Abstract
Littorella uniflora (Plantaginaceae) is a scarce and declining aquatic plant in Europe. Its population has been strongly reduced by changes in fishpond management (eutrophication) in the Czech Republic since the 1950s. We studied its seed bank in both recent (n = 8) and historical localities (the last found from 1972 to 2000; n = 10) and tested the effects of sediment type and burial depth on seed germination using extracted seeds from two recent populations. The seeds were found in 60% of the historical localities, mostly in low densities (≤6 seeds per 3.75 l of sediment), and also in 100% of the recent localities in various densities (8–1390 seeds per 3.75 l of sediment); however, low germination rates (0–13.3%) were estimated. The seeds germinated best on wet filter paper, followed by nutrient-rich fishpond sediment, but poorly on sand mixed with different substrates. Burial depth significantly affected seed germination. The seeds germinated only on the soil surface and at the depth of 1 cm but no seed germinated at the depth of 3 and 5 cm. Besides low water transparency, both high rate of sediment accumulation and the absence of summer drainage may endanger L. uniflora populations in fishponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Littorella uniflora (L.) Asch. (Plantaginaceae) is an evergreen, aquatic amphibious plant, with a typical small rosette of thick and stiff basal leaves. It belongs to the group of isoetids, which typically have a large proportion of porous root biomass and grow very slowly. They occur in wetlands and on sunny shorelines with low nutrient availability and in areas with low rates of sediment deposition (Pearsall, 1920).
Littorella uniflora occurs in oligotrophic and mesotrophic freshwaters of Northern and Western Europe (Murphy, 2002), especially in soft-water lakes, but also in reservoirs, rivers, streams, ponds and winter-flooded dune slacks to a depth of about 4 metres (Schoof-Van Pelt, 1973; Røslett & Brettum, 1989; Szmeja, 1997). Unlike the natural localities in Northern and Western Europe, L. uniflora occurs mainly in fishponds in the Czech Republic—artificial shallow water bodies primarily used for fish production. Many fishponds were constructed as early as the 16th century, in many cases at sites of former marshes, swamps, bogs or fens, ranging in size from less than one hectare to hundreds of hectares (Pokorný & Hauser, 2002). Until the second half of the 20th century, their water quality corresponded to that in soft-water lakes as their fish stock and the intensity of fertilization were low (IUCN, 1996). The application of periodic summer drainage, as a low-cost way to increase fish production, created optimal conditions for the development of amphiphytic isoetid populations (Hejný & Husák, 1978).
In the second half of the 20th century, a rapid decline occurred in the number of localities originally inhabited by isoetids (Arts, 2002). The loss of the sites mainly took place in the marginal areas of its distribution in Western and Central Europe. Acidification seems to be the main cause of their decline in Western Europe (Roelofs et al., 1984, 1994; Arts, 2002). A similar decline also occurred in the Czech part of the former Czechoslovakia (Hejný & Husák, 1978). However, the reasons were quite different. The overall intensification of the fishpond management since the 1960s has involved an application of high amounts of fertilizers, fish feeding, fish–duck farming, liming of fishpond bottoms and common carp (Cyprinus carpio) overstocking. As a result, water transparency dramatically decreased mainly because of high phytoplankton density (Hejný, 1967; Pokorný et al., 1990a; Procházka & Husák, 1999). Both low water transparency and the absence of regular summer drainage have led to the dramatic decline of a number of L. uniflora localities in the Czech Republic in the last century (Šumberová, 2011). Additionally, the composition of fishpond sediments significantly changed during the last 60 years (IUCN, 1996) and the rate of sediment deposition has increased substantially in the same period (Pokorný & Hauser, 2002).
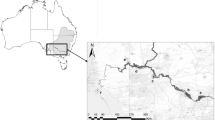
The Czech Republic is located on the south-eastern border of the continuous world distribution of L. uniflora (Procházka & Husák, 1999). Historically, L. uniflora was reported in about seventy fishponds in the Czech Republic (Klika, 1935; Jílek, 1956; Hejný, 1967; Husák & Adamec, 1998). Recent populations can be found only in eight localities (Šumberová, 2011; Kolář & Kolář, 2014)—two drinking water reservoirs and six extensively managed fishponds (Table 1; Fig. 1)—most of them located in the southern part of the Czech Republic. Therefore, L. uniflora is categorized as C1 species (i.e. critically endangered) in the Czech Red List of Vascular Plants (Grulich, 2012) and protected as a critically endangered species under the law (Decree no. 395/1992 Coll.). However, very few practical measures for its conservation have been undertaken to date. For example, there are only two nature reserves in the Czech Republic (Králek and Horní Mrzatec fishponds) where some measures for the preservation of L. uniflora population have been adopted (Hesoun et al., 2008; Marhoul et al., 2014). The species is also protected by the government law in France and Poland (Act, 1982, 2004) and categorized as LC (i.e. “least concern”) in the European Red List of Vascular Plants (Bilz et al., 2011).
Location of the historical and recent localities of Littorella uniflora in the Czech Republic where the sediment samples were taken. All localities are numbered and a detailed description is given in Table 1 and in the text
Littorella uniflora with evergreen leaves and CAM photosynthesis (Smolders et al., 2002) is able to grow under both submerged and emergent conditions. The submerged individuals are sterile and reproduce by runners (West, 1905). Sexual organs develop only under emergent conditions. The plant flowers from May to September (Slavík, 2000) as early as three or four weeks after the emergence (Robe & Griffiths, 1998). Its fruit is a yellow–brown to auburn, minute (1.5- to 2.5-mm-long), one-seeded nut produced in small quantities, with the maximum of about twenty seeds per plant per year (Arts & van der Heijden, 1990). John & Richert (2011) found very limited fruit buoyancy and suggested that hydrochory as generative dispersal mechanism was not important for this species, especially for its long-distance dispersal. According to the sediment age, seeds can be found in the sediment depth from 5 to 15 cm (Thompson et al., 1997; Bekker et al., 1999). L. uniflora has a persistent seed bank (Wynhoff, 1988; Bekker et al., 1999). Wynhoff (1988) mentioned that seeds kept the germination viability for more than 30 years. Bekker et al. (1999) found the Littorella seeds in an 80-year-old sediment in their study of seed bank in a dune slack, although without evaluating their germinability.
Seed density in the seed bank, seed vitality and germination ecology with respect to management may play a decisive role in the effort to support the Littorella uniflora populations in the Czech Republic. Therefore, we quantified seed bank densities at 10 historical and 8 recent localities of L. uniflora in the Czech Republic (1) and tested germination rates of the collected seeds (2). Additionally, we tested whether different sediment types (sand, clay, peat, pond sediment, topsoil, their mixtures and wet filter paper as a control) affected the germination of L. uniflora seeds (3) and whether the burial depth (1–5 cm) could reduce the germination of L. uniflora seeds both under controlled laboratory and near-natural conditions (4).
Materials and methods
Study area
The study was carried out in the Czech Republic, where recent localities of L. uniflora are concentrated in South Bohemia and Bohemian-Moravian Highlands, with the only exception being in the Central Bohemia (Fig. 1). Their altitudes range from 485 to 685 m a.s.l. (Table 1). The landscape in these regions consists of flat basins with large fishpond systems, some stretching over an area of several hundreds of hectares, or hilly country with chains of smaller fishponds in stream valleys. Geology of the basins and stream valleys is dominated by unconsolidated non-calcareous limnic sediments. Surrounding hills are mainly formed by granitoids and other crystalline bedrocks (Demek & Mackovčin, 2006). The mean annual air temperatures range between 6 and 8°C, and mean (April–September) growing season temperatures reach about 11–14°C. The mean annual precipitation ranges between approximately 500 and 700 mm, out of which approx. 350–500 mm falls during the April–September period (Tolasz, 2007).
Seed bank in the historical localities
We selected 10 localities with historical occurrence of L. uniflora according to an online database of the Nature Conservation Agency of the Czech Republic (Table 1; Fig. 1). Having accepted that seeds can be viable in sediment for more than 30 years (Wynhoff, 1988), we selected those localities where the species was present in the period from 1970 to 2000. We used both field prospection and aerial photography to select the most appropriate sites for sediment collection. In each locality, six 2-m-long transects perpendicular to the shoreline were set up. The sediment was sampled at a distance of 0.5 m apart along each transect. In total, we collected 30 sediment cores (4 cm in diameter, 10 cm in length, volume of 125 ml) in each locality. Sampling was performed by a custom sampler (type Beeker; Eijkelkamp Soil & Water, The Netherlands) in autumn 2014. The seed banks of Littorella uniflora were estimated by the rinsing method (Bernhardt, 1993; Devictor et al., 2007) which was recommended as a reliable method for the estimation of seed bank of rare species (Bernhardt et al., 2008). The sediment samples were air dried for two weeks, then sieved to reduce the volume by sieves of different sizes. All Littorella seeds were collected from sediments and counted using a stereomicroscope. The seeds were determined using Beijerinck (1976) determination key and online databases (Cappres et al., 2006).
Seed bank in the recent localities
In each recent locality (Table 1; Fig. 1), six 2-m-long transects perpendicular to the shoreline were set up. The transects were placed as close as possible to L. uniflora stands without harming the plants. The sediment sampling, collecting of the seeds and determination of the seed banks were carried out using the same techniques as in historical localities.
Germination rate of seeds from the recent localities
The germination rate of collected seeds was tested in a climatic chamber (custom-made by the CIRIS Ltd.; light source characteristics: 4000 K, 385 lm, 18 W m−2 PAR). A total of 75 seeds from each locality were sown directly on a wet filter paper in three Petri dishes, except for the locality no. 13 where only eight seeds were available. The experimental conditions were the same as recommended by Arts & van der Heijden (1990), i.e. 12/12 photoperiod, day/night temperatures 20/8°C. The samples were regularly watered by tap water in order to prevent desiccation.
Effect of sediment type
To test the effect of sediment type we used clay, sand, peat, topsoil, fishpond sediment and the following combinations: sand/peat; sand/fishpond sediment; sand/clay as germination substrates. The substrates were analysed for the following chemical parameters: pH, electrical conductivity of elution, concentrations of total organic carbon (TOC), total reduced nitrogen (TN), total phosphorus (TP), water soluble concentrations of Ca2+, Mg2+ and Al3+ according to the standard analytical methods (Zbíral, 1995). Strips of filter paper were placed in plastic dishes and covered with a 1-cm layer of a given substrate. Ten seeds of L. uniflora were sown on the surface of each substrate or directly on wet filter paper without any substrate (a control). Dishes with eight different sediment types and one control dish were randomly placed in one large plate (40 × 50 cm). Each sediment type was repeated ten times. The experiment was conducted in an open greenhouse in Třeboň from April to June 2015. Air temperature 15 cm above the germinated seeds was measured by a data logger (TMS 3; TOMST Ltd.). For sample irrigation, we used rain water instead of tap water to prevent sediment salinization during the long sample incubation in rather dry weather conditions. The seed with the emerged plumules from the seed coat was defined as a successfully germinated seed. Germinated seeds were counted twice per week and removed from the experiment. The experiment was terminated after 60 days.
Effect of burial depth tested in near-natural conditions
The effect of burial depth (1, 3 and 5 cm) on L. uniflora seed germination was tested between August and September 2014. Near-natural conditions were approximated in an open, naturally lit greenhouse in the Institute of Botany in Třeboň. In this pilot experiment, we used seeds extracted from two outdoor cultivation tanks in the Institute of Botany in Třeboň. L. uniflora plants originated from the Králek fishpond (Table 1; Fig. 1). The sediment samples were air dried for 2 weeks, seeds were manually collected using a stereomicroscope. The collected seeds were stored at 4 °C in a closed tube in the dark until the start of the experiment. Strips of filter paper were put on the bottom of small plastic dishes (10 cm in diameter). Then 25 seeds were sown in each plastic dish and covered by 1-, 3- or 5-cm-thick layer of sterilized sand, which had been heated to 250°C for 1 h. As a control, we sowed 25 seeds directly on a wet filter paper. Each treatment was replicated three times. All plastic dishes were placed in a randomized complete block design and exposed in an open greenhouse. The samples were irrigated by tap water regularly to prevent sample desiccation. During the experiment, the number of seedlings was counted twice per week and removed from the experiment. Those seedlings that were able to penetrate through the sediment up to the soil surface were considered as successfully germinated seeds. The experiment was terminated after 60 days.
Effect of burial depth tested in a climatic chamber
Additionally, the effect of burial depth (1, 3 and 5 cm) was tested in a climatic chamber from January to February 2015 in order to verify the results from the open greenhouse. Seeds from the Láz water reservoir (Table 1; Fig. 1) were used in this experiment due to the lack of seeds from the Třeboň cultivation. In this locality, the sediment samples close to the L. uniflora stands were sampled for seed collection in autumn 2014. Seed collecting, seed storage and the experiment setup (seed number, sterilized sand) were the same as described in the paragraph above.
All plastic dishes with the seeds were placed in a randomized complete block design and exposed in a climatic chamber (custom-made by the CIRIS Ltd.; light source characteristics: 4000 K, 385 lm, 18 W m−2 PAR). The experimental conditions were the same as recommended by Arts & van der Heijden (1990), i.e. 12/12 photoperiod, day/night temperatures 20/8°C. The samples were irrigated by tap water regularly in order to prevent sample desiccation. During the experiment, the number of seedlings was counted twice per week and removed from the experiment. Those seedlings that were able to penetrate through the sediment up to the soil surface were considered as successfully germinated seeds. The experiment was terminated after 60 days.
Statistical analyses
We used the generalized linear model (GLM) with binomial distribution of errors for the quantification of the effects of environmental variables (seed origin, sediment type or burial depth) on germination ability of L. uniflora seeds. The significance of the explanatory variables was tested by a Chi square test. The results are presented as estimates of mean probability of success ± their standard errors (SE). The significance level was set to 5%. Data management and all analyses were carried out using the R statistical program (R Core Team, 2015).
The original data and data analyses were made publicly available at https://github.com/jakubecp/Littorella-uniflora.
Results
Seed bank in the historical localities
In total, 173 seeds were found in the sediments at 10 sites with the historical occurrence of L. uniflora. The sums differed significantly among the sites (Table 1). The most seeds (n = 157; 90.7% in total) were found in the Svět fishpond. No seed of Littorella was found in four localities and only very low numbers (1 or 2 seeds) in other three localities.
Seed bank in the recent localities
In total, 2466 seeds were found at eight recent sites of L. uniflora (Table 1). The largest number of seeds (n = 1390) was found at the Láz water reservoir where the size of L. uniflora population was estimated to represent millions of individuals, many of them flowering every year. The lowest amount of seeds (n = 8) was found at the Nový fishpond where the population counted only hundreds of individuals and the last flowering was observed more than 10 years ago (Table 2). No seed from this locality was able to germinate during the germination test made in a climatic chamber (Table 2). On the other hand, the highest number of germinated seeds (13.3 ± 3.5%, z-value 2.176, P = 0.0296) came from the Osika fishpond with the population of thousands of individuals, which last prolifically flowered in 2007 (Table 2).
Germination on different substrates
The mean air temperature was 17.8 °C 15 cm above the sediment surface, the mean maximum and mean minimum air temperatures were 27.4 and 10.0 °C, respectively. The sediment type had a highly significant effect on the germination of L. uniflora seeds (Chi2 = 142.88, P < 0.001***). No seed germinated on topsoil. Low numbers of seeds germinated on the mixture of sand + peat (3.0 ± 1.5%) and sand + clay (4.0 ± 3.1%), while the higher numbers of seeds germinated on a fishpond sediment (24.0 ± 5.4%). However, the significantly highest number of seeds germinated in the control, i.e. on a wet filter paper (49.0 ± 6.2%) (Fig. 2).
Mean germination rate (in %) of Littorella uniflora seeds tested on different sediment types in an open greenhouse (near-natural conditions), substrates with germination rates significantly different from expected values are marked by *P < 0.05 and ***P < 0.001. The effect of sediment type was highly significant (z-values = 107.05, ***P < 2.2e–16 , n = 9). No seed germinated on topsoil
Germination in a different burial depth
The burial depth had a highly significant effect on the germination of L. uniflora seeds in both open greenhouse (χ2 = 108.74, P < 0.001***) and climatic chamber (χ2 = 22.354, P < 0.001***). No seed was able to germinate from the depth of 3 or 5 cm in either test (Table 3). The mean germination from the depth of 1 cm was 34.7 ± 5.3% in an open greenhouse and 9.3 ± 4.8% in a climatic chamber. In both tests, the highest number of seeds germinated in the control, i.e. on a wet filter paper (almost 53.3 ± 3.5% in an open greenhouse and 13.3 ± 7.4% in a climatic chamber).
Discussion
Seed bank in the historical localities
The L. uniflora seeds were found in 60% of the historical localities even though the populations disappeared more than 35 years ago. However, the seed number was low. The only exception was the Svět fishpond where the seed number was close to the numbers found in the recent localities (Table 1). These seeds could be produced by plants experimentally planted at the same microsite in this fishpond in 2002 (Kučerová et al., 2016). Thanks to favourable conditions (exposed bottoms due to lower water level), the plants were flowering during the whole growing season 2003. However, they completely disappeared in the next year after flooding. Natural recovery of the L. uniflora population from the seed bank in the historical localities may not be possible due to low numbers of seeds, their high age and burial in sediments. Nevertheless, as only a very limited number of seeds were collected, we could not estimate their germinability. Additionally, almost all historical localities are still used for intensive fish production. Therefore, high fish stock, low water transparency and highly eutrophic water (Pokorný et al., 1990b; Šumberová, 2011) make them unsuitable for both the regeneration and the long-term survival of the species. This assumption was confirmed during the experimental reintroduction of L. uniflora to the Svět fishpond (Kučerová et al., 2016).
Seed bank and long-term stability of L. uniflora in the recent localities
The L. uniflora seeds were found in all recent localities. The seed number was very variable (min. 8 compared to 1390 seeds in 3.75 l of sediment, Table 1). The large variability in seed numbers was also found for other rare Isoëto-Nanojuncetea species such as Carex bohemica, Coleanthus subtilis, Elatine hexandra, Eleocharis ovata or Tillaea aquatica by Bernhardt et al. (2008) or Šumberová et al. (2012). The seed number was influenced both by the total cover of the species and the flowering frequency. A higher number of seeds were found in localities with more recent flowering (Table 2), as documented by Šumberová (2001) or Kolář et al. (2015). The seeds in most localities (except for Láz, Králek and Rytíř) were probably more than 10 years old and their germinability was rather low (Table 2).
Regular or more frequent summer drainage of fishponds seems to be the crucial requirement for the long-term stability of the L. uniflora population (every 7th year at the minimum, cf. Hejný & Husák, 1978)—frequent flowering will produce more seeds which may help to restore the population after the unsuitable conditions (lower water transparency, occurrence of herbivorous fishes or high fish stock). Unlike many other species, L. uniflora may profit from extreme warm weather conditions during the growing season. Especially, dry summers may support its populations due to lower water level in fishponds, more frequent exposition of bottoms, followed by both the flowering of exposed plants and the germination from the seed bank, as noticed in hot and dry summer 2015.
Two out of the recent localities are drinking water reservoirs, one with the largest population in the Czech Republic (Láz reservoir), the second one (Karhov) with the rapidly declining population since 2007. Both reservoirs were adapted from the former fishponds and L. uniflora historically occurred in their vicinity. High water transparency, low fish stock and regular summer drainage offer optimal conditions for the long-term persistence of L. uniflora populations. However, the large-scale forest logging in the watershed followed by the lower water transparency and high rate of sedimentation negatively influenced the population in the Karhov reservoir and probably also in one of the historical localities (Pilská reservoir).
Specific requirements of L. uniflora seeds for germination
The generally lower germination rates found in a climatic chamber as compared to an open greenhouse were probably caused by lower light intensity in a climatic chamber and/or different seed origin (seeds from Láz versus from the Collection); however, the results were consistent in both experiments (Table 3).
The L. uniflora seeds were able to successfully germinate only when exposed on the sediment surface or immediately below it (up to 1 cm). The seeds sown in the depth of only 3 cm did not germinate at all. Similarly, sediment depth of 1 cm significantly reduced germination of a number of other aquatics (e.g. Eleocharis acicularis, Jurik et al., 1994). Therefore, the seeds may not germinate in places with high sedimentation rate or litter accumulation. In fishponds, the seeds could be buried to the depth of 50 cm (Šumberová, 2013, pers. comm.). Therefore, surface disturbances (shallow ploughing, removal of a part of the sediment) may support the germination from the seed bank in localities with a long-term, decreasing population of L. uniflora, as documented by Kolář et al. (2015) in the Králek fishpond.
In the Czech Republic, L. uniflora occurs mainly on sandy bottoms with small admixture of clay, peat or gravel (Šumberová, 2011). Surprisingly, seeds germinated most frequently in the control (a wet filter paper saturated with rain water) followed by nutrient-rich fishpond sediment. However, no seed germinated in similarly nutrient-rich topsoil (Fig. 2; Table 4). Soil from an intensively managed field may contain some residuals of herbicides which could negatively influence the germination; however, this was not measured in our study. Nutrient-rich fishpond sediment may stimulate the germination due to either high organic or high mineral nutrient content. However, such a sediment type is unfavourable for the long-term survival of L. uniflora plants as it usually occurs in places with a high rate of sediment accumulation (Pokorný & Hauser, 2002), which may cause rapid covering and shading of plants with sediment particles. Spierenburg et al. (2013) documented a higher production of the above-ground biomass as compared to the below-ground biomass in L. uniflora in high organic sediments, which led to frequent uprooting of plants during harsh weather conditions (strong wind followed by strong waves). Additionally, more rapidly growing and robust plants (e.g. Bidens spp., Juncus spp., young Typha spp., Phragmites australis), which are able to utilize the high soil nutrient content more efficiently, will outcompete the slowly growing L. uniflora (Arts & Leuven, 1988; Szmeja, 1994). Surprisingly, the highest water-soluble Ca concentration (238.7 mg/kg) was measured in the peat substrate (Table 4). This concentration is unlikely to negatively influence the germination as the Ca concentrations may reach similar values in the sediment samples from the Littorella stands in the Czech Republic (Kučerová & Kolář, unpubl. data). High water-soluble Al concentrations were measured in clay and sand/clay substrates (26.9 and 31.4 mg/kg dry weight, respectively). Such concentrations, however, are not expected to be toxic to the plant growth (cf. Cronan & Grigal, 1995).
We confirmed the findings of Wynhoff (1988) and Bekker et al. (1999) that L. uniflora had a long-term persistent seed bank (more than 30 years). However, germination rate may decrease after approx. 10–15 years. L. uniflora populations at permanently flooded sites are primarily maintained through clonal reproduction. The seeds can provide important functions for the recovery of populations after episodic declines as was observed in the Karhov water reservoir in 2015 where no plant was found in spring and summer. New plants (approx. 60 individuals), which germinated on exposed bottoms from the seed bank, were observed at the same site in late September, and more than 400 individuals in autumn 2016.
Conclusions
This study confirmed the presence of a seed bank of L. uniflora in all recent and some historical localities in the Czech Republic. Nevertheless, seeds were found at low densities in most of the historical localities. The germination rates were relatively low in the recent localities. The seeds germinated most frequently on a wet filter paper saturated with rain water, followed by a nutrient-rich fishpond sediment. An increasing burial depth had a significant negative effect on seed germination. Regular summer drainage is a necessary requirement for the long-term and abundant seed bank in fishponds.
References
Act, 1982: Decree from January 20th 1982 on the list of protected plant species. [Accessed 22 April 2013] http://legifrance.gouv.fr/jopdf/common/jo_pdf.jsp?numJO=0&dateJO=19820513&numTexte=&pageDebut=54559&pageFin= (in French).
Act, 2004: Regulation of the Minister of Environment from July 9th 2004 on the protected wild plants species. [Accessed 22 April 2013] http://isip.sejm.gov.pl/DetailsServlet?id=WDU20041681764&min=1 (in Polish).
Arts, G. H. P., 2002. Deterioration of Atlantic soft water macrophyte communities by acidification, eutrophication and alkalinisation. Aquatic Botany 73: 373–393.
Arts, G. H. P. & R. A. J. M. van der Heijden, 1990. Germination ecology of Littorella uniflora (L.) Aschers. Aquatic Botany 37: 139–151.
Arts, G. H. P. & R. S. E. W. Leuven, 1988. Floristic changes in soft water in relation to underlying environmental factors. Freshwater Biology 20: 97–111.
Beijerick, W., 1976. Seed Atlas of the Dutch Flora in Favor of Botany, Palaeontology, Soil Cultivation and Knowledge of Commodities, Including, in Addition to Native Flora, Our Main Crops. Backhuys & Meesters, Amsterdam. (in Dutch).
Bekker, R. M., E. J. Lammerts, A. Schmutter & A. P. Grootjans, 1999. Vegetation development in dune slacks: The role of persistent seed banks. Journal of Vegetation Science 10: 745–754.
Bernhardt, K.-G., 1993. Untersuchungen zur Besiedlung und Dynamik der Vegetation von Sand- und Schlickpionierstandorten. Dissertationes Botanicae 202: 1–223. (in German).
Bernhardt, K.-G., M. Koch, M. Kropf, E. Ulbel & J. Webhofer, 2008. Comparison of two methods characterising the seed bank of amphibious plants in submerged sediments. Aquatic Botany 88: 171–177.
Bilz, M., Kell, S.P., Maxted, N. & R.V. Lansdown, 2011. European Red List of Vascular Plants. Luxembourg: Publication Office of the European Union. [Accessed 22 June 2015] http://ec.europa.eu/environment/nature/conservation/species/redlist/downloads/European_vascular_plants.pdf.
Cappers, R. T. J., Neef, R.& R.M. Bekker, 2006. Digital seed atlas of the Netherlands. Groningen Instituut voor Archeologie. [Accessed 22 August 2015] http://seeds.eldoc.ub.rug.nl/.
Chán, V. (ed.), 1999. Annotated Red list of the southern Bohemian flora. Nature Conservation Agency of the Czech Republic, Praha. (in Czech).
Cronan, C. S. & D. F. Grigal, 1995. Use of calcium aluminium ratios as indicators of stress in forest ecosystems. Journal of Environmental Quality 24: 209–226.
Decree 395/92 Ministry of the Environment of the Czech Republic: List of the protected plants and animals. [Accessed 22 December 2014] http://www.mzp.cz/www/platnalegislativa.nsf/d79c09c54250df0dc1256e8900296e32/7698185c778da46fc125654b0044ddbc? (in Czech).
Demek, J. & P. Mackovčin, 2006. Geographic Lexicon of the Czech Republic: Mountains and Valleys. Nature Conservation Agency of the Czech Republic, Praha. (in Czech).
Devictor, V., J. Moret & N. Machon, 2007. Impact of ploughing on soil seed bank dynamics in temporary pools. Plant Ecology 192: 45–53.
Grulich, V., 2012. Red list of vascular plants of the Czech Republic: 3rd edition. Preslia 84: 631–645.
Hašková, J., 1990. Materials to flora of Devil´sburden (Votická vrchovina). Zprávy Československé botanické společnosti 25(2): 59–69. (in Czech).
Hejný, S., 1967. Problems of the conservation and zoning of pond reservoirs from the hydrobotanical point of view. Ochrana přírody 22: 83–90. (in Czech).
Hejný, S., 1978. Herbarium sheet. National Museum in Prague.
Hejný, S. & Š. Husák, 1978. Ecological Effect of Fishpond Amelioration. In Dykyjová, D. & J. Květ (eds), Pond Littoral Ecosystems. Springer Verlag, Heidelberg: 409–415.
Hesoun, P., S. Husák, I. Přikryl, O. Skácelová & K. Šumberová, 2008. Nature monument - Králek fishpond – monitoring of L. uniflora development.. Ms. Depon in: South Bohemian Regional Authority, České Budějovice (in Czech).
Hroudová, Z., 1972. Contribution to the phytosociological and floristic research of the fishponds near Jarošov nad Nežárkou. Sborník Jihočeského muzea v Českých Budějovicích 12(3): 129–143. (in Czech).
Husák, Š., 2000. Monitoring of Littorella uniflora Localities for Natura 2000 Programme. Ms. Depon. in South Bohemian Regional Authority, České Budějovice. (in Czech).
Husák, Š. & L. Adamec, 1998. Rescue cultivation of endangered aquatic and wetland plant species in the Institute of Botany in Třeboň. Příroda 12: 7–26. (in Czech).
IUCN, 1996. Importance of the Fishponds for the Landscape of Central Europe. Sustainable Exploitation of Fishponds in the Třeboňsko Protected Landscape Area and Biosphere Reserve. České koordinační středisko IUCN a IUCN Gland, Švýcarsko a Cambridge, Velká Británie. (in Czech).
Jílek, B., 1956. The phytosociology of fishpond communities. Preslia 28: 66–77. (in Czech).
John, H. & E. Richert, 2011. Hydrochory of Littorelletea and Isoëto-Nanojuncetea species in the Erzgebirge (Germany). Tuexenia 31: 87–104.
Jurik, T. W., S. C. Wang & A. G. van der Valk, 1994. Effects of sediment load on seedling emergence from wetland seed bank. Wetlands 14: 159–165.
Klika, J., 1935. The plant communities of the pond exposed bottoms in Central Europe. Beihefte zum Botanischen Centralblatt 53: 286–310. (in German).
Kolář, F. & J. Kolář, 2014. Littorella uniflora L. (Asch.). In: Lepší, M. & P. Lepší. Findings of Interesting and New Species in the Southern Part of Bohemia XX. Sborník Jihočeského Muzea České Budějovice Přírodní Vědy: 108–109 (in Czech).
Kolář, J., Kučerová, A. & P. Hesoun, 2015. Experience With Measures to Support the Populations of Littorella uniflora at the Nature Monument Králek. In: David, V. & T. Davidová, (eds). Collection of Abstracts from the Conference “Fishponds - Our Heritage and Wealth for the Future”. June 18–19th, 2015, Prague (in Czech).
Kučerová, A., L. Adamec, Š. Husák, E. Koutecká & M. Sosnová, 2016. Rescue introductions of endangered aquatic and wetland plant species to the Třeboňsko protected landscape area during 2005–2014. Sborník Jihočeského Muzea (in Czech) 56: 36–54.
Marhoul, P., Křesina, J. & O. Čížek, 2014. Nature monument - Horní Mrzatec fishpond, conservation plan for the period 2016–2025. [Accessed 19 January 2016] http://portal.gov.cz/portal/publikujici/ksab3eu/informace/16656_p2.pdf. (in Czech).
Murphy, K. J., 2002. Plant communities and plant diversity in softwater lakes of Northern Europe. Aquatic Botany 73: 287–324.
Pearsall, W. H., 1920. The aquatic vegetation of the English lakes. Journal of Ecology 6: 75–84.
Pokorný, J., Š. Husák & J. Květ, 1990a. Perspective of Fishpond Multi-Use. In Rambousková, H. & P. Trpák (eds), The Sustainable Development Strategy, Vol. 2. Collection of abstracts from the conference of the Study information and documents to the environment Department of Ecology of the Czech Botanical Society and the Ministry of the Environment, Prague. (in Czech).
Pokorný, J., J. Květ & P. Ondok, 1990b. Functioning of the plant component in densely stocked fishpond. Bulletin d’Ecologie 21: 44–48.
Procházka, F. & Š. Husák, 1999. Littorella uniflora (L.) Ascherson. In Čerovský, J., V. Feraková, J. Holub, S. Maglocký & F. Procházka, (eds), Red List of endangered and rare species of the Czech and Slovak Republics. 5. Vascular plants. Príroda, Bratislava (in Czech).
Pokorný, J. & V. Hauser, 2002. The restoration of fish ponds in agricultural landscapes. Ecological Engineering 18: 555–574.
R Core Team, 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Accessed 19 January 2016] http://www.R-project.org/.
Robe, W. E. & H. Griffiths, 1998. Adaptations for an amphibious life: changes in leaf morphology, growth rate, carbon and nitrogen investment, and reproduction during adjustment to emersion by the freshwater macrophyte Littorella uniflora. New Phytologist 140: 9–23.
Roelofs, J. G. M., J. A. A. R. Schmuurkes & A. J. M. Smits, 1984. Impact of acidification and eutrophication on macrophyte communities in soft waters. Aquatic Botany 18: 389–411.
Roelofs, J. G. M., T. E. Brandrud & A. J. P. Smolders, 1994. Massive expansion of Juncus bulbosus L. after liming of acidified SW Norwegian lakes. Aquatic Botany 48: 187–202.
Røslett, B. & P. Brettum, 1989. The genus Isoëtes in Scandinavia: an ecological review and perspectives. Aquatic Botany 35: 223–261.
Schoof-Van Pelt, M.M., 1973. Littorelletea: A Study of the Vegetation of Some Amphiphytic Communities of Western Europe, Thesis, Catholic University Nijmegen.
Skalický, V. & J. Vaněček, 1980. Contribution to the flora of the Blatensko region and adjacent areas III. Sborník Západočeského Muzea, Příroda, Plzeň, 361–132 (in Czech).
Skalický, V. & M. Štech, (eds), 2000. Results of the floristic course of the Botanical society of Czechoslovakia in 1974 in Humpolec., Praha (in Czech).
Slavík, B. (ed.), 2000. Flora of the Czech Republic, part 6. Academia, Praha. (in Czech).
Smolders, A. J. P., E. C. H. E. T. Lucassen & J. G. M. Roelofs, 2002. The isoetid environment: biogeochemistry and threats. Aquatic Botany 73: 325–350.
Spierenburg, P., E. C. H. E. T. Lucassen, C. Pulido, A. J. P. Smolders & J. G. M. Roelofs, 2013. Massive uprooting of Littorella uniflora (L.) Asch. during a storm event and its relation to sediment and plant characteristics. Plant Biology 15: 955–962.
Szmeja, J., 1994. Dynamics of the abundance and spatial organisation of isoetid population in an oligotrophic lake. Aquatic Botany 49: 19–32.
Szmeja, J., 1997. Evolution and conservation of Lobelia lakes in Poland. Fragm. Flor. Geobot. 42: 89–94.
Šumberová, K., 2001. Assessment of the Littorella uniflora Population at the Osika Fishpond and its Potential Threat. Ms. Depon. in Municipality of Jindřichův Hradec, Jindřichův Hradec. (in Czech).
Šumberová, K., 2011. Amphibious Vegetation with Littorella uniflora. In Chytrý, M. (ed.), Vegetation of the Czech Republic 3. Aquatic and Wetland Vegetation Academia, Praha: 282–286. (in Czech).
Šumberová, K., M. Ducháček & Z. Lososová, 2012. Life-history traits controlling the survival of Tillaea aquatica: a threatened wetland plant species in intensively managed fishpond landscapes of the Czech Republic. Hydrobiologia 689: 91–110.
Thompson, K., J. P. Bakker & R. M. Bekker, 1997. The Soil Seed Banks of North West Europe: Methodology, Density and Longevity. Cambridge University Press, Cambridge.
Tolasz, R., 2007. Climatic atlas of Czechia. Czech hydrometeorological institute and Palackého University in Olomouc, Praha, Olomouc. (in English and Czech).
West, G., 1905. A comparative study of the dominant phanerogamic and higher cryptogamic flora of aquatic habitat in three lake areas of Scotland. Proceedings of the Royal Society of Edinburg 25: 927–1023.
Wynhoff, I., 1988. Germination and vegetation development in berenings experiments with sediment. Report 248, Laboratory of Aquatic ecology, Catholic university, Nijmegen (in Dutch).
Zbíral, J., 1995. Soil analysis I-III. Unified laboratory procedures, Brno (in Czech).
Acknowledgements
This study was supported by the Grants No. 20144224, 20154213 from the Internal Grant Agency of the Faculty of Environmental Sciences, Czech University of Life Sciences Prague. It was also partly supported by the Long-term research development project no. RVO 67985939. The monitoring of the recent localities was supported by EEA and Norway grants (project nr. MGSII-11) in 2015. The authors are most grateful to Dr. Kateřina Šumberová and Dr. Lubomír Adamec for their valuable and helpful comments on the first version of the manuscript. We also thank two anonymous referees for their valuable comments. Thanks are also due to Jana Kinský for English revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Experiments were done on the critically endangered species and its parts; therefore, the permission from the Czech Ministry of Environment was needed. Numbers of the authorizations are SR/0008/TR/2014_3 and KUJI 33224/2014.
Additional information
Handling editor: Chris Joyce
Rights and permissions
About this article
Cite this article
Kolář, J., Kučerová, A., Jakubec, P. et al. Seed bank of Littorella uniflora (L.) Asch. in the Czech Republic, Central Europe: does burial depth and sediment type influence seed germination?. Hydrobiologia 794, 347–358 (2017). https://doi.org/10.1007/s10750-017-3109-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3109-3