Abstract
Liming programmes aiming to restore fish populations are being implemented in many acidified aquatic systems in northern Europe. We studied Odonata communities in 47 forest lakes in SW Sweden, 13 that are currently being limed, and 8 that have previously been limed. Thirty-one species were recorded, with the highest mean number in untreated lakes, followed by previously treated lakes and currently treated lakes. Species communities differed between untreated and limed lakes, but only few rare species found in the untreated lakes were absent in the treated lakes. Likewise, species known to thrive in acid environments were either rare or showed no preferences. Comparing the number of records of odonate species within a large regional area to the proportion of lakes inhabited in our study, we found that seven of the most commonly observed species occurred less frequently in limed lakes than in the untreated ones, including two of the three most common taxa. Reduced species numbers in limed lakes might be due to conditions on other trophic levels, including fish predation. We argue that Odonata should be considered when developing new biological indices of water quality, although the causes of the observed occurrence patterns need to be studied further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adverse changes often encountered in aquatic ecosystems as a result of acidification have been recognized and thoroughly studied since the 1970s (Bradley & Ormerod, 2002). Acidification and reduced pH levels are followed by a steady decline of biota (Herrmann & Svensson, 1995). Due to acid deposition, pH and acid-neutralizing capacity decrease and aluminium concentrations increase (Driscoll et al., 2003). Acidification often occurs due to acid deposition, which is mainly of a trans-boundary origin (Odén, 1968; Likens & Bormann, 1974; Bengtsson et al., 1980; Schindler, 1988). This applies also in Sweden, where many ecosystems are affected. The situation is well known, well documented and, moreover, improving during the past decades, as emissions generally decrease in most parts of Europe (Menza & Seip, 2004; Naturvårdsverket, 2011).
Water systems in Sweden (especially the western parts) are acidified, with 47% of the lakes in the south-west being affected. The percentages are lower in the eastern and northern parts of the country (9 and 2%, respectively; Naturvårdsverket, 2016). There is a limited natural recovery of acidified waters in Sweden, since most of the bedrock and moraine soil is low in buffering capacity (Håkanson, 2003) and the ecosystem sensitivity to acid deposition is high. Henrikson et al. (2005) assumed that acidification will continue to be a problem, especially in large, base-poor basins, for many decades to come. Today, estimates say that there are still some 12,000 acidified lakes in Sweden, but numbers have decreased by 41% since 1990, partly due to a slow natural recovery (Futter et al., 2014; Naturvårdsverket, 2016).
In aquatic environments, some invertebrates show a reduced abundance already at pH 6.0 (Schindler, 1988). In many parts of Scandinavia, the natural pH level in lakes and rivers is below 6 due to acidic bedrock, generally acid soils and coniferous forests, which further increases the impact of additional acid deposition. In order to restore acidified waters in Sweden, various liming programmes with governmental funding have been initiated, starting in 1977 (Naturvårdsverket, 2011). Early on, the negative economic impacts of acidification (e.g. the disappearance of fish populations and later the release of metal ions in acidified waters) became known (Jensen & Snekvik, 1972; Almer et al., 1974; Grahn et al., 1974), spurring a Swedish national liming strategy. Liming was initially set up to restore fish and fishing, keeping costs as low as possible (Bengtsson et al., 1980). These objectives were only later broadened to include the preservation or recovery of biodiversity (e.g. Henrikson et al., 2005).
The results from adding limestone powder and/or dolomite to waters are considered ‘beneficial’ in the majority of cases (Håkanson, 2003; Henrikson et al., 2005; Holmgren, 2014, but see Mant et al., 2013). There are several examples of biological improvements due to liming. For instance, Eriksson et al. (1983) found a positive response in populations of phytoplankton, zooplankton and larvae of benthic insects a few years after the liming of 22 lakes in various parts of Sweden. The authors noted successful reproduction of the remaining fish species immediately after the pH increase, but the picture is multi-faceted with different outcomes generated from studies over longer periods. Herrmann & Svensson (1995) found no significant changes when benthic macro-invertebrate lake communities were sampled in 1981 and 1994, following ongoing liming. Bradley & Ormerod (2002) reported only modest changes in invertebrate communities over 10 years in streams after catchment liming. Herrmann & Svensson (1995) suggested depletion of the soil mineral reservoir over the long years of acidification as a possible factor preventing full recovery of limed ecosystems. In Norway, Raddum & Fjellheim (2003) showed that liming of rivers resulted in species recoveries and changes in the community structure. It seemed quite easy for an acid-sensitive species to return to a recently limed system (Fjellheim et al., 2001), but the influx of acid surface water to a limed lake may still prevent the establishment of large populations of macroinvertebrates (Raddum & Fjellheim, 1995). Today, more than half of the acidified Swedish lakes, rivers and streams are not limed, and the Swedish EPA (Naturvårdsverket) considers that new liming programmes generally entail higher costs and yield less benefit than ongoing liming (Naturvårdsverket, 2011).
The impact of liming on benthic diversity can be studied in many ways. As community changes are more pronounced at the top of the food chains, we have chosen to focus on a group of insect predators, the Odonata (dragonflies and damselflies), whose larvae live in freshwater. It is known that odonates generally survive better than many other invertebrates and fish species in acidic environments (Suhling et al., 2015). The Odonata are often used as model organisms in conservation studies as they are sensitive to human disturbances, e.g. farming and forestry, and may serve as indicators of general species richness, or as indicators of the overall condition of aquatic ecosystems (Samways & Steytler, 1996; Sahlén & Ekestubbe, 2001; Sahlén, 2006; Flenner & Sahlén, 2008; Koch et al., 2014). Moreover, the group has previously been used to monitor habitat and water quality as well as the recovery of restored habitats (e.g. Clausnitzer, 2003; Suhling et al., 2006; Oertli, 2008; Simaika & Samways, 2009). Dragonflies have previously been included in some studies of the effects of liming on water bodies; Eriksson et al. (1980) found that larvae of Odonata, along with other predatory invertebrates, were more abundant in acidified lakes due to the reduced populations of insectivorous fish, but they noted no difference in species numbers. Later studies, however, showed that a higher number of dragonfly species inhabit limed lakes than untreated ones, even when including lakes with fish (Eriksson et al., 1983; cf. also Johansson & Brodin, 2003; Johansson et al., 2006). It is known that dragonflies show a higher tolerance to acidity, and a greater sensitivity to fish predation, than many other commonly assessed insect groups (Carbone et al., 1998).
In this paper, we aim at analysing the effects of liming on the dragonfly species composition in a forested area in SW Sweden. The area is strongly affected by acidification. The pH was reduced in more than 90% of 239 surveyed lakes according to a large-scale study in 1985 (Stibe & Schibli, 2010), and more recent data indicate that some 70% of the lakes in the region are still acidified. As species differ in ecology, with some being more tolerant (adapted) to acid conditions than others (Suhling et al., 2015), we expect a different species community in acid waters compared to limed ones. Our hypothesis is that a certain set of species reacts (directly or indirectly) to liming. These species being identified, we proceed to a discussion of their ecology and the implications for their conservation, and from there to the effects of liming programmes in general.
Materials and methods
Study area
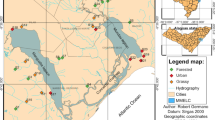
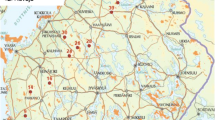
This study covers 47 small lakes (mean lake area 7.14 ha, range 0.18–49 ha) located in Halmstad and Hylte communities, southern Sweden (Fig. 1). The area is a mosaic of wetlands and forests, but the lakes in the western part are to a greater extent surrounded by fields and pastures. The forests are mainly coniferous production forests with spruce and pine trees planted approximately 100–150 years ago. Using data from other ongoing surveys, we could establish that the shorelines of the lakes were very similar to each other, comprising on average 40.7% mature forest, 15.7% young forest (<15 years), 40.2% clear cuts and only 3.4% urban areas.
Map of southern Sweden with Halland, Jönköping, Kronoberg and Västra Götaland (HJKV) administrative regions in grey. Map below right show Halmstad and Hylte region in Halland where white dots mark the locations of the 47 lakes in the study. Grey areas at the top correspond to the naturally acidic region (left) surveyed by Sahlén & Ekestubbe (2001) and the calcareous region (right) surveyed by Flenner & Sahlén (2008); lakes are indicated by white dots
Liming data
We used liming data freely available on the official website of the Swedish Agency for Marine and Water Management, Liming Information System (Nationella Kalkdatabasen, accessed on May 15th 2012). We divided the lakes into three categories: untreated (n = 26), previously treated (limed no less than 6 years prior to sampling; n = 8) and currently treated sites (n = 13), pooling the last two categories in some analyses.
pH
Only continuous monitoring of pH from spring to late autumn could produce fully comparable data, but due to limited sampling time we had to content ourselves with measuring pH in a selected number of lakes (n = 24), distributed evenly between lake categories, during April and May 2012, after the spring acid pulse. Our objective was not to get an exact value for each lake, but to obtain a comparable mean value for the lake categories, and to observe any differences between the categories. In addition, four of our eight currently treated lakes were included in a regional evaluation of liming programmes (Stibe, 2014), which enabled us to compare the data from these lakes to our own.
Recording of dragonflies
The lakes were sampled for dragonfly larvae during June, July and August of 2011, with additional samples taken in May 2012. These sampling dates enabled us to register the total species composition of the lakes, including the univoltine species, which are otherwise easily overlooked due to their short larval development time. The sampling was conducted using a standard student’s D-shaped water net, with 1.5 mm mesh size and 22 cm diameter, suitable for dragonfly surveys (Sahlén & Ekestubbe, 2001). We tried to sample all types of vegetation/substrate present along the shoreline up to a depth of about 0.5 m. The method implies sweeping the net through the aquatic vegetation, or along the bottom, three consecutive times, thus collecting most of the larvae present along about 1 m of the shoreline. At each lake, we did 15–40 such triple sweeps depending on lake size: fewer triple sweeps at smaller lakes and where the vegetation was sparse. Rich and varied vegetation necessitated a larger number of swipes. All larvae collected were preserved in 80% ethanol and punctured ventrally to prevent inner putrefaction and deformation of the abdomen. All larvae down to a body size of 5 mm were determined to species according to Norling & Sahlén (1997). The species Coenagrion pulchellum (Vander Linden, 1825) and C. puella (Linnaeus, 1758) cannot be separated as larvae and are here treated as a single taxon, as they have a somewhat similar ecology (Sahlén, 1996; Wildermuth & Martens, 2014). We used both presence/absence values and relative abundance of larvae encountered per lake (number of individuals per sample and per taxon corrected for sampling effort, i.e. number of triple sweeps with the net per site) for the calculations below; the latter was also used to calculate an evenness index for all lakes sampled. We decided to use the Calibrated modified Hill’s ratio (G 2,1) (Molinari, 1989), which works relatively well for both rare and common species in the dataset (Biesel et al., 2003). As our study focused on lakes, we excluded any running water specimens accidentally caught near the inlets and outlets of the lakes.
Patterns of species associations
In order to compare untreated lakes to (currently or previously) limed lakes, it is necessary to establish whether or not the species compositions differ between these categories. We did this in two steps:
First, we used the Nestedness Temperature Calculator (NTC; Atmar & Patterson, 1995) to establish if the species communities encountered were non-random sets of the regional species pool, where the species can be arranged along a scale from ‘most common’ to ‘rarest’. We chose to make two analyses: untreated vs. limed (currently or previously treated) lakes, assuming that species associations and, hence, any structured communities would differ between limed and unlimed habitats. Nestedness is a debated tool for evaluating species composition on islands in an archipelago or, as we use it, in a predominately forested landscape with scattered lakes of varying habitat quality. An overview of the use of nestedness can be found in, e.g., Simberloff & Martin (1991), Wright & Reeves (1992) and Atmar & Patterson (1993). Sahlén & Ekestubbe (2001), Suhling et al. (2006) and Flenner & Sahlén (2008) used the NTC to analyse whether or not odonate species were randomly distributed within a region, in order to select indicators of species richness or special habitats, or to evaluate changes in species composition over time. If species assemblages are nested, i.e. not randomly distributed, we can expect patterns of species associations.
Second, having established the existence of patterns, we used ANOVA to analyse differences between the lake categories in terms of species number. We also carried out discriminant function analyses to determine if the three liming categories differed markedly in odonate species composition. In the first analysis, we used a presence–absence matrix of species as the independent variable in the analysis, where the linear combinations of the variables that rendered the clearest separation between the lake categories are shown. In this case, we used three lake categories, meaning that two functions were calculated. The analysis produces Eigenvalues for each function, giving a relative measure of how much of the total variance is explained. In addition, it yielded Wilks’ lambda values, which measure how well each function separates variables into groups; small values indicate a better discriminatory ability of the function. The associated Chi-square statistics test was used to check if the means of the functions are equal across the categories used. We also pooled the two categories ‘previously limed’ and ‘currently limed’ into a single category, and carried out a second discriminant function analysis as above, to evaluate if the liming effects are temporary or of a more permanent kind. Both analyses provide classification results, in our case presenting to what extent the lake categories can be predicted by their species composition. In the second analysis, we used relative abundance data for all localities (number of larvae found adjusted to 40 triple sweeps per lake) and further log-transformed it (+1) to compensate for a few mass occurrences in the dataset, using three categories of lakes. The analyses were carried out using IBM SPSS statistics release 20.0.0.2.
In a final step, we compared the untreated lakes to the limed lakes, looking at the presence/absence of odonate species in the lake categories as a relative measurement of abundance. If all lakes in one category were inhabited, this constituted 100% occupancy, compared to, e.g., 50% (only half the lakes occupied) in another case. We are aware that the number of lakes differs between classes, and hence we only took into account species which occurred in at least five of the 47 lakes (~10%) to minimize random effects. Furthermore, pooling the currently and previously limed lakes in a second step of the comparison gave more even group sizes.
Comparison of regional species abundance
To evaluate our observations, we compared the regional species abundance to that found in three other datasets:
First, we used data from the nestedness matrix in Flenner & Sahlén (2008: Fig. 3, p. 178), where the regional species pool of an area with naturally calcareous soil (Fig. 1) is presented; no acidification occurs in that area, nor are the lakes naturally acidic. Although this area is situated 500 km to the north-east of Halland, it is the nearest available area with calcareous soil where the occurrence of Odonata has been thoroughly surveyed through sampling of larvae. We are aware that the comparison is made difficult by the fact that the regional fauna is different (a 70% species overlap), and we can expect climate and other environmental variables to differ. But if we assume that a low pH in itself is a strong determining factor for species occurrence, a different pattern should be present on calcareous soil. We measured occurrence as the number of sites (lakes) a species occupies in the dataset.
Second, we used data from the area ‘Bergslagen’ in Sahlén & Ekestubbe (2001: Fig. 3, p. 680), adding unpublished data on univoltine species from the original dataset for a comparison similar to those mentioned above. This is an area with naturally acidic lakes, which is also affected by acidification. There are a few ongoing liming programmes also in that area, but not in any of the lakes included in our dataset (Fig. 1). If the distribution patterns are general, we would expect the same species selection there as in the untreated lakes of the area covered in this study.
Third, we used postings on the Species Observation System (2015), an independent site for the collection of species sightings in Sweden, and ranked the number of observations (of one or several specimens) of all the 43 lentic species of Odonata observed in the south-western parts of Sweden (corresponding to the administrative regions of Halland, Jönköping, Kronoberg and Västra Götaland, HJKV; Fig. 1) during the years 2000–2014. In this case, abundance was measured as the cumulated number of observations for each species at all sites within the area. Any differences between the abovementioned datasets are discussed. As the vast majority of observations in the Species Observation System concern imagoes, a substantial component of vagrant specimens is probably included and the site specificity somewhat reduced. Thus, it may not be directly comparable to our data on larvae, but we use it here for a coarse comparison.
Results
Baseline data
In total, 31 species and 1,320 specimens (Supplementary table) were found in our lakes, with 1 to 16 species occurring in a single lake. Untreated lakes harboured 29 species (evenness G 2,1 = 0.51), currently treated lakes 21 species (0.45) and previously treated lakes 19 species (0.55). G 2,1 values indicate medium evenness in all three categories. There was a significant difference in species numbers between the lake categories (ANOVA, F = 4.18, P = 0.025), with the mean number being 8.23 ± 2.98 (SD; range 4–16 species) for untreated lakes, 5.08 ± 3.40 (1–12) for currently treated lakes and 6.13 ± 4.64 (2–16) for previously treated lakes. Post hoc tests (Tukey’s) revealed a significant difference in species numbers between untreated and currently treated lakes (P = 0.025), but not between untreated and previously treated, nor between currently and previously treated lakes (P > 0.05). The untreated lakes had a pH of 5.21 ± 0.58, the previously treated lakes 5.67 ± 0.57 and the currently treated lakes 6.50 ± 0.46. The average value calculated from four of the currently treated lakes in the regional evaluation by Stibe (2014) was 6.57 ± 0.37, i.e. at the same level as our measurements. Liming seems to increase the pH by more than 1 unit in our lakes.
Species associations
The nestedness matrix for untreated lakes (29 species, 26 lakes; fill 28.2%) was nested with a temperature of 32.46°, significantly lower than the temperature derived from 1,000 Monte Carlo simulations of random distributions (59.72° ± 4.56°, P < 0.001). For the currently and previously limed lakes together (21 + 19 = 24 species together, 21 lakes; fill 22.6%), the temperature was 16.55°, also lower than the randomly generated temperature (49.06° ± 5.57°, P < 0.001). Both matrices were highly nested, and patterns of species associations may therefore be present.
In our first discriminant analysis of three lake categories based on the presence/absence data, the two discriminant functions were not significant. The first function (Eigenvalue = 5.77, Wilks’ lambda = 0.080, χ 2 = 74.45, df = 60, P = 0.099) explained 87.2% of the variance. Function 2 (Eigenvalue = 0.84, Wilks’ lambda = 0.54, χ 2 = 18.05, df = 329, P = 0.94) explained 12.8%. In total, 91.5% of the lakes were classified to the correct lake category according to their odonate species composition. While all lakes belonging to the untreated category were correctly classified, 3 out of 13 (23%) of the currently treated and 1 out of 8 (12%) of the previously limed lakes were not correctly classified. There was a clear separation between the untreated lakes and the other two categories, but not between the currently and previously treated lakes, as shown in Fig. 2a. This was confirmed when pooling the two treated categories: As expected, the resulting discriminant function explained 100% of the variation and was highly significant (Eigenvalue = 5.47, Wilks’ lambda = 0.15, χ 2 = 56.03, df = 30, P = 0.003). The comparatively high Wilks’ lambda indicates that the function still has only a moderate discriminatory ability. Using log-transformed relative abundance data, the discriminant functions were likewise not significant. The first function (Eigenvalue = 6.08, Wilks’ lambda = 0.076, χ 2 = 76.04, P = 0.079) explained 87.6% of the variance. Function 2 (Eigenvalue = 0.859, Wilks’ lambda = 0.54, χ 2 = 18.30, P = 0.94) explained 12.4%. In total, 91.5% of the lakes were correctly classified according to their odonate species composition (Fig. 2b), as in the presence/absence analysis. The currently (2 out of 13) and previously limed lakes (2 out of 8) were not separated along the function 2 axis, indicating that these categories have a more similar species composition compared to the untreated sites. The patterns observed were consistent both for presence/absence and log-transformed relative abundance data.
Scatter-plot of the discriminant analyses performed for the datasets: a presence/absence and b log + 1 transformed abundance data with (1) white dots representing untreated lakes (n = 26), (2) black dots for currently treated lakes (n = 13) and (3) grey dots for previously limed lakes (n = 8); crosses mark group centroids, some dots overlap. While currently and previously treated lakes have species combinations which partly merge along function 2, there is a clear separation between these two categories and untreated lakes along function 1 in both cases
Regional species abundance
Looking into the species community and pooling the two treated categories, we saw that ten relatively abundant species had a lower frequency of occurrence in limed lakes compared to untreated lakes (Table 1), and that six of them: Lestes sponsa (Hansemann, 1823), Pyrrhosoma nymphula (Sulzer, 1776), Coenagrion puella/pulchellum, Aeshna juncea (Linnaeus, 1758), Cordulia aenea (Linnaeus, 1758) and Libellula quadrimaculata (Linnaeus, 1758), occurred in more than 15 lakes within the region. The differences between lake categories were pronounced (Table 1). A series of species, including Enallagma cyathigerum (Charpentier, 1840), Coenagrion lunulatum (Charpentier, 1840), Aeshna cyanea (Müller, 1764) and Somatochlora flavomaculata (Vander Linden, 1825), were present in untreated lakes. The three latter species were absent in the currently treated lakes, but they occurred in one out of eight lakes belonging to the previously treated category (cf., Table 2). The rest of the species differed either by only a few percent between categories: Erythromma najas (Hansemann, 1823), Coenagrion hastulatum (Charpentier, 1825), Aeshna grandis (Linnaeus, 1758), Somatochlora metallica (Vander Linden, 1825), Sympetrum danae (Sulzer, 1776) and S. sanguineum (Müller, 1764), or occurred in a total of less than five lakes in the dataset, which made them more prone to stochasticity: Coenagrion armatum (Charpentier, 1840), Aeshna mixta Latreille, 1805, Brachytron pratense (Müller, 1764), Epitheca bimaculata (Charpentier, 1825), Leucorrhinia caudalis (Charpentier, 1840), L. pectoralis (Burmeister, 1839), L. rubicunda (Linnaeus, 1758), Orthetrum cancellatum (Linnaeus, 1758), O. coerulescens (Fabricius, 1798), Sympetrum striolatum (Charpentier, 1840) and S. vulgatum (Linnaeus, 1758).
Comparing the frequency of species occurrence between limed (currently and previously) lakes and untreated lakes/general observations within the other regions (Fig. 1), we noted the following: Four of the species which occurred less frequently in limed lakes were counted among the more abundant species (top third) in the calcareous area, and five of the species were also among the top third in the acid soil area (Table 2). Among the ten most frequently observed species in the administrative regions, four occurred less frequently in currently or previously limed lakes. Furthermore, seven out of the ten species in Table 1 were found among the 20 most frequently observed in the administrative regions in question.
Discussion
In this study, we present evidence that some of the most abundant species in southern Sweden (Dannelid & Sahlén, 2008; Species Observation System, 2015) have a lower regional abundance in limed lakes than in untreated lakes within our area. Of these, Libellula quadrimaculata and Coenagrion puella/pulchellum were number two and three on the list of most commonly observed species in the administrative regions of Halland, Kronoberg, Jönköping and Västra Götaland, and the first and third most abundant species in the calcareous area, respectively. In the naturally acid regions, they were, however, less abundant (Tables 1, 2). A rough comparison between our data from the naturally acid region and the observations from 2000 to 2014 registered in the Species Observation System (2015) from the same area (N Västmanland and S Dalarna) gives at hand that the two species in question were ranked the 2nd and the 6th most abundant. This implies that these species are indeed among the most abundant also in the acid region, but not in the lakes sampled by Sahlén & Ekestubbe (2001). There may be several reasons for this; one may be that several ongoing liming programmes exist in the area (Länsstyrelsen Örebro län, 2010) affecting part of the lakes in the survey—a pattern observed also in the current investigation. Although there is no conclusive evidence, we believe that there are factors in limed water bodies which directly or indirectly affect the occurrence and/or abundance of some, but far from all, dragonfly species.
Within the European Union, the Water Framework Directive requires the identification of ‘favourable conservation status’ for all types of water bodies (Moe et al., 2010). Today’s communities are strongly influenced by humans, the lakes in our study being no exceptions, as the surrounding forest in the entire region was planted on open Calluna heathlands from approx. 1880 and onwards (Osbeck, 1996). A justified question is therefore: What is a ‘natural’, a ‘recovered’ or for that matter an ‘affected’ community in the anthropogenically modified landscape of today? And how do you obtain a favourable conservation status, and for which organisms.
It is well known that many organism groups respond differently to disturbances and other environmental factors (e.g. Faith & Walker, 1996; Lawton et al., 1998; Birk et al., 2012; Timm & Moels, 2012), a fact that has been thoroughly discussed in the context of using indicator species (Sahlén & Ekestubbe, 2001). Dragonflies are no exceptions to this rule, as they respond to forestry, agriculture and structures in the water (Sahlén, 1999, 2006; Koch et al., 2014). In our area, the samples were taken in scattered lakes within a mosaic landscape, with no aggregation of lakes belonging to a single category in any part of the area. We therefore expect that the observed differences in the surroundings, with more agriculture in the western part of the area, will affect all three categories equally. Assuming that land use is not the key to the differences observed, we suggest that liming, or simply acidity, may be a factor regulating the occurrence of Odonata which is worth focussing on.
Liming is known to reduce the amount of Sphagnum moss as the pH rises (Eriksson et al., 1983), and this should reduce the amount of Sphagnum-dependent species in limed and previously limed lakes. Among the Odonata this foremost applies to the genus Leucorrhinia (Henrikson, 1988, 1993; Dijkstra & Lewington, 2006). The five species in this genus are very common in Sweden (Sahlén, 1996; Dannelid & Sahlén, 2008; Species Observation System, 2015), but in our study the Leucorrhinia species inhabited only a small selection of the lakes. The species which seemed to ‘avoid’ currently or previously limed lakes were, however, not the Sphagnum specialists, but some of the most common generalist species in the area. As the observations in the administrative regions (Table 2) are based on adults, a fair amount of vagrant specimens are probably included in these data, as always when studying flying organisms (cf. Dennis & Hardy, 1999; Suhling et al., 2006). Bearing this in mind, liming still seems to reduce the occurrence of several common species, indicating that limed odonate communities may not be natural communities and certainly not good examples of ‘restored’ ones.
It has been shown that pH is one of the most important factors influencing freshwater species richness (Biggs et al., 2005; Hindar & Wright, 2005). Friday (1987) showed that few species are found in water bodies with a pH below 5.5. Wilander & Fölster (2007) reported that a pH below 6.2 would affect the species composition in Swedish lakes. Also, the target pH of liming programmes in Sweden is often set at 6.0, with a minimum of pH 5.6 (Naturvårdsverket, 2010; Stibe, 2014), depending on the type of water body and the organisms living therein. In the area studied here, the untreated lakes were rather acidic, at least early in the season, with a mean pH around 5.2—i.e. probably low enough to affect at least some fish populations (Simmons et al., 1996; Heibo & Vollestad, 2002) and other water organisms. The currently treated lakes had a pH of 6.5, in line with official figures from measurements at other seasons (Stibe, 2014), and the previously treated lakes had an intermediate pH. The mean number of species differed between the categories, whereas the evenness values were all within the same range (0.45–0.55). Our interpretation of this is that the lakes belonging to the different categories have a comparable structure of regionally abundant and rare species. Furthermore, our discriminant analyses showed that the species compositions were somewhat weakly defined and therefore to a certain extent similar. Hence, although certain species seem to be affected in limed lakes, the overall community structure is not affected, at least not with regard to the Odonata. But certain large-scale changes can be expected to occur elsewhere in the food web. McKie et al. (2006) reported an overall reduction in species evenness following liming, along with reduced species richness with regard to shredders. Descriptions of altered producer communities (e.g. Herrmann et al., 1993; Schneider & Lindstrom, 2009) are abundant. Here we may speculate that the reduced occurrence of common odonate species in limed lakes may be caused by changes at other trophic levels, affecting a set of species but not the odonate community structure as a whole. Further studies on the ecological niche of these species may be rewarding.
A common reason for initiating and continuing liming programmes is that positive changes in water quality may be achieved. Communities can to some extent recover from acidification through liming (Angeler & Goedkoop, 2010), but invasive species and global change may cause other effects. Natural recovery is also possible, but with highly varying final results (Stendera & Johnson, 2008; Holmgren, 2014; Murphy et al., 2014). It is argued that many acidified lakes and rivers will require liming for many decades to come (e.g. Larssen et al., 2010). In that context, nature conservation could either focus on target species like fishes or be based on a wider ecosystem approach. We know that fish diversity will increase at a higher pH (e.g. Russel et al., 2011), but for invertebrates the pattern is much more complex. For instance, McKie et al. (2006) reported that the general diversity was reduced in 4 out of 6 streams following liming, but that certain groups (some smaller Plecoptera) increased in numbers. Looking at lakes only, Eriksson et al. (1980) found that there was an increasing abundance of dragonfly larvae in acid waters, but Eriksson et al. (1983) observed the opposite pattern, i.e. a higher number of dragonfly species in limed lakes than in untreated ones. In North America, Pollard & Berrill (1992) noted a higher dragonfly species diversity in lakes with lower pH, i.e. the same pattern observed by us, but in their study species numbers were not correlated to pH. Strong & Robinson (2004) showed that the abundance of frequently found taxa was higher in lakes with fish than in fishless lakes, regardless of pH. As we have not sampled all our lakes for fish, we cannot exclude this as a determining factor (cf. Wittwer et al., 2010; Nummi et al., 2012) as we generally found common odonate species more often in lakes with a low pH, where we expect to find little or no fish. Hence, we must consider how much of the observed variation in the Odonata community is due to predation (fish) or intraguild predation from other Odonata and large Dytiscidae beetles (cf. e.g. Yee, 2010; Suhling & Suhling, 2013; Worthen & Horacek, 2015) rather than to pH. As proposed by, e.g., Moore (1984), Clark & Samways (1996) and Sahlén & Ekestubbe (2001), odonates are expected to react rapidly to environmental change and may serve as good indicators when evaluating habitat restoration (D’Amico et al., 2004). If pH is a major factor, we see a pattern where restored habitats become depleted of diversity, despite improvements in water quality. At least, it seems that a ‘favourable conservation status’ is not achieved solely by liming in the case of Odonata. Water chemistry may naturally play a large role here, considering that the humic acid levels change with both acidification and liming. As far as we know, very little research has been made on how Odonata larvae react to changes in water chemistry (Corbet, 1999).
In a perfectly nested set of lakes, those with few species would only harbour regionally ‘common’ species that are also present at the more species-rich sites (Cutler, 1994). This pattern is slightly blurred when sampling natural sites, but in this study we observed a highly significant nested species composition in both our datasets. This implies that the Odonata species do not occur randomly within the region. Instead, we may expect patterns created by environmental variables (cf., Suhling et al., 2006), in our case liming. McAbendroth et al. (2005), analysing pond macro-invertebrate assemblages, noted that the observed nestedness pattern was best explained by pond area, with other habitat parameters and isolation being of less importance. Our lakes differ in area by a factor of 100 (0.18–49 ha) which certainly has an influence on the species composition; lakes with a longer shoreline have room for more varied plant structures and, hence, more niche space (Sahlén, 2006); odonates respond to the size, form and structure of the habitat. Hassall et al. (2011), looking at pond volume as a measure of size, found no significant relationship between this and invertebrate species richness, whereas Biggs et al. (2005) reported a strong correlation between pond size and macro-invertebrate diversity. Diversions from conventional species–area relationships may be due to ecological interactions as second-order effects (Hassall et al., 2011). Treating different groups of organisms separately would give a better understanding of species–area relationships under varying environmental conditions. Gray & Arnott (2011) identified limited dispersal and population connectivity as an important factor determining the formation of communities. Our study organisms are generally regarded as good dispersers (Corbet, 1999; Andersen et al., 2016) and should therefore be able to fly between all lakes included in this study. It is therefore more likely that our nested communities are shaped by acidity on different trophic levels, and/or by interactions with predators, than by dispersal issues.
There are many different approaches to assessing the ecological impact of acidification, and methods differ between countries (Moe et al., 2010). Several different metrics are in use, especially for river assessment, while fewer attempts have been made to assess acid stress effects on invertebrates in lakes (Schartau et al., 2008). Most indices use species of Ephemeroptera, Plecoptera, Trichoptera, Gastropoda, Hirudinea and Crustacea, while excluding most other invertebrates, including the Odonata. We know that Odonata species respond more to the form and structure of the habitat than to its acidity or general nutrient level (Cannings & Cannings, 1994; Foster, 1995), but in this study we show that some of the most abundant Odonata species react, at least indirectly, to the reduced acidity in a habitat subjected to liming. As stated, this may either be a direct effect caused by changes in the food chain/species community as a whole, thus comparable to predation, or due to other factors. This has interesting implications for future revisions of indices, indicating that new taxa might be considered. Moe et al. (2010) pointed out that there is a research challenge to find biological metrics able to distinguish between naturally acidic habitats and anthropogenically acidified ones. We suggest that the Odonata may be a good candidate taxon, at least in fishless habitats, but further research is needed to evaluate causes and effects on other trophic levels and in naturally acidic lakes.
References
Almer, B., W. Dickson, C. Ekström, E. Hörnström & U. Miller, 1974. Effects of acidification on Swedish lakes. Ambio 3: 30–36.
Andersen, E., B. Nilsson & G. Sahlén, 2016. Survival possibilities of the dragonfly Aeshna viridis (Insecta, Odonata) in southern Sweden predicted from dispersal possibilities. Journal of Insect Conservation 20: 179–188.
Angeler, D. G. & W. Goedkoop, 2010. Biological responses to liming in boreal lakes: an assessment using plankton, macroinvertebrate and fish communities. Journal of Applied Ecology 47: 478–486.
Atmar, W. & B. D. Patterson, 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96: 373–382.
Atmar, W. & B. D. Patterson, 1995. The Nestedness Temperature Calculator: A Visual Basic Program, Including 294 Presence-Absence Matrices. AICS Research, Inc., University Park, New Mexico, and The Field Museum, Chicago.
Bengtsson, B., W. Dickson & P. Nyberg, 1980. Liming acid lakes in Sweden. Ambio 9: 34–36.
Biesel, J.-N., P. Usseglio-Polatera, V. Bachmann & J.-C. Morteau, 2003. A comparative analysis of evenness index sensitivity. International Review of Hydrobiology 88: 3–15.
Biggs, J., P. Williams, M. Whitfield, P. Nicolet & A. Weatherby, 2005. 15 years of pond assessment in Britain: results and lessons learned from the work of Pond Conservation. Aquatic Conservation Marine and Freshwater Ecosystems 15: 693–714.
Birk, S., W. Bonne, A. Borja, S. Brucet, A. Courrat, S. Poikane, A. Solimini, W. van de Bund, N. Zampoukas & D. Hering, 2012. Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators 18: 31–41.
Bradley, D. C. & S. J. Ormerod, 2002. Long-term effects of catchment liming on invertebrates in upland streams. Freshwater Biology 47: 161–171.
Cannings, S. G. & R. A. Cannings, 1994. The Odonata of the northern cordilleran peatlands of North America. Memoirs of the Entomological Society of Canada 126(Supplement S169): 89–110.
Carbone, J., W. Keller & R. W. Griffiths, 1998. Effects of changes in acidity on aquatic insects in Rocky littoral habitats of lakes near Sudburg, Ontario. Restoration Ecology 6: 376–389.
Clark, T. E. & M. J. Samways, 1996. Dragonflies (Odonata) as indicators of biotope quality in the Krüger National Park, South Africa. Journal of Applied Ecology 33: 1001–1012.
Clausnitzer, V., 2003. Dragonfly communities in coastal habitats of Kenya: indication of biotope quality and the need of conservation measures. Biodiversity and Conservation 12: 333–356.
Corbet, P. S., 1999. Dragonflies: Behavior and Ecology of Odonata. Harley Books, Colchester.
Cutler, A. H., 1994. Nested biotas and biological conservation: metrics, mechanisms, and meaning of nestedness. Landscape and Urban Planning 28: 73–82.
D’Amico, F., S. Darblade, S. Avignon, S. Blanc-Manel & S. J. Ormerod, 2004. Odonates as indicators of shallow lake restoration by liming: comparing adult and larval responses. Restoration Ecology 12: 439–446.
Dannelid, E. & G. Sahlén, 2008. Trollsländor i Sverige, en fälthandbok, 2nd ed. Länsstyrelssen i Södermanlands län, Nyköping.
Dennis, R. L. H. & P. B. Hardy, 1999. Targeting squares far survey: predicting species richness and incidence of species for a butterfly atlas. Global Ecology and Biogeography 8: 443–454.
Dijkstra, K.-D. B. & R. Lewington, 2006. Field Guide to the Dragonflies of Britain and Europe. British Wildlife Publishing, Gillingham.
Driscoll, C. T., K. M. Driscoll, M. J. Mitchell & D. J. Raynal, 2003. Effects of acidic deposition on forest and aquatic ecosystems in New York State. Environmental Pollution 123: 327–336.
Eriksson, M. O. G., L. Henrikson, B. I. Nilsson, G. Nyman, H. G. Oscarson, A. E. Stenson & K. Larsson, 1980. Predator-prey relations important for the biotic changes in acidified lakes. Ambio 9: 248–249.
Eriksson, F., E. Hörnström, P. Mossberg & P. Nyberg, 1983. Ecological effects of lime treatment of acidified lakes and rivers in Sweden. Hydrobiologia 101: 145–164.
Faith, D. P. & P. A. Walker, 1996. Environmental diversity: on the best-possible use of surrogate data for assessing the relative biodiversity of sets of areas. Biodiversity and Conservation 5: 399–415.
Fjellheim, A., A. Tysse & V. Bjerknes, 2001. Reappearance of highly acid-sensitive invertebrates after liming of an alpine lake ecosystem. Water, Air and Soil Pollution 130: 1391–1396.
Flenner, I. & G. Sahlén, 2008. Dragonfly community re-organization in boreal forest lakes: rapid species turnover driven by climate change? Insect Conservation and Diversity 1: 169–179.
Foster, G. N., 1995. Evidence for pH insensitivity in some insects inhabiting peat pools in the Loch Fleet catchment. Chemistry and Ecology 9: 207–215.
Friday, L. E., 1987. The diversity of macroinvertebrate and macrophyte communities in ponds. Freshwater Biology 18: 87–104.
Futter, M. N., S. Valinia, S. Löfgren, S. J. Köhler & J. Fölster, 2014. Long-term trends in water chemistry of acid-sensitive Swedish lakes show slow recovery from historic acidification. Ambio 43: 77–90.
Grahn, O., H. Hultberg & L. Landner, 1974. Oligotrophication: a self-accelerating process in lakes subjected to excessive supply of acid substances. Ambio 3: 93–94.
Gray, D. K. & S. E. Arnott, 2011. Does dispersal limitation impact the recovery of zooplankton communities damaged by a regional stressor? Ecological Applications 21: 1241–1256.
Håkanson, L., 2003. Consequences and correctives related to lake acidification, liming and mercury in fish: a case-study for Lake Huljesjön, Sweden, using the Lake Web-model. Environmental Modelling and Assessment 8: 275–283.
Hassall, C., J. Hollinshead & A. Hull, 2011. Environmental correlates of plant and invertebrate species richness in ponds. Biodiversity and Conservation 20: 3189–3222.
Heibo, E. & L. A. Vollestad, 2002. Life-history variation in perch (Perca fluviatilis L.) in five neighbouring Norwegian lakes. Ecology of Freshwater Fish 11: 270–280.
Henrikson, B. I., 1988. The absence of antipredator behavior in the larvae of Leucorrhinia dubia (Odonata) and the consequences for their distribution. Oikos 51: 179–183.
Henrikson, B. I., 1993. Sphagnum mosses as a microhabitat for invertebrates in acidified lakes and the color adaptation and substrate preference in Leucorrhinia dubia (Odonata, Anisoptera). Ecography 16: 143–153.
Henrikson, L., A. Hindar & I. Abrahamsson, 2005. Restoring acidified lakes: an overview. In O`Sullivan, P. E. & C. S. Reynolds (eds), The Lakes Handbook: Lake Restoration and Rehabilitation, Vol. 2. Blackwell Publishing, Malden, MA: 483–499.
Herrmann, J. & B. S. Svensson, 1995. Resilience of macroinvertebrate communities in acidified and limed streams. Water, Air and Soil Pollution 85: 413–418.
Herrmann, J., E. Degerman, A. Gerhardt, C. Johansson & I. Muniz, 1993. Acid-stress effects on stream biology. Ambio 22: 298–307.
Hindar, A. & R. F. Wright, 2005. Long-term records and modelling of acidification, recovery, and liming at Lake Hovvatn, Norway. Canadian Journal of Fisheries and Aquatic Sciences 62: 2620–2631.
Holmgren, K., 2014. Challenges in assessing biological recovery from acidification in Swedish lakes. Ambio 43: 19–29.
Jensen, K. W. & E. Snekvik, 1972. Low pH levels wipe out salmon and trout populations in southern Norway. Ambio 1: 223–225.
Johansson, F. & T. Brodin, 2003. Effects of fish predators and abiotic factors on dragonfly community structure. Journal of Freshwater Ecology 18: 415–423.
Johansson, F., G. Englund, T. Brodin & H. Gardfjell, 2006. Species abundance models and patterns in dragonfly communities: effects of fish predators. Oikos 114: 27–36.
Koch, K., C. Wagner & G. Sahlén, 2014. Farmland versus forest: comparing changes in Odonata species composition in western and eastern Sweden. Insect Conservation and Diversity 7: 22–31.
Länsstyrelsen Örebro län, 2010. Örebro Adiminstrative Region. Åtgärdsplan för kalkningsverksamheten i Örebro läns sjöar och vattendrag 2010–2015. Örebro, Sweden.
Larssen, T., B. J. Cosby, E. Lund & R. F. Wright, 2010. Modeling future acidification and fish populations in Norwegian surface waters. Environmental Science and Technology 44: 5345–5351.
Lawton, J. H., D. E. Bignell, B. Bolton, G. F. Bloemers, P. Eggleton, P. M. Hammond, M. Hodda, R. D. Holt, T. B. Larsen, N. A. Mawdsley, T. E. Stork, D. S. Srivastava & A. D. Watt, 1998. Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391: 72–76.
Likens, G. E. & F. H. Bormann, 1974. Acid rain: a serious regional environmental problem. Science 184: 1176–1179.
Mant, R. C., D. L. Jones, B. Reynolds, S. J. Ormerod & A. S. Pullin, 2013. A systematic review of the effectiveness of liming to mitigate impacts of river acidification on fish and macro-invertebrates. Environmental Pollution 179: 285–293.
McAbendroth, L., A. Foggo, S. D. Rundle & D. T. Bilton, 2005. Unravelling nestedness and spatial pattern in pond assemblages. Journal of Animal Ecology 74: 41–49.
McKie, B. G., Z. Petrin & B. Malmqvist, 2006. Mitigation or disturbance? Effects of liming on macroinvertebrate assemblage structure and leaf-litter decomposition in the humic streams of northern Sweden. Journal of Applied Ecology 43: 780–791.
Menza, F. C. & H. M. Seip, 2004. Acid rain in Europe and the United States: an update. Environmental Science and Policy 7: 253–265.
Moe, S. J., A. K. Schartau, T. Bækken & B. McFarland, 2010. Assessing macroinvertebrate metrics for classifying acidified rivers across northern Europe. Freshwater Biology 55: 1382–1404.
Molinari, J., 1989. A calibrated index for the measurement of evenness. Oikos 56: 319–326.
Moore, N. W., 1984. Dragonflies as indicators of environmental health. IUCN Species Survival Commission Newsletter 1984: 7–8.
Murphy, J. F., J. H. Winterbottom, S. Ortona, G. L. Simpson, E. M. Shillan & A. G. Hildrew, 2014. Evidence of recovery from acidification in the macroinvertebrate assemblages of UK freshwaters: a 20-year time series. Ecological Indicators 37: 330–340.
Nationella kalkdatabasen, 2012. Swedish Agency for Marine and Water Management, Liming Information System. http://kalkdatabasen.lansstyrelsen.se/
Naturvårdsverket, 2010. Kalkning av sjöar och vattendrag, handbok 2010: 2. Swedish Environmental Protection Agency, Stockholm
Naturvårdsverket, 2011. Nationell plan för kalkning 2011–2015. Report 6449, Swedish Environmental Protection Agency, Stockholm.
Naturvårdsverket, 2016. Swedish Environmental Protection Agency. Färre försurade sjöar. http://www.naturvardsverket.se/Sa-mar-miljon/Vatten/Forsurade-sjoar/.
Norling, U. & G. Sahlén, 1997. Odonata, dragonflies and damselflies. In Nilsson, A. (ed.), The Aquatic Insects of North Europe, Vol. 2. Apollo Books, Stenstrup: 13–66.
Nummi, P., V.-M. Vaananen, M. Rask, K. Nyberg & K. Taskinen, 2012. Competitive effects of fish in structurally simple habitats: perch, invertebrates, and goldeneye in small boreal lakes. Aquatic Sciences 74: 343–350.
Odén, S., 1968. Nederbördens och luftens försurning: dess orsaker, förlopp och verkan i olika miljöer. Ekologikommittén, Statens naturvetenskapliga forskningsråd, Stockholm.
Oertli, B., 2008. The use of dragonflies in the assessment and monitoring of aquatic habitats. In Córdoba-Aguilar, A. (ed.), Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research. Oxford University Press, New York: 79–95.
Osbeck, P., 1996. Djur och natur i södra Halland under 1700-talet. Spektra, Halmstad.
Pollard, J. B. & M. Berrill, 1992. The distribution of dragonfly nymphs across a pH gradient in south-central Ontario lakes. Canadian Journal of Zoology 70: 878–885.
Raddum, G. G. & A. Fjellheim, 1995. Effects of liming and acid surface water on the mayfly Leptophlebia vespertina in Lake Hovvatn. Water, Air and Soil Pollution 85: 961–966.
Raddum, G. G. & A. Fjellheim, 2003. Liming of River Audna, southern Norway: a large-scale experiment of benthic invertebrate recovery. Ambio 32: 230–234.
Russell, D. J., K. M. Preston & R. J. Mayer, 2011. Recovery of fish and crustacean communities during remediation of tidal wetlands affected by leachate from acid sulfate soils in north-eastern Australia. Wetlands Ecology and Management 19: 89–108.
Sahlén, G., 1996. Sveriges trollsländor, 2nd ed. Fältbiologerna, Sollentuna.
Sahlén, G., 1999. The impact of forestry on dragonfly diversity in central Sweden. International Journal of Odontology 2: 177–186.
Sahlén, G., 2006. Specialists vs. generalists among dragonflies: the importance of forest environments to form diverse species pools. In Cordero, A. (ed.), Forests and Dragonflies. Pensoft, Moscow and Sofia: 153–179.
Sahlén, G. & K. Ekestubbe, 2001. Identification of dragonflies (Odonata) as indicators of general species richness in boreal forest lakes. Biodiversity and Conservation 10: 673–690.
Samways, M. J. & N. S. Steytler, 1996. Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biological Conservation 78: 279–288.
Schartau, A. K., S. J. Moe, L. Sandin, B. McFarland & G. G. Raddum, 2008. Macroinvertebrate indicators of lake acidification: analysis of monitoring data from UK, Norway and Sweden. Aquatic Ecology 42: 293–305.
Schindler, D. W., 1988. Effects of acid rain on freshwater ecosystems. Science 239: 149–153.
Schneider, S. & E.-A. Lindstrom, 2009. Bioindication in Norwegian rivers using non-diatomaceous benthic algae: the acidification index periphyton (AIP). Ecological Indicators 9: 1206–1211.
Simaika, J. P. & M. J. Samways, 2009. An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodiversity and Conservation 18: 1171–1185.
Simberloff, D. & J. L. Martin, 1991. Nestedness of insular avifaunas: simple summary statistics masking complex species patterns. Ornis Fennica 68: 178–192.
Simmons, K. R., P. G. Cieslewicz & K. Zajicek, 1996. Limestone treatment of Whetstone Brook, Massachusetts. 2. Changes in the brown trout (Salmo trutta) and brook trout (Salvelinus fontinalis) fishery. Restoration Ecology 4: 273–283.
Species Observation System, 2015. An independent internet site for collecting sightings of species. http://www.artportalen.se
Stendera, S. & R. K. Johnson, 2008. Tracking recovery trends of boreal lakes: use of multiple indicators and habitats. Journal of the North American Benthological Society 27: 529–540.
Stibe, L., 2014. Vattenkemisk effektuppföljning i kalkade sjöar och vattendrag i Hallands län 2009–2013. Meddelande 2014: 18. Länsstyrelsen i Hallands län, Enheten för naturvård & miljöövervakning, Halmstad, Sweden.
Stibe, L. & H. Schibli, 2010. Vattenkemisk effektuppföljning i kalkade sjöar och vattendrag i Hallands län 2004–2008. Meddelande 2010: 4, Länsstyrelsen i Hallands län, Halmstad, Sweden
Strong, K. F. & G. Robinson, 2004. Odonate communities of acidic Adirondack Mountain lakes. Journal of the North American Benthological Society 23: 839–852.
Suhling, I. & F. Suhling, 2013. Thermal adaptation affects interactions between a range-expanding and a native odonate species. Freshwater Biology 58: 705–714.
Suhling, F., G. Sahlén, A. Martens, E. Marais & C. Schütte, 2006. Dragonfly assemblages in arid tropical environments: a case study from western Namibia. Biodiversity and Conservation 15: 311–332.
Suhling, F., G. Sahlén, S. Gorb, V. J. Kalkman, K. D. B. Dijkstra & J. van Tol, 2015. Order Odonata. In Thorp, J. & D. C. Rogers (eds), Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge, MA: 893–932.
Timm, H. & T. Moels, 2012. Littoral macroinvertebrates in Estonian lowland lakes: the effects of habitat, season, eutrophication and land use on some metrics of biological quality. Fundamental and Applied Limnology 180: 145–156.
Wilander, A. & J. Fölster, 2007. Sjöinventeringen 2005; en synoptisk vattenkemisk undersökning av Sveriges sjöar. Report 2007: 16, Institutionen för Miljöanalys, SLU, Uppsala
Wildermuth, H. & A. Martens, 2014. Taschenlexikon der Libellen Europas: Alle Arten von den Azoren bis zum Ural im Porträt Gebundene Ausgabe. Quelle & Meyer Verlag GmbH & Co., Wiebelsheim.
Wittwer, T., G. Sahlén & F. Suhling, 2010. Does one community shape the other? Dragonflies and fish in Swedish lakes. Insect Conservation and Diversity 3: 124–133.
Worthen, W. B. & H. J. Horacek, 2015. The distribution of dragonfly larvae in a South Carolina stream: relationships with sediment type, body size, and the presence of other larvae. Journal of Insect Science 15: 31.
Wright, D. H. & J. H. Reeves, 1992. On the meaning and measurement of nestedness of species assemblages. Oecologia 92: 416–428.
Yee, D. A., 2010. Behavior and aquatic plants as factors affecting predation by three species of larval predaceous diving beetles (Coleoptera: Dytiscidae). Hydrobiologia 637: 33–43.
Acknowledgments
We wish to thank Costanza Uboni and Sanna Persson, who assisted us with part of the larval sampling. Anna Lejfelt-Sahlén helped us to improve our English. This study is based on part of a Master’s Thesis in EU Inland Water Quality Assessment by RAJ in a co-operation between Universidad Autónoma de Madrid, Spain and Halmstad University 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Marcelo S. Moretti
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al Jawaheri, R., Sahlén, G. Negative impact of lake liming programmes on the species richness of dragonflies (Odonata): a study from southern Sweden. Hydrobiologia 788, 99–113 (2017). https://doi.org/10.1007/s10750-016-2990-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2990-5