Abstract
We use public records from 1980 to 2014 to analyse survival of the EU Annex IV species Aeshna viridis in Sweden, a dragonfly strongly associated with the plant Stratiotes aloides. We clustered localities with S. aloides based on assumed dispersal abilities of A. viridis, using a dispersing radius of 2–100 km, calculating the proportion of sites with S. aloides that A. viridis is able to reach. If mean dispersal capability is high (40 km or above) 92.6 % or more of the localities are connected. For a good disperser, the probability of long-time survival is good. We further analysed the species richness of other Odonata and aquatic plants at 98 localities from the dataset. A. viridis co-occurred with more Odonata in the presence of S. aloides and running water but not in lakes. S. aloides sites had a higher number of other aquatic plants. Area had no impact on the occurrence of the species. For the present situation we surveyed 32 localities with known occurrence of the species. Only half of the sites for S. aloides contained any specimens while A. viridis occurred in the same number of sites. The species co-occurred in only 8 of 32 sites. In four sites A. viridis larvae appeared among Menyanthes trifoliata, Phragmites australis, Potamogeton natans and Sphagnum spp., indicating that at high latitudes A. viridis breeds among other species. Indirect monitoring based only on S. aloides would underestimate the number of populations of the dragonfly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities have expanded and intensified under the last centuries as industrial, agricultural and urban areas have grown, initiating both structural and functional changes on the environment (Smith et al. 1999). This has resulted in habitat loss and fragmentation (Liao et al. 2013) leading to substantial biodiversity loss (Scherr and McNeely 2008; Raebel et al. 2012). In European forest and farmland landscapes, intense land-use has resulted in a homogenization of, especially, aquatic habitats (Wagner et al. 2000; Thiere et al. 2009), leading to a pronounced decline in biodiversity (Krebs et al. 1999; Benton et al. 2003; Koch et al. 2014). Freshwater ecosystems are particularly subject to ever-increasing anthropogenic pressure (Ward 1998; Suhling et al. 2006).
Like all aquatic organisms, dragonflies (Odonata) have suffered and declined severely throughout Europe in the past half century (Keller et al. 2010; Kalkman et al. 2010, 2015). One of the foremost factors behind this decline is the decreasing number of suitable water bodies for dragonfly reproduction (Keller et al. 2010; Raebel et al. 2012). Being semi-aquatic insects, dragonflies oviposit in water-courses, lakes, ponds and fens and their larval stage occurs in water (Corbet 1999; Clausnitzer et al. 2009; Keller et al. 2010). Specialist species, which are particularly dependent on specific habitat features, and therefore often rarely occurring, can easily go extinct if their habitat disappears (Harabiš and Dolný 2012; Sahlén 2006a, b, c; Suutari et al. 2008).
One such specialist species suffering from habitat loss and degradation, at least in the western parts of its distribution, is Aeshna viridis Eversmann 1836, a dragonfly considered near threatened on the red list of European dragonflies (Kalkman et al. 2010), but, due to many populations in the north and the east of its range, listed as ‘least concern on’ the global red list (Sahlén 2006a). The larva of this species is strongly associated with Stratiotes aloides (Rantala et al. 2004; Suutari et al. 2008; de Vries 2010; Jaeschke et al. 2012; Kalkman et al. 2015), an insect-plant association long known (e.g., Robert 1958) and well studied. The rosette of the plant, with its spine-covered leaves and its dense populations, makes a good shelter for the larvae of the dragonfly both against fish predation (Corbet 1999; Rantala et al. 2004), intraguild predation and interference competition by larvae from other dragonfly species (Corbet 1999; Hopper 2001; Suutari et al. 2004).
Although often regarded as having a strict dependence on S. aloides (Kalkman et al. 2015), it is known that female A. viridis also, though rarely, use other water plants as egg-laying substrate, e.g., Typha spp. and Sparganium spp. (Rantala et al. 2004; Suutari et al. 2008). It has been shown that larvae can be found among other plants, but only if S. aloides is present in the same environment (Rantala et al. 2004). There are only a few reports from continental Europe of A. viridis larvae being found in bodies of water without S. aloides (e.g., Münchberg 1956; Robert 1958).
S. aloides is a water macrophyte with a rosette that hibernates on the bottom of ponds, lakes and ditches through the winter. The rosette rises to the surface in the spring and then stays floating until the autumn when it sinks to the bottom again (Smolders et al. 2003; Strzałek and Koperski 2009), but in some populations the rosettes remain submerged throughout the season (Renman 1989). S. aloides is typically found in eu- and mesotrophic shallow parts of stagnant streams, ponds, lakes and ditches. Because there are mainly only female specimens growing in northern temperate areas the reproduction is mainly asexual; the mother plant sends out tillers and turions which create dense populations (Nielsen and Borum 2008; Strzałek and Koperski 2009; Smolders et al. 2003). The special life strategy and asexual reproduction makes the species sensitive to, among other things, eutrophication caused by modern agriculture and changes in the hydrological structure. The species is known to have declined in Europe (and Sweden) during the last century (Smolders et al. 2003; Rantala et al. 2004), recently declared extinct in Spain (Aedo et al. 2015), but is not considered threatened in Sweden (ArtDatabanken 2015). The reason for its decline is often eutrophication with an accumulation of organic material and intense growth of other aquatic plants.
The European Union Habitats Directive, approved by the Council of the European Communities in 1992, is together with the Birds Directive the main legislation in Europe regarding policies for nature conservation. The annexes list all protected habitats and species in the EU (cf., e.g. Cardoso 2012). Most member states have adopted annexes II and IV as national lists of protected species. Further, these directives are the basis of the Natura 2000 network of protected areas in Europe for which member states regularly report the status of annex species within the areas covered by the directive (European Commission 2013a). The creation of the Natura 2000 network is considered one of the major actions for maintaining biodiversity on the European level (Cantarello and Newton 2008). This includes making standardized ecological monitoring of Annex II an IV species legally binding for the member states (Bock et al. 2005). Being an Annex IV species, A. viridis has been monitored by field surveys in Sweden where adults were observed and larvae are netted, foremost at Natura 2000 sites (Sahlén 2006b), but the methods are currently under revision. Such monitoring is time consuming and, moreover, adults can only be studied on sunny days when the species is on wing (Norling and Sahlén 1997; Corbet 1999). In addition, to identify larvae of this species a certain amount of knowledge of the colour pattern variation of the larvae in the family is required. Developing alternate and more straightforward methods to monitor this and other species is therefore needed.
Looking in general at species monitoring programmes, there is often a voluntary component with citizens reporting observations directly into a database (reviewed by Tulloch et al. 2013) and datasets on species distribution are frequently used to investigate species dispersal ability, ecology and habitat choice in conservation management (Pressey et al. 2007; Reside et al. 2011). Records in such public databases often contain presence-only data, reported by private individuals, universities or received when scientific collections are digitized. As the data therefore is gathered by different collectors and with different intentions, methods and knowledge (Graham et al. 2004; Elith et al. 2006; De Ornellas et al. 2011; Reside et al. 2011) there may be bias or other errors in it, something to be aware of when making decisions in conservation planning (Graham et al. 2004; Rae et al. 2007; De Ornellas et al. 2011). Nevertheless, using publicly available datasets could be a major advantage when monitoring A. viridis as costs would be reduced, and the timing of surveys and good weather as well as the need for taxonomic expertise for identifying larvae can be discarded.
To test the possible use of public records for the monitoring of A. viridis we did the following: We used all data available for A. viridis and S. aloides on the web-based Species Information System to evaluate the overlap in observations. Are the species observed at the same locations or are they not as strongly associated as previously believed? Further, estimating different capabilities for dispersal in A. viridis enabled us to investigate how many populations of A. viridis there exists in Sweden today under different dispersal scenarios. If the populations are connected in a metapopulation structure they should have the possibility to survive in a longer time perspective, in this case based on the possibility of specimens to reach (other) S. aloides populations. From the same dataset we also evaluated other factors important for sites with or without S. aloides, namely the size of the water bodies inhabited by the species, the diversity at the sites (as measured by the occurrence of other Odonata species) and the number of water plants observed at the sites. As observations in a database stretch over many years, the situation may look different when viewed in a narrow time window. We therefore sampled a number of known localities for the two species to see how strong the association between them is at present and at the same time note occurrence of the dragonfly among other plants at sites without S. aloides.
Materials and methods
Data from the Species Observation System
We used data available on the Species Observation System, a web-based and independent site for collecting sightings of species, foremost in Sweden (Species Observation System 2015). All Swedish records of A. viridis and S. aloides up to 61° north in the system from January 1 1980 to December 31, 2014 were downloaded on January 31, 2015. In total 304 and 735 records respectively of the species were available. Both sets of records were each reported by between some 90 and 100 different persons, organisations or county administrative boards. As reports in the system are given coordinates during input there is no consensus as to a single locality always getting the same coordinates and records sometimes overlap by some tens of metres up to a kilometre. When processing the records, localities close to each other will therefore be superimposed, showing up as a single dot in the matrix, as will observations from the same locality but from different years.
From the records we were able to use 98 different locations for A. viridis where we could obtain information on the occurrence of other Odonata species and species of aquatic plants, 35 with S. aloides present and 63 without. We merged sites closer to each other than 2 km and excluded all but five sites where no information on other species was given.
Analysis of dispersal and occupancy
The dispersal ability of A. viridis has not been studied in detail, as is the case with most Odonata. Studies on Zygoptera (damselflies) have revealed that some species rarely disperse at all (Watts et al. 2007), while others can fly several kilometres. For Anisoptera (dragonflies) a study on Leucorrhinia caudalis (Bolliger et al. 2011) noted dispersal at least over 5 km, independent of landscape structures, while Dolný et al. (2014) studying Sympetrum depressiusculum, noted 1200 m as the longest dispersal distance based on almost 2900 marked individuals. Larger species are known to sometimes migrate many tens of kilometres, sometimes several hundred (Ólafsson 1975; Corbet 1999), the longest yearly expansion rate being ~80 km (Anax imperator moving north/east due to climate change; Flenner and Sahlén 2008). This constant northern expansion might well correspond to the average dispersal possibility of the species, certain individuals probably moving much longer distances but rarely establishing colonies. Hence, we would expect, assuming A. viridis to be a fairly mobile species, that 80 km should be close to the upper dispersal range. We therefore chose five possible mean dispersal ranges for the species: 2, 5, 20, 40 and 100 km, the longest one well over 80 km. As the accuracy of the coordinates for A. viridis and S. aloides allow for some variation, the lower dispersal range lies within the expected distance should little or no dispersal take place. Further, in these scenarios we assumed that the normal condition for A. viridis is to inhabit waters where S. aloides is present and, subsequently, coordinates for observations of the dragonfly should not be farther away from a S. aloides locality than 2, 5, 20, 40 and 100 km respectively. In line with this reasoning, if there are other S. aloides localities within these distances from the closest one, A. viridis would have a continual possibility to disperse in its successive generations. A possible range where A. viridis can live and disperse would then constitute an area whose outer shape is limited by the set distances to localities where S. aloides is growing. Such an area may be inhabited by A. viridis or not. We also assume that there are no major barriers for dispersal within the area. Apart from the big lakes which can easily be circumnavigated if not transversed, all other terrain should not prevent dispersal in a dragonfly (Bolliger et al. 2011).
We put forward a method to group or cluster the localities with S. aloides together into meaningful communities depending on the dispersal abilities of A. viridis. The aggregation of S. aloides is based upon the dispersal distance r. Let \(\bar{B}_{r} (x_{i} ) = \{ x;|x - x_{i} |\, \le r\}\) be the closed ball with the usual Euclidian norm \(| \cdot |\) centred at location \(x_{i}\) for the \(i{\text{th}}\) observation of n at hand. Let \(I = \{ 1,2, \ldots ,n\}\) be the index set of them, and make a disjoint partition of it into index sets \(J_{k} = \{ i;i \in I\}\) forming m mutually disjoint connected and maximized communities \(S_{k} = \bigcup\nolimits_{{i \in J_{k} }} {\bar{B}_{r} (x_{i} )}\). The process of forming the m communities is hierarchical and m is not known or prescribed a priori. Also note that \(\{ S_{k} \}\) is unique for a given r under the requirements given. We present results for dispersing radius r = 2, 5, 20, 40 and 100 km projected on a map of southern Sweden. Finally, based on the map data, we calculated the proportion of sites with S. aloides that A. viridis is able to reach when dispersing 2, 5, 20, 40 or 100 km. The computations and graphics were done in Mathematica.
Field survey
To obtain an estimate of current co-occurrence in southern Sweden, we selected 34 locations ranging from SW to NE for field surveys where one or both species had been found previously; either based on recent (<10 years) observations in the Species Observation System or on unpublished records from the same period from GS (Fig. 1, blue dots). We based our selection on us being able to survey these locations within a few weeks at a time and that they were easy to reach by car. We judge the localities to constitute a good representative subgroup of all localities, including a wide range of habitats from both running and standing water. S. aloides had been observed in 31 of the chosen sites, A. viridis in 12 with mutual occurrence in 9. The localities were distributed over a large part of the total distribution area for A. viridis and visited during late May and June 2013 when the rosettes of S. aloides were clearly visible in the water and once more in July 2015. At each of the localities we surveyed a 2 m wide expanse of water along the shoreline netting dragonfly larvae. We used a standard student’s D-shaped water net with a diameter of 22 cm and 1.5 mm mesh size swiping it through the water vegetation for a stretch of approximately 1 m, three times in a row, a method which has proved to work well collecting dragonfly larvae (Sahlén and Ekestubbe 2001). Three to four such nettings were done at each type of vegetation (general structure and species composition) present at the water’s edge, the amount of netting therefore varying between sites. We actively searched for S. aloides at all localities and, if present, netting always took place among these plants. Further, we noted among what plant species any A viridis larvae were found. All larvae were determined to species in the field according to the characters given in Norling and Sahlén (1997). As A. viridis is under protection of both Swedish and European law (SFS 2007: 845; European Commission 2013b) all larvae of the species were released on-site after identification.
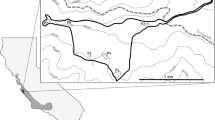
Observations of Aeshna viridis (cerise; n = 268) and S. aloides (green; n = 716) in southern Sweden, coordinates downloaded from the Species Observation System on January 31, 2015. Observations range from January 1980 to December 2014. Note that a majority of the dots appear superimposed of each other. Blue dots represent 34 localities which were surveyed in May–June 2013 and July 2015
Associations with other species and habitat size
For the 98 sites extracted from the public data we analysed differences in the number of dragonflies and number of aquatic plants present at S. aloides and non-S. aloides sites using t tests, including the size of the sites obtained from several online map services. We further examined differences in the number of observed aquatic plant species in the same way and also compared the presence/absence of the plant species where larvae were found during the field survey between the same groups to better understand the association of A. viridis with other plant species.
Results
If the mean dispersal capability of A. viridis is 2 km, 50.6 % of the localities with S. aloides could be reached (Fig. 2a), corresponding to a low overlap in occurrence. For 5, 20, 40 and 100 km dispersal the proportion of sites increased to 62.5, 86.2, 92.6 (Fig. 2b–d) and 100 % respectively. We note that the two shorter dispersal ranges result in many small and isolated populations of A. viridis and that only the three higher dispersal capabilities gives the possibility of large, coherent populations in Sweden.
Possibilities for Aeshna viridis (cerise) to disperse between S. aloides localities (green) under different scenarios. a 2 km mean dispersal range; b 5 km; c 20 km; and d 40 km. The colouring is as follows: If there is an observation of one or more A. viridis in a community its interior is painted in cerise and its boundary slightly darker in the same colour. If not, it is painted green in a similar fashion. For singleton communities the interior colour is inherited from the boundary. At low dispersal ranges the continuity is low and, hence, long time survival is uncertain (many green areas with only S. aloides). At the highest dispersal scenario most localities are connected, forming a metapopulation network covering most of the range of the species (all the area is cerise meaning A. viridis is able to disperse in it). The 100 km dispersal scenario is not shown
Field survey
Both visits to the 34 locations gave the same results for occurrence of the species: We found S. aloides at 16 sites, 51.6 % of those from which it was originally reported. At the first visit we noted that at three other locations, small S. aloides specimens were found washed-up on the shoreline, but no population was found in the lake/river section. We tentatively assumed that these specimens were transported to the sites via adjacent water courses. We found A. viridis at 12 sites, 10 of which were originally reported (83.3 %) and at two from where it was previously not reported. Both species co-occurred at eight locations; at six old (66.7 %) and at the two new. Further, at five of the A. viridis locations, larvae were found in other vegetation than S. aloides. These other water plants were Menyanthes trifoliata, Phragmites australis, Potamogeton natans and Sphagnum spp. S aloides was not present in the environment at four of these locations.
Associations with other species and habitat size
The number of other Odonata species did not differ significantly between sites with or without Stratiotes (t test, p = 0.058). The number of species varied more at sites without S. aloides (range 1–32) that at sites with the species (2–24). Looking only at running water sites (n = 18), the numbers of other Odonata differed significantly between sites without (mean 8.60 ± 6.68 standard deviation) and with (15.50 ± 5.68; t = −2.278, df = 16, p = 0.037) S. aloides; the water soldier sites having double the number of other species. Lake sites (n = 80) did not differ (p = 0.177). The number of other water plant species differed significantly between sites without S. aloides (5.65 ± 7.72) and sites with the species (9.77 ± 11.06; t = −2.16, df = 96, p = 0.033). The difference is stronger when looking only at lake sites (t = −3.291, df = 78, p = 0.001) but not significant for running water sites (p = 0.102). At 22 (34.9 %) of the localities without S. aloides, no water plants were reported while no other plants were reported at only 2 (5.7 %) of the sites with S. aloides.
Menyanthes trifoliata occurred at 16.7 % of the sites with S. aloides and at 9.4 % of those without. The numbers for the other species among which larvae were found in the field survey was 50 and 34.4 % for Phragmites australis, 16.7 and 12.5 % for Potamogeton natans and 22.2 and 50 % for Sphagnum sp., respectively. In 34.4 % of the localities without S. aloides none of the four plants occurred.
We found no significant differences between site area when comparing S. aloides sites to non-S. aloides sites (t test, p = 0.177). The habitat varied from small overgrown ponds of a few hundred square metres, via ponds and lakes of varying size up to bays and islands in three of the greatest lakes in Sweden (Hjälmaren, Mälaren and Vänern) as well as rivers of varying size.
Discussion
Several interesting results emerge from our study: First, even if dispersing adults move only 2 km A. viridis specimens would be able to reach over 50 % of the sites inhabited by S. aloides. It is not, however, until a much higher dispersal capability is assumed that the species will have the possibility to occur in coherent populations over large areas of southern Sweden (Fig. 2c, d). Second, we noted that localities with S. aloides harboured a higher number of other water plant species than sites without S. aloides; lakes, especially, had higher numbers. Habitat selection due to factors created by the rich plant community may therefore have a strong impact on the occurrence of this species. We further noted that a high percentage of localities without S. aloides had no other plants reported while the opposite was true for localities with S. aloides which indicate that visitors searching for and recording A. viridis may only to some extent note any other water plants than S. aloides, while visitors in search of S. aloides seem also to note A. viridis (and maybe other Odonata) indicating again the problems arising when using databases based on volunteer reports (van Strien et al. 2010). Third, in our field surveys we observed different rates of recapture, indicating that the turnover rate of S. aloides populations is fairly high with local populations persisting only over a certain number of years (Hanski 1999; Harveson et al. 2006; Koch et al. 2014), while A. viridis appears more persistent at locations although the numbers in our study are low. Suhonen et al. (2013) assumed that the persistence of A. viridis populations might depend on the presence of a few S. aloides patches of larger size rather than a network of small ones in the region. We are not aware of any neighbouring large patches to the surveyed sites, nor could the sites we visited be considered large patches, but detailed knowledge on the occurrence of both species in Sweden (and Europe) is lacking (Sahlén 2006a; Lansdown 2014; Kalkman et al. 2015). S. aloides is reported to occur only in communities with a high species richness of aquatic vegetation and is said to indicate good status of the ecosystem (Sugier et al. 2010), the habitat choice of A. viridis coinciding well with this type of environment (cf., Kalkman et al. 2015). If water quality is not affected, S. aloides populations may be stable over time (Renman 1989), and preserving or restoring such sites might be the best method of ensuring long time survival of A. viridis in many regions as the co-occurrence of the species is shown to be high in this study. Such protection and restoration programs have already been carried out in the Netherlands (de Vries 2010) and Germany (BFN 2010), both by protecting existing areas with S. aloides and creating new suitable habitats. We noted the disappearance of slightly less than half the populations in a 30-year timespan but we cannot say if this points to a decline or if it is the normal turnover rate for a thriving metapopulation at northern latitudes.
The decline of S. aloides in areas of Western Europe, is believed to be due to hydrological changes where alkaline river water and eutrophication together produces iron deficiency, sulphate and ammonium toxicity and competition by non-rooted species (Smolders et al. 1996, 2003). Here there are many attempts to create new areas for the species (and the dragonfly) but a problem is the high nitrogen levels in large areas of the distribution of this species which would be a severe problem if reintroducing in areas where it was previously extinct (Abeli et al. 2014). This general change of European aquatic communities is shown over large areas, e.g., from Poland where Gołdyn (2009) pointed out a general change of species composition in many bodies of water over a 30 year perspective. Specialised species like S. aloides were replaced by widespread generalists, seemingly increasing the species richness in many locations but, in fact, reducing the regional diversity, resulting in a trivial flora in most locations. The same pattern has been observed for dragonflies in Sweden (Flenner and Sahlén 2008; Koch et al. 2014), where specialists like A. viridis have disappeared from certain regions. To ensure the survival of both A. viridis and S. aloides, in areas of decline, other conservation approaches might be necessary and further studies are needed.
The presence of A. viridis larvae among other plant structures (M. trifoliata, P. australis, P. natans and Sphagnum spp.) in five out of the 12 sites suggests that the association with S. aloides structures is weaker in Sweden than has been reported from elsewhere (Tarkowska-Kukuryk 2006; Jaeschke et al. 2012; Kalkman et al. 2015). The prevailing understanding of edge populations is otherwise that they may be restricted to suboptimal environments distinct from the global range of the species (e.g. Vale et al. 2014), becoming increasingly rare and, hence, occupying a more narrow niche than in the core areas (Thomas et al. 1998). One effect of this was shown by Oliver et al. (2012) in that increases in population variability occur towards (climatic) range boundaries. Studies in Finland, also edge populations for A. viridis, showed that the species inhabited S. aloides sites only (Rantala et al. 2004). Our observations of alternate plant communities serving as larval habitat are not unique, as earlier studies have shown that adult female A. viridis to some extent oviposit in other vegetation than S. aloides (Münchberg 1956; Robert 1958; Rantala et al. 2004; Suutari et al. 2008). Münchberg (1956) reports a typical species composition of a S. aloides locality where the following plants are often present (synonyms revised): Glyceria maxima, Menyanthes trifoliata, Comarum palustre, Sparganium emersum, S. erectum, Acorus calamus, and in open water areas also Nuphar lutea or Nymphaea alba. Whether egg-laying in other plants than Stratiotes implies a suboptimal habitat or not can be debated, but it is safe to assume that the structures in the water formed by these plants resemble those of Stratiotes, as structures in the water are among the most important cues for an egg-laying female (Sahlén 2006c). We can also note that in our analysis of plants observed at A. viridis localities extracted from the species Observation System, at least one of the alternate species encountered during the field survey was found at 65 % of the sites where S. aloides was absent, thus giving ample possibilities for finding an alternate larval habitat. It is probably also safe to assume that there might a big number of vagrant specimens in the dataset, comprising part of the localities where no S. aloides was found.
Physical environmental factors and biotic interactions limit the survival and niche breadth of all aquatic species (Wellborn et al. 1996) and it is plausible that e.g. predation, is not more severe in these alternate plant communities than in Stratiotes localities. The presence of Sphagnum in our localities may prove as good protection against fish as Stratiotes does (cf., Henrikson 1993). Sphagnum is common, especially in acid or acidified bodies of water, a common case in Sweden (Håkanson 2003; Henrikson et al. 2005). Fish predation is one of the most important regulating mechanisms affecting the survival of aquatic organisms, including dragonfly larvae (Brooks and Dodson 1965; McPeek 1990; Johansson and Brodin 2003) and the effect of fish on dragonfly populations is evaluated by Wohlfahrt et al. (2006) and Wittwer et al. (2010). We tentatively assume that the alternate plant composition observed in a few Swedish lakes seems to provide other means of fish-free conditions for the larvae, something worth further studies in the Sphagnum rich lakes of Sweden.
Though is it credible to see the decline of S. aloides as the biggest threat to A. viridis on a European scale, we show that populations at northern latitudes may not be as dependent on the presence of the plant. The number of possible sites for A. viridis populations will then be considerably higher than the number of S. aloides populations, which in turn are several times more abundant than the dragonfly. As pointed out by e.g., Poniatowski and Fartmann (2010), habitat quality along with patch size and degree of isolation have been identified as the most important factors determining the occurrence of species. Not all suitable habitat patches will be used by any species (Ehrlen and Eriksson 2000), normally regulated by limited dispersal. We see this pattern in that the localities with A. viridis are much fewer than those with S. aloides. As we used a volunteer database the actual number of available substrate and of dragonfly populations within the area is probably much higher. Korkeamäki and Suhonen (2002) were able to show that species with a narrow distribution had lower long-term survival than species with a broad distribution. Applying this on the present species will imply that species with restricted habitat preferences, like A. viridis, are less good survivors. Despite this, no decline in the species has been seen in the north and east of its distribution area although it is decreasing in Europe as a whole (Kalkman et al. 2010, 2015), perhaps due to the number of possible habitat patches being much higher than the number observed.
As for indirect monitoring of A. viridis we find that the number of S. aloides populations alone does not give a reliable measure of how many A. viridis populations there might be expected, at least not in Sweden. The occurrence of the dragonfly in other plant communities implies that the number of such populations might be very high, but our field survey included too few localities to extrapolate a reliable percentage. Further, our field survey showed that records of S. aloides cannot be assumed to exist over a 10-year period, as almost half of the sites visited were empty at our sampling. Nevertheless, we see advantages in the method despite the problem that data generated by the public on a voluntary basis inevitably is incomplete and that species occurrence analyses based on such lists are less accurate than those derived from monitoring programs (van Strien et al. 2010). But in this case we deal with a conspicuous and, therefore, interesting, species which people will report if encountered, thus generating a relatively high number of observations. And, as the species cannot be mixed up with anything else, all observations should be regarded as correct, at least all observations at short distances. A directed search for the species as part of a regular monitoring scheme requires expertise on larvae or sunny weather on demand or both. Hence, an indirect monitoring will produce lower than expected numbers but still higher numbers than a monitoring scheme with limited funding would produce.
References
Abeli T, Rossi G, Smolders AJP, Simone O (2014) Nitrogen pollution negatively affects Stratiotes aloides in Central-Eastern Europe. implications for translocation actions. Aquat Conserv Mar Freshw Ecosyst 24:724–729
Aedo C, Medina L, Barbera P, Fernandez-Albert M (2015) Extinctions of vascular plants in Spain. Nord J Bot 33:83–100
ArtDatabanken (2015) Rödlistade arter i Sverige 2015. ArtDatabanken, SLU, Uppsala
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
BFN, Federal Agency for Nature Conservation (2010) Hamme lowlands (Hammeniederung). http://www.nachhaltigkeitsindikator.bfn.de/0203_hammerniederung+M5054de7a952.html. Accessed 15 Jan 2014
Bock M, Rossner G, Wissen M, Remm K, Langanke T, Lang S, Klug H, Blaschke T, Vrščaj B (2005) Spatial indicators for nature conservation from European to local scale. Ecol Indic 5:322–338
Bolliger J, Keller D, Holderegger R (2011) When landscape variables do not explain migration rates: an example from an endangered dragonfly, Leucorrhinia caudalis (Odonata: Libellulidae). Eur J Entomol 108:327–330
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35
Cantarello E, Newton AC (2008) Identifying cost-effective indicators to assess the conservation status of forested habitats in Natura 2000 sites. For Ecol Manag 256:815–826
Cardoso P (2012) Habitats Directive species lists: urgent need of revision. Insect Conserv Divers 5:169–174
Clausnitzer V, Kalkman VJ, Ram M, Collen B, Baillie JEM, Bedjanič M, Darwall WRT, Dijkstra K-DB, Dow R, Hawking J, Karube H, Malikova E, Paulson D, Schütte K, Suhling F, Villanueva RJ, von Ellenrieder N, Wilson K (2009) Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biol Conserv 142:1864–1865
Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Harley Books, Colchester
de Ornellas P, Milner-Gulland EJ, Nicholson E (2011) The impact of data realities on conservation planning. Biol Conserv 144:1980–1986
de Vries H (2010) Species protection plan for Aeshna viridis. Brachyton 12:25–31
Dolný A, Harabiš F, Mižičová H (2014) Home range, movement, and distribution patterns of the threatened dragonfly Sympetrum depressiusculum (Odonata: Libellulidae): a thousand times greater territory to protect? PLoS ONE 9:e100408. doi:10.1371/journal.pone.0100408
Ehrlen J, Eriksson O (2000) Dispersal limitation and patch occupancy in forest herbs. Ecology 81:1667–1674
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McCoverton J, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve predictions of species’ distributions from occurrence data. Ecography 29:129–151
European Commission (2013a) Environment, Habitats Directive reporting. http://ec.europa.eu/environment/nature/knowledge/rep_habitats/index_en.htm. Accessed 8 Jan 2014
European Commission (2013b) Environment, species protected under the Habitats Directive. http://ec.europa.eu/environment/nature/conservation/species/habitats_dir_en.htm. Accessed 8 Jan 2014
Flenner I, Sahlén G (2008) Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conserv Divers 1:169–179
Gołdyn H (2009) Changes in plant species diversity of aquatic ecosystems in the agricultural landscape in West Poland in the last 30 years. Biodiv Conserv 19:61–80
Graham CH, Ferrier S, Huettman F, Moritz C, Peterson AT (2004) New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol Evol 19:497–500
Håkanson L (2003) Consequences and correctives related to lake acidification, liming and mercury in fish—a case-study for Lake Huljesjön, Sweden, using the Lake Web-model. Environ Model Assess 8:275–283
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Harabiš F, Dolný A (2012) Human altered ecosystems: suitable habitats as well as ecological traps for dragonflies (Odonata): the matter of scale. J Insect Conserv 16:121–130
Harveson PM, Grant WE, Lopez RR, Silvy NJ, Frank PA (2006) The role of dispersal in Florida Key deer metapopulation dynamics. Ecol Model 195:393–401
Henrikson B-I (1993) Sphagnum mosses as a microhabitat for invertebrates in acidified lakes and the colour adaptation and substrate preference in Leucorrhinia dubia (Odonata, Anisoptera). Ecography 16:143–153
Henrikson LA, Hindar A, Abrahamsson I (2005) Restoring acidified lakes: an overview. In: O’Sullivan PE, Reynolds CS (eds) The lakes handbook. Lake restoration and rehabilitation, vol 2. Blackwell, Malden, pp 483–499
Hopper KR (2001) Flexible antipredator behavior in a dragonfly species that coexists with different predator types. Oikos 93:470–476
Jaeschke A, Bittner T, Jentsch A, Reineking B, Schlumpprecht H, Beierkuhnlein C (2012) Biotic Interactions in the face of climate change: a comparison of three modelling approaches. PLoS ONE 7(12):e51472. doi:10.1371/journal.pone.0051472
Johansson F, Brodin T (2003) Effects of fish predators and abiotic factors on dragonfly community structure. J Freshw Ecol 18:415–424
Kalkman VJ, Boudot J-P, Bernard R, Conze K-J, De Knijf G, Dyatlova E, Ferreira S, Jović M, Ott J, Riservato E, Sahlén G (2010) European red list of dragonflies. European Invertebrate Survey—The Netherlands; IUCN Species Programme; IUCN Species Survival Commission; IUCN Regional Office for Pan-Europe
Kalkman VJ, Kalniņš M, Bernard R (2015) Aeshna viridis Eversmann, 1836. In: Boudot J-P, Kalkman VJ (eds) Atlas of the European dragonflies and damselflies. KNNV Publishing, Zeist, pp 167–168
Keller D, Brodbeck S, Flöss I, Vonwil G, Holderegger R (2010) Ecological and genetic measurements of dispersal in a threatened dragonfly. Biol Conserv 143:2658–2663
Koch K, Wagner C, Sahlén G (2014) Farmland versus forest: comparing changes in Odonata species composition in western and eastern Sweden. Insect Conserv Divers 7:22–31
Korkeamäki E, Suhonen J (2002) Distribution and habitat specialization of species affect local extinction in dragonfly Odonata populations. Ecography 25:459–465
Krebs JR, Wilson JD, Bradbury RB, Siriwardena GM (1999) The second silent spring? Nature 400:611–612
Lansdown RV (2014) Stratiotes aloides. The IUCN red list of threatened species 2014: e.T167872A42387458
Liao J, Li Z, Hiebeler DE, El-Bana M, Deckmyn G, Nijs I (2013) Modelling plant population size and extinction thresholds from habitat loss and habitat fragmentation: effects of neighbouring competition and dispersal strategy. Ecol Model 268:9–15
McPeek MA (1990) Behavioral differences between Enallagma species (Odonata) influencing differential vulnerability to predators. Ecology 71:1714–1726
Münchberg P (1956) Zur Bindung der Libelle Aeschna viridis Eversm. an die Pflanze Stratiotes aloides L. (Odon.). Nachr bl Bayer Entomol 5:113–118
Nielsen LT, Borum J (2008) Why the free floating macrophyte Stratiotes aloides mainly grows in highly CO2-supersaturated waters. Aquat Bot 89:379–384
Norling U, Sahlén G (1997) Odonata, dragonflies and damselflies. In: Nilsson A (ed) The aquatic insects of North Europe, vol 2. Apollo Books, Stenstrup, pp 13–65
Ólafsson E (1975) Drekaflugan Hemianax ephippiger (Burm.) (Odonata) óvæntur gestur á Íslandi. Náttúrufræðingurinn 45:209–212
Oliver TH, Roy DB, Brereton TT, Thomas JA (2012) Reduced variability in range-edge butterfly populations over three decades of climate warming. Glob Change Biol 18:1531–1539
Poniatowski D, Fartmann T (2010) What determines the distribution of a flightless bush-cricket (Metrioptera brachyptera) in a fragmented landscape? J Insect Cons 14:637–645
Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA (2007) Conservation planning in a changing world. Trends Ecol Evol 22:583–590
Rae C, Rothley K, Dragicevic S (2007) Implications of error and uncertainty for an environmental planning scenario: a sensitivity analysis of GIS-based variables in a reserve design exercise. Landsc Urban Plan 79:210–217
Raebel EM, Merck T, Feber RE, Riordan P, Thompson DJ, Macdonald DW (2012) Multi-scale effects of farmland management on dragonfly and damselfly assemblages of farmland ponds. Agric Ecosyst Environ 161:80–81
Rantala MJ, Ilmonen J, Koskimäki J, Suhonen J, Tynkkynen K (2004) The macrophyte, Stratiotes aloides, protects larvae of dragonfly Aeshna viridis against fish predation. Aquat Ecol 38:7–81
Renman G (1989) Life histories of two clonal populations of Stratiotes aloides L. Hydrobiologia 185:211–222
Reside AE, Watson I, VanDer WJ, Kutt AS (2011) Incorporating low-resolution historic species location data decreases performance of distribution models. Ecol Model 222:3444–3447
Robert P-A (1958) Les Libellules. Delachaux & Niestlié, Neuchâtel
Sahlén G (2006a) Aeshna viridis. In: IUCN (2013) IUCN red list of threatened species, version 2013.2. http://www.iucnredlist.org. Accessed 1 Sept 2015
Sahlén G (2006b) Manual för basinventering av trollsländor. Naturvårdsverket, Stockholm
Sahlén G (2006c) Specialists vs. generalists among dragonflies—the importance of forest environments to form diverse species pools. In: Cordero A (ed) Forests and dragonflies. Pensoft, Moscow, pp 153–179
Sahlén G, Ekestubbe K (2001) Identifications of dragonflies (Odonata) as indicators of general species richness in boreal forest lake. Biodivers Conserv 10:673–687
Scherr SJ, McNeely JA (2008) Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes. Philos Trans R Soc B 363:477–491
SFS, Svensk författningssamling (2007) Artskyddsförordning 2007:845. Stockholm, Miljödepartementet
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196
Smolders AJP, Roelofs JGM, den Hartog C (1996) Possible causes for the decline of the water soldier (Stratiotes aloides L) in the Netherlands. Arch Hydrobiol 136:327–342
Smolders AJP, Lamers LPM, den Hartog C, Roelofs JGM (2003) Mechanisms involved in decline of Stratiotes aloides L. in The Netherlands: sulphate as a key variable. Hydrobiologia 506–509:603–610
Species Observation System (2015) An independent internet site for collecting sightings of species. http://www.artportalen.se/. Accessed 31 Jan 2015
Strzałek M, Koperski P (2009) The Stratiotes aloides L. stand as a habitat in oxbow lake Bużysko. Aquat Bot 90:1–6
Sugier P, Lorens B, Chmiel S, Turczynski M (2010) The influence of Ceratophyllum demersum L. and Stratiotes aloides L. on richness and diversity of aquatic vegetation in the lakes of mid-eastern Poland. Hydrobiologia 656:43–53
Suhling F, Sahlén G, Martens A, Marais E, Schütte C (2006) Dragonfly assemblage composition and diversity in arid tropical environments: a case study from western Namibia. Biodivers Conserv 15:311–332
Suhonen J, Suutari E, Kaunisto KM, Krams I (2013) Patch area of macrophyte Stratioites aloides as a critical resource for declining dragonfly Aeshna viridis. J Insect Conserv 17:393–398
Suutari E, Rantala MJ, Salmela J, Suhonen J (2004) Intraguild predation and interference competition on the endangered dragonfly Aeshna viridis. Oecologica 140:135–138
Suutari E, Salmela J, Paasivirta L, Rantala MJ, Tynkkynen K, Luojumäki M, Suhonen J (2008) Macroarthropod species richness and conservation priorities in Stratiotes aloides (L.) lakes. J Insect Conserv 13:413–414
Tarkowska-Kukuryk M (2006) Water soldier Stratiotes aloides L. (Hydrocharitaceae) as a substratum for macroinvertebrates in a shallow eutrophic lake. Pol J Ecol 54:441–451
Thiere G, Milenkovski S, Lindgren P-E, Sahlén G, Berglund O, Weisner SEB (2009) Wetland creation in agricultural landscapes: biodiversity benefits on local and regional scales. Biol Conserv 142:964–973
Thomas JA, Simcox DJ, Wardlaw JC, Elmes GW, Hochberg ME, Clarke RT (1998) Effects of latitude, altitude and climate on the habitat and conservation of the endangered butterfly Maculinea arion and its Myrmica ant hosts. J Insect Conserv 2:39–46
Tulloch AIT, Possingham HP, Joseph LN, Szabo J, Martin TG (2013) Realising the full potential of citizen science monitoring programs. Biol Conserv 165:128–138
Vale CG, Tarroso P, Brito JC (2014) Predicting species distribution at range margins: testing the effects of study area extent, resolution and threshold selection in the Sahara-Sahel transition zone. Divers Distrib 20:20–33
van Strien AJ, Termaat T, Groenendijk D, Mensing V, Kéry M (2010) Site-occupancy models may offer new opportunities for dragonfly monitoring based on daily species lists. Basic Appl Ecol 11:495–503
Wagner H, Wildi O, Ewald KC (2000) Additive partitioning of plant species diversity in an agricultural mosaic landscape. Landsc Ecol 15:219–227
Ward JV (1998) Riverine landscapes: biodiversity patterns, disturbance regimes, and aquatic conservation. Biol Conserv 83:269–278
Watts PC, Saccheri IJ, Kemp SJ, Thompson DJ (2007) Effective population sizes and migration rates in fragmented populations of an endangered insect (Coenagrion mercuriale: Odonata). J Anim Ecol 76:790–800
Wellborn D, Skelly K, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Wittwer T, Sahlén G, Suhling F (2010) Does one community shape the other? Dragonflies and fish in Swedish lakes. Insect Conserv Divers 3:124–133
Wohlfahrt B, Mikolajewski DJ, Joop G, Suhling F (2006) Are behavioural traits in prey sensitive to the risk imposed by predatory fish? Freshw Biol 51:76–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersen, E., Nilsson, B. & Sahlén, G. Survival possibilities of the dragonfly Aeshna viridis (Insecta, Odonata) in southern Sweden predicted from dispersal possibilities. J Insect Conserv 20, 179–188 (2016). https://doi.org/10.1007/s10841-016-9850-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-016-9850-5