Abstract
The 2011 Tohoku-oki tsunami damaged most sandy coastlines in Tohoku, Japan, and sandy beach vegetation was extensively disturbed. Buried seeds contribute greatly to vegetation recovery and ecological succession in general. Knowing the impact of tsunamis on seed banks and drift seeds is important in estimating the recovery of destroyed vegetation, but such information is lacking. Therefore, we examined buried seed and drift seed populations to elucidate the potential for the recovery of species diversity on sandy beaches. We collected samples of sandy sediment and debris in Iwate and Aomori prefectures, northern Tohoku, Japan. We estimated the seed populations in these samples using both the germination and seed-floating methods. In December 2011, the species composition of buried seeds in sandy sediment was very simple; ruderal plants comprising Chenopodium, Compositae, and Gramineae were remarkable. Although we found no coastal plant seeds in December 2011 sediment, eight coastal plants, Atriplex subcordata, Salsola komarovii, Setaria viridis var. pachystachys, Boehmeria splitgerbera, Leymus mollis, Linaria japonica, Carex kobomugi, and Glehnia littoralis, emerged in November 2012. Concerning alien plants, four species were observed in 2011—Bidens frondosa, Erigeron canadensis, Erigeron annuus, and Atriplex prostrata—and Plantago lanceolata was seen in 2012. The coastal species S. komarovii and the alien species Cakile edentula occurred in a debris sample in 2011. In the following year, we identified many types of coastal plants, alien plants, and other species. The seed bank that existed before the tsunami appears to have been mostly lost, and its contribution to vegetation recovery in 2011 would therefore have been small. The species composition and diversity of the seed bank after the tsunami will depend on the introduction of dispersed seeds directly from living vegetation and/or via the ocean.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Most sandy coastlines in northern Tohoku, Japan, were deleteriously affected by the tsunami caused by the Great East Japan Earthquake, with coastal vegetation suffering extensive damage. Some reports have clarified the impact of the tsunami on coastal vegetation (e.g., Hara et al. 2012; Ooue 2012; Hara 2014; Shimada 2014) and described the floristic changes in beach vegetation since the tsunami (Hayasaka et al. 2012). Ecological evaluations have been reported and conservation measures presented (Shibuya et al. 2014; Shimada 2014). Determining the resilience mechanisms of vegetation after disturbance by a tsunami is important and will contribute to the conservation of coastal ecosystems.
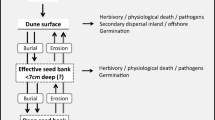
Buried seeds play an important role in vegetation restoration (Tsuyuzaki 1989; Bakker et al. 1996; Baskin and Baskin 2014) and ecological succession in general (Oosting and Humphreys 1940; Ishizuka 1962; Numata et al. 1964). The existing research on seed banks will be useful for estimating the resilience mechanisms of destroyed vegetation. However, most studies on seed banks have been conducted in grasslands and forests, and few have examined sandy coastal habitats. Furthermore, information regarding the impact of tsunamis on the seed banks of sandy beach vegetation is lacking due to the infrequency of these events.
Various seeds dispersed by seawater drift ashore (Bakker et al. 1996; Nakanishi 1988, 2013; Tokura et al. 1988). In addition, some studies have reported that seedlings of coastal plants, such as Carex kobomugi, become established in drift lines where much debris is deposited (Ishizuka 1962; Oka et al. 2009b). Ishizuka (1962) suggested that the establishment of coastal plants from seeds often starts in drift lines because the sediment includes many drifted seeds and provides a good germination bed. Although the relationship between drift seeds and seedlings in drift lines was unclear in these studies, drifted seeds may have the potential to restore damaged populations. Some species may be on the verge of local extinction due to the catastrophic disturbance resulting from a tsunami. In such cases, the introduction of seeds from the sea may contribute to the recovery of populations and increase species diversity.
Therefore, we examined buried seed populations in sandy sediment and in debris using germination tests to clarify the potential capacity for the recovery of species diversity on sandy beaches.
2 Study Sites
We sampled beach sediments on six sandy beaches—Yoshihama, Nebama, Akedo, Tofugaura, Kuji in Iwate Prefecture, and Oosuga in Aomori Prefecture, northern Tohoku—in December 2011 and November 2012 (Fig. 18.1). In Nebama (including the Murohama area) and Oosuga, sampling was conducted only in 2012.
All study sites were severely damaged by the tsunami on 11 March 2011 (Table 18.1). Wave heights reached over 10 m at every site. In Tofugaura and Akedo, waves over 20 m were observed. Seawalls and other structures were completely destroyed at Yoshihama. At Nebama, Akedo, and Tofugaura, seawalls were severely damaged as a result of the tsunami, with fracturing and collapse of protective structures. No artificial structures existed beyond the sandy beaches at Kuji (sandspit) and Oosuga (natural coast). At Yoshihama and Nebama, all areas of the sandy beach became bare; all vegetation vanished due to the tsunami and ground subsidence. At the other sites, coastal vegetation partially or mostly survived. The dominant species were Leymus mollis, C. kobomugi, and Rosa rugosa, among others. Coastal forests dominated by Pinus were not included in this investigation.
3 Methods
3.1 Sampling and Analysis
We performed a detailed analysis of buried seed communities in December 2011 with the view that seeds present at that time might strongly affect subsequent recovery of vegetation. We collected sediment samples from transects on each beach. To obtain buried seeds from various coastal habitats, we collected samples from several types of plant communities, as well as from bare ground.
To discriminate tsunami deposits from the collection samples, we tried digging a ~50–70-cm-deep pit, but we were unable to identify whether each sample corresponded to tsunami deposits. Thus, sand samples were collected from depths of 0–5 cm (surface) and 30–35 cm (underground) at each sampling point. These sampling units were 20 × 20 cm2 × 5 cm thick in each sampling layer; i.e., one sample was 2 liters (L). These 2-L samples were divided into two 1-L samples, one of which was used in the germination test and the other in the environmental analysis. In the 2012 survey, we obtained sediment only from 0 to 5 cm depth because few seeds were buried in the deeper sediment (30–35 cm depth), as described below. We collected samples from five to eight points per beach at Yoshihama, Akedo, Tofugaura, and Kuji on 3–4 December 2011. In November 2012, we collected samples at the same points as in the 2011 investigation, plus some additional points. Furthermore, we collected some samples at Nebama and Oosuga. In total, we obtained 8–12 samples on six coastlines: Yoshihama, Nebama, Akedo, Tofugaura, Kuji, and Oosuga.
Samples of debris were also collected from drift lines (linear structures formed by the deposition of drifted materials) along sand dunes (Fig. 18.2). We collected two samples per beach at Akedo and Kuji on 3–4 December 2011 and in November 2012; then we collected three to eight samples from each coastline (Yoshihama, Nebama, Akedo, Tofugaura, Kuji, and Oosuga) on 3–4 December 2011. The sampling units were again 20 cm2. All debris within the square (except anthropogenic garbage and large algae) was collected. One liter (1 L) of the samples was used for the germination test after removing large organic materials.SPSS Statistics (IBM) was used for statistical analysis.
3.2 Germination Method
Samples were placed on seed-free vermiculite in containers and set under lights in a greenhouse at the Korimoto Experimental Field of the Nature Education and Research Department, Faculty of Education, Kagoshima University, Kagoshima, Japan. These samples in containers were covered by nonwoven cloth (white in color, translucency 90 %, mesh size about 0.2–0.5 mm) to prevent outside seeds from entering the sample. Samples were watered twice daily (early morning and evening) using a water sprinkler. The test was conducted for about 1 year, from 26 December 2011 to 2 December 2012 for the samples collected in 2011 and 30 December 2012 to 29 November 2013 for those collected in 2012. If we could not identify a species from the morphological characteristics of the stem and leaves (e.g., Gramineae, Cyperaceae), the seedlings were transplanted and cultivated until they flowered.
3.3 Floating Seed Method
To detect seeds that did not emerge in the germination test, the sample from the container used in the germination test was immersed in a solution of 50 % K2CO3 (Tsuyuzaki and Goto 2001). This test was performed only for the 2011 samples and followed the germination test, i.e., from December 2012 to June 2013.
The samples were immersed in 50 % K2CO3 solution and agitated; organic deposits that floated to the surface were then scooped off and washed with water. This operation was repeated about five times, until no further deposits floated to the surface. Seeds were identified based on their morphological characteristics and size, according to Nakayama et al. (2000), Asano (2005), Shimizu et al. (2001), Suzuki et al. (2012), and Uemura et al. (2010). When species determination was difficult, identification was completed to the genus or family level. After identification, we estimated seed viability using the crushing technique (Naka and Yoda 1984) and TTC (2,3,5-triphenyltetrazolium chloride) methods (Weed Science Society of Japan 2001). If the embryo and albumen were empty or brown, the seeds were considered dead (Naka and Yoda 1984); if white, viability was validated using the 0.5 % TTC solution, and if pink the seed was considered alive.
3.4 Ecological Character of Identified Plants
We categorized species identified in the germination and flotation tests into three types: coastal plants, alien plants, and other species. The list of coastal plants in Japan was derived from Sawada et al. (2007) and that of alien species was based on YList (Yonekura and Kajita 2003).
4 Results and Discussion
4.1 Species Composition of the Seed Bank in December 2011
4.1.1 Buried Seeds in the Sandy Surface Sediment (0–5 cm Depth)
We identified 18 species in the germination test for all study sites, not counting unknown species: six species at Yoshihama and Akedo, four at Tofugaura, and nine at Kuji. Seed density was highest at Akedo, at 6.4 ± 11.8 L−1 (Table 18.2). The densities at other sites were about 3 L−1 (Yoshihama and Kuji) or 1 L−1 (Tofugaura). The overall seed density in these samples was low. Chenopodium album emerged at every study site. Other Chenopodium species (C. glaucum and C. ambrosioides) were also observed. In addition, Compositae (e.g., Artemisia indica var. maximowiczii, Youngia japonica), Gramineae (e.g., Digitaria ciliaris, Digitaria violascens, Panicum dichotomiflorum, Zoysia japonica), and Oenothera (perhaps including O. biennis, O. parviflora, O. glazioviana) were remarkable.
The floating seed method detected live seeds of Chenopodium spp. and some freshly dead seeds of Chenopodium spp., Cyperus sp., Compositae sp., Eleusine indica, and Salsola komarovii. Some of these freshly dead seeds may have died during the germination or floating tests, as they still contained white, apparently living tissue. Although the cause of death of these seeds was unclear, they might have survived if they had been preserved in good condition on the beach. Because the tests in our study took a long time, seed survivorship might have been underestimated. Some studies have reported that the seed burial depth affects seedling emergence in some annual plants (Yamanaka et al. 2000; Kondo et al. 2002; Tobe et al. 2007). Although we observed some species that comprise the vegetation of sandy coasts (e.g., Sagina maxima and Sonchus brachyotus), coastal species (Sawada et al. 2007) were lacking. Also, we observed many species as dead seeds, e.g., members of Rosa (perhaps the dominant species in living vegetation, R. rugosa), common species of the Polygonaceae (Persicaria, Rumex), and deciduous trees distributed on mountain slopes—Acer, Actinidia, Zelkova, and Tilia, among others.
As a general trend at these study sites, alien species and other species consisting of ruderal plants belonging to the Chenopodiaceae, Compositae, and Gramineae dominated the buried seed community. Coastal plants were absent from the seed bank in the surface layer of sandy sediment.
In this study, we could not determine whether the sandy sediment on the ground surface was deposited by the tsunami. Szczuciński et al. (2012a) reported the existence of tsunami deposits (a few cm thick) on a beach on the Sendai Plain. These tsunami deposits comprised mainly of medium and coarse sand. This tendency was also reported following the 2004 Indian Ocean tsunami (Szczuciński et al. 2012b). In general, medium and coarse sand were found along the coastlines examined. In addition, coastal sand is frequently moved by wind, and thus distinguishing tsunami deposits is difficult.
4.1.2 Buried Seeds in Underground Sediment (30–35 cm Depth)
We found very few seeds in samples collected in sediment from 30 to 35 cm depth (Table 18.2). The total seed density at each site was below 1.0 L−1 (Table 18.2), significantly lower than on the surface (Mann–Whitney U test, p < 0.01). We found four species (A. indica var. maximowiczii, D. ciliaris, Eragrostis multicaulis, and Poa annua), but the number of species in each sample was also smaller than on the surface (p < 0.01).
Floating revealed a few seeds in samples from 30 to 35 cm (Table 18.3). Freshly dead Chenopodium spp. and Cyperus spp. seeds were confirmed, but the other species’ seeds were dead. We found no living seeds.
As a result, the number of living seeds (germinated seeds + living seeds) from the 30 to 35-cm layer was significantly less than on the surface (p < 0.01). Although buried seeds of some non-coastal species (e.g., Chenopodium, Artemisia, Digitaria, and Poa) were found in the deeper layers, their regeneration would have been unlikely as the seeds were very few in number and almost dead.
4.2 Changes in Buried Seed Diversity and Arrival of Drifted Seeds
We examined changes in buried seeds’ composition and the arrival of drifted seeds after 1 year, as these factors will greatly affect vegetation recovery. At that time, the seeds buried in the surface sandy sediment and the drifted seeds in the debris as determined by the germination test in 2012 will also be present (Table 18.2, Figs. 18.3 and 18.4).
4.2.1 Changes in Buried Seed Composition in Sandy Sediments
The mean density of seeds at Yoshihama, Akedo, and Kuji increased greatly in 2012, to 96.6 ± 177.3, 24.3 ± 32.1, and 30.7 ± 75.3 L−1, respectively (Fig. 18.3). Increases in the density of alien species and other species were responsible for this result. We found almost no change at Tofugaura. Species density (d; d = S/log10A) had the same trend. Although d values were <10 at all sites, the values increased at Yoshihama (48.0), Akedo (26.2), Tofugaura (4.4), and Kuji (20.2). The d values at Nebama and Oosuga were 18.2 and 3.7, respectively.
We found no coastal plants’ seeds in December 2011 sediment, as noted above. However, eight coastal plants (Atriplex subcordata, S. komarovii, Setaria viridis var. pachystachys, Boehmeria splitgerbera, L. mollis, Linaria japonica, C. kobomugi, and Glehnia littoralis) emerged in November 2012 (Table 18.2). Four alien plant species were observed in 2011, comprising only Chenopodium and Oenothera species. In 2012, Compositae (Bidens frondosa, Erigeron canadensis, Erigeron annuus), A. prostrata, and Plantago lanceolata were also observed. Although the “other species” groups in 2011 consisted mainly of weeds from an upland field that belonged to Compositae, Gramineae, and Oenothera, many species were added to the buried seed communities in 2012, e.g., paddy field weeds (Rorippa palustris, Echinochloa crus-galli var. crus-galli, and Eclipta thermalis), Rumex spp., and Setaria pumila, among others.
The number of species observed in each species group increased, probably because of seeds provided from surrounding vegetation and/or other shore plants.
4.2.2 Seeds That Drifted Ashore
In 2011, the mean densities of drifted seeds (mixed in debris) at Akedo and Kuji were 11.0 ± 11.3 and 3.0 ± 2.8 (no. L−1), respectively (Fig. 18.4). Coastal plants accounted for more than half of the seeds at both sites. The coastal species S. komarovii and the alien species Cakile edentula occurred at Akedo (Table 18.2). Additionally, we also observed some ruderal herbs (Rumex spp., Y. japonica) and riparian trees (e.g., Zelkova serrata and Pterocarya rhoifolia).
During 2012, we obtained samples at the other four sites; the mean density of drifted seeds was below 15 L−1 at every site (Fig. 18.4). We could not confirm the seeds in the Akedo debris samples. Many kinds of coastal plants, alien plants, and species were confirmed at five sites, except Akedo (Table 18.2). Species density, d, was higher at Nebama, Kuji, and Oosuga than at the other sites.
Coastal plants observed in debris included C. kobomugi, S. komarovii, L. mollis, Atriplex patens, and Artemisia stelleriana. Among alien species, various Compositae (e.g., B. frondosa and E. canadensis) and Chenopodiaceae (e.g., C. glaucum and A. prostrata) had relatively high densities. Additionally, ruderal plants (e.g., A. indica var. maximowiczii, D. ciliaris, and Rumex spp.) and some crop seeds (e.g., Momordica charantia and Zea mays) were also observed. At Akedo, we found no buried seeds in debris in 2012, despite finding many seeds in the 2011 samples, perhaps because local people had cleaned up the beach in the interim.
4.3 Contribution of the Seed Bank to Vegetation Recovery After the Tsunami Disturbance
In this study, we detected buried seeds that had been dispersed before December 2011. However, as it is not possible to know the distribution of buried seeds immediately after and before the tsunami, the origin of seeds buried in sandy sediments has been estimated in some reports.
The existence of seed banks on sandy coastlines has been demonstrated previously (e.g., Pierce and Cowling 1991; Looney and Gibson 1995; Aziz and Khan 1996). In Japan, Fujiki et al. (2001) reported the seed bank species composition at Tottori Sand Dunes. That study showed that the C. kobomugi community seed bank consisted of C. kobomugi, Ischaemum anthephoroides, and E. indica (mean seed density: 6330.0 ± 8385.5 m−2 × 10 cm in November). Sawada and Tsuda (2005b) clarified the formation potential of a persistent seed bank of Lathyrus japonicus, G. littoralis, Calystegia soldanella, C. kobomugi, Carex pumila, Oenothera laciniata, Ixeris repens, I. anthephoroides, and others. C. kobomugi had a seed bank and viable seeds concentrated in the surface layer (0–4 cm depth; Yamanaka et al. 2000). The above-cited species were widely distributed at our study sites. Consequently, coastal vegetation may have had seed banks in the surface sand before the tsunami.
According to vegetation survey data collected by Hayasaka et al. (2012) before the tsunami, major coastal plants, such as L. mollis, C. kobomugi, C. pumila, L. japonicas, I. repens, and C. soldanella, were widely distributed at these study sites. Chenopodium album (the dominant species among the buried seeds in our study) was not found at Yoshihama, Akedo, Tofugaura, or Kuji before the tsunami. At our study sites, however, C. album emerged at every site, and coastal plants were absent from the seed bank in the surface layer of sandy sediment.
In addition, some reports have shown erosion of sandy beaches and dunes in Miyagi Prefecture (Nishi et al. 2012; Richmond et al. 2012). According to aerial photographs and reports by Ooue (2012) and Shimada (2014), coastal Pinus forests at Yoshihama, Nebama, and Akedo vanished, probably due to erosion and ground subsidence.
Given these observations, it is likely that the sandy beach vegetation and topsoil at our study sites were severely eroded by the tsunami. Therefore, the seed bank that existed before the tsunami was mostly lost, and the contribution of a seed bank to vegetation recovery in 2011 would have been small. The species composition and diversity of the seed bank after the tsunami will depend on the introduction of dispersed seeds directly from living vegetation and/or through the ocean.
The dispersal route of seeds shows three general patterns: (1) dispersal from coastal vegetation on the same beach, (2) dispersal from inland vegetation in the same region, and (3) dispersal from coastal vegetation on another beach. At Oosuga, Kuji, Tofugaura, and Akedo, all three dispersal routes might be in effect because coastal vegetation remained only partially after the tsunami disturbance. If some perennial plants survive, recovery of vegetation after a tsunami may be relatively rapid. The vegetation used to occupy a broader area in these regions (Kawanishi, unpublished data). Many surviving perennial plants seem to be growing by vegetative reproduction. These plants will also produce seeds, and some dispersed seeds will become dormant and comprise the seed bank. However, the regeneration of plants that have vanished from a beach, especially in the case of bare beaches such as Yoshihama and Nebama, will depend largely on routes (2) and (3). In this case, from the viewpoint of the recovery of coastal plants, route (3) (i.e., drifted seeds) will be very important.
In this study, we observed many drifted seeds of S. komarovii (a coastal plant) at Akedo and Kuji in 2011. This species can disperse continuously via the sea and form communities on drift lines (Nakanishi 2013). Additionally, we observed A. subcordata, C. kobomugi, L. mollis, G. littoralis, A. patens, A. stelleriana, I. anthephoroides, and I. repens in 2012. Sawada and Tsuda (2005a) clarified the potential sea dispersal of A. subcordata, A. patens, L. japonicas, G. littoralis, C. soldanella, I. repens, C. kobomugi, and C. pumila due to their ability to float on seawater for extended periods. Additionally, C. pumila, C. kobomugi, C. soldanella, and Tetragonia expansa can disperse to remote beaches (Oka et al. 2009a). These species (distributed at our study sites) may disperse widely on sandy coastlines. However, some coastal plants cannot float for long, and dispersal to distant locations will be difficult for them (Sawada and Tsuda 2005a).
Therefore, if local vegetation has survived a tsunami, it should be preserved because it will have a very important role as a source of seeds and contribute to the efficient recovery of damaged populations.
Additionally, attention should be given to the behavior of alien species. Ajima (2001) clarified the seed accumulation of alien species in seed banks in many types of plant communities. After extensive disturbance by the tsunami, some alien species, such as the Chenopodiaceae, Compositae, and Cruciferae, formed a community (Hayasaka et al. 2012; Kanno et al. 2014; Oka and Hirabuki 2014). Although alien species’ communities have declined over the years since the tsunami (Kanno et al. 2014), we found alien seeds, especially Chenopodiaceae (C. album, C. ambrosioides, C. glaucum, and A. prostrata), Compositae (B. frondosa, E. canadensis, and E. annuus), and other taxa (Oenothera spp., P. lanceolata, and Trifolium repens, Table 18.2, Fig. 18.4), buried in sediment and debris. Hence, these alien species have the potential to accumulate in the seed bank. The species composition of buried and drifted seeds will be important to conservation. Our results indicate the importance of the remaining living vegetation and seed inputs from the sea. Considering the recovery of coastal vegetation, we must estimate the ecological succession at each site. To recognize the process and mechanism of succession, we have to continue to monitor, survey, and analyze the relationships between seed banks and vegetation.
References
Ajima M (2001) Accumulation of seeds of exotic species in the soil seed bank in Japan. Jpn J Conserv Ecol 6:155–177 (in Japanese)
Asano S (2005) Seeds/fruits and seedling of plants in Japan. Zenkoku Noson Kyoiku Kyokai, Tokyo, 279 pp (in Japanese)
Aziz S, Khan MA (1996) Seed bank dynamics of a semi-arid coastal shrub community in Pakistan. J Arid Environ 34:81–87
Bakker JP, Poschlod P, Strykstra RJ, Bakker RM, Thompson K (1996) Seed banks and seed dispersal: important topics in restoration ecology. Acta Bot Neerlandica 45(4):461–490
Baskin C, Baskin J (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Academic, San Diego, 1586 pp
Fujiki D, Yamanaka N, Tamai S (2001) Relationship between vegetation types and seed banks on Tottori Sand Dune. J Jpn Soc Revegetation Technol 26(3):209–222 (in Japanese with English summary)
Hara M (2014) Results of a survey of tsunami effects. Veg Sci News 18:21–40 (in Japanese)
Hara M, Asami K, Tomita M (2012) The society of vegetation science report on the great east Japan earthquake in the south sanriku region. Veg Sci News 16:40–48 (in Japanese)
Hayasaka D, Shimada N, Konno H, Sudayama H, Kawanishi M, Uchida T, Goka K (2012) Floristic variation of beach vegetation caused by the 2011 Tohoku-oki tsunami in northern Tohoku, Japan. Ecol Eng 44:227–232
Ishizuka K (1962) Ecological studies on the vegetation of coastal sand bars II. Succession in vegetation and developmental processes of dunes. Ann Rep Gakugei Fac Iwate Univ 20:140–168
Kanno H, Hirabuki Y, Sugiyama T, Tomita M, Hara K (2014) Vegetation change in various coastal forest habitats after a huge tsunami: a three-year study. In: Disturbance and recovery of the coastal-ecotone vegetation following the Great East Japan Earthquake and Tsunami. Jpn J Conserv Ecol 19:201–220
Kondo T, Sakai A, Sasaki S (2002) Effects of sowing dates and burial depth on seedling emergence and survival of Calystegia soldanella Roem. et Schult. and Lathyrus japonicus Willd. J Jpn Soc Revegetation Technol 28(2):330–341 (in Japanese with English abstract)
Looney PB, Gibson DJ (1995) The relationship between the soil seed bank and above-ground vegetation of a coastal barrier island. J Veg Sci 6:825–836
Naka K, Yoda K (1984) Community dynamics of evergreen broadleaf forests in south western Japan–2–Species composition and density of seeds buried in the soil of a climax evergreen oak forest. Bot Mag Tokyo 97:61–79
Nakanishi H (1988) Dispersal ecology of the maritime plants in the Ryukyu Islands, Japan. Ecol Res 3:163–173
Nakanishi H (2013) Seedling populations from sea-borne seeds on drift debris in northern and western Kyushu, Japan. Veg Sci 30:17–24 (in Japanese with English abstract)
Nakayama S, Marehide I, Tadashi M (2000) Seeds of wild plants in Japan. Tohoku University Press, Sendai, 678 pp (in Japanese)
Nishi R, Manu J, Jansen T, Hayashi K (2012) Scarp generation behind a dune and shore protection structure by tsunami on March 11, 2011. Proc Civ Eng 68(2):198–203 (in Japanese with English summary)
Numata M, Hayashi I, Komura T, Oki K (1964) Ecological studies on the buried-seed population in the soil as related to plant succession I. Jpn J Ecol 14:207–215 (in Japanese with English synopsis)
Oka K, Hirabuki Y (2014) Revegetation of coastal plants damaged by the 2011 Tohoku tsunami. In: Disturbance and recovery of the coastal-ecotone vegetation following the Great East Japan Earthquake and Tsunami. Jpn J Conserv Ecol 19:189–199 (in Japanese with English abstract)
Oka K, Okuma M, Yoshizaki S (2009a) Estimation of the source of seed supply by thalassochory for the Higashimachi district of the Shonan coast, Kanagawa Prefecture. Bull Int Assoc Landsc Ecol Jpn 13:45–54 (in Japanese with English abstract)
Oka K, Yoshizaki S, Kobori H (2009b) The emergence and establishment of seedlings of five coastal dune plants on the Enshu-nada coast, Shizuoka Prefecture, central Japan. Veg Sci 26:9–20 (in Japanese with English abstract)
Oosting HJ, Humphreys ME (1940) Buried viable seeds in a successional series of old field and forest soils. Bull Torrey Bot Club 67:253–273
Ooue M (2012) Circumstance of coastal vegetation affected by tsunami in northern and mid area of Kitakami Mountains. Veg Sci News 16:49–58 (in Japanese)
Pierce SM, Cowling RM (1991) Disturbance regimes as determinants of seed banks in coastal dune vegetation of the southeastern Cape. J Veg Sci 2:403–412
Richmond B, Szczuciński W, Chagué-Goff C, Goto K, Sugawara D, Witter R, Tappin DR, Bruce J, Fujino S, Nishimura Y, Goff J (2012) Erosion, deposition and landscape change on the Sendai coastal plain, Japan, resulting from the March 11, 2011 Tohoku-oki tsunami. Sediment Geol 282:27–39
Sawada Y, Tsuda S (2005a) Thalassochory potential in 14 species of coastal plants in the warm temperate zone of Japan. Veg Sci 22:53–61 (in Japanese with English abstract)
Sawada Y, Tsuda S (2005b) Potential for persistent seed bank formation in 14 coastal dune plants in the warm temperate zone in Japan. Veg Sci 22:135–146 (in Japanese with English abstract)
Sawada Y, Nakanishi H, Oshida K, Hattori T (2007) A check list of coastal plants in Japan. Hum Nat 17:85–101 (in Japanese with English abstract)
Shibuya K, Shimada N, Suzuki M (2014) Study of the present condition of the Sanriku coastal eco-tone and environmental conservation measures in Iwate Prefecture. J Policy Stud 15:181–199 (in Japanese with English abstract)
Shimada N (2014) Effect of tsunami with the Great East Japan earthquake to coastal vegetation and circumstance of conservation, in Iwate Pref. Veg Sci News 18:44–54 (in Japanese)
Shimizu N, Morita H, Hirota S (2001) Picture book of alien plants in Japan 1. Zenkoku Noson Kyoiku Kyokai, Tokyo, 554 pp (in Japanese)
Suzuki I, Takahashi F, Yasunobu N (2012) Seeds and fruits illustrated. Seibundo-Shinkosha, Tokyo, 272 pp (in Japanese)
Szczuciński W, Kokociński M, Rzeszewski M, Chagué-Goff C, Cachão M, Goto K, Sugawara D (2012a) Sediment sources and sedimentation processes of 2011 Tohoku-oki tsunami deposits on the Sendai Plain, Japan—insights from diatoms, nannoliths and grain size distribution. Sedimentol Geol 282:40–56
Szczuciński W, Rachlewicz G, Chaimanee N, Saisuttichai D, Tepsuwan T, Lorenc S (2012b) 26 December 2004 tsunami deposits left in areas of various tsunami runup in coastal zone of Thailand. Earth, Planets and Space 64:843–858
Tobe K, Liping Z, Omasa K (2007) Seed germination and seedling emergence of three annuals growing on desert sand dunes in China. Ann Bot 95:649–659
Tokura R, Tanaka T, Yanobu N (1988) Current-dispersed seeds from Kumihama Sand Dune. Bulletin of Kyoto University of Education. Series B. Math Nat Sci 73:25–30 (in Japanese with English Abstract)
Tsuyuzaki S (1989) Contribution of buried seeds to revegetation after eruptions of the volcano Usu, northern Japan. Bot Mag Tokyo 102:511–520
Tsuyuzaki S, Goto M (2001) Persistence of seed bank under thick volcanic deposits twenty years after eruptions of Mount Usu, Hokkaido Island, Japan. Am J Bot 88:1813–1817
Uemura S, Katsuyama T, Shimizu N, Mizuta M, Morita H, Hirota S, Ikehara N (2010) Picture book of alien plants in Japan, vol 2. Zenkoku Noson Kyoiku Kyokai, Tokyo, 579 pp (in Japanese)
Weed Science Society of Japan (ed) (2001) Method in weed science. Weed Science Society of Japan, Tokyo, 463 pp (in Japanese)
Yamanaka N, Imada M, Tamai S (2000) Effects of sand movement on the seed bank formation and seedling dynamics of Carex kobomugi Ohwi. Sand Dune Res 47(1):1–11 (in Japanese with English abstract)
Yonekura K, Kajita T 2003 BG plants (YList). http://ylist.info/. Last accessed 22 July 2015 (in Japanese)
Acknowledgments
We thank Mr. Mitsuru Ikeda and Mrs. Miyo Ryuuno of the Experimental Field of the Nature Education and Research Department, Faculty of Education, Kagoshima University, for their technical support and helpful suggestions on the germination test. We also thank Ms. Mio Hidaka, Mr. Takuma Maeda, Mr. Teppei Hashimoto, Mr. Ryou Yamakita, and members of the Laboratory of Botany, Faculty of Education, Kagoshima University, for their assistance in the field survey. This study was supported by a research grant from the Mitsui & Co., Ltd. Environment Fund (R11-F1-015-1, Leader: Daisuke Hayasaka).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Kawanishi, M., Hayasaka, D., Shimada, N. (2016). The Species Composition of Buried Seeds of Seashore Vegetation Disturbed by the Great East Japan Earthquake and Tsunami in Northern Tohoku, Japan. In: Urabe, J., Nakashizuka, T. (eds) Ecological Impacts of Tsunamis on Coastal Ecosystems. Ecological Research Monographs. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56448-5_18
Download citation
DOI: https://doi.org/10.1007/978-4-431-56448-5_18
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56446-1
Online ISBN: 978-4-431-56448-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)