Abstract
Quantification of intra-specific morphological variability of aquatic biota along environmental gradients can produce biological proxies that can be applied to paleoenvironmental reconstructions. This morphology-derived proxy information can be especially valuable when dealing with low-diversity fossil assemblages, i.e. in situations when paleoenvironmental inference based on species composition of the assemblage is less effective. We analyzed valve size and outline shape of the widespread and highly environmentally tolerant ostracode species Limnocythere inopinata collected in 15 lakes and ponds of Western Mongolia. We quantified shape variability among and within these living populations in relation to water chemistry and physical habitat variables. Our results indicate that: (1) a population’s mean valve outline is related to habitat type, (2) surface water temperature, the alkalinity to sulphate ratio, specific conductance and total phosphorus together explain a high portion of the variance in mean valve outline between populations, and (3) a quantitative model inferring the alkalinity to sulphate ratio from mean valve outline has an R² of 0.88 and RMSEP of 0.17. These results corroborate the hypothesis that high morphological variability in this ostracode species is due to both ecophenotypic variance and high clonal diversity associated with a mixed reproductive strategy (a combination of sexual and parthenogenetically reproducing lineages), and underline the value of morphometric techniques in paleoecology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantitative inference models that exploit species distributions of aquatic biota along modern-day environmental gradients are being developed with increasing frequency. This includes quantitative reconstructions based on fossil ostracode species assemblages from lake sediments (Mezquita et al. 2005; Viehberg 2006; Mischke et al. 2007, 2010). Successful application of such models depends on species turnover along the selected environmental gradient being sufficiently high, a condition in ostracode communities that is mainly found in the freshwater range (De Deckker and Forester 1988). At higher salinities associated with evolved continental brine types, assemblage diversity typically declines and few eurytopic species become dominant. Yet, such low-diversity ostracode assemblages contain evidence of population response to environmental gradients, for example through intra-specific ecophenotypic changes. With regard to applications in paleoenvironmental research, those working with ostracodes have linked the size, shape and ornamentation of valves to different aspects of the environment (Babinot et al. 1991). Especially in conditions with reduced species turnover (such as at high salinities), it is rewarding to make maximal use of the available morphometric “signals”.
This study links the size and outline shape of ostracode valves from living populations of Limnocythere inopinata, a common Holarctic species with broad tolerances, to ambient water chemistry. This species is known to have a mixed reproductive strategy involving both sexual and parthenogenetic reproduction. This has resulted in high clonal diversity, with certain clones being restricted to ephemeral or perennial habitats and others restricted to hydrologically stable freshwater lakes (Geiger et al. 1998).
Ostracodology has a long tradition of studying valve morphometry, including the pioneering work by Richard H. Benson and Richard A. Reyment (Benson 1976; Reyment and Bookstein 1993). However, quantitative studies of morphometric variability in recent continental ostracodes, and the implications for paleoecological or evolutionary interpretation of fossil assemblages, have emerged only recently (Baltanás and Geiger 1998; Baltanás et al. 2002, 2003; Danielopol et al. 2008). Danielopol et al. (2002) and Baltanás et al. (2003) described how morphometric analyses can be applied to evolutionary and ecological studies of continental ostracodes. Whereas the morphometry of more strongly ornamented marine ostracodes is usually described using landmark analysis (control points in geometric morphometrics [Reyment 1996]), outline analysis is preferred for non-marine ostracodes because they generally have smooth valve outlines, lacking clear homologous sites (Baltanás et al. 2002).
Concerning the morphological variability of L. inopinata, Yin et al. (1999) first showed, albeit qualitatively, that both environment and genome, and their interactions, determine valve shape. Baltanás et al. (2003) illustrated the morphometric variation in outline shape using 11 populations of L. inopinata from Austria and China, and attributed the observed differences in valve shape to gradients in habitat stability and water chemistry.
We analyzed a dataset of L. inopinata from lakes and ponds in Western Mongolia, covering a broad range of environmental stability and water chemistry. Our main aims were to: (1) confirm that morphological changes along environmental gradients are consistent, (2) quantify the corresponding variation in relation to environmental variables, and (3) validate the potential of this technique for quantitative environmental reconstruction.
Materials and methods
Collection and digitization of ostracode valves
Ostracode samples were collected with a 250-μm-mesh net in the shallow littoral zone of 15 lakes and ponds in Western Mongolia, during field campaigns in 2004 and 2005. Complementary data on the aquatic environment were recorded: surface water temperature (SWT), pH, specific conductance (SC, μScm−1), Total Dissolved Solids (TDS, mg L−1) and the water chemistry variables Ca (meq L−1), SO4 (meq L−1), Cl (meq L−1), Total Phosphorus (TP, mg L−1), Total Nitrogen (TN, mg L−1), Organic Nitrogen (ON, mg L−1) and carbonate alkalinity (Alk, meq L−1). From these values we calculated %Cl, %SO4, %Ca, the ratios Alk/Cl, Alk/SO4, Alk/Ca, Mg/Ca, and the Calcite Saturation Index (CSI) (Van der Meeren et al. 2010). Environmental variables with strongly skewed distributions among sites were logarithmically transformed before further analysis. Using the available regional ostracode collections from 55 Western Mongolian lakes (Van der Meeren et al. 2010), we selected sites that: (1) yielded enough specimens of the target species Limnocythere inopinata (≥20 adult specimens) (2) span the complete regional water chemistry gradient from freshwater to hypersaline, and (3) represent the diversity of sampled aquatic habitats, including pools and different types of lakes (dilute, medium saline and saline). Fifteen sites were selected for analysis (Table 1), of which two contain sexual populations (male:female sex ratios <1:1) and all others contain parthenogenetic populations only. All available specimens were dissected, and valves were stored dry in plastic micro-paleontological slides. A total of 678 left valves were mounted in outer lateral view on glass microscope slides, and were photographed against translucent background light with a Leica® DFC420 digital camera. Valve length was measured on these original images with LAS® software. For each site, ten specimens (nine for population s1 of the eastern side of Uvs Nuur) were subjected to full morphometric analysis of the valve outline.
Morphometrics
Digital images were first edited manually by increasing contrast and removing occasional shadows or dirt particles that obscured the valve outlines. The resulting black and white images were converted to bitmap format, and the outlines were first digitized using tps software (Rohlf 2001), and then subjected to B-spline analyses using Morphomatica v 1.6 (Brauneis et al. 2006), which also allows computation of mean population shapes. Morphomatica was set to standardize lateral valve outlines for area prior to computation of control points, hence the observed variance in position of control points represents only shape differences (independent of size). This procedure resulted in coordinates for 24 control points along the computed mean valve outlines. Additional applications of Morphomatica software are described in Baltanás et al. (2003) and Danielopol et al. (2008). Variance in shape for each population was calculated as the square root of the sum of Euclidian distances between control points for all specimens of a population and their calculated mean specimen, divided by the number of control points. In this way, shape variance for each population was quantified as the average deviation of control points from the corresponding calculated mean specimen.
Statistical analysis
Non-metric multidimensional scaling (NMDS) in Primer v 6 (Clarke and Gorley 2006) visualized the morphological diversity as recorded by the coordinates of the control points of the computed mean shapes. A series of Redundancy Analyses (RDA, in Canoco v 4.5 software; ter Braak and Šmilauer 2002) with manual forward selection of variables and significance tested through 999 Monte Carlo permutations, allowed us to extract the portion of shape variance explained by the minimal set of measured physical and chemical variables. Single variables were added to the ordination to quantify their marginal effect (including co-variation), whereas their unique effect (explanatory power of the individual variable) was assessed by a series of partial RDAs. The percent variance explained by each variable was calculated from the proportion of the sum of canonical eigenvalues versus the total inertia. Based on the same set of morphometric and environmental data, inference models based on the Modern Analogue Technique (MAT) were constructed with PAST v 1.91 (Hammer et al. 2001) software, making use of Squared Euclidean distance measure, leave-one-out jackknifing, and interpolation based on the closest two analogues among the sampled modern populations.
Results
Non-metric multidimensional scaling (NMDS) analysis illustrates the mean morphological variation among the 15 Mongolian L. inopinata populations as measured by the control point coordinates of the calculated mean shapes (Fig. 1). The resulting spatial arrangement in two-dimensional morphospace is based solely on the mean shape differences between populations, but also orders the populations according to their general habitat type. The mean shape of populations from hydrologically stable freshwater habitats (dilute, d1–d3; Table 1) is consistently more rectangular, whereas populations from saline (s1–s4) or ephemeral (pond, p1–p4) environments display a more strongly sloping posterodorsal margin. The two freshwater populations with males (d1 and d2) plot close together with the third freshwater lake population and two of the pond populations. The electronic supplementary material shows more clearly the shape variance and computed mean shape for the total set of analyzed outlines, and for some populations representing extremes in morphospace as indicated by their position in NMDS (populations d3, m2, s1).
Nonmetric multidimensional scaling analysis of the morphometrical variability of the calculated mean valve shape of ostracode populations from Western Mongolia, with superimposed reconstructed mean population outlines; valve outlines were standardised for size and oriented with anterior margin to the left and ventral margin downwards. Abbreviations as in Table 1
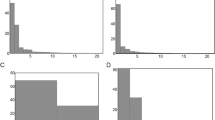
Populations from saline waters have on average smaller valves than those of dilute waters. The highest correlation (r = −0.67; p < 0.001) between mean valve length and an environmental variable was found with specific conductance (SC) (Fig. 2a). Morphological variability (shape variance) within a population is highest for warm, typically shallow, saline lakes (r = 0.58, p = 0.02 for SC and r = 0.59, p = 0.02 for surface water temperature [SWT] (Fig. 2b,c). Population m2 (illustration C of electronic supplementary material) displayed the lowest variance in shape, whereas population s1 had the maximum variance (illustration D of electronic supplementary material).
a Relationship between average valve length and SC (log curve indicates correlation with log SC; r = −0.67) b Morphometric variation within populations, in relation to salinity (as specific conductance, SC) c morphometric variation within populations, in relation to surface-water temperature d relationship between Alk/SO4 and salinity as TDS (double log curve indicates correlation between log TDS and log Alk/SO4; r = −0.76)
Redundancy (RDA) analyses indicated that TDS, SWT, the Alk/SO4 ratio and TP have the greatest influence on the mean shape of individual populations. In our dataset, Alk/SO4 itself is inversely correlated (r = −0.76; p < 0.001) with specific conductance (Fig. 2d). The selected four variables together explain 63% of the shape variance, as quantified by control points of the mean calculated shapes (Fig. 3). Their unique contributions to the observed morphometric variation ranged from TDS (27.1%), SWT (22.7%), Alk/SO4 (21.3%) to TP (12.0%), but taking into account the co-variance explained by the other co-variables (partial RDA), only SWT and Alk/SO4 showed significant contributions (Table 2). A Modern Analogue Technique (MAT) inference model for estimation of the Alk/SO4 ratio from mean shape variance gave the best performance over the other major variables, with an R² of 0.88, RMSEP of 0.170 and a mean error of 12% of the total gradient (Fig. 4). The skewed trend in the residuals of this model mainly results from underestimation of Alk/SO4 at the dilute end of the water chemistry gradient.
Redundancy analysis of the mean morphometric variation between 15 Mongolian L. inopinata populations, with indication of the loadings of selected environmental variables on the first two ordination axes. Habitat type abbreviations as in Table 1
a Inferred versus measured Alk/SO4 for the MAT inference model based on mean valve shape variance of Mongolian L. inopinata populations, with superimposed regression (solid line), 95% confidence interval (dotted lines) and 1:1 reference (broken line) b plot of model residuals with regression (solid line) and zero reference (broken line)
Discussion
Geiger et al. (1998) suggested that the high morphological diversity of L. inopinata, in combination with its ecological segregation (multiple asexual clones with different environmental tolerances), could eventually lead to taxonomic separation. However, from a phylogenetic point of view, such splitting makes little sense, because the sexual populations and the parthenogenetic clones of this species form a (semi-) continuous cluster (Yin et al. 1999). Although the extremes of this morphological gradient within L. inopinata can be easily discriminated visually, morphometric tools allow quantification of morphometric variation over its entire range (Baltanás et al. 2003).
In our dataset from Mongolia, outline shapes of populations from similar environments clustered together in multidimensional morpho-space, but the degree of clustering varied between the different environments: shape variance increased with increasing temperature and specific conductance, conditions which in our dataset are mainly associated with shallow saline lakes. This greater shape variance may reflect the higher clonal diversity of populations of this species inhabiting highly fluctuating environments (Geiger et al. 1998). As no rare males were observed, but sex ratios in sexual populations were <1:1 (male:female), the two ‘sexual’ populations could represent a combination of sexually reproducing and parthenogenetic lineages, as has been described for other ostracode species with mixed reproduction (Bode et al. 2010). The position of these at least partly sexually reproducing populations in the NMDS plot does not differ markedly from the exclusively parthenogenetic reproducing populations, especially not from the other freshwater population. However, our limited dataset does not allow proper comparison between analogous environments to discriminate morphometric differences between populations with different reproductive strategies.
Our data show a negative relationship between valve length and solute concentration, in agreement with previous observations that this species grows smaller in more saline waters. This aspect of morphometric variation has been studied with L. inopinata populations in China (Yin et al. 2001; Zhang et al. 2004) and Nigeria (Roberts et al. 2002). However, given the demonstrated impact of temperature on valve length in single clones (Yin et al. 1999) and on mean shape (this study), we recommend that length measurements for quantitative inference of past salinity be used only in situations where temperature variation through time can be assumed negligible.
Variation in mean valve outline (i.e. xy-coordinates of control points along the computed mean population shapes) showed good correlation with specific environmental variables. The similar relative position of individual populations in the NMDS and RDA plots (Figs. 1 and 3) indicates that the explained shape variance represents real morphological gradients, also in the ordination. Interestingly, the concentration of calcium—the environmental variable that best explained the presence/absence of L. inopinata in a larger regional dataset of Mongolian waters (Van der Meeren et al. 2010)—was not selected here as influencing its morphometric variation. Apparently, other variables than those affecting the distribution of the species as a whole govern the shape variance; morphometric variance is associated with ecophenotypic variability and occurrence of different clones within the species. Although salinity (as TDS) explained a sizable fraction (27.1%) of the variance when it was the single variable in the ordination, this decreased (10.1%) and dropped below significance when the effects of temperature, Alk/SO4 and TP were taken into account. Temperature and Alk/SO4 had high and significant individual contributions to the explained variance. Because temperature was measured as SWT at the moment of sampling, it gives little information about the real temperature regime of the sampled sites at the moment of valve calcification. Although SWT is related to physical aspects of both water body, depth, among others, and site, altitude, among others, it lacks the temporal integration that mean water temperature has during the growth phase. Diurnal changes and weather conditions presumably produced some scatter in the recorded SWT data. As such, it makes sense that the best-performing MAT inference model was obtained for Alk/SO4, which is probably more stable over the season than SWT. Because sulfate behaves more conservatively in Mongolian waters (its concentration increases more strongly with salinity than does alkalinity), Alk/SO4 is inversely related to solute concentration. As Alk/SO4 is linked to solute evolution, inferred changes could track changes in lake water balance or relative climatic moisture, or changes in the source of solutes delivered to the lake. Hydrological or climatological shifts resulting in more negative (local) water balance can thus produce a decrease in Alk/SO4.
The statistical performance of our MAT inference model, which is similar to that of models based on ostracode species assemblages (Mezquita et al. 2005; Mischke et al. 2007), suggests high potential for paleoecological applications. The skewed residuals largely result from underestimation of Alk/SO4 in the freshwater range, but few populations in our dataset cover this part of the gradient. A larger dataset would allow further identification of the distribution and plasticity of different morphotypes along environmental gradients.
Our results represent the morphological response of what could be considered the Limnocythere inopinata species complex, including sexual and parthenogenetic lineages, to variation in aquatic environments in the studied region of Western Mongolia. Whereas we expect a similar response over larger geographic scales, this would need to be confirmed for other regions before this morphometric proxy could be applied in paleohydrological reconstructions from those regions. Also, because the pattern of occupied geographical and ecological niches in such a species complex is probably quite dynamic (resulting from turnover of parthenogenetic lineages; Bode et al. 2010), interpretation of fossil ostracode data based on calibration of valve shape in recently occupied niches should always be done within the limitations of the “space for time substitution” of calibration data sets in mind.
Conclusions
Observed morphological variation of L. inopinata along a gradient of dissolved ion concentration and composition in Mongolian waters most likely represents both ecophenotypic responses and turnover of clones with slightly different shapes. Populations from similar habitats also displayed similarity in valve outlines, and morphological variance was highest for shallow saline lakes. The variables SWT, TDS, Alk/SO4 and TP explain a high portion of the observed morphological variation in mean population shapes. These patterns permit quantitative inference of past water chemistry, as illustrated by a MAT inference model for Alk/SO4.
We propose that the presented methodology is particularly relevant for fluctuating saline environments where ostracode shape variability is high (Geiger et al. 1998, this study) and species diversity is typically low. Future quantitative reconstructions based on ostracode morphometrics have most potential if: (1) interactions between morphological variation associated with different reproductive modes (including clonal ecology), and ecophenotypic variance within lineages are well understood (L. inopinata provides a good model organism for such research); (2) response to environmental change in reconstructions can be (partially) constrained with respect to temperature or hydrochemical changes. Because both variables affect ostracode size and shape, studying environments with specific hydrochemical or physical stability would help identify drivers and responses; and (3) timescales of the environmental change of interest, morphometric response and sample resolution are sub equal.
References
Babinot J-F, Carbonel P, Peypouquet J-P, Colin J-P, Tambereau Y (1991) Variations morphologiques et adaptations morphofonctionelles chez les ostracodes: signification environnementale. Geobios 13:135–145
Baltanás A, Geiger W (1998) Intraspecific morphological variability: morphometry of valve outlines. In: Martens K (ed) Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracods. Backhuys, Leiden, pp 127–142
Baltanás A, Alcorlo P, Danielopol DL (2002) Morphological disparity in populations with and without sexual reproduction: a case study in Eucypris virens (Crustacea, Ostracoda). Biol J Linn Soc 75:9–19
Baltanás A, Braunei W, Danielopol DL, Linhart J (2003) Morphometric methods for applied ostracodology: tools for outline analysis of nonmarine ostracodes. Paleontological Society Papers 9:101–117
Benson RH (1976) The evolution of the ostracode Costa analyzed by “Theta-Rho” difference. Abh Natwiss Ver Hambg 18(19):127–139
Bode SNS, Adolfsson S, Bautz ERD, Martins MJF, Schmit O, Vandekerkhove J, Mezquita F, Namiotko T, Rossetti G, Schön I, Butlin RK, Martens K (2010) Exceptional cryptic diversity and multiple origins of parthenogenesis in a freshwater ostracod. Mol Phylogenet Evol 54:542–552
Brauneis W, Linhart J, Stracke A, Danielopol DL, Neubauer W, Baltanás A (2006) Morphomatica (Version 1.6.0) user manual/tutorial. Limnological Institute of the Austrian Academy of Sciences, Mondsee, Austria
Clarke KR, Gorley RN (2006) Primer v. 6: computer program and user manual/tutorial. Primer-E Ltd, Plymouth, p 190
Danielopol DL, Ito E, Wansard G, Kamiya T, Cronin T, Baltanás A (2002) Techniques for collection and Study of Ostracoda. In: Holmes JA, Chivas AR (eds) The Ostracoda: Applications in Quaternary Research. The American Geophysical Union, Washington DC, pp 65–97
Danielopol DL, Baltanás A, Namiotko T, Geiger W, Pichler M, Reina M, Roidmayr G (2008) Developmental trajectories in geographically separated populations of non-marine ostracods: morphometric applications for palaeoecological studies. Senckenb Lethaea 88:183–193
De Deckker P, Forester RM (1988) The use of ostracods to reconstruct continental palaeoenvironmental records. In: De Deckker P, Colin JP, Peypouquet J-P (eds) Ostracoda in the Earth Sciences. Elsevier, New York, pp 175–199
Geiger W, Otero M, Rossi V (1998) Clonal ecological diversity. In: Martens K (ed) Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracods. Backhuys, Leiden, pp 243–256
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9 [downloaded at http://palaeo-electronica.org/2001_1/past/issue1_01.htm]
Mezquita F, Roca JR, Reed JM, Wansard G (2005) Quantifying species-environment relationships in non-marine Ostracoda for ecological and palaeoecological studies: examples using Iberian data. Palaeogeogr Palaeoclimatol Palaeoecol 225:93–117
Mischke S, Herzschuh U, Massmann G, Zhang C (2007) An ostracod-conductivity transfer function for Tibetan lakes. J Paleolimnol 38:509–524
Mischke S, Bößneck U, Diekmann B, Herzschuh U, Jin H, Kramer A, Wünnemann B, Zhang C (2010) Quantitative relationship between water-depth and sub-fossil ostracod assemblages in Lake Donggi Cona, Qinghai Province, China. J Paleolomnol 43:589–608
Reyment RA (1996) Some applications of geometric morphometrics to Ostracoda. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE (eds) Advances in Morphometrics. Plenum Press, New York, pp 387–398
Reyment RA, Bookstein FL (1993) Infraspecific variability in shape in Neobuntonia airella: an exposition of geometric morphometry. In: McKenzie KG, Jones PJ (eds) Ostracoda in the Earth and Life Sciences. AA Balkema, Rotterdam, pp 291–314
Roberts HR, Holmes JA, Swan ARH (2002) Ecophenotypy in Limnocythere inopinata (Ostracoda) from the late Holocene of Kajemarum Oasis (north-eastern Nigeria). Palaeogeogr Palaeoclimatol Palaeoecol 185:41–52
Rohlf FJ (2001) Tpsdig, Program version 1.43. Department of Ecology and Evolution, State University of New York, Stony Brook (http://life.bio.sunysb.edu/morph/soft-dataacq.html)
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community Ordination (version 4.5). Microcomputer Power, Ithaca NY, USA
Van der Meeren T, Almendinger JE, Ito E, Martens K (2010) The ecology of ostracodes (Ostracoda, Crustacea) in western Mongolia. Hydrobiologia 641:253–273
Viehberg FA (2006) Freshwater ostracod assemblages and their relationship to environmental variables in waters from northeast Germany. Hydrobiologia 571:213–224
Yin Y, Geiger W, Martens K (1999) Effects of genotype and environment on phenotypic variability in Limnocythere inopinata (Crustacea: Ostracoda). Hydrobiologia 400:85–114
Yin Y, Li W, Yang X, Wang S, Li S, Xia W (2001) Morphological responses of Limnocythere inopinata (Ostracoda) to hydrochemical environment factors. Sci China Ser D 44:316–323
Zhang E, Shen J, Wang S, Yin Y, Zhu Y, Xia W (2004) Quantitative reconstruction of the paleosalinity at Qinghai Lake in the past 900 years. Chin Sci Bull 49:730–734
Acknowledgments
We thank Y. Khand (Mongolian Academy of Sciences) and S.N. Soninkhishig (National University of Mongolia) for invaluable support during fieldwork and J.E. Almendinger for assembling and processing the water chemistry data. Collection of the study material was supported by the National Science Foundation (NSF) under grants DEB-0316503 to Mark Edlund and James E. Almendinger (Science Museum of Minnesota). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of NSF. The research was funded by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) to the first author. We also thank Mark Brenner, Finn Viehberg and one anonymous referee for helpful comments. This is contribution 10-06 of the Limnological Research Center of the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10933_2010_9463_MOESM1_ESM.pdf
Illustration of morphometric outline variance (standardized for area) for the full set of included outlines and for three selected populations (a) all included outlines (fifteen populations, n = 149) (b) population d3 (c) population m2 (d) population s1. Thin black lines indicate individual valve outlines by thin plate splines algoritm (see Materials and methods), thicker red lines indicate the corresponding computed mean population shape. The selected populations represent extremes in the observed morphospace as identified by NMDS (cf. Fig. 1 manuscript) (PDF 119 kb)
Rights and permissions
About this article
Cite this article
Van der Meeren, T., Verschuren, D., Ito, E. et al. Morphometric techniques allow environmental reconstructions from low-diversity continental ostracode assemblages. J Paleolimnol 44, 903–911 (2010). https://doi.org/10.1007/s10933-010-9463-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-010-9463-z