Abstract
The existence of a dormant “bank” of plant seeds plays an important role in maintaining and preserving species and genetic diversity. However, information on the spatial heterogeneity of the pool of dormant seeds among wetland complexes along riverine systems is limited. In this study we collected sediment from 18 wetlands within six wetland complexes along the Murray River, Australia. The germinable, residual and viable seed banks in each wetland complex were assessed by undertaking a germination trial, counting seeds (morphotypes) and viability testing. A diverse and viable seed bank exists within all the complexes however seed bank communities differed among wetland complexes. There was no difference in the viability of seeds within the sediment profile, however more seeds occurred in the surface layers of sediment and communities differed with sediment profile depth. In general, the number of species germinating was fewer than the number of seed morphotypes counted. Management actions need to be targeted not only at preserving the extant plant communities but also to ensure the seed bank is replenished. This may also involve allowing wetlands to dry sufficiently to enable cracking of wetland sediment and seeds to become buried to maximise the longevity of the seed store.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flow regimes associated with riverine landscapes create and sustain a mosaic of wetlands and habitat types. Within this heterogeneous landscape, diverse biotic communities are maintained, many of which are specific to individual wetlands (Boulton and Brock 1999; Capers et al. 2010; Nielsen and Brock 2009; Turner et al. 2001). The heterogeneity in wetland and habitat types is reflected in the composition of floodplain and wetland plant communities that vary spatially and temporally, depending on the availability of water and flow (Greet et al. 2012), season (Greet et al. 2013, 2011), availability of propagules (Andersson and Nilsson 2002; Boedeltje et al. 2004) and geomorphological factors such as; the shape, depth and size of habitats (Thoms et al. 2006, Barrett et al. 2010). These factors demonstrate that regional and local factors influence the composition of communities (Leibold et al. 2004) and that the movement and presence of propagules (seeds, fragments, root stock) is likely to be important to the persistence of biotic diversity (Jansson et al. 2005; Merritt et al. 2010; Nilsson et al. 2010).
Plants can be dispersed by multiple vectors - primarily water, animals (e.g. waterbirds) and wind (Howe and Smallwood 1982). The distance that a propagule will disperse may be dependent on its morphology and the mode of dispersal (Lacoul and Freedman 2006; Morris 2012; Raulings et al. 2011; Soons 2006), and each mode has the capacity to disperse propagules over large distances. For example, ducks are highly transient and are known to disperse seeds, either ingested or attached to feathers (Raulings et al. 2011; Soons et al. 2016) whereas seeds of some species can be dispersed many kilometres by wind (Soons 2006). In contrast to dispersal by animals and wind which move propagules in multiple directions, dispersal by water is unidirectional. In riverine-floodplain systems, transport by water is a well-recognised mode of dispersal that is important for maintenance of diversity in wetlands. The capacity of seeds and or propagules to disperse, and the distance they will disperse depends on morphology, physical characteristics such as the size of the seed or propagule (Carthey et al. 2016; Nilsson et al. 2010; Stromberg et al. 2011) and by the seasonality and duration of flow (Boedeltje et al. 2004; Riis 2008).
Wetlands associated with lowland rivers vary in the frequency and duration of inundation and connection and may experience periods of complete dryness (Boulton and Brock 1999). Many wetland plant species survive adverse environmental conditions, such as complete drying, by producing long-lived dormant seeds (Brock et al. 2003) which may persist for extended periods of time (Brock 2011; Brock et al. 2003; Leck and Brock 2000; Nielsen et al. 2013) and are generally referred to as a “seed bank” (Templeton and Levin 1979). The dynamics of the seed bank are primarily governed by the production of seeds and via inputs from dispersal. Seeds may be lost or gained via dispersal and seed germination as well as general ageing, predation and burial, inputs into the seed bank generally exceed losses (De Stasio 1989). In general, inputs into the seed bank exceed losses resulting in an accumulation of seeds in the sediments of wetlands (Brock et al. 2003; James et al. 2007; Porter et al. 2007). However, long term exposure to adverse environmental conditions such as drying (Nielsen et al. 2013) and burial of seeds will reduce the diversity and numbers of plants capable of germinating (Espinar and Clemente 2007; Grillas et al. 1993; Leck and Simpson 1987; van Der Valk and Davis 1979). The existence of a seed bank in the soil has been documented for many different types of plant communities and is important in maintaining ecological (species) and genetic diversity in plant populations and communities (Templeton and Levin 1979; Thompson and Grime 1979).
In this paper we explore changes in the abundance, species richness and community composition of plant seed banks associated with six wetland complexes associated with the Murray River in south eastern Australia and their distribution and viability down a sediment profile. We used two methods to compare seed banks; germination trials and direct counting of seeds. Two hypothesis are tested (1) if flooding promotes longitudinal dispersal of propagules along a river, seed bank plant communities within the sediment of each wetland complex will be similar, and (2) seed density and viability will decrease with depth in the sediment profile.
Materials and methods
Sediment collection
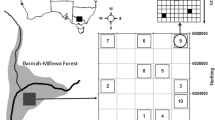
Sediment was collected from six wetland complexes along the length of the Murray River. Barmah Forest (BF) is the most easterly, then Gunbower Forest (GF—200 river kilometres downstream of Barmah), Hattah Lakes (HL—700 km), Lindsay-Mulcra-Wallpolla Islands (LMW—1000 km), Chowilla Floodplain (CF—1150 km) and the Lower Lakes (1750 km) at the mouth of the Murray River. Along the Murray River there is an environmental gradient, with rainfall decreasing and temperatures increasing in a westerly direction. Salinity levels and other water quality parameters also increase along the length of the river (Shafron et al. 1990). The Murray River flows through a relatively flat landscape falling from approximately 110 meters A.S.L. at Barmah Forest to sea level at the Lower Lakes. For the majority of wetlands in south-eastern Australia there is limited information on the frequency, duration or timing of inundation nor is there any information on variations over time in water quality (Boulton and Brock 1999).
All six wetland complexes have been recognised as either internationally or nationally significant due to their ecological values and characteristics (Environment Australia 2001) (Fig. 1) and in general, they all consist of mosaics of semi-permanent and ephemeral lakes, swamps and channels.
All wetlands within these complexes undergo periods of drying which may persist for months to years. Within these wetlands a viable seed bank can persist for at least 10 years before significant losses begin to occur (Brock 2011; Leck and Brock 2000; Nielsen et al. 2013; van Der Valk and Davis 1979). All six wetland complexes underwent significant flooding during 2010–2013. Prior to this south-eastern Australia had been significantly impacted by extended drought (Murphy and Timbal 2008).
Three wetlands were sampled within each wetland complex between November and December 2013. In each wetland, four transects were randomly located within the wetland, extending from the estimated high water mark towards the middle of the wetland. Along each transect, five equally spaced cores were collected giving a total of 20 cores per wetland. Each core was 10 cm in depth and 4 cm in diameter. Therefore, from each wetland, a surface area of 0.025 m2 and a volume of 0.003 m3 was sampled, giving a combined surface area of 0.075 m2 and volume of 0.008 m3 from each wetland complex. This compares favourably with combined surface areas required per treatment of 0.1 m2 reported by Forcella (1984) and 0.08 m2 reported by Brock et al. (1994). Cores were enclosed in cling wrap for protection and transported to the laboratory for processing.
In the laboratory, each core was sliced into 2.5 cm layers (labelled 2.5, 5, 7.5 and 10 cm). As drying after wetting has been demonstrated to promote dormancy breakage and increase germination from habitats that undergo unpredictable flooding (Bonis et al. 1995; Casanova and Brock 2000; Porter et al. 2007), sediment was dried and lightly crushed to break up clods, allow mixing of the sediment and to remove existing vegetation (Brock et al. 2005; Crosslé and Brock 2002). Slices of cores from each depth within each wetland were combined and well mixed to give a single sample per depth per wetland (i.e. for each wetland there were four sediment depth samples that were a composite of the 20 original cores). Sediment from each of the layers was then divided into three for use in three different trials: (1) assessment of seed banks using germination trials (germinable seed bank); (2) assessment of seed bank by direct counting of seeds identified to morphotypes (potential seed bank) and a subset of these seeds assessed for viability. To allow comparison between the abundance of seeds germinating and the abundance of seed morphotypes counted, all counts were converted to numbers of seeds per m2.
Germinable seed bank
The germinable seed bank is defined by (1) Species richness—the number of species germinating, and (2) Abundance of seeds germinating—the number of seeds that germinated (expressed as seeds per m2).
Sediment for the germination trial was separated into four replicate pots for each wetland at each of the four sediment depth. Pots (10 cm diameter and 6 cm deep) were filled with sand to a depth of 4 cm and a known weight of sediment added to the top to give a wetland depth of approximately 2 cm (Nielsen et al. 2003). As previous germination studies have indicated that fewer species germinate under flooded conditions compared to damp conditions, sediment in this study was exposed to a single damp treatment (Capon and Brock 2006). Therefore, the germination trial consisted of sediment collected from 18 wetlands each with four sediment depths with four replicates of each giving a total of 288 pots. Pots were randomly assigned to tanks within a glasshouse at Wonga Wetlands (Albury, NSW). At the end of 12 weeks all plants were counted and identified to lowest possible taxonomic resolution to give germinable richness and abundance. Identification and nomenclature of angiosperms and ferns follows the Australian Plant Name Index (http://www.anbg.gov.au/apni/) and the Australian Plant Census (http://www.anbg.gov.au/chah/apc). After harvesting, the numbers of plants germinating within each pot were converted to abundance (numbers per m2) and the average of each replicate sediment depth in each wetland obtained. Therefore, in the analysis there was a single sample from each sediment depth for each wetland.
Potential seed bank
The potential seed bank is the number of seeds that remain ungerminated under the experimental conditions. As the taxonomy of wetland seeds is poorly described, seeds were grouped by morphotype based on size, shape and appearance. The potential seed bank is defined by (1) Morphotype richness—the number of different seed morphotypes counted, and (2) Abundance of morphotypes—estimated number of each seed morphotype counted in the sediment (expressed as seeds per m2).
The potential seed bank was assessed by directly recovering seeds from the sediment samples using the floatation method developed by Malone (1967). From each sample, a 10 g subsample was added to 20 ml of the extraction solution in a centrifuge tube. The extraction solution was made by combining 10 g sodium hexametaphosphate (Calgon) with 5 g of sodium bicarbonate and 25 g of magnesium sulfate dissolved in 200 ml of water (Malone 1967). In brief, the sediment and extraction solution within the centrifuge tube were shaken vigorously for 10 s to form a slurry, before being mechanically agitated on a vortex mixer on medium setting for 5 min. The agitation causes separation of organic materials through floatation. The floating organic layer containing seeds is decanted from the solution and passed through a 90 µm sieve to retain seeds. The remaining soil residue was re-agitated with fresh extraction solution twice more to ensure that all organic material was extracted. Seeds and organic material were then transferred into specimen sorting trays and dried in an oven overnight at 40 °C.
As the taxonomy of aquatic seeds is poorly described, all seeds were sorted into morphotypes and the number of each morphotype counted with the aid of a Zeiss Stemi SV 6 stereomicroscope (Zeiss, Germany). Only those seeds that were deemed to be intact and undamaged were included in the analysis. Some of the morphotypes detected were “fruits” which when dissected contained multiple seeds. These “fruits,” were counted as a single seed. Therefore, the numbers reported are an underestimation of the actual number of seeds present. Numbers counted from each sample were converted to numbers per m2.
Residual seed bank
The residual seed bank is the estimated abundance of viable seeds within the potential seed bank, obtained by multiplying the estimated abundance of morphotypes by the estimated proportion of viable seeds.
The viability of seeds was determined using the tetrazolium test (TZ test) (Cottrell 1948). As every species requires its own specific instructions for handling, preparation, dissection and evaluation and due to lack of available information on aquatic and riparian species, a common method was compiled and applied to all seeds tested. Seeds were preconditioned to rehydrate the tissue and to activate the germination process by placing seeds on moist paper towel for 24 h. After preconditioning, embryos were either dissected from seeds or seeds were cut, so that the embryo was bisected and then placed in a 1% solution of 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) and placed in an oven at 30 °C for 24 h, after which the seeds were rinsed prior to evaluation. As viable seeds release hydrogen ions during respiration, viable seeds reduce the dye and viability is detected as a colour change (viable embryos appear pink).
Due to time constraints only one replicate set of samples from each wetland was analysed for viability.
Statistical analysis
Due to differences in the taxonomic resolution between the germination trials (species) and seed counts (morphotypes) there is no intent to statistically compare differences between the two communities.
Multivariate analyses of patterns in plant species community composition were carried out in PRIMER (V6.1.10), with the PERMANOVA + for PRIMER 6 (PRIMER-E, Plymouth, U.K.) (Anderson et al. 2008; Clarke et al. 2014).
For the germination and seed count data, data were square-root transformed. All subsequent analysis were derived from a Bray-Curtis similarity matrix. Patterns of community composition (based on species richness and abundance) among wetland complexes for both the germination and seed morphotypes count data were visualised using multivariate statistical procedures. Specifically metric multidimensional scaling (MDS) using boot-strap averages and displaying the regions in which 95% of the boot-strap averages fall were used to visualise patterns of community composition among the wetland complexes (Clarke et al. 2014). Two-way Permutational Multivariate Analysis of Variance (PERMANOVA) (Anderson et al. 2008) with wetland complex (WC) and sediment depth (SD) as fixed factors (Model = WC + SD + WC × SD) was used to determine whether significant differences could be detected among wetland complexes and between sediment depths. Where significant effects were indicated, pairwise PERMANOVA analyses were undertaken to identify which combinations differed significantly (Anderson et al. 2008).
Univariate analysis of the taxonomic richness and abundance were carried out in PRIMER (V7.0.13), with the PERMANOVA+ for PRIMER 7 (PRIMER-E, Plymouth, U.K.) (Anderson et al. 2008). Analysis of the species and morphotypes richness data was undertaken on untransformed data and abundance (germinating and morphotypes) data were square-root transformed. Analyses were then derived from a Euclidean similarity matrix. Two-way Permutational Multivariate Analysis of Variance (PERMANOVA) (Anderson et al. 2008) using the same model as above was used to determine whether significant differences could be detected between the six wetland complexes and sediment depth.
Results
Germinable seed-bank
The number of species germinating varied significantly among wetland complexes (P < 0.001) and between sediment depths (P = 0.003) (Tables 1, 2, 3) but there was no significant variation in the number of species germinating from each sediment depth among wetland complexes (P = 0.249) (Tables 1, 2).
Similarly there were differences in the abundance of seeds germinating among wetland complexes (P < 0.001) and between sediment depths (P < 0.001) with generally more seeds germinating from the surface layers (Tables 1, 2, 3). There was no variation in the abundance of seeds germinating at each sediment depth among wetland complexes (P = 0.249) (Tables 1, 2).
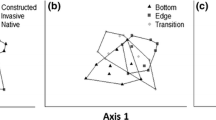
The differences in the number of species germinating and the abundance of germinable seeds was reflected in differences in the plant communities germinating from sediment from each wetland complex (Fig. 2a). These differences were confirmed by PERMANOVA and pairwise comparisons indicate that significant differences occurred in the communities germinating among all wetland complexes (P < 0.050) (Tables 2, 3).
Metric multidimensional scaling (bootstrap averages) of a germinating seeds community composition, and b morphotype community composition, observed from each wetland complex, triangle denotes Barmah Forest; square denotes Gunbower Forest; diamond denotes Hattah Lakes; circle denotes Lindsay-Mulcra-Wallpolla Island; inverted triangle denotes Chowilla Floodplain; plus denoted Lower Lakes. Black symbols represent the mean value of the boot-strap averages. Shaded areas represent the region in which 95% of the bootstrap averages occur
The germinating communities varied with sediment depth across all the wetland complexes (P = < 0.001) (Table 2; Fig. 3a). These differences were confirmed by pairwise comparisons (Table 4).
Metric multidimensional scaling (bootstrap averages) of a germinating seed communities from each sediment depth, and b the morphotypes communities at each sediment depth. Triangle = 2.5 cm, inverted triangle = 5 cm, square = 7.5 cm; diamond = 10 cm. Black symbols represent the mean value of the boot-strap averages. Shaded areas represent the region in which 95% of the bootstrap averages occur
Potential seed bank
One hundred and eighteen unique seed morphotypes were identified from this study. The number of morphotypes identified varied significantly among wetland complexes (P = 0.005) and between sediment depths (P = 0.010) (Tables 1, 2, 3). There was no variation in the number of morphotypes recorded at each sediment depth among wetland complexes (P = 0.791) (Tables 1, 2).
Similarly there were differences in the abundance of morphotypes among wetland complexes (P = 0.009) (Tables 1, 2, 3), and among sediment depths with more morphotypes found in the surface layers (P < 0.001). There was no variation in the abundance of morphotypes recorded from each sediment depth among wetland complexes (P = 0.628) (Table 2).
The differences in morphotypes richness and the abundance of morphotypes was reflected in differences in the morphotype communities within each wetland complex (P < 0.001) (Table 2; Fig. 2b). These differences were confirmed by PERMANOVA, and pairwise comparisons indicate that significant differences occurred in the morphotype communities among all wetland complexes (Table 3).
The seed morphotype communities varied with sediment depth across all the wetland complexes (P = < 0.001) (Table 2; Fig. 3b). These differences were confirmed by pairwise comparisons (Table 4).
Residual seed bank
There was no significant difference in the viability of seeds among wetland complexes (P = 0.149) with average viability ranging from 45% in Gunbower Forest to 22% in the Lower Lakes. In general, seed viability was greater in surface layers, but these differences were not significant (P = 0.127), and this response was consistent across the wetland complexes (WC x SD, P = 0.678) although in general viability was higher in the two top surface layers (0–5 cm) (Table 1).
The pool of viable seeds within each complex is large (Table 1) and within most wetland complexes the pool of viable seeds is largest in the surface sediment and decreases down the sediment profile (Table 1).
Discussion
In this study we tested two hypothesis. The first hypothesis that seed banks would be similar among wetland complexes was not supported. Significant differences occurred in the seed bank among each wetland complex indicating that dispersal longitudinally along a river did not overwhelm plant community differences among wetland complexes. The second hypothesis that seed abundance and viability would decrease with depth in the sediment profile was partially supported. More seeds were found in the surface layer of sediment compared to deeper sediment layers, however there was no difference in the viability of seeds between sediment depths.
Observed wetland plant communities associated along the Murray River are known to be species rich with approximately 800 species recorded along the length of the river with different communities occurring in each of the wetland complexes. This variability is consistent with other studies that have shown that plant communities vary spatially in response to environmental gradients (Campbell and Nielsen 2014; Lacoul and Freedman 2006), geomorphic features such as size and shape, and hydrological attributes such as depth, frequency and duration of inundations (Barrett et al. 2010) and local conditions required for deposition and subsequent germination (Riis and Sand-Jensen 2006). These environmental gradients and geomorphic features create a mosaic of habitats that support a diversity of plant species, many of which are often recorded from a single wetland (Alexander et al. 2008; Brock et al. 2003; Campbell et al. 2014).
In this study the number of species that germinated, and seed morphotypes identified from each wetland complex were considerably less than the number of plant species that have been recorded within each wetland complex summed over the preceding 10 years (Campbell and Nielsen 2014). In general, seed counting and identification of seed morphotypes indicated a greater number of species than reflected from germination alone. We found that published descriptions linking the morphology of wetland seeds to particular species are rare, requiring us to classify and differentiate seeds by their morphotype. Consequently the number of morphotypes provides a conservative estimate of the number of species present, as species with similar morphology are not always distinguishable. Despite these limitations in seed identification, consistent differences were detected among wetland complexes.
Separation of seeds and direct counting of seed morphotypes yielded greater taxa richness and abundance of seeds compared to numbers of species germinating even though only identified to morphotypes (Bernhardt et al. 2008; Durant et al. 2016; Gonzalez and Ghermandi 2012). Differences in the abundance and richness between communities identified using the two methods may be related to dormancy and specific requirements to germinate as well as seed size (Bernhardt et al. 2008; Gonzalez and Ghermandi 2012; Price et al. 2010). Germination studies underestimate the total number of viable seeds and species due to appropriate cues required to trigger germination not always being provided (Baskin and Baskin 2001; Brock et al. 2003; Brown 1992; Wright and Clarke 2009). For example, in germination studies using sediment collected from wetlands in Iowa, USA, but incubated under different conditions, the numbers of plants germinating varied from 145 per m2 under one set of conditions up to 20,000 per m2 under different conditions (van der Valk and Davis 1976, 1978). Conversely, direct counting of seeds possibly over represent the number of potentially viable seeds and species present, by including non-viable seeds (Baskin and Baskin 2001). Further research such as molecular analysis that links seed morphology to species is likely to reduce the differences between the numbers of observed species and numbers counted in the seed bank.
Estimates of seed bank sizes are highly variable among locations and can be related to increasing variability in environmental conditions (Bissels et al. 2005). In our germination trial, the number of plants germinating was low in comparison to many other studies, ranging from an average of 10,000 per m2 in Barmah Forest to fewer than 1000 per m2 in the Lower Lakes. However, as previously noted the number and types of plants that germinate will vary depending on the environmental conditions imposed (van der Valk and Davis 1976, 1978). The low numbers that occurred in this study are likely to be influenced by the conditions in which the germination experiment was undertaken. In comparison, the estimated number of seed morphotypes counted in the sediment of each wetland was substantially greater with numbers ranging from more than 175,000 seeds per m2 in Gunbower Forest down to 50,000 seeds per m2 in other wetland complexes.
The potential viable pool of seeds is the number of viable seeds that did not germinate under the imposed conditions. In our study only 5% of seeds germinated under the conditions in which the germination experiment was undertaken, which demonstrates that a significant pool of seeds remained dormant creating a potential reservoir of seeds present in all wetlands (van der Valk and Davis 1978).
Consistent with other studies, the number of seeds germinating and the number of morphotypes counted declined with increased burial depth (Gleason et al. 2003; Leck and Simpson 1987; Nicholson and Keddy 1983; van Der Valk and Davis 1979; Xiao et al. 2010). A decline is generally expected, reflecting a balance between seed deposition and the loss of seeds from the system through germination, decomposition (Grillas et al. 1993), dispersal, predation and aging (Espinar et al. 2005). These differences were also reflected in the composition of species and morphotypes down the sediment profiles (Espinar and Clemente 2007). However, even at the lower depths (10 cm), there were substantial numbers of morphotypes present in most wetlands. There were also a number of exceptions to the pattern of declining number of seeds germinating with increasing sediment depth (e.g. Barmah Forest) where more seeds germinated from 10 cm depth compared to either the 5 or 7.5 cm depths. Cracking of wetland sediments during the dry phase of wetlands is a common feature of the Murray River floodplain (Evans and McCabe 2010) and this is a likely mechanism to distribute seeds lower in the sediment profile (Burmeier et al. 2010b; Espinar and Clemente 2007). The fate of these buried seeds varies among species and environmental conditions with burial favouring the germination of larger seeds (Burmeier et al. 2010a). Burial of seeds in cracks may represent an important reserve of seeds, with the age of seeds increasing as sediment depth increases (Leck and Graveline 1979; McGraw 1987). These seeds are protected from severe dry periods, elevated temperatures and predation and may be especially important in wetlands that undergo unpredictable conditions that vary annually (Espinar and Clemente 2007; Leck and Graveline 1979). During subsequent dry periods, the cracks may reform and, if the seeds are light, they may float to the surface upon re-wetting where germination may occur (Espinar and Clemente 2007; Espinar et al. 2005).
In general, viability of seeds was higher in the surface sediment compared to viability at 10 cm sediment depth. The seed bank is a reserve of seeds comprising of both viable and non-viable seeds, and often the seed bank is dominated by non-viable seeds (Forcella 1992). It is likely that the viable seeds found at the 10 cm sediment depth are older and represent a method for extending the period of seed storage (Espinar et al. 2005).
If long distance dispersal of seeds is occurring along riverine corridors we would expect some degree of similarity among wetland complex seed banks. These results suggests that long distance dispersal of seeds over 100 s of kilometres is poor, even though the potential for seeds to disperse by water, wind or animals may be high (Boedeltje et al. 2003; Figuerola and Green 2002; Soons et al. 2016; Soons and Ozinga 2005; Tackenberg et al. 2003). Indeed the median dispersal distance for the majority of plants has been reported as not exceeding more than 15 meters and only a few plants had a 99 percentile distance that exceeded 1000 s of meters (Nathan et al. 2008; Soons and Ozinga 2005). Even if seeds were capable of dispersing over larger distances their ability to germinate and establish would depend on their individual habitat requirements being met (Soons and Ozinga 2005). Indeed dispersal over distances greater than 1000 s of meters is likely to be rare and driven by extreme events (Gillespie et al. 2012; Nathan et al. 2008).
Conclusion
Results from this study indicate that a large, diverse and viable seed bank exists within all the wetlands. Management actions aimed at watering floodplain complexes need to be targeted to ensure adequate time is allowed not only for seed germination but also for seed set and dispersal so that the seed bank is replenished. This may also involve allowing wetlands to dry sufficiently to enable cracking of wetland sediment and seeds to become buried to maximise the longevity of the seed store.
References
Alexander P, Nielsen DL, Nias D (2008) Response of wetland plant communities to inundation within floodplain landscapes. Ecol Manag Restor 9:187–195
Anderson MJ, Gorley RN, Clarke KR (2008) Permanova+ for Primer: guide to software and statistical methods. PRIMER-E, Plymouth
Andersson E, Nilsson C (2002) Temporal variation in the drift of plant litter and propagules in a small boreal river. Freshw Biol 47:1674–1684
Barrett R, Nielsen DL, Croome R (2010) Associations between the plant communities of floodplain wetlands, water regime and wetland type. River Res Appl 26:866–876
Baskin CC, Baskin JM (2001) Seeds: ecology, biogeography and evolution of dormancy and germination. Cambridge University Press, Cambridge
Bernhardt KG, Koch M, Kropf M, Ulbel E, Webhofer J (2008) Comparison of two methods characterising the seed bank of amphibious plants in submerged sediments. Aquat Bot 88:171–177
Bissels S, Donath TW, Hölzel N, Otte A (2005) Ephemeral wetland vegetation in irregularly flooded arable fields along the northern Upper Rhine: the importance of persistent seedbanks. Phytocoenologia 35:2–3
Boedeltje G, Bakker JP, Bekker RM, Van Groenendael JM, Soesbergen M (2003) Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J Ecol 91:855–866
Boedeltje GER, Bakker JP, Ten Brinke A, Van Groenendael JM, Soesbergen M (2004) Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported. J Ecol 92:786–796
Bonis A, Lepart J, Grillas P (1995) Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos 74:81–92
Boulton AJ, Brock MA (1999) Australian freshwater ecology: processes and management. Gleneagles Publishing, Glen Osmond SA
Brock MA (2011) Persistence of seed banks in Australian temporary wetlands. Freshw Biol 56:1312–1327
Brock MA, Theodore K, O’Donnell L (1994) Seed-bank methods for Australian wetlands. Aust J Mar Freshw Res 45:483–493
Brock MA, Nielsen DL, Shiel RJ, Green JD, Langley JD (2003) Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshw Biol 48:1207–1218
Brock MA, Nielsen DL, Crosslé K (2005) Changes in biotic communities developing from freshwater wetland sediments under experimental salinity and water regimes. Freshw Biol 50:1376–1390
Brown D (1992) Estimating the composition of a forest seed bank: a comparison of the seed extraction and seedling emergence methods. Can J Bot 70:1603–1612
Burmeier S, Donath TW, Otte A, Eckstein RL (2010a) Rapid burial has differential effects on germination and emergence of small- and large-seeded herbaceous plant species. Seed Sci Res 20:189–200
Burmeier S, Eckstein RL, Otte A, Donath T (2010b) Desiccation cracks act as natural seed traps in flood-meadow systems. Plant Soil 333:351–364
Campbell C, Nielsen D (2014) Understanding the effects of alterations to hydrological connectivity on the composition of aquatic and floodplain plant communities. The role of hydrological and riparian connectivity in maintaining biodiversity of river-floodplain ecosystems. Final Report prepared for Department of Environment’s National Environmental Research Program by the MDFRC and CSIRO, MDFRC Publication 38/2014, April, 245 pp, pp 51–68
Campbell CJ, Johns CV, Nielsen DL (2014) The value of plant functional groups in demonstrating and communicating vegetation responses to environmental flows. Freshw Biol 59:858–869
Capers RS, Selsky R, Bugbee GJ (2010) The relative importance of local conditions and regional processes in structuring aquatic plant communities. Freshw Biol 55:952–966
Capon SJ, Brock MA (2006) Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshw Biol 51:206–223
Carthey AJ, Fryirs KA, Ralph TJ, Bu H, Leishman MR (2016) How seed traits predict floating times: a biophysical process model for hydrochorous seed transport behaviour in fluvial systems. Freshw Biol 61:19–31
Casanova M, Brock MA (2000) How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecol 147:237–250
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. PRIMER-E: Plymouth
Cottrell HJ (1948) Tetrazolium salt as a seed germination indicator. Ann Appl Biol 35:123–131
Crosslé K, Brock MA (2002) How do water regime and clipping influence wetland plant establishment from seed banks and subsequent reproduction? Aquat Bot 74:43–56
De Stasio BT Jr (1989) The seedbank of a freshwater crustacean: copepodology for the plant ecologist. Ecology 70:1377–1389
Durant RA, Nielsen DL, Ward KA (2016) Evaluation of Pseudoraphis spinescens (Poaceae) seed bank from Barmah Forest floodplain. Aust J Bot 64:669–677
Environment Australia (2001) A directory of important wetlands in Australia, 3rd Environment Australia, Canberra
Espinar JL, Clemente L (2007) The impact of vertic soil cracks on submerged macrophyte diaspore bank depth distribution in Mediterranean temporary wetlands. Aquat Bot 87:325–328
Espinar JL, Thompson K, García LV (2005) Timing of seed dispersal generates a bimodal seed bank depth distribution. Am J Bot 92:1759–1763
Evans JP, McCabe MF (2010) Regional climate simulation over Australia’s Murray-Darling basin: a multitemporal assessment. J Geophys Res: Atmos 115:D14114
Figuerola J, Green AJ (2002) Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw Biol 47:483–494
Forcella F (1984) A species-area curve for buried viable seeds. Aust J Agric Res 35:645–652
Forcella F (1992) Prediction of weed seedling densities from buried seed reserves. Weed Res 32:29–38
Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK (2012) Long-distance dispersal: a framework for hypothesis testing. Trends Ecol Evol 27:47–56
Gleason RA, Euliss NHJ, Hubbard DE, Duffy WG (2003) Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil egg and seed banks. Wetlands 23:26–34
Gonzalez S, Ghermandi L (2012) Comparison of methods to estimate soil seed banks: the role of seed size and mass. Community Ecol 13:238–242
Greet J, Webb JA, Cousens RD (2011) The importance of seasonal flow timing for riparian vegetation dynamics: a systematic review using causal criteria analysis. Freshw Biol 56:1231–1247
Greet J, Cousens RD, Webb JA (2012) Flow regulation affects temporal patterns of riverine plant seed dispersal: potential implications for plant recruitment. Freshw Biol 57:2568–2579
Greet J, Cousens R, Webb JA (2013) Seasonal timing of inundation affects riparian plant growth and flowering: implications for riparian vegetation composition. Plant Ecol 214:87–101
Grillas P et al (1993) Submerged macrophyte seed bank in a Mediterranean temporary marsh: abundance and relationship with established vegetation. Oecologia 94:1–6
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
James CS, Capon SJ, White MG, Rayburg SC, Thoms MC (2007) Spatial variability of the soil seed bank in a heterogeneous ephemeral wetland system in semi-arid Australia. Plant Ecol 190:205–217
Jansson R, Zinko U, Merritt DM, Nilsson C (2005) Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river. J Ecol 93:1094–1103
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136
Leck MA, Brock MA (2000) Ecological and evolutionary trends in wetlands: evidence from seeds and seed banks in New South Wales., Australia and New Jersey. Plant Species Biol 15:97–112
Leck MA, Graveline KJ (1979) The seed bank of a freshwater tidal marsh. Am J Bot 66:1006–1015
Leck MA, Simpson RL (1987) Seed bank of a freshwater tidal wetland: turnover and relationship to vegetation change. Am J Bot 74:360–370
Leibold MA et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Malone CR (1967) A rapid method for enumeration of viable seeds in soil. Weeds 15:381–382
McGraw JB (1987) Seed-bank properties of an Appalachian sphagnum bog and a model of the depth distribution of viable seeds. Can J Bot 65:2028–2035
Merritt DM, Nilsson C, Jansson R (2010) Consequences of propagule dispersal and river fragmentation for riparian plant community diversity and turnover. Ecol Monogr 80:609–626
Morris K (2012) Wetland connectivity: understanding the dispersal of organisms that occur in Victoria’s wetlands. Arthur Rylah Institute for Environmental Research Technical Report Series No. 225. Department of Sustainability and Environment, Heidelberg, Victoria.
Murphy BF, Timbal B (2008) A review of recent climate variability and climate change in south-eastern Australia vol 28. Wiley https://doi.org/10.1002/joc.1627
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A (2008) Mechanisms of long-distance seed dispersal. Trends Ecol Evol 23:638–647
Nicholson A, Keddy PA (1983) The depth profile of a shoreline seed bank in Matchedash Lake, Ontario. Can J Bot 61:3293–3296
Nielsen DL, Brock MA (2009) Modified water regime and salinity as a consequence of climate change: prospects for wetlands of Southern Australia. Clim Change 95:523–533
Nielsen DL, Brock MA, Crosslé K, Harris K, Healey M, Jarosinski I (2003) The effects of salinity on aquatic plant germination and zooplankton hatching from two wetland sediments. Freshw Biol 48:2214–2223
Nielsen DL, Podnar K, Watts RJ, Wilson AL (2013) Empirical evidence linking increased hydrologic stability with decreased biotic diversity within wetlands. Hydrobiologia 708:81–96
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev 85:837–858
Porter JL, Kingsford RT, Brock MA (2007) Seed banks in arid wetlands with contrasting flooding, salinity and turbidity regimes. Plant Ecol 188:215–234
Price J, Wright N, Gross BR, Whalley CL WRDB (2010) Comparison of seedling emergence and seed extraction techniques for estimating the composition of soil seed banks. Methods Ecol Evol 1:151–157
Raulings E, Morris KAY, Thompson R, Nally RM (2011) Do birds of a feather disperse plants together? Freshw Biol 56:1390–1402
Riis T (2008) Dispersal and colonisation of plants in lowland streams: success rates and bottlenecks. Hydrobiologia 596:341–351
Riis T, Sand-Jensen K (2006) Dispersal of plant fragments in small streams. Freshw Biol 51:274–286
Shafron M, Croome R, Rolls J (1990) Water quality. In: Mackay N, Eastburn D (eds) The Murray. Murray-Darling Basin Commission, Canberra
Soons MB (2006) Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Appl Veg Sci 9:271–278
Soons MB, Ozinga WA (2005) How important is long-distance seed dispersal for the regional survival of plant species? Divers Distrib 11:165–172
Soons MB, Brochet A-L, Kleyheeg E, Green AJ (2016) Seed dispersal by dabbling ducks: an overlooked dispersal pathway for a broad spectrum of plant species. J Ecol 104:443–455
Stromberg JC, Butler L, Hazelton AF, Boudell JA (2011) Seed size, sediment, and spatial heterogeneity: post-flood species coexistence in dryland riparian ecosystems. Wetlands 31:1187–1197
Tackenberg O, Poschlod P, Bonn S (2003) Assessment of wind dispersal potential in plant species. Ecol Monogr 73:191–205
Templeton AR, Levin DA (1979) Evolutionary consequences of seed pools. Am Nat 114:232–249
Thompson K, Grime JP (1979) Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J Ecol 67:893–921
Turner MG, Gardner RH, O’Neill RV (2001) Landscape ecology in theory and practice: pattern and process. Springer, New York
van der Valk A, Davis C (1976) The seed banks of prairie glacial marshes. Can J Bot 54:1832–1838
van der Valk AG, Davis CB (1978) The role of seed banks in the vegetation dynamics of prairie glacial marshes. Ecology 59:322–335
van Der Valk AG, Davis CB (1979) A reconstruction of the recent vegetational history of a prairie marsh, Eagle Lake, Iowa, from its seed bank. Aquat Bot 6:29–51
Wright BR, Clarke PJ (2009) Fire, aridity and seed banks. What does seed bank composition reveal about community processes in fire-prone desert? J Veg Sci 20:663–674
Xiao C, Wang X, Xia J, Liu G (2010) The effect of temperature, water level and burial depth on seed germination of Myriophyllum spicatum and Potamogeton malaianus. Aquat Bot 92:28–32
Acknowledgements
The authors gratefully acknowledge the use of data managed and collected by numerous organisations and individuals. In particular, for each location, the authors thank: Keith Ward and other staff from the Goulburn Broken CMA (Barmah Forest); Kathryn Stanislawski and other staff from the North Central CMA and staff from Australian Ecosystems Pty Ltd (Gunbower Forest); staff at The Murray-Darling Freshwater Research Centre and the Mallee CMA (Hattah Lakes and Lindsay-Mulcra-Wallpolla Islands); Jason Nicol and other staff at South Australian Research and Development Institute, (Chowilla Floodplain and the Lower Lakes). Data was collected and funded by the Murray-Darling Basin Authority as part of The Living Murray Monitoring program (contract number MD2556). The Living Murray is a joint initiative funded by the New South Wales, Victorian, South Australian, Australian Capital Territory and Commonwealth governments and coordinated by the Murray-Darling Basin Authority. The authors also gratefully acknowledge The Raukkan Natural Resources Management Team and Michael Diplock at Ngarrindjeri Regional Authority, Adrienne Rumbelow (SA DEWNR), Jan Whittle (SA DEWNR), Jason Nicol (SARDI), Keith Ward (GB CMA), Andrew Greenfield (Mallee CMA), Kathryn Stanislawki (NCCMA), Louisa Davis (DEPI) and Kate Bennetts for invaluable site related information. Paul Brown and Paul McInerney for their comments and feedback on early drafts of this paper and staff at the MDFRC, Jorge Portinho, Katherine Oliver and Cassie King for assistance with field work and sample processing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nielsen, D.L., Campbell, C., Rees, G.N. et al. Seed bank dynamics in wetland complexes associated with a lowland river. Aquat Sci 80, 23 (2018). https://doi.org/10.1007/s00027-018-0574-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0574-3