Abstract
Beta diversity is the variability in species composition among sampling units for a given area and can be influenced by several environmental drivers, including environmental heterogeneity. Here, we considered the contribution of seven tributaries flowing into the Upper Paraná River channel as the mains drivers of environmental heterogeneity and zooplankton beta diversity. We used Mantel test to examine the relationships between zooplankton functional beta diversity components (total, turnover, and nestedness) and environmental and geographical distance. Generalized dissimilarity modeling (GDM) was run to test which environmental variables were the best predictors of beta diversity components. Mantel’s test results revealed that total beta diversity was positively related with environmental heterogeneity in almost all periods. GDM analysis results showed that total beta and turnover were related to temperature, organic suspended matter, dissolved oxygen, NH4, and pH, while nestedness was influenced by depth and geographic distances. Our results support the idea that smaller rivers are a main source of diversity for large rivers, especially the ones with cascading reservoirs. Overall, our study shoes that variation in limnological variables results in higher dissimilarity in zooplankton communities and that environmental change filters and sorts species according to their functional traits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species diversity is a multifaceted concept that can be decomposed into γ (regional species richness), α (local species richness), and β components (Whittaker, 1972). Beta diversity is defined by Anderson et al. (2006) as the variability in species composition among sampling units for a given area, which can be partitioned into two components: turnover and nestedness. The turnover component reflects changes in species identities (or relative abundances) between sites, whereas nestedness reflects to what extent the species-poor sites contain a proper subset of the species-rich sites (Baselga & Orme, 2012; Villéger et al., 2013; Legendre, 2014). Complementary to taxonomic variability, which considers all species as functionally equivalent (Swenson et al., 2011; Villéger et al., 2013), functional variability considers species functional traits, i.e., morphological, physiological, and ecological characteristics directly linked to ecosystem functions (Hébert et al., 2017).
Beta diversity may be influenced by several environmental drivers, including connectivity, environmental heterogeneity, and seasonality (Lopes et al., 2014; Heino et al., 2015; Gianuca et al., 2017). Furthermore, differences in the contribution of nestedness and turnover to total beta diversity can reflect metacommunity ecological processes such as mass effects and species sorting (Heino et al., 2015; Gianuca et al., 2017). Environmental heterogeneity is defined as the variability in abiotic conditions among localities within a region (Anderson et al., 2006; Heino et al., 2015). More heterogeneous regions usually support a greater number of functionally distinct species (Veech & Crist, 2007; Bozelli et al., 2015; Soares et al., 2015). This is observed because the conditions of each site within a region allow the presence of a particular set of species, with environmental filtering thus producing differences in composition among sites (Leibold et al., 2004; Astorga et al., 2014; Bini et al., 2014; Lansac-Tôha et al., 2019).
However, environmental heterogeneity and beta diversity patterns across space are strongly dependent on the size of the sampling unit (‘grain’) and the size of the region encompassing all sampling units (‘extent’, i.e., stream, basin, and ecoregion) (Wiens, 1989; Barton et al., 2013; Heino et al., 2015). The increase in grain and spatial extent leads to an increase in environmental gradients, which also increase the strength of environmental filtering (i.e., species sorting) causing variation on species and functional traits composition (Heino, 2011; Heino et al., 2015). High dispersal rates (i.e., mass effects) are also scale dependent and under large spatial extent their effects may lose strength (Gianuca et al., 2017). Thus, it is expected that mass effects act more strongly on beta diversity at small spatial extents and species sorting at large spatial extents. Species sorting linked to high environmental heterogeneity would especially change species functional traits causing functional turnover (Mouchet et al., 2010), whereas functional nestedness is expected to be observed in spatial isolation (i.e., low strength of mass effect) (Henriques-Silva et al., 2013; Bender et al., 2016; Gianuca et al., 2017). However, nestedness may also be produced by environmental constraints (Henriques-Silva et al., 2013) and local extinctions (Matthews et al., 2015), a topic that may need further investigation (Henriques-Silva et al., 2013).
Large rivers display great environmental variability (Heino et al., 2004; Siqueira et al., 2009) that result in differences in community composition among different sites in a same river (Brown & Swan, 2010). Such systems provide good models for testing spatial patterns in beta diversity (Heino et al., 2013). The Paraná River has a large and long channel that receives several tributaries along its course. These tributaries contribute organic and inorganic matter, as well as propagules and individuals of several biological communities (Bomfim et al., 2017). The natural flow of the Paraná River has been altered by the numerous dams that have been constructed along its course, which have altered water flow, physical–chemical characteristics, and biological communities, finally changing its trophic status to meso-oligotrophic (Roberto et al., 2009; Bomfim et al., 2017). The fragmentation of rivers by dams are a critical threat to freshwater biodiversity (Winemiller et al., 2016; Zhang et al., 2019), causing impacts that range from reductions in water flow and nutrient availability to changes in species interactions and composition (Winemiller et al., 2016; Zhao et al., 2017; Bruno et al., 2019). Such impacts can cause spatial and temporal environmental, species, and trait homogenization (Martínez et al., 2013; Braghin et al., 2018; Bomfim et al., 2021), in addition to local reorganization of communities when species are selected from the regional pool to co-occur locally (Wang et al., 2021). This affects the entire river ecosystem function. Nevertheless, the tributaries that flow into the Paraná River’s main channel are a way of recovering from the dam effects. These smaller rivers can also be an important source of environmental variability along the main channel, as they have distinct natural history, physical–chemical characteristics, and biological identity (Roberto et al., 2009; Bomfim et al., 2017).
The zooplankton community, which includes Rotifera, Cladocera, and Copepoda, plays an important role in ecosystem functioning (Litchman et al., 2013; Dias et al., 2014) linking primary producers to higher trophic levels and acting in the energy flow (Allan, 1976). Zooplankton became an important tool to access the link between environmental heterogeneity and beta diversity patterns (Gianuca et al., 2017; Braghin et al., 2018; Bomfim et al., 2021). Also, studies tracking changes in zooplankton functional traits can help us to understand ecosystem processes, such as productivity and nutrient cycling (Diaz & Cabido, 2001). Therefore, partitioning functional beta diversity into its components (replacement and nestedness) and associating such patterns to environmental and metacommunity drivers will provide additional understanding as to how these mechanisms shape biodiversity patterns across spatial scales and affect ecosystem functioning (Nogueira et al., 2010; Ewers et al. 2013).
Here, we considered the contribution of tributaries to the environmental variability of the Upper Paraná River main channel. We sampled zooplankton along the river channel, after the entrance of each of its tributaries, and tested whether environmental heterogeneity and/or spatial distances between sites drove zooplankton functional beta diversity and its nestedness and turnover components along a 230-km stretch of the Upper the Paraná River. We tested these relationships in four moments along the year. As each tributary brings its own biotic and abiotic contributions to the main river, we expected (1) the seven distinct tributaries flowing into Paraná River along its course to create meaningful changes in biotic and abiotic characteristics leading to different degrees of environmental heterogeneity and functional beta diversity; (2) high environmental heterogeneity among sampled sites to induce high total functional beta diversity; and (3) functional turnover to be explained by environmental heterogeneity due to species sorting created by environmental heterogeneity. As the river flow is constantly changing biological communities along the river channel and as tributaries are the main source of variation to this system, we expected (4) the spatial distances between sites to have no importance in determining beta diversity or its components. Finally, we investigated which where the limnological variables most associated to changes in total functional beta diversity and functional nestedness and turnover.
Materials and methods
Study area
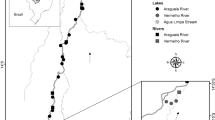
This study was carried out in the Upper Paraná River floodplain (22° 37′–23° 55′ S e 53° 06′–54° 80′ W), located between Porto Primavera reservoir (São Paulo state) and the Itaipu reservoir (Paraná state) (Fig. 1). The Paraná River is the main river of this basin, it is the tenth largest in the world in relation to discharge (5, 0.108 m3 year−1) and the fourth in drainage area (2, 8.106 km2) (Petri & Fulfaro, 1983). This area has great ecological importance due to its high biodiversity, as well as a high economic and social importance and is thus protected by three conservation units (Protected Area of the Islands and Várzeas of the Paraná River, National Park of Ilha Grande, and the State Park of Ivinhema). This floodplain is also characterized by diverse aquatic environments with high heterogeneity (backwaters, connected, and isolated lakes, rivers, and channels) and is under influence of seven tributaries, the rivers Baia, Paranapanema, Ivaí, Ivinheima, Amambai, Iguatemi, and Piquiri (Fig. 1) (Thomaz et al., 2007; Agostinho et al., 2004). Due to these characteristics the preservation and understanding of these complex environments are indispensable.
Sampling and laboratory analysis
Ten sampling points were selected along a transect, in the Paraná River (Fig. 1). Samplings of the zooplankton community occurred quarterly, in August and November of 2013 and February and May of 2014, for a total of four samplings dates, carried out from point 10 to point 1, against the flow of the river to avoid spatial autocorrelation. Samples were collected on the center, also right and left margins of the Parana River (three samplings per point, which provided 120 samples overall). As the river is an environment with high flow velocity, the transect collection was chosen in order to obtain more representative samples of each point. Sampling sites in the Paraná River were always located upstream of the mouths of the main tributaries. On the left of the river the main tributaries are the rivers Paranapanema, Ivaí, and Piquiri and on the right, Baia, Iguatemi, Ivinheima, and Amambaí.
The zooplanktonic community was sampled at the sub-surface of the pelagic region in each site. Using a motorized pump and plankton net (68 µm mesh) and by filtering 600 l of water per sample. The collected material was kept in flasks with formalin 4% tamponed with calcium carbonate. The identification of Rotifera, Cladocera, and Copepoda species was based on specialized literature (Koste, 1978; Reid, 1985; Matsumura-Tundisi, 1986; Segers, 1995; Elmoor-Loureiro, 1997, 2010; Lansac-Tôha et al., 2002), and the specific richness of each sample was analyzed until the stabilization curve of the species increment. These results were used to estimate the composition of the community in each sampled environment and period.
Environmental characterization
For the analysis of environmental heterogeneity, the data from abiotic variables were collected simultaneously with zooplankton sampling with portable digital devices. These included measures of depth (m), water temperature (°C), pH, ammonium (μg l−1), phosphate (μg l−1), conductivity (mg l−1), turbidity (NTU), inorganic (mg l−1), and organic suspended matter (mg L−1), all following the methodology described by Roberto et al. (2009). All values collected from the abiotic variables are presented in Supplementary Material 1.
Functional characterization of the species
In total, 166 species were found (103 Rotifera, 44 Cladocera, and 19 Copepoda) and functionally characterized (Supplementary Material 2). The information of each functional characteristic of the species was obtained from studies carried out in or near the floodplain of the Upper Paraná River (Paggi, 1978; Bonecker et al., 1998; Lansac-Tôha et al., 2002; Barnett et al., 2007; Perbiche-Neves et al., 2015; Braghin et al., 2018). For the functional characterization of the zooplankton community, six functional traits were used: habitat, feeding type, mean body length, reproduction, lifespan, and predatory escape response. These traits indicate the influence on ecosystem processes and the response of organisms to environmental conditions (Barnett et al., 2007). These traits were selected within the specified types and categories (Table 1).
The habitat trait was considered the place where species are found most frequently, in the distribution and occurrence of the species, being classified in pelagic or littoral. Pelagic species live in the water column and have adaptations to stay in this location, for example, body shape and higher surface volume ratio. Littoral species are considered to be those associated with some type of substrate, such as macrophytes. Each category contributes efficiently to the transfer of energy in the region where it is found.
The feeding type represents a wide diversity of behavioral, physiological, morphological, and lifespan characteristics of organisms (Litchman et al., 2013). This trait indicates the ability to acquire food, the efficiency of the feeding, and the type of food the organisms ingest. Differences in these characteristics affect in different ways secondary productivity and nutrient cycling via difference in efficiency and filtration rate, for example (Andersen & Hessen, 1991).
The mean body length can influence in the energy transfer, secondary production (Litchman et al., 2013), and the amount of energy allocated by these organisms (Hébert et al., 2016), with bigger organisms being able to allocate more energy than the smaller ones. Therefore, species with larger size, in general, present higher productivity, reflecting on the secondary productivity (Dias et al., 2014). The reproduction trait indicates, mainly, the investment in reproduction and energy consumed, but also show environmental condition that will influence on the type of reproduction of the species (Villéger et al., 2017). Sexually reproducing species will contribute more to the secondary productivity of environments, and asexual reproducer, in turn, can contribute more to the cycling of nutrients by the rapid introduction of new organisms in the community (Braghin et al., 2018). Reproduction also influences the morphology, behavior, quality, and concentration of food (Melão, 1999).
Lifespan represents the duration of the organism’s life cycle and also influences colonization processes. Thus, organisms with a short life cycle colonize the environment more quickly and contribute to the constant entry of energy and new organisms into the environment. Rotifers and cladocerans have short cycle, obtaining a greater number of generations in a same amount of time (Allan, 1976). Copepods, on the other hand, have two stages of development, therefore, they are classified with long life cycle (Dodson & Brooks 1965). However, long life cycles contribute more to the secondary production of the environment (Dias et al., 2014). The predatory escape response, considered as the ability of species to escape from predator attack, can affect body size and shape (Dodson & Brooks, 1965) and influence the swimming agility and visibility of organisms (Allan, 1976). Thus, rotifers were classified as low response to predator and cladocerans with medium response. Among the copepods, the Cyclopoids present a big response and the Calanoid the maximum response (Braghin et al., 2018).
Data analysis
Beta diversity indexes were computed for each month sampled individually. In order to determine the relative contribution of turnover and nestedness to overall functional beta diversity, the additive partitioning of the pairwise Jaccard dissimilarity proposed by Baselga (2010), Baselga & Orme (2012) and Villéger et al. (2013) was used. Total functional beta diversity (FD-total) was decomposed into two components: turnover (FD-turn) and nestedness (FD-nest). Before calculating the functional pairwise dissimilarities, a functional space was first constructed with a PCoA (Villéger et al., 2008), using the Gower distance between species, which is suitable for mixed data (in this case a trait matrix with numerical and categorical data) (Gower, 1966). As some species had zero occurrence in a particular sampling time, we excluded then from both taxonomic and trait data. From the volume of multidimensional space occupied by the species, we calculated the functional beta diversity. Because the number of species in each site must be strictly higher than number of dimensions, we used the first three PCoA axes.
In order to eliminate multicollinearity among the environmental variables, we calculated Spearman rank correlations to identify those pairs of variables that were too highly correlated. We considered that two variables were highly correlated for a pairwise Spearman correlation coefficient of more than 0.7. At all sampling times, we found inorganic and organic suspended matter highly correlated and the first was excluded. In contrast, all other eight variables described above were weakly correlated and therefore kept for further statistical analyses.
We used the Mantel test to examine for each sampled month the relationships between functional beta diversity components (FD-total, FD-turn, and FD-nest) and the following explanatory matrices: environmental (standardized Euclidean distance) and geographical distance (Euclidean distance). A significant and positive correlation indicates that the patterns of functional dissimilarities among sites are coherent with environmental variation and/or geographical distances. We also performed a partial Mantel test to verify the importance of environmental heterogeneity while controlling the spatial effect. In all cases, 10,000 random permutations were used to test if the concordances between distance matrices differed from the random relationships and ensure the stability of the probability estimates (Jackson & Somers, 1989).
Generalized dissimilarity modeling (GDM, Ferrier et al., 2007) was run to test which environmental variables were the best predictors of beta diversities and their partitioning components, and how much beta diversity was explained by geographical distances among sites in each sampled month. The GDM examines the beta diversity among pairs of sites as a non-linear multivariate function of the environmental variables of these sites. It can overcome two major problems: non-linearity in beta diversity among sites and environmental dissimilarities and uneven rates of beta diversity along environmental gradients (Ferrier et al., 2007; Fitzpatrick et al., 2013). GDM uses flexible splines (constrained to be positively monotonic, Ferrier et al., 2007) instead of parametric transformations of the variables. In this case, the functional dissimilarity matrices (FD-total, FD-turn, and FD-nest) were used as response variables and as inter-site distances of predictor variables based on values of the environmental variables, as well as geographical distance between sites.
All computations implemented in this study were performed with the R software (R Core Team, 2021) and in particular with ‘functional.beta.pair’ function of the ‘Betapart’ package (Baselga & Orme, 2012). Mantel and partial Mantel tests were performed using ‘mantel’ function of the ‘Ecodist’ package. GDMs were performed using ‘GDM’ package (Ferrier et al., 2007).
Results
Total beta diversity (FD-total) was similar in all sampled months, with a higher contribution of the nestedness component that was maintained through time (Fig. 2). According to the Mantel test, FD-total was positively related to environmental heterogeneity (EH) in all periods except for February 2014. In the first 2 months that were sampled, results of the partial mantel test revealed a relationship between FD-total and EH independent of the geographical distance. Only in the first sampled month, August 2013, did geographical distance relate to FD-total (Table 2). Functional turnover (FD-turn) showed a positive relationship with EH in two sampled months (August 2013 and May 2014), and according to the partial mantel showed a relationship to EH independent of space in August 2013 (Table 2). Functional nestedness (FD-nest) was positively related to EH (also when the spatial effect was removed in the partial Mantel) only in November 2013 (Table 2). The Mantel tests revealed no significant relationship between geographical distance and the two components of functional beta diversity (Table 2).
The GDM analysis showed significant relationships between functional beta diversity and environmental variables in the same months and for the same beta components as did the Mantel tests (Table 3). According to these significant models, different variables were most important in explaining total beta diversity and its components in each month. In general, FD-total (for which models were significant in August 2013 and February and May 2014) was mainly related to changes in temperature, organic suspension material (MSO), dissolved oxygen (DO), and NH4. FD-turn (significant models in August 2013 and May 2014) was mainly related to changes in the same variables as FD-total, while FD-nest (only one significant model, in November 2013) was mainly influenced by depth and NH4 (Fig. 3).
Discussion
The results of our analysis on the relationship between environmental heterogeneity and functional beta diversity of zooplankton along the longitudinal axis of the Upper Paraná River support the hypothesis that the contribution of the tributaries is important to the maintenance of biotic and abiotic variation along the longitudinal axis of this main river. We found that functional beta diversity of zooplankton was driven by environmental heterogeneity along the Upper Paraná river, even though this relationship was not present in all sampled months. Moreover, evidencing the effect of species sorting in the sampled sites, environmental heterogeneity explained variation in functional turnover in half of the sampled months. However, we found unexpected associations between heterogeneity and functional nestedness and between the spatial distribution of the sampled sites and total beta diversity, each in one of the sampled months. The GDMs were significant only in the same months (and for the same components) as the tests for environmental heterogeneity, indicating that the effect of environmental heterogeneity on beta diversity was linked to the spatial variation of particular variables. Furthermore, we found that the importance of each variable differed between the sampled months.
Total functional beta diversity of zooplankton showed similar levels in all the sampled months, and nestedness was its most important component in all months. The high contribution of nestedness is not common for taxonomic beta diversity, but is an expected result when evaluating functional trait composition because even if there is a turnover in species composition, most species usually share similar functional traits, which produces a lower functional turnover (Braghin et al., 2018; Coccia et al., 2021). Even if beta diversity, nestedness and turnover levels were maintained over time, the importance of environmental heterogeneity in explaining these diversity aspects varied between months, as did the identity of the particular variables found to influence beta diversity and its components. This temporal variation in the importance of the environmental heterogeneity and of particular variables for beta diversity may be linked to the dynamics innate to lotic systems. Rivers are flowing waterbodies that may present changing limnological conditions due to changes that occur in their respective drainage basins (Vannote et al., 1980). Seasonal variations occur, e.g., in the water level, and flood pulses are known to homogenize biotic and abiotic factors in such systems (Thomaz et al., 2007; Bozelli et al., 2015). This could be related to the absence of a relationship between heterogeneity (and particular environmental variables) and beta diversity in February 2014, when the water level of the Paraná was higher.
The main environmental variables to have affected beta diversity were dissolved oxygen, NH4, depth, temperature, and suspended organic material. These variables influence beta diversity because they are related to seasonality and food availability, which will favor the development of populations in time and space. However, as lotic environments are very dynamic, due to the constant flow of water and rapid cycling of organic matter, a synergy between these effects is often observed. Therefore, food availability is related to seasonal variation in nutrients, depth, temperature, and dissolved oxygen. Furthermore, the entry of tributaries influences the spatial dynamics of the main channel (Vannote et al., 1980), interfering with a synergy between the effects. Long-term ecological studies in the upper Paraná River floodplain (20 years) have shown a clear synergy between the amount of available nutrients, seasonal variation in water volume, and connectivity between environments as a structuring factor for the zooplankton community (Bonecker et al., 2020).
Although we found an overall effect of environmental heterogeneity and particular variables in beta diversity and its components, we found that environmental heterogeneity was to a certain extent spatially structured in our first sampled month (August 2013). This was the only signal of influence of the geographical variable in beta diversity or its components in our results, and it only occurred for total beta diversity in this month. Nevertheless, even if the Mantel test showed a (weak) spatial effect, the geographic coordinates were not important to explain diversity according to the GDM. Thus, considering the strong effect of the environmental variables on beta diversity, our results still support the idea that the tributaries are the main source of variation to this river system.
The positive relationship between environmental heterogeneity and beta diversity has been widely recognized in different systems and for different biological groups (Alahuhta et al., 2017; Liu et al., 2018; López-Delgado et al., 2020). Maintaining high levels of composition dissimilarity among assemblages is a way of maintaining regional diversity. In such context, exploring the environmental heterogeneity–beta diversity relationship informs on mechanisms that maintain regional diversity in a highly impacted ecosystem (Socolar et al., 2016). Here, we have explored the environmental heterogeneity produced by tributaries of a large and highly dammed Neotropical river and its effects in the main river’s functional beta diversity. The construction of dams in the Paraná River, both upstream and downstream of the floodplain, has deeply impacted its ecosystem, causing the loss of local species and traits and favoring the occurrence of already common ones (Baeten et al., 2012). Such changes caused species and trait homogenization in the habitats linked to the Paraná River (Braghin et al., 2018). Our results show that the environmental heterogeneity introduced by tributaries in this main river may in part counteract the effects of the reservoir cascade, supporting higher spatial dissimilarity in zooplankton functional traits along the longitudinal axis of the river. As zooplankton communities are the main secondary producers in the water column, being essential supporters of higher trophic levels in the aquatic food chain (Allan, 1976), supporting their functional diversity is also supporting this essential ecosystem function.
We recognize two caveats in our study. From an ecological viewpoint, we recognize that, although our results clearly showed the crucial role of tributary-induced spatial heterogeneity in mitigating the effects of anthropogenic activity on the ecosystem dynamics, more information about zooplankton community in each of the tributaries would be necessary for a deeper understanding of what is the contribution of each of them to the main river. In addition, from a statistical viewpoint, we used Mantel and GDM analyse to verify the relationship between biological and environmental variables. Both analyses are appropriate to perform and popular among ecologists for this propose. However, critiques have been addressed to both of them regarding their power to detect effects, which can be lower than that of other techniques (Legendre et al., 2015, Peres-Neto & Jackson, 2011). Still, considering how conservative these analyses may be, this does not invalidate our results.
Our results support the idea that smaller rivers may be a main source of biotic and abiotic diversity for large rivers with cascading reservoirs. We show that tributaries contribute to the Upper Paraná River, providing variation in limnological variables to this main river that leads to higher dissimilarity in the zooplankton communities along its channel. Trait turnover was the beta diversity component that depended most on environmental heterogeneity, evidencing that environmental variables in each tributary act as filters and sort species according to their functional traits. The species sorted in each small river are then exported to the Paraná River, contributing to its overall diversity. The weak effect of the spatial proximity on the river channel’s beta diversity also supports the tributaries as main sources of zooplankton species. We conclude that dammed rivers depend on their non-dammed tributaries to maintain their diversity. These smaller affluents are the keepers of the diversity that can still be found in such deeply altered ecosystems.
Data availability
All data generated or analyzed during this study are included in this article.
Code availability
Not applicable.
References
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2004. Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Ecohydrology and Hydrobiology 4: 255–256.
Alahuhta, J., S. Kosten, M. Akasaka, et al., 2017. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. Journal of Biogeography 44: 1758–1769.
Allan, J. D., 1976. The University of Chicago Life History Patterns in Zooplankton. The University of Chicago Press for the American Society of Naturalists 110: 165–180.
Andersen, T. & D. O. Hessen, 1991. Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnology and Oceanography 36: 807–814.
Anderson, M. J., K. E. Ellingsen & B. H. Mcardle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
Astorga, A., R. Death, F. Death, R. Paavola, M. Chakraborty & T. Muotka, 2014. Habitat heterogeneity drives the geographical distribution of beta diversity: the case of New Zealand stream invertebrates. Ecology and Evolution 4: 2693–2702.
Baeten, L., P. Vagansbeke, M. Hermy, G. Petrken, K. Vanhuyse & K. Verheyen, 2012. Distinguishing between turnover and nestedness in the quantification of biotic homogenization. Biodiversity and Conservation 21: 1399–1409.
Barnett, A. J., K. Finlay & B. E. Beisner, 2007. Functional diversity of crustacean zooplankton communities: Towards a trait-based classification. Freshwater Biology 52: 796–813.
Barton, P. S., S. A. Cunningham, A. D. Manning, H. Gigg, D. B. Lindenmayer & R. K. Didham, 2013. The spatial scaling of beta diversity. Global Ecology and Biogeography 22: 639–647.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A. & C. D. L. Orme, 2012. Betapart: an R package for the study of beta diversity. Methods in Ecology and Evolution 3: 808–812.
Bender, M. G., F. Leprieur, D. Mouillot, M. Kulbicki, V. Parravicini, M. R. Pie, D. R. Barneche, L. G. R. Oliveira-Santos & S. R. Floeter, 2016. Isolation drives taxonomic and functional nestedness in tropical reef fish faunas. Ecography 40: 425–435.
Bini, L. M., V. L. Landeiro, A. A. Padial, T. Siqueira & J. Heino, 2014. Nutrient enrichment is related to two facets of beta diversity for stream invertebrates across the United States. Ecology 95: 1569–1578.
Bomfim, F. F., T. Mantovano, D. C. Amaral, W. S. Palhiarini, C. C. Bonecker & F. A. Lansac-Tôha, 2017. Adjacent environments contribute to the increase of zooplankton species in a neotropical river. Acta Limnologica Brasiliensia (online) 29: 1–103.
Bomfim, F. F., F. M. Lansac-Tôha, C. C. Bonecker & F. A. Lansac-Tôha, 2021. Determinants of zooplankton functional dissimilarity during years of El Niño and La Niña in floodplain shallow lakes. Aquatic Sciences 83: 41.
Bonecker, C. C., F. A. Lansac-Tôha & D. C. Rossa, 1998. Planktonic and non-planktonic rotifers in two environments of the Upper Parana River floodplain, State of Mate Grosso do Sul, Brazil. Brazilian Archives of Biology and Technology 41: 447–456.
Bonecker, C. C., L. P. Diniz, L. S. M. Braghin, T. Mantovano, J. V. F. Silva, F. F. Bomfim, D. A. Moi, S. Deosti, G. N. T. Santos, D. A. Candeias, A. J. M. M. Mota, L. F. M. Velho & F. A. Lansac-Tôha, 2020. Synergistic effects of natural and anthropogenic impacts on zooplankton diversity in a subtropical floodplain: a long-term study. Oecologia Australis 24: 524–537.
Bozelli, R. L., S. M. Thomaz, A. A. Padial, P. M. Lopes & L. M. Bini, 2015. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753: 233–241.
Braghin, L. S. M., B. A. Almeida, D. C. Amaral, T. F. Canella, B. C. G. Garcia & C. C. Bonecker, 2018. Effects of dams decrease zooplankton functional b-diversity in river-associated lakes. Freshwater Biology 63: 721–730.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
Bruno, D., O. Belmar, A. Maire, A. Morel, B. Dumont & T. Datry, 2019. Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Global Change Biology 25: 1612–1628.
Coccia, C., B. A. Almeida, A. J. Green, A. B. Gutiérrez & J. A. Carbonell, 2021. Functional diversity of macroinvertebrates as a tool to evaluate wetland restoration. Journal of Applied Ecology 58: 2999.
Dias, J. D., C. C. Bonecker & M. R. Miracle, 2014. The rotifer community and its functional role in lakes of a neotropical floodplain. International Review of Hydrobiology 99: 72–83.
Diaz, S. & M. Cabido, 2001. Vive la difference: plant functional diversity matters to ecosystem processes: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution 16: 646–655.
Dodson, S. I. & J. L. Brooks, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Elmoor-Loureiro, L. M. A., 1997. Manual de identificação de cladóceros límnicos do. Brasil Universa, Brasília.
Elmoor-Loureiro, L. M. A., 2010. Cladóceros do Brasil: Famílias Chydoridae e Eurycercidae. Disponível em: http://cladocera.wordpress.com. Acesso em 19 de setembro 2017.
Ewers, R. M., R. K. Didham, W. D. Pearse, V. Lefebvre, I. M. D. Rosa, J. M. B. Carreiras, R. M. Lucas & D. C. Reuman, 2013. Using landscape history to predict biodiversity patterns in fragmented landscapes. Ecology Letters 16(10): 1221–1233.
Ferrier, S., G. Manion, J. Elith & K. Richardson, 2007. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Diversity and Distributions 13: 252–264.
Fitzpatrick, S., G. Bramley & S. Johnsen, 2013. Pathways into multiple exclusion homelessness in seven UK cities. Urban Studies 50(1): 148–168.
Gianuca, A. T., S. A. J. Declerck, P. Lemmens & L. De Meester, 2017. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology 98(2): 525–533.
Gower, J. C., 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53: 325–338.
Hébert, M. P., B. E. Beisner & R. A. Maranger, 2016. Meta-analysis of zooplankton functional traits influencing ecosystem function. Ecology 97: 1069–1080.
Hébert, M. P., B. E. Beisner & R. Maranger, 2017. Linking zooplankton communities to ecosystem functioning: toward an effect-Trait framework. Journal of Plankton Research 39: 3–12.
Heino, J., 2011. A macroecological perspective of diversity patterns in the freshwater realm. Freshwater Biology 56: 1703–1722.
Heino, J., P. Louhi & T. Muotka, 2004. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49: 1230–1239.
Heino, J., M. Grönroos, J. Ilmonen, et al., 2013. Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate spatial scales. Freshwater Science 32: 142–154.
Heino, J., A. S. Melo & L. M. Bini, 2015. Reconceptualising the beta diversity–environmental heterogeneity relationship in running water systems. Freshwater Biology 60: 223–235.
Henriques-Silva, R., Z. Lindo & P. R. Peres-Neto, 2013. A community of metacommunities: exploring patterns in species distributions across large geographical areas. Ecology 94: 627–639.
Jackson, D. A. & K. M. Somers, 1989. Are probability estimates from the permutation model of Mantel’s test stable? Canadian Journal of Zoology 67(3): 766–769.
Koste, W., 1978. Rotatoria die Rädertiere Mitteleuropas begründet von Max Voight. Monogononta, Vol. I: 673. Vol. II: 474. Gebrüder Borntraeger, Berlin.
Lansac-Tôha, F. A., L. F. M. Velho, J. Higuti & E. M. Takahashi, 2002. Cyclopidae (Crustacea, Copepoda) from the Upper Paraná River Floodplain, Brazil. Revista Brasileira de Biologia = Brazilian Journal of Biology 62: 125−133.
Lansac-Tôha, F. M., J. Heino, B. A. Quirino, G. A. Moresco, O. Peláez, B. R. Meira, L. C. Rodrigues, S. Jati, F. A. Lansac-Tôha & L. F. M. Velho, 2019. Differently dispersing organismo groups show contrasting beta diversity patterns in a dammed subtropical river basin. Science of the Total Environment 691: 1271–1281.
Legendre, P., 2014. Replacement and richness difference components. Global Ecology and Biogeography 23: 1324–1334.
Legendre, P., M. J. Fortin & D. Borcard, 2015. Should the Mantel test be used in spatial analysis? Methods in Ecology and Evolution 6(11): 1239–1247.
Leibold, M. A., M. Holyoak, N. Mouquet &, et al., 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Litchman, E., M. D. Ohman & T. Kiørboe, 2013. Trait-based approaches to zooplankton communities. Journal of Plankton Research 35: 473–484.
Liu, J., M. Vellend, Z. Wang & M. Yu, 2018. High beta diversity among small islands is due to environmental heterogeneity rather than ecological drift. Journal of Biogeography 45(10): 2252–2261.
Lopes, P. M., L. M. Bini, S. A. J. Declerck, et al., 2014. Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE 9(10): e109581.
López-Delgado, E. O., K. O. Winemiller & F. A. Villa-Navarro, 2020. Local environmental factors influence beta-diversity patterns of tropical fish assemblages more than spatial factors. Ecology 101(2): e02940.
Martínez, A., A. Larranaga, A. Basaguren, J. Petez, C. Mendoza-lera & J. Pozo, 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
Matsumura-Tundisi, T., 1986. Latitudinal distribution of Calanoida copepods in freshwater aquatic systems of Brazil. Revista Brasileira de Biologia = Brazilian Journal of Biology 46: 527−553.
Matthews, T. J., C. Sheard, H. E. W. Cottee-Jones, T. P. Bregman, J. A. Tobias & R. J. Whittaker, 2015. Ecological traits reveal functional nestedness of bird communities in habitat islands: a global survey. Oikos 124: 817–826.
Melão, M., 1999. Desenvolvimento e aspectos reprodutivos de Cladóceros e Copépodos de águas continentais brasileiras. In: Pompêo M. (Ed.) Perspectivas da Limnologia no Brasil. Gráfica e Editora União, São Luís: 45−57.
Mouchet, M. A., S. Villéger, N. W. Mason & D. Mouillot, 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24: 867–876.
Nogueira, I. S., J. C. Nabout, M. S. R. Ibanez & L. M. Bourgoin, 2010. Determinants of beta diversity: the relative importance of environmental and spatial processes in structuring phytoplankton communities in an Amazonian floodplain. Acta Limnologica Brasiliensia 22: 247–256.
Paggi, S. J., 1978. Introduccion al estúdio de lós rotíferos. Revista De La Asociación Ciencias Naturales Del Litoral 9: 19–49.
Perbiche-Neves, G., G. A. Boxshall, D. Previattelli, M. G. Nogueira & C. E. F. Rocha, 2015. Identification guide to some Diaptomid species (Crustacea, Copepoda, Calanoida, Diaptomidae) of “de la Plata” River Basin (South America). ZooKeys 111: 1–111.
Peres-Neto, P. R. & D. A. Jackson, 2001. How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia 129(2): 169–178.
Petri, S. & V. J. Fulfaro, 1983. Geologia da Chapada dos Parecis, Mato Grosso, Brasil. Revista Brasileira De Geociências 7: 274–282.
R Core Team, 2021. R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna.
Reid, J. W., 1985. Chave de identificação e lista de referências bibliograficas para as espécies continentais sulamericanas de vida livre da ordem Cyclopoida (Crustacea, Copepoda). Boletim De Zoologia 9: 17–143.
Roberto, M. C., N. N. Santana & S. M. Thomaz, 2009. Limnology in the Upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Brazilian Journal of Biology 69: 717–725.
Segers, H., 1995. Rotifera: the Lecanidae (Monogonta). Guides to the identification of the micro invertebrates of the continental water of the world, Vol. 6. SPB Academic, The Hague: 226.
Siqueira, T., L. M. Bini, M. C. Cianciaruso, F. O. Roque & S. Trivinho-Strixino, 2009. The role of niche measures in explaining the abundance–distribution relationship in tropical lotic chironomids. Hydrobiologia 636: 163–172.
Soares, C. E. A., L. F. M. Velho, F. A. Lansac-Tôha, C. C. Bonecker, V. L. Landeiro & L. M. Bini, 2015. The likely effects of river impoundment on beta-diversity of a floodplain zooplankton metacommunity. Natureza & Conservação 13: 74–79.
Socolar, J. B., J. J. Gilroy, W. E. Kunin & D. P. Edwards, 2016. How should beta-diversity inform biodiversity conservation? Trends in Ecology & Evolution 31: 67–80.
Swenson, J. J., C. E. Carter, J. C. Domec & C. I. Delgado, 2011. Gold Mining in the Peruvian Amazon: Global Prices, Deforestation, and Mercury Imports. PLoS ONE 6(4): e18875.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Veech, J. A. & T. O. Crist, 2007. Habitat and climate heterogeneity maintain beta-diversity of birds among landscapes within ecoregions. Global Ecology and Biogeography 16: 650–656.
Villéger, S., N. W. H. Mason & D. Mouillot, 2008. New multidimensional functional diversity indices for a multifaced framework in functional ecology. Ecology 89: 2290–2301.
Villéger, S., G. Grenouillet & S. Brosse, 2013. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Global Ecology and Biogeography 22: 671–681.
Villéger, S., S. Brosse, M. Mouchet, D. Mouillot & M. J. Vanni, 2017. Functional ecology of fish: current approaches and future challenges. Aquatic Sciences 79: 783–801.
Wang, J., C. Z. Ding, J. Tao, X. M. Jiang, J. Heino, L. Y. Ding, W. Su, M. L. Chen, K. Zhang & D. M. He, 2021. Damming affects riverine macroinvertebrate metacommunity dynamics: insights from taxonomic and functional beta diversity. Science of the Total Environment 763: 142945.
Whittaker, R. H., 1972. Evolution and measurement of species diversity. Taxon 21: 213–251.
Wiens, J. A., 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Winemiller, K. O., P. B. Mcintyre, L. Castello &, et al., 2016. Balancing hydropower and biodiversity in the Amazon. Congo and Mekong. Science 351(6269): 128–129.
Zhang, Y., C. R. Peng, S. Huang, J. Wang, X. Xiong & D. H. Li, 2019. The relative role of spatial and environmental processes on seasonal variations of phytoplankton beta diversity along different anthropogenic disturbances of subtropical rivers in China. Environmental Science and Pollution Research 26: 1422–1434.
Zhao, K., K. Song, Y. D. Pan, L. Z. Wang, L. J. Da & Q. X. Wang, 2017. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecological Indicators 73: 96–104.
Acknowledgements
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for granting a scholarships for the authors. Nupelia/UEM, CORIPA, and ICMBio for providing access to infrastructure and sampling facilities.
Funding
This work was supported by the Universal CNPq Project “Upper Paraná River: Longitudinal gradient of environmental variables and aquatic communities in the last non-dammed stretch (between Porto Primavera and Itaipu reservoirs)” (Process 478629/2012-5).
Author information
Authors and Affiliations
Contributions
Conceptualization: (BIOR, FALT, and CCB). Methodology, data collection, and laboratory analysis: (BIOR and FdFB); species identification: (BIOR and LdSMB); statistical analyses: (FMLT, FdFB, and BdAA). Writing and original draft preparation: (BIOR); writing, reviewing, and editing of the manuscript: (LdSMB, FMLT, FdFB, BdAA, CCB, and FALT); funding acquisition: (FALT); and supervision: (FALT and CCB). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: Bernadette Pinel-Alloul
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ribeiro, B.I.O., Braghin, L.d.S.M., Lansac-Tôha, F.M. et al. Environmental heterogeneity increases dissimilarity in zooplankton functional traits along a large Neotropical river. Hydrobiologia 849, 3135–3147 (2022). https://doi.org/10.1007/s10750-022-04917-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04917-6