Abstract
This study describes and quantifies morphological valve traits of the ostracod Limnocythere rionegroensis from Patagonian steppe lakes and explores their association with lake water characteristics. Surface ornamentation was examined by scanning electron and transmitted light microscopy, and valve size and shape were analyzed using morphometric techniques. Limnocythere rionegroensis shows remarkable variations in surface ornamentation, based on which three morphotypes (MI, MII, and MIII) were identified. Valves of morphotypes MI and MIII are larger, show slight to moderate external reticulation, and a higher shape variability, whereas MII is characterized by a very conspicuous reticulation, lower shape variability, and smaller valves. Outline analysis yielded a great shape disparity related to the dorsal margin slope. MI was found in sexual populations from euhaline to mesohaline ephemeral lake; MII occurs in parthenogenetic populations from mesohaline to oligohaline permanent or ephemeral lakes; and MIII, from both sexual and asexual populations, inhabits a broad range of environmental conditions in terms of salinity and stability. Limnocythere rionegroensis intraspecific variations may be caused by environmental parameters and genetic factors associated to reproductive strategies. These results contribute to the knowledge of extant L. rionegroensis morphological variability and provide additional clues to improve the environmental interpretation of fossil assemblages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ostracods (Class Ostracoda) are small aquatic crustaceans with low-Mg calcite bivalve carapaces which are often well preserved in Quaternary sediments and can be used as environmental proxies (Holmes, 2001). Their species-specific ecological tolerances and habitat preferences make them excellent bioindicators of the surrounding environmental conditions (Forester, 1986, 1991; Carbonel et al., 1988; De Deckker & Forester, 1988; Forester et al., 1994). Based on this environmental sensibility, ostracod abundance and diversity in Quaternary sediments have been intensively used as paleoecological and paleoclimatological indicators of climate changes and past environments (Palacios-Fest et al., 1994; Schwalb, 2003). Anatomical features of soft parts and carapaces such as size, shape, muscle scar patterns, hinge articulation, and ornamentation are useful for species identification (Karanovic, 2012). At species level, however, the carapaces may also exhibit morphological variations; their size, shape, and ornamentation may be influenced and altered by growth, sexual dimorphism as well as environmental and genetic factors (Van Harten, 1975; Yin et al., 1999; Danielopol et al., 2008; De Deckker & Martens, 2013; Ruiz et al., 2013). Because of their taxonomic implications and paleoecological information, the study of intraspecific variations in ostracods has received continued attention during approximately the last 35 years (Neil, 2000).
Geometric morphometry is a more recent technique to quantify and evaluate morphological variations (Rohlf, 1990). Geometric morphometry focuses on shapes as entire configuration points associated with the biological form (“landmarks”) or a sequence of points along the outline, allowing a complete reconstruction of the shape (Foster & Kaesler, 1988; Baltanás & Danielopol, 2011). Outline analysis is a more appropriate geometric morphometric method applied to nonmarine ostracods, because it usually needs few landmarks for the characterization of their morphology (Baltanás et al., 2003). Many studies have been carried out using outline analysis, including, for example, the pioneering work of Kaesler & Waters, (1972), Kaesler & Maddocks (1984), Schweitzer et al. (1986), and Maness & Kaesler (1987), among others.
This study focuses on the morphological variations of the calcified valves from the nonmarine ostracod Limnocythere rionegroensis Cusminsky & Whatley 1996 from surface sediments of Patagonian lakes using morphometric and statistical analyses. This species has been found in Patagonian Steppe lakes and in Quaternary sediments of Cuyo, Pampean, and Patagonian regions of Argentina (Cusminsky & Whatley, 1996; Whatley & Cusminsky, 2000; Markgraf et al., 2003; Cusminsky et al., 2005, 2011; D’Ambrosio, 2014; Ohlendorf et al., 2014; Ramón-Mercau et al., 2014). The aim of this paper is to compare the intraspecific variability such as morphological traits including valve ornamentation, outline shape, and size of L. rionegroensis from different inland aquatic environments of Patagonia with the modern hydrochemical conditions of the host waters in order to contribute to their potential as proxy in paleoenvironmental reconstructions.
Materials and methods
Species under analysis
Limnocythere rionegroensis was first described from Quaternary sediments from La Salina, Patagonia (Cusminsky & Whatley, 1996), but its soft part morphology remains unknown. This species presents a distinct sexual dimorphism and is represented by parthenogenetic and sexual populations in Patagonia (Cusminsky & Whatley, 1996; Schwalb et al., 2002; Schwalb, 2003; Cusminsky et al., 2005). Morphologically, this species resembles Limnocythere bradbury Forester recorded and described by Richard Forester (1985) from the Quaternary of the United States and recent sediments of Mexico (Cusminsky & Whatley, 1996; Schwalb et al., 2002; Cusminsky et al., 2005). Cusminsky & Whatley (1996) compared L. rionegroensis specimens from the La Salina core to L. bradbury and pointed out that the former is smaller with less concentrically orientated reticulation and a noncrenulated hinge. Both species occur in similar environments such as fresh to saline temporary and permanent lakes with Na+ as the dominant cation and are considered as Quaternary paleoclimatic indicators of environments with high evaporation rates (Forester, 1985; Cusminsky et al., 2005). Limnocythere rionegroensis is typical of ephemeral environments because it can tolerate high solute contents of host waters but also occurs in some permanent lakes (Schwalb et al., 2002; Cusminsky et al., 2005; Ramón-Mercau et al., 2012). This species inhabits sodic waters enriched in chlorine and/or sulfate and/or bicarbonate and reaching salinities above 2,300 mg l−1 TDS, mostly mesohaline waters (Cusminsky et al., 2005; Ramón-Mercau et al., 2012). Cusminsky et al. (2011) recorded two varieties of L. rionegroensis in a sediment core from Lago Cardiel (CAR 99-7P) that were associated with different postulated ionic concentrations at different stages during lake evolution. Var. 1 was related to a higher ionic concentration than var. 2, suggesting a change in the hydrological balance of Lago Cardiel at the transition of the late Pleistocene to the Holocene (Cusminsky et al., 2011).
Study area
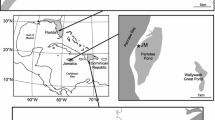
Valves of L. rionegroensis were collected from six sites in four lakes, located in the Patagonian steppe between the Andes and the Atlantic coast. Patagonia exhibits a strong precipitation gradient ranging from West to East from 2000 to 200 mm year−1, respectively. The rainy season occurs primarily during the austral winter (May–August). Laguna Cari-Laufquen Grande (41°S, 69°W, 810 m a.s.l.) is located in the northern part of Patagonia (Fig. 1a). The mean annual precipitation in this area is approximately 200 mm year−1, and annual mean temperature is 4°C (Ariztegui et al., 2008). Cari-Laufquen Grande is a brackish, sodium bicarbonate (pH 8.6, 4,000 ppm total dissolved solids) ephemeral lake with an average water depth of 3 m during the rainy season (Galloway et al., 1988; Cusminsky et al., 2005; Ariztegui et al., 2008). In Laguna Cari-Laufquen Grande two sites were sampled: Cari-Laufquen Grande (CG; 41° 10′S, 69°29′W, 795 m a.s.l.) and Cari-Laufquen Grande N Bay (CGB; 41°10′S, 69°28′W, 810 m a.s.l.). The other three water bodies are located in the southern region of Patagonia at 49°S (Fig. 1b). There, the mean annual precipitation is about 160 mm near Lago Cardiel, and the mean annual temperature is about 8°C (Heinsheimer, 1959; Markgraf et al., 2003). As a result of low precipitation near the lake area only a few smaller, permanent, and ephemeral streams originate around it (Gilli et al., 2005). In this area, two small ephemeral water bodies, Laguna Marrón Grande (MG, 48°46′S, 71°16′W, 525 m a.s.l.) and Laguna Marrón Chica (MC, 48°46′S, 71°16′W, 525 m a.s.l.) and a permanent environment, Lago Cardiel, were sampled. Lago Cardiel, is large lake located in a closed basin in the center of the southern Patagonia plateau about 100 km east of the Andean Cordillera. The lake has a surface area of about 370 km2, a maximum depth of 76 m (Gilli et al., 2005). In Lago Cardiel two sites were sampled: The “Cardiel Transect” in 1998 (CT; 48° 48′S, 71°12′W, 276 m a.s.l.) and “Cardiel Bay” in 2001 (CB; 48°49′S, 71°12′W, 276 m a.s.l.).
Locality map indicating the six sites in four lakes where L. rionegroensis was collected. Sites codes are as follows: CG Laguna Cari-Laufquen Grande, CGB Laguna Cari-Laufquen Grande Bay (Fig. 1a), MG Laguna Marrón Grande, MC, Laguna Marrón Chica, CT Lago Cardiel Transect, CB Lago Cardiel Bay (Fig. 1b)
Data collection
Water temperature, pH, and conductivity were measured in situ during collection of surface sediments in the austral spring–summers of 1998 and 2001 (Table 1). In the laboratory, ion concentrations (K+, Na+, Ca2+ and Mg2+) were measured using atomic absorbance spectrometry (Perkin Elmer Analyst 100). In CGB and CG, the presence of male and female individuals indicates sexual reproduction, whereas all other populations consisted exclusively of females, suggesting parthenogenetic populations.
Adult valves of L. rionegroensis were separated from surface sediment samples, counted, and grouped according to gender and body size. In order to describe and illustrate the variations in valve ornamentation, best preserved left valves from female adult specimen were examined under a scanning electron microscope (SEM). For morphometric analysis, left female valves were photographed in external lateral view against translucent background with a digital camera (Sony NSSC-DC50A) fitted to a standard light microscope (×40 magnification) (Table 1). Thus, in both analysis left–right valve asymmetry and morphological variations related to sexual dimorphism were avoided.
Morphometric and statistical analysis
Outline analysis was applied to the standard light microscope images of 130 female valves (Table 1), which were digitized using TpsDig software (Rohlf, 2010). Geometric morphometric analysis of valve outlines was performed using Linhart’s B-spline algorithm (Neubauer & Linhart, 2008) with Morphomatica v1.6 software (Linhart et al., 2007). This method fits elementary polynomial curves to the actual outline, with each curve defined by its corresponding control points. Thirty-two control points (16 dorsal and 16 ventral) were used to describe the outline of each specimen. The differences between any two outlines were estimated as the area deviation which is the area ‘between’ the outlines once they were superimposed, after they had been normalized for position, rotation, and size (outline size was normalized to unit area) (Neubauer & Linhart, 2008). In addition, virtual mean outlines (consensus shape) for each morphotype were computed using the Morphomatica software.
Based on area deviation, a pairwise dissimilarity index was computed (Neubauer & Linhart, 2008) and the resulting resemblance matrix was submitted to nonMetric Multidimensional Scaling (n-MDS) in order to visualize patterns in morphological variability. To quantify the degree of morphological variability of the valve shape within (dispersion) and between (disparity) morphotypes, a test of the homogeneity of dispersion was performed (PERMDISP) using Primer v.7 software (Clarke & Gorley, 2015). This test implemented by Namiotko et al. (2015) is based on the dissimilarities of the Euclidian distance and expresses the shape dispersion in a given morphotype as the mean area deviation to consensus (MDC) ± standard error (SE).
Valve length and height (µm) were measured on the images of the valves with Image-Pro software. The differences in length and height of morphotypes were analyzed by a Kruskal–Wallis nonparametric test, and Z’s post hoc test for pairwise comparisons was performed using Statistica v.10 (StatSoft, Inc. 2011).
Finally, to explore the relation between the intraspecific variability and the abiotic variables, the range (minimum–maximum) of environmental variables in which morphotypes occur was examined.
Results
Ornamentation patterns
Based on SEM images and microscopic analysis of valves, three morphotypes of L. rionegroensis were distinguished (Fig. 2).
Morphotype I (MI) (n = 45). The fundamental ornamentation pattern is a slight reticulation showing polygonal and shallow depressions, or fossae, separated by thin walls, or muri (first-order reticulation). Valves also present several small pits inside fossae (second-order reticulation). Valve ornamentation is almost absent or very smooth in the center of the valves (Fig. 2a–d). This morphotype is characteristic of CG (100%) (Fig. 2c, d) and CGB (98%) (Fig. 2a, b) sites from Laguna Cari-Laufquen Grande (Fig. 1; Table 1).
Morphotype II (MII) (n = 73). Valves display a prominent reticulation with deep and generally irregular fossae, in general rounded in the center of the valves, to polygonal near the outline of the valves, and bounded by thick and elevated muri. Most of the valves (~67%) present a second-order reticulation with pits inside fossae (Fig. 2j–l). In other specimens, this second-order ornamentation is absent (Fig. 2h). All valves from CB (Fig. 2k, l), 92% of CT (Fig. 2i, j), 89% of MG (Fig. 2g, h), and 29% of MC lakes display MII (Fig. 1; Table 1).
Morphotype III (MIII) (n = 12). This morphotype is similar to MI but shows a more distinct reticulation. Polygonal fossae are separated by thin muri, covering the entire valve surface (Fig. 2e). Second-order reticulation is also present with several pits inside fossae (Fig. 2f). This morphotype was found for 71% of valves from MC (Figs. 2e, f), 11% of valves from MG, 8% of valves from CT, and in 2% of valves in CGB (Fig. 1; Table 1).
Shape and size variations
The nonmetric multidimensional analysis (n-MDS) plot displays the morphological variations among the normalized 130 valve outlines of the six L. rionegroensis populations (Fig. 3). A morphological gradient is recognized. MI valves are spread over the entire morphospace, exhibiting higher dispersion (MDC ± SE = 22.1 ± 1.0). MII extend from the center to the left side of the morphospace and the valves present the lowest dispersion of the three morphotypes (MDC ± SE = 18.9 ± 0.7). Finally, MIII valves are also placed from the center to slightly to the left side of the n-MDS plot, their morphological dispersions are between MI and MII (MDC ± SE = 20.5 ± 1.2). Figure 4 shows the mean valve outline and the digitized outlines of each morphotype. MI valves have the dorsal margin almost straight to highly arched or with a pronounced slope of the dorsal margin. MII have valve shapes with straight to slightly arched dorsal margins and the valves of MIII have generally slightly arched dorsal margins. Valve shape disparity of MI was significantly higher than MII (test of homogeneity of dispersions PERMDISP t = 2.648, P(perm) = 0.01), MIII are similar to MI and MII (t ≈ 0.8, P(perm) ≈ 0.5).
Nonmetric Multidimensional Scaling plot (normalized for area) for 130 left female valves of L. rionegroensis with superposed reconstructed outlines at extremes (right = MI; left = MII) and center (MIII) of morphospace. For site codes see caption of Fig. 1
Regarding body size, lengths and heights of the three morphotypes differ significantly (Kruskal–Wallis test: length H2, N=130 = 41.6, height H2, N=130 = 45.6, P < 0.001). MI and MIII valves are larger than MII, and MI valves are higher than MII (Multiple Comparisons Z′ values; P < 0.001) (Table 2). Fig. 5 displays the relation between length and height of valves with indication of the three morphotypes; MIII has valves proportionately less high (length/height = 1.85) than MI and MII (length/height = 1.82).
The relationship between morphotypes and environmental variables indicates that the characteristics of host waters inhabited by MI (i.e., CGB and CG sites) are very different from MII (i.e., MC, MG, CT, and CB sites). Waters with morphotype MI present higher values for temperature, conductivity, K+, Mg2+, and Na+ concentrations. MII inhabiting waters are characterized by lower temperature, conductivity, K+, Mg2+, and Na+ concentrations. Moreover, morphotype MIII covers a broad range between MI and MII environmental variables (Table 3).
Discussion
Three morphotypes (MI, MII, and MIII) of L. rionegroensis were characterized based on their morphological traits. MI individuals display a range from a nearly smooth surface to a slightly primary reticulation pattern, larger adult sizes (mean ± SD length, 661 ± 29 µm) and have valves with the dorsal margin almost straight to highly arched (Fig. 4). It was found in very high percentages in ephemeral euhaline to mixoeuhaline (mesohaline) environments such as CG and CGB sites from Laguna Cari-Laufquen. MII morphotype is characterized by valves with a strong reticulation pattern, shorter valves (mean ± SD length, 626 ± 24 µm), and have in general valves with a straight to slightly arched dorsal margin (Fig. 4). This morphotype occurs in high percentages in mixoeuhaline (mesohaline) environments to oligohaline, including ephemeral lakes (MG and MC) and a permanent lake (CT and CB sites) (see Table 1). The third morphotype, MIII, displays a similar but more pronounced reticulation than MI, has adult sizes similar to MI (mean ± SD length, 656 ± 33 µm), and has valves with a straighter dorsal margin than MI (Fig. 4). MIII was found in ephemeral and permanent water bodies, generally associated with MII. All three morphotypes show broad variation of size and shape (Figs. 3, 4, 5).
The ornamentation patterns of Limnocythere species may be variable, with various types of ornamentation, as well as shape and size variations between populations (Yin et al., 1999). Limnocythere bradbury from lakes of the central Mexico Plateau, where waters change seasonally from fresh to slightly saline, shows an ornamentation very similar to L. rionegroensis, highly variable and ranging from nearly smooth to conspicuously reticulated (Forester, 1985). Limnocythere inopinata (Baird) valves can be either noded or free of nodes, and valves present a pattern of reticulation that varies from reticulated, with secondarily pitted fossae, to partly or completely smooth (Yin et al., 1999; Zhai et al., 2010). No direct correlation between ornamentation for L. inopinata and the effects of salinity or temperature was found (Yin et al., 1999). The intensity of ornamentation, the development of reticulation, and the presence of tubercles may be controlled by the environmental conditions during the biomineralization of the carapace, or by genetics, or by a combination of both (Yin et al., 1999; Neil, 2000; Laprida & Ballent, 2008).
Limnocythere rionegroensis shows remarkable differences of its surface ornamentation that include variation in intensity, thickness, and height of the muri, shape of the fossae, and number of pits inside them (second ornamentation order) (Fig. 2). These variations seem to correspond with the valve aggradation–degradation concept defined by Peypouquet et al. (1980); individuals with thin muri and shallow polygonal fossae (MI and MIII) are the “degraded” morphotypes, whereas specimens with thick muri and rounded to polygonal fossae (MII) correspond to the “aggraded” type. The aggradation–degradation phenomenon proposes that the intensity of surface ornamentation may be mainly influenced by the carbonate equilibrium (Mg2+/Ca2+ ratios) at the water/sediment interface where ostracods molt. In general, a higher carbonate ion concentration in the water, generally favors stronger calcification and reticulation (Carbonel & Hoibian, 1988; Carbonel et al., 1990; Babinot et al., 1991). In our dataset, more strongly ornamented valves occur in high percentages in median to slightly saline waters (i.e., CB, CT, and MG sites), whereas weakly ornamented valves were found mainly in higher saline environments (i.e., CG, CGB, and MC sites) (see Table 3). For Limnocythere africana, Klie stated that their reticulation is developed in less concentrated bicarbonate–sodic waters but with abundant dissolved Ca2+. The smooth forms of this species are a sign of the Ca2+ ion precipitation and announce the arrival of carbonate evaporites (Carbonel & Peypouquet, 1983).
The analysis of the outlines reveals a morphological gradient in which one extreme are located valves with dorsal margin straight and in the other extreme are valves with highly arched dorsal margin. The three morphotypes of L. rionegroensis display a high morphological variability and overlap in the morphospace. MI exhibits the highest morphological variability (Fig. 3), this morphotype occurs in sexual populations from Laguna Cari-Laufquen (CG and CGB sites) a euhaline to mesohaline, ephemeral and unpredictable environment. MII valves, that show smaller shape disparity than MI, are present in mesohaline to oligohaline both ephemeral and permanent lakes and are associated to parthenogenetic reproduction. Valves of the transitional morphotype III are related to a broad environmental range (salinity and stability). Thus, the high shape disparity of valves from Laguna Cari-Laufquen may be due to the high genetic diversity due the sexual reproduction or to environmental factors (stability/instability, predictability/unpredictability, and salinity of water bodies) or a combination of both. Van der Meeren et al. (2010) indicated that the shape variance of sexual and parthenogenetic populations of L. inopinata from ponds and lakes in Mongolian increased with temperature and specific conductance, conditions associated with shallow saline lakes. Furthermore, they found that the mean shape outline was more rectangular in populations from hydrologically stable freshwater habitats, whereas populations from environments with higher salinities or ephemeral lakes display a more distinctly sloping dorsal margin (Van der Meeren et al., 2010). The individuals of MI have valve outlines with the dorsal margin more arched than the rest of the morphotypes (Figs. 3, 4). Similarly, Yin et al. (1999) studied the effects of genotype and environment on morphological variability in valves of L. inopinata from Austrian and Chinese populations and clones and suggested that valves with rounded dorsal margin are indicative of moderate to highly saline waters. Valve morphology (shape) can be affected by genotype and environmental factors (mainly temperature and salinity), while interactive factors can contribute to morphological variability (Baltanás & Geiger, 1998; Yin et al., 1999).
Regarding body size, MI and MIII, saline and weakly ornamented morphotypes of L. rionegroensis, tend to have larger valves than the strongly ornamented morphotype, MII. It is known that the environmental characteristics of host waters affect the adult size of ostracod valves (e.g., Yin et al., 1999), however, the evidence provided is not conclusive. For instance, morphometric investigations carried out on populations of L. inopinata from China, Austria, and Western Mongolia showed a reverse trend; in saline waters adults were on average smaller than those of dilute waters (Yin et al., 1999; Van der Meeren et al., 2010). These authors attribute this relation to genetics and/or water chemistry, mainly water specific conductance.
In relation to the type of habitat preference, MI and MIII were mainly found in ephemeral lakes (CGB, CG, MC, and MG sites), whereas MII occurs in both permanent (CT and CB sites from Lago Cardiel) and ephemeral lakes (MG and MC) (Table 1). In ephemeral environments from the Patagonian steppe, L. rionegroensis is the dominant species (>80%) (Schwalb et al., 2002; Cusminsky et al., 2005, p. 445; Table 2). Likely, in this type of water bodies, poorly diversified food sources and niches, together with strong impacts of abiotic factors such as salinity and temperature, are responsible for survival of only a few species (Geiger, 1998). In permanent lakes, including Lago Cardiel (CT and CB sites), characterized by moderate to high degrees of evaporation of lake waters. L. rionegroensis (MII) occurs in lower percentages (<45%) and is mainly associated with Eucypris fontana (Graf), Limnocythere patagonica Cusminsky and Whatley, Eucypris virgata Cusminsky and Whatley, Kapcypridopsis megapodus Cusminsky et al., Limnocythere sp. and Eucypris labyrinthica Cusminsky and Whatley (Cusminsky et al., 2005, p. 445; Table 2). This species assemblage is typical of permanent ponds and lakes from Patagonia (Schwalb et al. 2002).
Our dataset shows that MII is associated to parthenogenesis lineages since only female populations were found, whereas MI, collected in populations with a significant ratio of males, seems to be linked to sexual lineages. Furthermore, MIII was found in parthenogenetic and sexual populations. In general, parthenogens are often dominant in habitats with no significant environmental fluctuations, short-lived, or predictable unstable environments, whereas sexuality seems to be the more successful reproduction strategy to persist in unpredictably unstable habitats (Martens, 1998). Only L. rionegroensis females were found in mixohaline (mesohaline) to oligohaline environments, and males were found in euhaline to mesohaline host waters such as Laguna Cari-Laufquen (Schwalb et al., 2002; Cusminsky et al., 2011). Our data agree with the observations made by Löffler (1990) who found males of L. inopinata only in strongly alkaline, and consequently with elevated solute concentrations, water bodies. More recently, studies of field populations of L. inopinata found bisexual populations from Chinese saline lakes and a asexual population from freshwater lake Mondsee (Yin et al., 1999) but in Turkey lakes, Reed et al. (2012) did not find a simple relationship between salinity and the reproductive mode of L. inopinata.
Cusminsky et al. (2011) found two varieties of L. rionegroensis, distinguished by their ornamentation, in sediment core CAR 99-7P retrieved from Lago Cardiel, which were related to different ionic concentrations. Preliminary studies of L. rionegroensis from the Lago Cardiel sediment core suggest that MI and MIII are more similar to L. rionegroensis var. 1, weakly ornamented, and MII belongs with L. rionegroensis var. 2, strongly ornamented. The presence of both varieties within the fossil species assemblage in different sections of the core suggests a change in hydrological balance in Lago Cardiel between the late Pleistocene and the late Holocene (Cusminsky et al., 2011). Before 12.7 cal. kyr BP, L. rionegroensis var. 1 was the dominant species in the core and thus suggested higher ionic concentration. After 12.7 cal. kyr BP, L. rionegroensis var. 2 was present together with L. patagonica and E. fontana, suggesting a decrease in ionic concentration and thus an increase in humidity causing a decrease in salinity (Cusminsky et al., 2011). These interpretations inferred from fossil ostracod assemblages are consistent with lake-level fluctuations and changes in the regional water balance reconstructed from Lago Cardiel sediments and seismic profiling (Gilli et al., 2001, 2005; Ariztegui et al., 2008).
Conclusions
The analysis of Limnocythere rionegroensis morphological variability from ephemeral and permanent Patagonian steppe lakes shows remarkable differences in surface ornamentation from weakly to heavily reticulated, shape changes, and variations in adult sizes. Based on valve ornamentation, three morphotypes MI, MII, and MIII were identified, and additional morphometric studies allowed us to describe these morphotypes. A gradient in ornamentation, shape, and size was recognized. There was a great shape variation related to valve dorsal margin and MI presented higher shape variability than MII. Weakly ornamented and largest valves of MI were found in sexual populations from ephemeral environments with euhaline to mesohaline waters, whereas smallest but distinctly ornamented valves of MII seem to be associated with parthenogenetic populations and mesohaline to oligohaline waters of ephemeral and permanent lakes. The results of this work suggest that morphological variations and reproduction modes of L. rionegroensis deserve special attention since they would provide tools to improve the analysis of actual and fossil species assemblages. However, further studies of Patagonian saline lakes and laboratory experiments should obtain more actual data of this paleo-indicator species in order to better understand the environmental significance of L. rionegroensis morphotypes.
References
Ariztegui, D., F. S. Anselmetti, A. Gilli & N. Waldmann, 2008. Late Pleistocene environmental change in Eastern Patagonia and Tierra del Fuego – A limnogeological approach. In Rabassa, J. (ed.), The Late Cenozoic of Patagonia and Tierra del Fuego. Elsevier, London: 241–253.
Babinot, J. F., P. Carbonel, J. P. Peypouquet, J.-P. Colin & Y. Tambareau, 1991. Variations morphologiques et adaptations morphofonctionnelles chez les ostracodes: Signification environnementale. Geobios 24(Supplement 1): 135–145.
Baltanás, A. & D. L. Danielopol, 2011. Geometric morphometrics and its use in ostracod research: a short guide. Joannea Geologie and Paläontologie 272: 235–272.
Baltanás, A. & W. Geiger, 1998. Intraspecific morphological variability: morphometry of valve outlines. In Martens, K. (ed.), Sex Parthenogenesis: Evolutionary Ecology of Reproductive Modes in Non-Marine Ostracods. Backhuys Publishers, Leiden: 127–142.
Baltanás, A., W. Brauneis, D. L. Danielopol & J. Linhart, 2003. Morphometric methods for applied ostracodology: tools for outline analysis of nonmarine ostracodes. In Park L. E. & A. J. Smith (eds), Bridging the Gap: Trends in the ostracod biological and geological sciences. The Paleontological Society Papers, 9: 101–118.
Carbonel, P. & T. Hoibian, 1988. The impact of organic matter on ostracods from an equatorial Deltaic area, the Mahakam Delta-Southeastern Kalimantan. In Hanai, T., N. Ikeya & K. Ishizaki (eds), Evolutionary Biology of Ostracoda—Its Fundamentals and Applications. Kodansha, Tokio: 353–366.
Carbonel, P. & J. P. Peypouquet, 1983. Ostracoda as indicators of ionic concentrations and dynamic variations: Methodology (Lake Bogoria, Kenya). In Maddocks, R. F. (ed.), Applications of Ostracoda. University of Houston Geoscience, Houston: 264–276.
Carbonel, P., J. P. Colin & D. L. Danielopol, 1988. Paleoecology of limnic ostracodes: a review of some major topics. Palaeogeography, Palaeoclimatology, Palaeoecology 62: 413–461.
Carbonel, P., P. Mourguiart & J. P. Peypouquet, 1990. The external mechanisms responsible for morphological variability in Recent Ostracoda: seasonality and biotope situation: an example from Lake Titicaca. In Whatley, R. C. & C. Maybury (eds), Ostracoda and Global Events. British Micropalaeontological Society Publication Series. Chapman and Hall, London: 331–340.
Clarke, K. R. & R. N. Gorley, 2015. PRIMER v7: User Manual/Tutorial. PRIMER-E, Plymouth.
Cusminsky, G. C. & R. C. Whatley, 1996. Quaternary non-marine ostracods from lake beds in Northern Patagonia. Revista Española de Paleontología 11: 143–152.
Cusminsky, G. C., A. P. Pérez, A. Schwalb & R. Whatley, 2005. Recent lacustrine ostracods from Patagonia, Argentina. Revista Española de Micropaleontología 37: 431–450.
Cusminsky, G. C., A. Schwalb, A. P. Pérez, D. Pineda, F. A. Viehberg, R. Whatley, V. Markgraf, A. Gilli, D. Ariztegui & F. S. Anselmetti, 2011. Late Quaternary environmental changes in Patagonia as inferred from lacustrine fossil and extant ostracods. Biological Journal of the Linnean Society 103: 397–408.
D’Ambrosio, S., 2014. Reconstrucción paleolimnológica de la laguna Llancanelo (Mendoza, Argentina) a través del estudio de ostrácodos del Cuaternario. PhD Thesis, Universidad Nacional de La Plata-Facultad de Ciencias Naturales y Museo, La Plata.
Danielopol, D. L., A. Baltanás, T. Namiotko, W. Geiger, M. Pichler, M. Reina & G. Roidmayr, 2008. Developmental trajectories in geographically separated populations of non-marine ostracods: morphometric applications for palaeoecological studies. Senckenbergiana lethaea 88: 183–193.
De Deckker, P. & R. M. Forester, 1988. The use of ostracods to reconstruct continental palaeoenvironmental records. In De Deckker, P., J. P. Colin & J. P. Peypouquet (eds), Ostracoda in the Earth Sciences. Elsevier, Amsterdam: 175–199.
De Deckker, P. & K. Martens, 2013. Extraordinary morphological changes in valve morphology during the ontogeny of several species of the Australian ostracod genus Bennelongia (Crustacea, Ostracoda). European Journal of Taxonomy 36: 1–37.
Forester, R. M., 1985. Limnocythere bradburyi n. sp.: a modern ostracode from Central Mexico and a possible Quaternary paleoclimatic indicator. Journal of Paleontology 59: 8–20.
Forester, R. M., 1986. Determination of the dissolved anion composition of ancient lakes from fossil ostracodes. Geology 14: 796–798.
Forester, R. M., 1991. Pliocene-climate history of the western United States derived from lacustrine ostracodes. Quaternary Science Reviews 10: 133–146.
Forester, R. M., S. M. Colman, R. L. Reynolds & L. D. Keigwin, 1994. Lake Michigan’s late Quaternary limnological and climate history from ostracode, oxygen isotope and magnetic susceptibility. Journal of Great Lakes Research Elsevier 20: 93–107.
Foster, D. W. & R. L. Kaesler, 1988. Shape analysis: Ideas from Ostracoda. In McKinney, L. M. (ed.), Heterochrony in Evolution. Plenun Press, New York: 53–69.
Galloway, R. M., V. Markgraf & J. P. Bradbury, 1988. Dating shorelines of lakes in Patagonia, Argentina. Journal of South America Earth Sciences 1: 195–198.
Geiger, W., 1998. Population dynamics, life histories and reproductive modes. In Martens, K. (ed.), Sex and Parthenogenesis: Evolutionary Ecology of Reproductive Modes in Non-Marine Ostracods. Backhuys Publishers, Leiden: 215–228.
Gilli, A., F. S. Anselmetti, D. Ariztegui, J. P. Bradbury, K. R. Kelts, V. Markgraf & J. A. McKenzie, 2001. Tracking abrupt climate change in the Southern Hemisphere: a seismic stratigraphic study of Lago Cardiel, Argentina (49oS). Terra Nova 13: 443–448.
Gilli, A., F. S. Anselmetti, D. Ariztegui, M. Beres, J. A. McKenzie & V. Markgraf, 2005. Seismic stratigraphy, buried beach ridges and contourite drifts: the Late Quaternary history of the closed Lago Cardiel basin, Argentina (49°S). Sedimentology 52: 1–23.
Heinsheimer, J. J., 1959. El Lago Cardiel. Anales Academia Argentina Geografia 3: 86–132.
Holmes, J. A., 2001. Ostracoda. In Smol, J., H. J. B. Birks & W. Last (eds), Tracking Environmental Change Using Lake Sediments. Kluwer Academic Published, Dordrecht: 125–151.
Kaesler, R. L., & R. F. Maddocks, 1984. Preliminary harmonic analysis of outlines of recent macrocypridid Ostracoda. In Krstić, N. (ed.), Symposium on ostracodes. Taxonomy, Biostratigraphy and Distribution of Ostracodes. Serbian Geological Society, Beograd: 169–174.
Kaesler, R. L. & J. A. Waters, 1972. Fourier analysis of the ostracode margin. Bulletin of the Geological Society of America 83: 1169–1178.
Karanovic, I., 2012. Recent Freshwater Ostracods of the World. Springer, Berlin.
Laprida, C. & S. Ballent, 2008. Capítulo 21: Ostracoda. In Camacho, H. H. & M. I. Longobucco (eds), Los Invertebrados Fósiles. Fundación de Historia Natural, Buenos Aires: 599–624.
Linhart, J., W. Brauneis, W. Neubauer & D. L. Danielopol, 2007. Morphomatica software, Version 1.6. Springer, Berlin.
Löffler, H., 1990. Paleolimnology of Neusiedlersee, Austria. Hydrobiologia 214: 229–238.
Maness, T. R. & R. L. Kaesler, 1987. Ontogenetic changes in the carapace of Tyrrhenocythere amnicola (Sars) A hemicytherid ostracode. The University of Kansas Paleontological Contributions 118: 69–72.
Markgraf, V., J. P. Bradbury, A. Schwalb, S. J. Burns, C. Stern, D. Ariztegui, A. Gilli, F. S. Anselmetti, S. Stine & N. Maidana, 2003. Holocene palaeoclimates of southern Patagonia: limnological and environmental history of Lago Cardiel, Argentina. The Holocene 13: 581–591.
Martens, K., 1998. Sex and ostracods: a new synthesis. In Martens, K. (ed.), Sex and Parthenogenesis: Evolutionary Ecology of Reproductive Modes in Non-marine Ostracods. Backhuys Publishers, Leiden: 295–321.
Namiotko, T., D. L. Danielopol, U. von Grafenstein, S. Lauterbach, A. Brauer, N. Andersen, M. Hüls, K. Milecka, A. Baltanás & W. Geiger, 2015. Palaeoecology of Late Glacial and Holocene profundal Ostracoda of pre-Alpine lake Mondsee (Austria) – A base for further (palaeo-)biological research. Palaeogeography, Palaeoclimatology, Palaeoecology 419: 23–36.
Neil, J. V., 2000. Factors influencing intraspecific variation and polymorphism in marine podocopid Ostracoda, with particular reference to Tertiary species from southeastern Australia. Hydrobiologia 419: 161–180.
Neubauer, W. & J. Linhart, 2008. Approximating and distinguishing Ostracoda by the use of B-Splines. In Danielopol, D. L., M. Gross & W. E. Piller (eds), Contribution to Geometric Morphometric. Karl Franzens Universität, Graz: 21–42.
Ohlendorf, C., M. Fey, J. Massaferro, T. Haberzettl, C. Laprida, A. Lücke, N. Maidana, C. Mayr, M. Oehlerich, J. Ramón Mercau, M. Wille, H. Corbella, G. St-Onge, F. Schäbitz & B. Zolitschka, 2014. Late Holocene hydrology inferred from lacustrine sediments of Laguna Cháltel (southeastern Argentina). Palaeogeography, Palaeoclimatology, Palaeoecology 411: 229–248.
Palacios-Fest, M. R., A. S. Cohen & P. Anadón, 1994. Use of ostracodes as paleoenvironmental tools in the interpretation of ancient lacustrine records. Revista Española de Micropaleontología 9: 145–164.
Peypouquet, J. P., O. Ducasse, J. Gayet & L. Pratviel, 1980. Agradation et dégradation des tests d’ostracodes. Intéret pour la connaissance de l’évolution paléohydrologique des domaines margino-littoraux. Actes Réunion “Cristallisation, Déformation, Dissolution des Carbonates”, Groupe d'étude des Systèmes Carbonates. Université Bordeaux III, Bordeaux: 357–369.
Ramón-Mercau, J., C. Laprida, J. Massaferro, M. Rogora, G. Tartari, N. I. Maidana & N. I. Mainada, 2012. Patagonian ostracods as indicators of climate-related hydrological variables: implications for paleoenvironmental reconstructions in Southern South America. Hydrobiologia 694: 235–251.
Ramón-Mercau, J., M. S. Plastani & C. Laprida, 2014. A review of the genus Limnocythere (Podocopida: Limnocytheridae) in the Pampean region (Argentina), with the description of a new species. Limnocythere cusminskyae sp. nov. Zootaxa 3821: 26–36.
Reed, J. M., F. Mesquita-Joanes & H. I. Griffiths, 2012. Multi-indicator conductivity transfer functions for Quaternary palaeoclimate reconstruction. Journal of Paleolimnology 47: 251–275.
Rohlf, F. J., 1990. Morphometrics. Annual Review of Ecology and Systematics 21: 299–316.
Rohlf, F. J., 2010. TpsDig digitize landmarks from image files, scanner, or video, version 2.16. Department of Ecology and Evolution, State University of New York at Stony Brook.
Ruiz, F., M. Abad, A. M. Bodergat, P. Carbonel, J. Rodríguez-Lázaro, M. L. González-Regalado, A. Toscano, E. X. García & J. Prenda, 2013. Freshwater ostracods as environmental tracers. International Journal of Environmental Science and Technology 10: 1115–1128.
Schwalb, A., 2003. Lacustrine ostracodes as stable isotope recorders of late-glacial and Holocene environmental dynamics and climate. Journal of Paleolimnology 29: 267–352.
Schwalb, A., S. J. Burns, G. C. Cusminsky, K. Kelts & V. Markgraf, 2002. Assemblage diversity and isotopic signals of modern ostracodes and host waters from Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 187: 323–339.
Schweitzer, P. N., R. L. Kaesler & G. P. Lohmann, 1986. Ontogeny and heterochrony in the ostracode Cavellina Coryell from Lower Permian rocks in Kansas. Paleobiology 12: 290–301.
StatSoft. Inc., 2011. STATISTICA (data analysis software system), version 10. StatSoft. Inc., Cape Town.
Van der Meeren, T., D. Verschuren, E. Ito & K. Martens, 2010. Morphometric techniques allow environmental reconstructions from low-diversity continental ostracode assemblages. Journal of Paleolimnology 44: 903–911.
Van Harten, D., 1975. Size and environmental salinity in the modern euryhaline ostracod Cyprideis torosa (Jones, 1850), a biometrical study. Palaeogeography, Palaeoclimatology, Palaeoecology 17: 35–48.
Whatley, R., & G. C. Cusminsky, 2000. Quaternary lacustrine Ostracoda from Northern Patagonia: A review. In Gierlowski-Kordesch, E. H., & K. R. Kelts (eds), Late Basins Through Space and Time: American Association of Petroleum Geologist, Studies in Geology. Tulsa 46: 581–590.
Yin, Y., W. Geiger & K. Martens, 1999. Effects of genotype and environment on phenotypic variability in Limnocythere inopinata (Crustacea: Ostracoda). Hydrobiologia 400: 85–114.
Zhai, D., J. Xiao, L. Zhou, R. Wen, Z. Chang & Q. Pang, 2010. Similar distribution pattern of different phenotypes of Limnocythere inopinata (Baird) in a brackish-water lake in Inner Mongolia. Hydrobiologia 651: 185–197.
Acknowledgements
We thank the Patagonian Lake Drilling Project (PATO/PALATRA) team members for providing lake water chemistry data. We also appreciate the help and suggestion of anonymous reviewers, which improved this work significantly. This study was funded by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) PIP 00819 and 00021, the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), PICT 2010-0082 and 2014-1271, the Universidad Nacional del Comahue (Proyecto B166 and B194), the German Climate Research Program (DEKLIM E: PROSIMUL III, FKZ 01 LD 003), and the DAAD (PROALAR D/07/09565).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Alison J. Smith, Emi Ito, B. Brandon Curry & Patrick De Deckker / Multidisciplinary aspects of aquatic science: the legacy of Rick Forester
Rights and permissions
About this article
Cite this article
Ramos, L., Cusminsky, G., Schwalb, A. et al. Morphotypes of the lacustrine ostracod Limnocythere rionegroensis Cusminsky & Whatley from Patagonia, Argentina, shaped by aquatic environments. Hydrobiologia 786, 137–148 (2017). https://doi.org/10.1007/s10750-016-2870-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2870-z