viviane Firmino for encouraging us to submit our paper to this issue. Special thanks to group “Girls can do Science”: Rayssa Silva do Carmo, Francieli Fátima Bomfim, and Fabiane Barral Sampaio for helping with sample and measure of the plants in the field, Flávia Ribeiro and Jéssica Damasceno for the help in measuring the plants in the laboratory, and Grazielle Sales Teodoro for discussing the results with us. We also thank Mayra N. Barral Neves and Rosiane Araújo at Hydro Paragominas for the support in the field and the two anonymous reviewers that read, made comments, and improve our work.
Funding
The research leading to these results received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (processes: 433125/2018-7), Brazilian Biodiversity Fund (Bolsas FUNBIO Conservando o Futuro) by the project “Functional response of aquatic macrophytes to water stress conditions caused by climate change: evidences of intraspecific variability and trade-offs” (Project number 0092020), Hydro Paragominas Company, Brazilian Research Consortium (this paper is number BRC0063 in the publication series) by the project “Effects of soil use on diversity and ecophysiology on the riparian vegetation, aquatic macrophytes, and plankton in streams and lagoons in mining areas of Paragominas, Pará, Brazil” and Award For Women in Science Brazil 2021—L’Oréal Brasil, UNESCO and Academia Brasileira de Ciências.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material collection and preparation were performed by both authors, analyses were performed by ALB Fares. The first draft of the manuscript was written by ALB Fares, and both authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Rossano Bolpagni, Lars Lønsmann Iversen, Mattia Martin Azzella & Andreas Hussner / A Unified Understanding of Macrophyte Ecology and Adaptations: Plant Functional Traits and Trait-based Approaches.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fares, A.L.B., Michelan, T.S. Intraspecific variation in leaf traits and evolutionary plant strategies of three macrophytes across an environmental gradient in Eastern Amazon. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05593-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05593-4