Abstract
The phytoplankton community structure is potentially influenced by both environmental and spatial processes. In addition, the relative importance of these two processes to phytoplankton assemblage will be affected by hydrological connectivity. However, the impacts of anthropogenic activities on phytoplankton beta diversity and the relative importance of these two processes to phytoplankton are still poorly understood, especially in water conservation areas. Here, we examined the relative importance of local and regional environmental control and spatial structuring of phytoplankton communities in five rivers with different degrees of disturbance during wet and dry seasons. We found that community structure and local environmental conditions varied greatly in seasons and rivers. The reference river (with minimum disturbance) had the highest homogeneity of environmental conditions and phytoplankton assemblage, while the excessive disturbance rivers (sand mining activities) had the greatest environmental heterogeneity and species dissimilarity between sites. Variation partitioning analysis showed that the phytoplankton community variation was mainly explained by the spatial variables in the wet season (summer and autumn) and winter, while the local environmental variables explained the largest variation of phytoplankton community in the dry season (spring). However, broad-scale variables were selected by redundancy analysis in both dry and wet seasons, which indicates that long-distance scales always have low river connectivity, regardless of whether the river is overflowing or drying up. Local environmental processes explained the most variation in phytoplankton community within all of the rivers, suggesting that deterministic processes usually work on relatively small spatial scales. However, this effect would be weakened by anthropogenic activities, especially sand mining activities. We inferred that sand mining activities increased the environmental heterogeneity and species dissimilarity between sites by causing watercourse habitat patches and obstructing river connectivity. On the other hand, as the excessive disturbance, sand mining activities significantly reduced the species richness and abundance of phytoplankton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological diversity can be classified into three levels: alpha, beta, and gamma (Magurran 2004). The beta diversity reflects the spatial or temporal differences in community structure (Soininen 2010). It is an important concept for understanding the functioning of ecosystems and for the conservation of biodiversity (Nogueira et al. 2010). At present, ecologists are not only concerned on the beta diversity itself but also on interpreting the causes for beta diversity. Biogeography and community ecology are two disciplines that combine stochastic, dispersal processes and deterministic, environmental filtering as determinants of beta diversity (Heino et al. 2017). The relative importance of spatial and local and regional environmental processes in determining beta diversity has been evaluated in various metacommunities, but assessing their relative significance and understanding these perspectives have been a long-standing challenge in ecological studies (Heino et al. 2015b), because their relative roles on structuring communities depend primarily on species functional traits, local and regional environmental dynamics, and hydrological and general ecosystem properties (spatial scales, geographical gradients, geographical location, dispersal routes, and patch history) (Devercelli et al. 2016; Niño-García et al. 2016). Generally, deterministic processes decrease in importance at very broad spatial extents, as dispersal limitation precludes species from tracking environmental variation, while stochastic processes likely to be more effective at very broad spatial scale, as increasing spatial scale should lead to increasing dispersal limitation and spatially structured variation in community structure (Hájek et al. 2011).
Phytoplankton constitutes the base of aquatic food webs. Unlike other larger aquatic organisms, small algae are constantly undergoing rapid temporal and spatial changes (Yang et al. 2017b). Phytoplankton is sensitive to the variation of environmental conditions; thus, it may be susceptible to environmental filtering. Because of the planktonic lifestyle, it has a strong ability of dispersal, but it should depend on the connectivity of the watercourse. Therefore, hydrological changes across temporal scales play a key role in the phytoplankton dispersal processes. In the wet season, phytoplankton has higher dispersal ability due to the better connectivity within watercourse, and the mass effects may occur with species flooding into unfavorable sites; on the contrary, in the dry season, the watercourse is highly disconnected and the dispersal limitation will shape communities, that is, species do not fully track preferred conditions (Heino et al. 2015b). Therefore, the relative importance of spatial and environmental processes in structuring phytoplankton composition or beta diversity is also often linked to the temporal scale (flood regime). In riverine environments, there is no general consensus on how phytoplankton is assembled given that studies have currently provided divergent and equivocal evidence (Isabwe et al. 2018).
Human activities along rivers cause substantial hydrological alterations that affect riverine community structure and function (Yang et al. 2017a). Agricultural and industrial activities and domestic sewage change the water quality through nutrient and pollutant inputs, which further affects the species composition and abundance of phytoplankton. Sand mining practices are widely adopted in developing countries to meet the demand of sand for infrastructure construction. Concern about the impacts of sand dredging on environments and metacommunities is increasingly reported in China (Wu et al. 2007; Lai et al. 2014). Extraction of sand not only leads to bank erosion, riverbed degradation, and buffer zone encroachment but also causes serious ecological problems of river water, such as turbidity increase, diversion of water flow, weakened riverine connectivity and interaction, and habitat loss and fragmentation, which ultimately disrupt the integrity of riverine ecosystem (Isabwe et al. 2018). The increasing turbidity reduces the utilization of light energy for algae and may directly decrease the abundance and number of species of phytoplankton. The broken riverine ecosystem will obstruct river connectivity and limit species dispersal, which may affect the importance of spatial processes in structuring metacommunities. The enhanced site isolation will cause large differences of environmental conditions and species composition between sites. Sand mining reduces the variety and abundance of benthic species has been reported by a number of authors (Boyd et al. 2005; Meng et al. 2018); however, its effects on phytoplankton community structure are poorly understood. Understanding the key ecological processes that govern phytoplankton community assembly in rivers under human pressure is clearly important for sustainable watershed management.
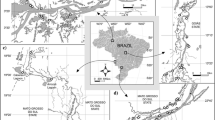
Our study area consists of five tributaries, all of which are located in the upper reaches of the Hanjiang River. The Hanjiang River is the largest tributary of the Yangtze River and the water source of the middle route of South-to-North Water Diversion Project (SNWDP) in China. The SNWDP was designed to divert water from rivers in South China to North China and alleviate the severe shortage of water resources in North China. The planned area of this project involves 438 million people. The middle route of SNWDP has a total length of 1432 km, and it extracts 9.5 × 109 m3 water a year from the Danjiangkou Reservoir in the middle reaches of the Hanjiang River. Therefore, the water quality of this important protected area has attracted more and more attention from many researchers. At present, many studies have focused on the spatiotemporal dynamics of phytoplankton and its driving environmental factors in the lower reaches of the Hanjiang River. However, few studies have been done on the factors that drive the variation of phytoplankton in the upper reaches of the Hanjiang River, especially those that combine spatial processes with anthropogenic activities.
In this study, we examined the relative importance of local and regional environmental control and spatial structuring of phytoplankton communities along five rivers with different degrees of disturbance across four seasons. We aimed to answer the questions: (1) whether the main community assembly processes underlying phytoplankton is different across contrasting hydrologic regimes (wet and dry seasons); (2) whether environmental and spatial processes play different roles within and among rivers; and (3) whether the different patterns and intensities of anthropogenic activities affect the relative importance of environmental and spatial processes in structuring phytoplankton communities. Based on previous studies, we hypothesized that deterministic processes determine the community structure in wet seasons and small spatial scales (within rivers), while stochastic processes play a more important role in dry seasons and large spatial scales (among rivers). Finally, we hypothesized that anthropogenic activities, especially sand mining, have a significant impact on the structure and beta diversity of phytoplankton communities.
Materials and methods
Study area
The five rivers in the study area all originated from the southern Qinling Mountains in Central China, and they are disturbed by human activities in different patterns and degrees. The Jinshui River (JSR) is located in a national nature reserve and is rarely disturbed by human activities, so it was served as a reference river in this study. The tributaries of Yue River (YR) are mainly affected by agricultural non-point source pollution, and the main stream is mainly affected by urban areas. The Qi River (QR) is seriously affected by agricultural activities, and the downstream is also affected by dams. The upper reaches of Si River (SR) are less disturbed, but the middle and lower reaches are polluted by severe domestic sewage and industrial wastewater. The whole watercourse of the Jinqian River (JQR) is affected by the sand mining activities. Sand mining activities began in 2007 and have become increasingly common throughout the river since 2013, causing serious ecological problems, but no measures have been taken to alleviate this situation. Furthermore, the upstream of JQR is also affected by domestic sewage pollution. The geographical coordinates, total length, and drainage area of the five rivers were listed in Table 1. The study area has a typical subtropical monsoon climate with obvious seasonal precipitation. The wet season is from May to October, and the heaviest rainfall occurs in July and August (summer) (Lu et al. 2014). According to historic data, we defined summer (July) and autumn (September to October) as wet seasons, and winter (December) and spring (April) were defined as dry seasons in this study.
Biological data
The data in this study were collected seasonally from July 2016 to April 2017. A total of 295 samples (85 in summer, 70 in autumn, 70 in winter, and 70 in spring) from the five rivers were collected. The sampling sites were shown in Fig. 1. The numbers of sampling sites in JSR, YR, JQR, QR, and SR were 16, 19, 22, 16, and 12, respectively. One liter of water sample (0–0.5 m) for cell counting and species identification of phytoplankton was preserved with Lugol’s solution and was concentrated to a final volume of 30 mL after 48 h of sedimentation. Algal abundance was counted at ×400 magnification under a light microscope using a counting chamber (0.1 mL) (at least 400 algal cells were counted and identified). In order to accurately identify diatom species, the algal cells were treated with sulfuric acid to remove organic matter. Cleaned frustule suspensions were settled onto microscopy glass slides and dried, and then the slides were mounted using Naphrax (refractive index, 1.703) and observed in light microscopy at ×1000 magnification (Stockner and Benson 1967).
Explanatory variables
Three types of explanatory variables were collected: environmental (local scale) variables, land-cover (regional scale) variables, and spatial variables. The environmental variables were getting from in situ measurement and laboratory analysis. Water temperature (WT), dissolved oxygen (DO), electric conductivity (EC), pH, and turbidity (TUB) were measured in situ using a multiparameter probe (YSI-2000, USA). Permanganate index (CODMn), chemical oxygen demand (CODCr), total phosphorus (TP), soluble reactive phosphorus (SRP), total nitrogen (TN), nitrate (NO3−), nitrite (NO2−), ammonium (NH4+), silicate (Si), sulfate (SO42−), sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), fluoride (F−), and chloride (Cl−) were determined following standard methods (APHA 1999).
Land-cover variables consisted of forestland, shrub and grassland, bare areas, agricultural areas, developed areas, water areas, and drawdown areas (Fig. A1 of Appendix 1). The sub-basin area for each sample site was delineated by ArcGIS 10.3 (Esri, Inc.) using Digital Elevation Models (DEM) with 30-m resolution which provided by the Chinese Academy of Sciences (http://www.cnic.cn/) for land-cover analysis. For each sample site, land-cover data used included available remote sensing images of Landsat images, Sentinel 2, and ASTER with 30-m resolution. The images were then interpreted and expressed as the percentage of above seven principal land-cover types (Liu 1996) using ENVI 5.3 (Exelis Visual Information Solutions, Inc.) within a sub-basin, and the map’s overall classification accuracy is more than 85% (Huang et al. 2016).
Spatial variables were derived using Principal Coordinates of Neighbour Matrix (PCNM) (Borcard and Legendre 2002; Borcard et al. 2004). The PCNM analysis creates a number of spatial variables based on the actual distances on the river networks. This is an eigenvector-based approach that allows for the modeling of spatial structures as predictor variables of variation in species abundance from broad to fine scales. The first eigenvectors represent broad-scale variation, whereas the ones with small eigenvalues represent finer-scale variation (Diniz-Filho and Bini 2005). Eigenfunction-based procedures allow an analysis at different spatial scales and are able to address complex patterns of spatial variation. Despite the problems encountered with the eigenvector techniques, for example, eigenvector methods can inflate the variation explained by a given causal process (Gilbert and Bennett 2010), they have a number of desirable aspects (Borcard et al. 2004), and have been used widely. In this study, only eigenvectors with positive eigenvalues were used as explanatory variables (Borcard et al. 2011). Separate PCNM analyses were run for each season and each river.
Statistical methods
Canonical analysis of principal coordinates (CAP) (Anderson and Robinson 2003, Anderson and Willis 2003) was used to test difference in community structure and environmental conditions among seasons and rivers. CAP is a variant of principal coordinate analysis (PCO), aiming to find axis through the multivariate cloud of points that are best at discriminating among a priori defined groups (Clarke and Warwick 2001). It allows a constrained ordination to be done on the basis of any distance or dissimilarity measure. In this study, we used Bray-Curtis coefficients for algal abundance data and Euclidean distances for standardized environmental data. The significant dissimilarities of communities and environmental conditions among seasons and rivers were tested by applying two-way Analysis of Similarity (ANOSIM) (Clarke 1993) based on permutation procedures with 999 runs.
We also used permutation tests for homogeneity of multivariate dispersion (PERMDISP) (Anderson 2006; Anderson et al. 2006) to examine the multivariate dispersions within seasons and rivers. In this study, species abundance data were used to measure the total beta diversity across seasons and rivers. The ANOVA F statistic was used to compare the differences among seasons and rivers in the distance from observations to their group centroid. Significant differences among groups were tested through permutation of least-squares residuals. The analysis was performed using Bray-Curtis coefficients for biological data and Euclidean distances on standardized environmental data. All tests were run using 999 permutations. CAP, ANOSIM, and PERMDISP were carried out with the software package Primer 6.0, including the addon package PERMANOVA.
Constrained ordination was used to analyze the relationship between phytoplankton community structure and environmental data (local scale variables), land-cover data (regional scale variables), and spatial variables. Redundancy Analysis (RDA) was used to select the significant driving factors related to phytoplankton community structure. Multicollinearity of variables was evaluated by variance inflation factors (VIF, variables were excluded with VIF > 20). Then, significant driving factors were selected by the forward selection with Monte Carlo permutations for further analysis. All biological abundance data and environmental data (except pH) were log (x + 1) transformed before analysis, and the land-use type data were transformed by inverse sine square root transformation. RDA was run using the “vegan” package (Oksanen et al. 2013) in the R environment (R version 3.4.3).
Variation partitioning analysis with partial redundancy analyses (pRDA) was conducted to estimate the fractions of phytoplankton community variation that could be explained solely by each type of explanatory variables and their shared variation. The variations were estimated using adjusted R2, which provides unbiased estimates of the explained variation (Peres-Neto et al. 2006). The contributions of the three explanatory variable groups at each season and each river were estimated. The variation partitioning was performed using the function varpart in the R package vegan (Oksanen et al. 2013). All these analyses were performed separately for each species matrices in the same way.
Results
The variation of phytoplankton community structure
A total of 166 phytoplankton taxa were identified. Bacillariophyceae (89) was the dominant class, followed by Chlorophyceae (47), and other classes had less number of species. One hundred fourteen phytoplankton taxa were found in both summer and autumn, followed by spring (95) and winter (71). The numbers of species, cell density, and dominance index (Y = ni/N × fi, where Y represents the dominance index, ni represents the abundance of species i, N represents the total abundance, fi represents the frequency of species i occurring at sampling sites) of dominant species in each river were listed in Table 2.
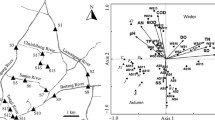
According to CAP and ANOSIM analysis, both of phytoplankton community structure and local environmental conditions differed significantly among seasons (R = 0.336, p = 0.001, Fig. 2a; R = 0.386, p = 0.001, Fig. 2c) and rivers (R = 0.354, p = 0.001, Fig. 2b; R = 0.446, p = 0.001, Fig. 2d). For environmental data, correct classification rates were higher than those for community data (Fig. 2). Summer and JSR had the highest percentage of correct classifications for seasons and rivers, respectively.
The PERMDISP analysis indicated that beta diversity had no significant difference among seasons with both combined data (Fig. 3a) and separate data (Fig. A2 of Appendix 4) but was significantly (p < 0.001) different among rivers (Fig. 3b). Beta diversity in JSR and QR was significantly lower (p < 0.01) than that of other three rivers. The rivers also differed significantly (p < 0.001) in terms of their environmental heterogeneity (Fig. 3d), and the relative environmental heterogeneity in each river was corresponded to phytoplankton beta diversity. The environmental heterogeneity had no significant difference among seasons with combined data (Fig. 3c), and only JQR showed significant difference of environmental heterogeneity among seasons with separate data (Fig. A2 of Appendix 4).
Boxplots based on tests of homogeneity of dispersion analysis representing mean distances from group centroids for phytoplankton community abundance data in seasons (a) and rivers (b) using Bray-Curtis dissimilarity and for local environmental data in seasons (c) and rivers (d) using standardized Euclidean distance. The x-axis abbreviations in (b) and (d) represent individual rivers
Through the PCNM analysis, 47, 40, 39, and 39 spatial variables showing positive eigenvalues were formed in summer, autumn, winter, and spring, respectively. But for rivers, few positive spatial variables were formed. The significant local variables, regional variables, and spatial variables included in the RDAs determined by the forward selections in different seasons and rivers were listed in Table 3.
For local variables, turbidity was selected in three seasons and all rivers made for the species matrices. The chemical oxygen demand, water temperature, and silicate were also selected with relatively high frequency. For regional variables, developed areas and drawdown areas were selected in all seasons for the species matrices. The spatial variables representing the broad-scale relations among the sites (PCNM1, 2, 3, 4) were more commonly selected than the finer-scale spatial variables in all seasons. For the analysis of rivers, few regional variables and spatial variables were selected.
Variation partitioning
Seasons
The local and regional environmental variables and the spatial variables all explained the phytoplankton community structure, and their importance varied from season to season (Fig. 4a–d). When examining the dependent variable groups separately, the results showed that spatial variables explained the largest proportion of phytoplankton variations in summer (9.4%), autumn (5.4%), and winter (6.4%), while local environmental variables explained the largest variations in spring (8.4%). The pure regional variables explained the least proportion of phytoplankton variations in all seasons. The shared fractions among all variables groups ranged from 3.4 to 6.2% across four seasons, and the largest proportion was in summer. The variations explained jointly by the different pairs of variable groups varied in different seasons. The shared fraction between local and regional variables was the largest in winter (3.8%) and the smallest in summer (0.6%). The shared fraction between local and spatial variables was the largest in winter (5.5%) and the smallest in spring (2.7%). The shared fraction between regional and spatial variables was small in all seasons and was not even detected in summer. The amount of unexplained variation was relatively large in all seasons, with similar residuals in summer, winter, and spring, and relative larger residual in autumn.
Venn diagrams showing the fractions of phytoplankton community structure explained by the local environmental variables (Local), regional environmental variables (Regional), and spatial variables (Spatial). All fractions are based on adjusted R2 values shown as percentages of total variation. Values < 0 are not shown. a Summer. b Autumn. c Winter. d Spring. e Jinshui River (JSH). f Yue River (YR). g Jinqian River (JQR). h Qi River (QR). i Si River (SR)
Rivers
The pure local variable group contributed much more variation of phytoplankton community than regional variables and spatial variables in all rivers (Fig. 4e–i). It explained the largest variation in JSR (20.1%) and the smallest variation in JQR (10.4%). The pure regional variables and spatial variables explained the very small variation of phytoplankton community in all rivers. The shared fractions among all variables groups explained more of the variation in JSR and SR than in other three rivers. The variations explained jointly by the different pairs of variable groups were small for all rivers.
Discussion
CAP analysis showed that phytoplankton community structure and environmental conditions differed among seasons and rivers. However, the phytoplankton beta diversity and environmental heterogeneity had no significant differences among seasons. This illustrated that although environmental factors varied obviously with seasons, resulting in significant changes in species composition and abundance, the changes of sampling sites were homogeneous, so the differences of spatial dissimilarity in phytoplankton community were not detected among seasons. However, the phytoplankton beta diversity as well as the environmental heterogeneity differed among rivers. Beta diversity is previously considered dependent on environmental heterogeneity (Astorga et al. 2014), and high environmental heterogeneity in water bodies is expected to cause high beta diversity (Tonkin et al. 2016). In this study, the phytoplankton beta diversity was consistent with the trend of environmental heterogeneity, indicating that environmental heterogeneity played an important role in driving the variation of phytoplankton beta diversity.
Determinants of phytoplankton community structure in different seasons
We examined the effects of environmental variables (physical and chemical variables, land-cover variables) and spatial variables on phytoplankton community structure, which can be translated into environmental filtering and dispersal limitation (Heino et al. 2017). In this study, these two processes played their roles in determining the phytoplankton community structure in different seasons.
Seasonal sampling collection is usually used to discern the impact of seasonal changes in environmental variables on metacommunity structure (Dini-Andreote et al. 2015). High and low water-level river stages (in wet and dry seasons) are often considered because these periods appear to be a good proxy for riverine connectivity (Isabwe et al. 2018). Considering among-site connectivity via actual watercourses is essential for better understanding spatial coexistence mechanisms for phytoplankton communities (Meier and Soininen 2014). Given the fact that dry seasons are characterized by slower and less flushing water regimes and resulting in poor connectivity and dispersal limitation (Campbell et al. 2015), which therefore can indicate a more spatially structured phytoplankton community. On the contrary, there should be less spatially structured phytoplankton communities in wet seasons. However, no such phenomenon was found in this study. The pure spatial variables explained the largest variation of phytoplankton community in summer, autumn (wet seasons), and winter (dry season), while local environmental variables explained the largest variation of phytoplankton community in spring (dry season). This finding may be due to the extensive sampling scale among the five rivers. Spatial variables (PCNM1, 2, 3, 4) representing the broad-scale relations among the sites were selected in all seasons in our models. This may indicate that long-distance scales always have low riverine connectivity regardless of flooding or drying up. The spatially structured phytoplankton community at small scales during the dry season was also not supported by the analysis. This result is partially in agreement with that phytoplankton is able to cross dispersal barriers at small spatial scales (Finlay 2002). Beisner et al. (2006) reported that overland dispersal (via wind or animal vectors) was also important to phytoplankton community structure. Also, the likely effects of actual hydrological (such as runoff, flow velocity, residence time) and morphological (such as sinuosity) variables as drivers of phytoplankton community structure which are lacking in our study area cannot be ignored.
The local environmental variables were more pronounced in spring (dry season). It may be because the weak site-to-site connectivity and longer water residence time during dry season limit the overall habitat availability and therefore may act as “natural” environmental filter (Chase 2007). John et al. (2007) also suggested that the different environment-controlled effect might be attributed to the involvement of different environmental variables among the analysis in different seasons. In our analysis, from summer to spring, different significant environment-controlled variables were selected to participate in the variation partitioning analysis. However, land-cover types and spatial geographic coordinates did not change with the seasons in a year; thus, the local environmental variables might be the most important influencing factors for the variations of phytoplankton community structure. Spatial variables were more pronounced in winter may be related to freezing, especially in the case of less water.
Local environmental variables explained more variance than regional variables (land-cover variables) in all analyses. This was congruent with many studies (e.g., Özkan et al. 2013; Lindholm et al. 2018). Local environmental variables, such as chemical oxygen demand and nutrients, are often strongly influenced by land-cover types, particularly in agricultural areas and developed areas (Maberly et al. 2003). In this study, agricultural areas and developed areas significantly positively correlated with most local environmental factors (Table A3 of Appendix 5), such as CODMn, most nutrients, and ions. This may explain the relatively weak effect of land-cover, as it has probably been accounted for, in part, by including local environmental variables (Özkan et al. 2013). Therefore, the local and regional environmental variables are often analyzed as the same dependent variable group (Alahuhta and Heino 2013; Isabwe et al. 2018).
Determinants of phytoplankton community structure in different rivers
The variation partitioning analysis for rivers indicated that local environmental variables group played a much more important role in structuring phytoplankton community than pure regional variables group and spatial group in all rivers. Although eigenvectors representing broad-scale variation (PCNM1 or PCNM2) were selected in each river, the pure spatial group had small contribution for the variation of phytoplankton community structure. This was consistent with many studies, which showed that environmental filtering was potentially an essential driver of variation in phytoplankton community structure at small spatial scales, where dispersal limitation was not strong (Jyrkänkallio-Mikkola et al. 2016). Previous findings in metacommunity studies have shown that species can better track environmental heterogeneity among sites at small spatial scales (Heino 2011). ANOSIM analysis based on abundance data indicated that phytoplankton community structure of the five rivers differed in pairs. Environmental heterogeneity between rivers might drive individual rivers to encompass a specific community (Jyrkänkallio-Mikkola et al. 2016). Specifically, the concentration of SRP, Na+, K+, Mg2+, Ca2+, Cl−, and EC and TUB varied considerably among these five rivers (Table A1 of Appendix 2), and the turbidity was selected as the driving factor for each river. Turbidity attenuates light penetrating the water column by scattering and absorbing solar radiation, thereby interfering with photosynthesis of phytoplankton (Guenther and Bozelli 2004). Also, phytoplankton can act as adhesion surfaces, and the adhesion of clay particles onto algal cells may lead to an increase in algal sinking (Cuker et al. 1990). These might be reflected in our study to some extent. JQR had the significantly highest turbidity among all rivers and the lowest phytoplankton abundance. The environmental heterogeneity among these rivers may result from anthropogenic activities.
The influence of human activities on environmental conditions and phytoplankton community structures
Land-cover types reflect the patterns and degrees of anthropogenic activities. Agricultural areas and developed areas represent human activities such as runoff containing agrochemicals, industrial effluents, and municipal wastewater. The nutrient input from these activities has a great effect on water quality and sediment properties and further affects biodiversity (Shayo et al. 2011). In this study, JSR was most covered by forestland, which represented the pristine ecological conditions. It had the lowest concentration of nutrients, ions, EC, and turbidity, as well as the lowest beta diversity of phytoplankton and environmental heterogeneity. This is inconsistent with many studies that pristine ecosystem has high environmental heterogeneity and community dissimilarity (Wyzga et al. 2011; Zeni and Casatti 2014; Jyrkänkallio-Mikkola et al. 2016). The highest classification rate of community structure and environmental conditions was found in JSR, which suggested that the phytoplankton communities and environmental conditions between the sampling sites were homogenous within JSR. This might be related to better connectivity without artificial blockage within pristine river. In addition, the variation of phytoplankton in JSR was well explained by local environmental variables. This indicated that in species sorting, species were expected to closely track preferred environmental conditions without disturbances. YR and QR were mainly disturbed by agricultural activities and SR was mainly affected by urban activities (domestic sewage and industrial wastewater). These three rivers had higher beta diversity and environmental heterogeneity compared with JSR. The non-point pollution from agricultural activities and the point pollution from domestic sewage and industrial wastewater caused an uneven distribution of chemicals and suspended materials within a river, resulting in great differences in environmental conditions and species composition between sites.
Sand mining activities have seriously disturbed the waters and riverside ecosystems and increased erosion by directly removing sand and disrupting the sediment budget (Chaussard and Kerosky 2016). These activities increased runoff containing sand into waterbodies and significantly increased the turbidity of water (Ashraf et al. 2011). The turbidity itself reduces the use of light energy by phytoplankton, and in addition, the transport of suspended solids causes siltation and reduces the flow rate, which limits the dispersal of phytoplankton. In this study, the whole watercourse of JQR was disturbed by sand mining, and our results showed that JQR had the highest phytoplankton beta diversity and environmental heterogeneity. Maloufi et al. (2016) attributed the elevated phytoplankton beta diversity to elevated environmental heterogeneity, mainly due to anthropic effects. Sand mining activities may lead to extensive loss of phytoplankton and habitats fragmentation (Walther et al. 2002), and the limited dispersal capacity and the high environmental heterogeneity support the high beta diversity of phytoplankton. Mass effects may prevent perfect species sorting to take place effectively, and species may thus be temporarily present at unfavorable sites (Heino et al. 2015a). In addition, the significant difference of environmental heterogeneity in JQR among seasons (Fig. A2 of Appendix 4) also indicated that anthropogenic activities changed environmental conditions in a short period of time. We noticed that JQR had the highest residual variation of explanation, which may be due to some unknown factors, such as unmeasured environmental factors, total sediment load, patch history, as well as other impacts connected with human activities (Vilmi et al. 2016).
Anthropogenic activities significantly increased the concentration of nitrogen, phosphorus, and various ions (Na+, K+, Mg2+, Ca2+, Cl−, and SO42−), and the most notable was the increases of EC and turbidity. These excessive substances have a negative effect on river water quality (Taka et al. 2017) and also influence the growth and diversity of phytoplankton. Sand mining activities reduced the species richness and abundance of phytoplankton by increasing turbidity of rivers. It had the greatest effect on beta diversity and environmental heterogeneity, specifically increased the difference in species composition and environmental conditions between sites, which might be due to habitat fragmentation and geographical isolation. Agricultural activities and urban activities increased the species richness and abundance of phytoplankton via nutrient and pollutant runoff. These might be explained by the Intermediate Disturbance Hypothesis, where intermediate disturbance (agricultural activities and urban activities) had higher species diversity than excessive disturbance (sand mining activities) and mild disturbance. Agricultural and urban activities also increased the differences in species composition and environmental conditions between sites, mainly due to uneven point source and non-point source pollution.
Conclusion
Long-distance scales always have low riverine connectivity regardless of whether the river is overflowing or drying up; this resulted from that spatial process underlay phytoplankton communities only on large scale in both wet seasons and dry seasons. Local environmental processes explained the most variation of phytoplankton community in all rivers, suggesting that deterministic processes usually work on relatively small spatial scales. However, this effect would be weakened by anthropogenic activities, especially sand mining activities. Our results showed that the river with limited disturbance (the reference river) had the highest homogeneity of environment conditions and phytoplankton community structure. However, anthropogenic activities, especially sand mining, greatly increased the environmental heterogeneity and species dissimilarity between sites by causing watercourse habitat patches and obstructing river connectivity. On the other hand, as the excessive disturbance, sand mining significantly decreased the species richness and abundance of phytoplankton via increasing turbidity. Therefore, sand mining activities should be controlled in case there is continuous degradation and fragmentation of habitats and continuous loss of species richness diversity.
References
Alahuhta J, Heino J (2013) Spatial extent, regional specificity and metacommunity structuring in lake macrophytes. J Biogeogr 40:1572–1582
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson MJ, Robinson J (2003) Generalized discriminant analysis based on distances. Aust N Z J Stat 45:301–318
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
APHA (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC
Ashraf MA, Maah MJ, Yusoff I, Wajid A, Mahmood K (2011) Sand mining effects, causes and concerns: a case study from Bestari Jaya, Selangor, Peninsular Malaysia. Sci Res Essays 6:1216–1231
Astorga A, Death R, Death F, Paavola R, Chakraborty M, Muotka T (2014) Habitat heterogeneity drives the geographical distribution of beta diversity: the case of New Zealand stream invertebrates. Ecol Evol 4:2693–2702
Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, Longhi ML (2006) The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87:2985–2991
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153:51–68
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85:1826–1832
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer. Springer International Publishing
Boyd SE, Limpenny DS, Rees HL, Cooper KM (2005) The effects of marine sand and gravel extraction on the macrobenthos at a commercial dredging site (results 6 years post-dredging). ICES J Mar Sci 62:145–162
Campbell RE, Winterbourn MJ, Cochrane TA, McIntosh AR (2015) Flow-related disturbance creates a gradient of metacommunity types within stream networks. Landsc Ecol 30:667–680
Chase JM (2007) Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci U S A 104:17430–17434
Chaussard E, Kerosky S (2016) Characterization of black sand mining activities and their environmental impacts in the Philippines using remote sensing. Remote Sens Basel 8:100
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E. Plymouth Marine Laboratory Press
Cuker BE, Gama PT, Burkholder JM (1990) Type of suspended clay influences lake productivity and phytoplankton community response to phosphorus loading. Limnol Oceanogr 35:830–839
Devercelli M, Scarabotti P, Mayora G, Schneider B, Giri F (2016) Unravelling the role of determinism and stochasticity in structuring the phytoplanktonic metacommunity of the Paraná River floodplain. Hydrobiologia 764:139–156
Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF (2015) Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc Natl Acad Sci U S A 112:1326–1332
Diniz-Filho JAF, Bini LM (2005) Modelling geographical patterns in species richness using eigenvector-based spatial filters. Glob Ecol Biogeogr 14:177–185
Finlay BJ (2002) Global dispersal of free-living microbial eukaryote species. Science 296:1061–1063
Gilbert B, Bennett JR (2010) Partitioning variation in ecological communities: do the numbers add up? J Appl Ecol 47:1071–1082
Guenther M, Bozelli R (2004) Effects of inorganic turbidity on the phytoplankton of an Amazonian lake impacted by bauxite tailings. Hydrobiologia 511:151–159
Hájek M, Roleček J, Cottenie K, Kintrová K, Horsák M, Poulíčková A, Hájková P, Fránková M, Dítě D (2011) Environmental and spatial controls of biotic assemblages in a discrete semi-terrestrial habitat: comparison of organisms with different dispersal abilities sampled in the same plots. J Biogeogr 38:1683–1693
Heino J (2011) A macroecological perspective of diversity patterns in the freshwater realm. Freshw Biol 56:1703–1722
Heino J, Melo AS, Bini LM (2015a) Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw Biol 60:223–235
Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015b) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869
Heino J, Soininen J, Alahuhta J, Lappalainen J, Virtanen R (2017) Metacommunity ecology meets biogeography: effects of geographical region, spatial dynamics and environmental filtering on community structure in aquatic organisms. Oecologia 183:121–137
Huang C, Zhou Z, Wang D, Dian Y (2016) Monitoring forest dynamics with multi-scale and time series imagery. Environ Monit Assess 188:273
Isabwe A, Yang JR, Wang Y, Liu L, Chen H, Yang J (2018) Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci Total Environ 630:658–667
John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallejo M, Foster RB (2007) Soil nutrients influence spatial distributions of tropical tree species. Proc Natl Acad Sci U S A 104:864–869
Jyrkänkallio-Mikkola J, Heino J, Soininen J (2016) Beta diversity of stream diatoms at two hierarchical spatial scales: implications for biomonitoring. Freshw Biol 61:239–250
Lai X, Shankman D, Huber C, Yesou H, Huang Q, Jiang J (2014) Sand mining and increasing Poyang Lake’s discharge ability: a reassessment of causes for lake decline in China. J Hydrol 519:1698–1706
Lindholm M, Grönroos M, Hjort J, Karjalainen SM, Tokola L, Heino J (2018) Different species trait groups of stream diatoms show divergent responses to spatial and environmental factors in a subarctic drainage basin. Hydrobiologia 816:213–230
Liu J (1996) Macro-scale survey and dynamic study of natural resources and environment of China by remote sensing. China Science and Technology Press (in Chinese)
Lu S, Wang BP, He H, Li JK, Gao HY (2014) Climate characteristics of area precipitation in flood season in upper reaches of the Hanjiang River. J Arid Meteorol 32:201–206 (in Chinese with English abstract)
Maberly SC, King L, Gibson CE, May L, Jones RI, Dent MM, Jordan C (2003) Linking nutrient limitation and water chemistry in upland lakes to catchment characteristics. Hydrobiologia 506:83–91
Magurran AF (2004) Measuring biological diversity. Blackwell, Oxford, p 256
Maloufi S, Catherine A, Mouillot D, Louvard C, Couté A, Bernard C, Troussellier M (2016) Environmental heterogeneity among lakes promotes hyper β-diversity across phytoplankton communities. Freshw Biol 61:633–645
Meier S, Soininen J (2014) Phytoplankton metacommunity structure in subarctic rock pools. Aquat Microb Ecol 73:81–91
Meng XL, Jiang XM, Li ZF, Wang J, Cooper KM, Xie ZC (2018) Responses of macroinvertebrates and local environment to short-term commercial sand dredging practices in a flood-plain lake. Sci Total Environ 631:1350–1359
Niño-García JP, Ruiz-González C, del Giorgio PA (2016) Interactions between hydrology and water chemistry shape bacterioplankton biogeography across boreal freshwater networks. ISME J 10:1755–1766
Nogueira SID, Nabout JC, Ibañez MDSR, Bourgoin LM (2010) Determinants of beta diversity: the relative importance of environmental and spatial processes in structuring phytoplankton communities in an Amazonian floodplain. Acta Limnol Bras 22:247–256
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P (2013) Vegan: community ecology package. R package version 2.0–9
Özkan K, Jeppesen E, Søndergaard M, Lauridsen TL, Liboriussen L, J-Christian S (2013) Contrasting roles of water chemistry, lake morphology, land-use, climate; and spatial processes in driving phytoplankton richness in the Danish landscape. Hydrobiologia 710:173–187
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Shayo S, Lugomela C, Machiwa J (2011) Influence of land use patterns on some limnological characteristics in the south-eastern part of Lake Victoria, Tanzania. Aquatic Ecosyst Health 14:246–251
Soininen J (2010) Species turnover along abiotic and biotic gradients: patterns in space equal patterns in time? Bioscience 60:433–439
Stockner JG, Benson WW (1967) The succession of diatom assemblages in the recent sediments of Lake Washington. Limnol Oceanogr 12:513–532
Taka M, Kokkonen T, Kuoppamäki K, Niemi T, Sillanpää N, Valtanen M, Warsta L, Setälä H (2017) Spatio-temporal patterns of major ions in urban stormwater under cold climate. Hydrol Process 31:1564–1577
Tonkin JD, Stoll S, Jähnig SC, Haase P (2016) Contrasting metacommunity structure and beta diversity in an aquatic-floodplain system. Oikos 125:686–697
Vilmi A, Karjalainen SM, Hellsten S, Heino J (2016) Bioassessment in a metacommunity context: are diatom communities structured solely by species sorting? Ecol Indic 62:86–94
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, J-Marc F, Hoehg-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wu G, de Leeuw J, Skidmore AK, Prins HHT, Liu Y (2007) Concurrent monitoring of vessels and water turbidity enhances the strength of evidence in remotely sensed dredging impact assessment. Water Res 41:3271–3280
Wyzga B, Oglecki P, Radecki-Pawlik A, Zawiejska J (2011) Diversity of macroinvertebrate communities as a reflection of habitat heterogeneity in a mountain river subjected to variable human impacts. In: Simon A, Bennett SJ, Castro JM (eds) Stream restoration in dynamic fluvial systems. American Geophysical Union, Washington, D. C, pp 189–207
Yang JR, Lv H, Isabwe A, Liu L, Yu X, Chen H, Yang J (2017a) Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res 120:52–63
Yang W, Zheng Z, Zheng C, Lu K, Ding D, Zhu J (2017b) Temporal variations in a phytoplankton community in a subtropical reservoir: an interplay of extrinsic and intrinsic community effects. Sci Total Environ 612:720
Zeni JO, Casatti L (2014) The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 726:259–270
Funding
This work was supported by Major Science and Technology Program for Water Pollution Control and Treatment (No. 2017ZX07108-001) and the key deployment project of the Chinese Academy of Sciences (ZDRW-ZS-2016-7-1). We are grateful to Yintao Jia, Kang Chen, and Zhengfei Li for their assistance during the field survey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOC 3.03 mb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Peng, C., Huang, S. et al. The relative role of spatial and environmental processes on seasonal variations of phytoplankton beta diversity along different anthropogenic disturbances of subtropical rivers in China. Environ Sci Pollut Res 26, 1422–1434 (2019). https://doi.org/10.1007/s11356-018-3632-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3632-4