Abstract
Limnoperna fortunei first came to scientific attention when it was introduced into the potable freshwater supply system of Hong Kong around 1968. The initial occurrence in Hong Kong was related to the commencement of supplies of water from the East River in China. Aspects of the biology and anatomy of L. fortunei were investigated in 1973 (Morton, Malacologia 12:265–281, 1973). Then, comparisons were made with other mytiloids and with the invasive Dreissena polymorpha (Dreissenidae), which L. fortunei resembles superficially. The suggestion of a relationship between the two taxa was discounted on anatomical grounds—both species being convergently heteromyarian, adapting them to similar lives in the freshwater habitats of Eastern and, subsequently, Western Europe and North America, and mainland Asia plus Japan and Taiwan and latterly South America, respectively. The present study re-examines aspects of the biology and anatomy of L. fortunei commensurate upon its recent range extensions, specifically with regard to its occupation of a variety of freshwater habitats in order to better understand the reasons for its opportunistic success. It is concluded that L. fortunei probably evolved from a brackish water either Xenostrobus-like or Perna-like ancestor—the question of Limnoperna’s ancestry still unresolved. Regardless, it was the evolution of the heteromyarian form in the, indisputedly mytiloidean, ancestor that opened up the hitherto bivalve-unoccupied hard-surface epibenthic environment for colonisation in both lentic and lotic freshwaters ecosystems. This was probably associated with the adoption of osmoregulation and efficient systems for the collection, selection and digestion of ample seston resources. The energy thereby obtained has been focussed into rapid fecundity at the expense of shell growth and the adoption, thereby, of a r-selected sexual strategy and life history trait.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
In recent years, the attentions of water supply engineers and biologists in Europe and North and South America have been drawn to the problems of fouling caused by introduced freshwater bivalve molluscs. Since the nineteenth century, Dreissena polymorpha (Pallas 1771) has expanded its range from an initial restricted area focussed on the Caspian and Black seas to one encompassing much of the European continent. Although the initial spread of this species may have started in the eighteenth century (Karatayev et al. 2007b), the subsequent dramatic range expansion is attributed to the construction of waterways for the transport of goods and raw materials during the Industrial Revolution. This is certainly the reason for the range expansion that D. polymorpha experienced in Great Britain in the nineteenth century and subsequently (Kerney and Morton 1970), and it is still being transported this way, along canals and rivers, in Ireland (Hayden and Caffrey 2013). In the twentieth century, however, D. polymorpha (McMahon 1982) and a second species, Dreissena rostriformis bugensis Andrusov, 1897 (Mills et al. 1996), were introduced into North America, probably both within the ballast water of ships arriving in the St. Lawrence Seaway around 1989 (see Chapter “Parallels and Contrasts Between Limnoperna fortunei and Species of Dreissena” in this volume). Subsequently, D. rostriformis bugensis has been back-introduced into Europe, but in the west, that is, the freshwater section of the Rhine–Meuse Estuary in The Netherlands in 2006 and, later, upstream into the River Rhine and its tributaries, the Main and Moselle in Germany, and the River Meuse in Belgium and France (Mathews et al. 2014; see Fig. 3 in Chapter “Parallels and Contrasts Between Limnoperna fortunei and Species of Dreissena”). Both dreissenid species possess a byssus and are thus adapted to the colonisation of solid surfaces, such as are found in the pipes and conduits of water supply systems, typically inaccessible to the endobenthic bivalve constituents of freshwater faunas, for example, representatives of the Unionoidea and Corbiculoidea.
That said, however, the Asian corbiculid Corbicula fluminea (Müller 1774) has been introduced into North American waterways and caused similar fouling problems as the two species of Dreissena. First reported upon in 1938 from the Columbia River in the state of Washington (Burch 1944), this species has since expanded its range to encompass all of the major North American river basins (McMahon 1982). The fouling problems caused by C. fluminea are somewhat different from those caused by Dreissena in that as an endobenthic species it became a particular nuisance when occurring in sands dredged for ultimate use in the manufacture of concrete. Although, it does clog pipes and power station condensers. As with the species of Dreissena, the rapid range expansion experienced by C. fluminea is attributable to man’s development of artificial waterways for water supply purposes. Corbicula fluminea has been introduced into European waters being first reported upon from the Rhine in 1991 (Kinzelbach 1991). It has subsequently spread throughout much of Western Europe and invaded Great Britain being recorded first from the River Chet in the Norfolk Broads in 1998 (Howlett and Baker 1999). It has subsequently spread as far east as the Czech Republic (Beran 2000; 2006), colonising all the countries in-between. Like Limnoperna fortunei, C. fluminea has also been introduced into South America, being recorded (as Corbicula manilensis) from Brazilian rivers in 1978 by Veitenheimer-Mendes (1981) and from the Río de la Plata estuary, Uruguay (as C. largillierti), by Ituarte (1994), in 1982. Beasley et al. (2003) recorded C. fluminea as occurring first in the lower Amazon basin and the Pará and Tocantins rivers, Brazil, in 1997–1998.
Recently too, another corbiculid, that is, Corbicula fluminalis (Müller 1774) has been introduced into Venezuela (Martínez 1987; as C. manilensis (Philippi 1844), which is a junior synonym of C. fluminalis). This species also occurs in Asia, but is estuarine occupying the lower reaches of major rivers such as the Pearl River in southern China where it was first studied (Morton 1982). As an estuarine species, it is unlikely that C. fluminalis will impact water supply systems to the same extent as the wholly freshwater species of Dreissena and C. fluminea; although where such waters are tapped for industrial cooling water systems this species too may become a problem in its now, new, introduced range.
A similar problem has arisen with the Chinese pond mussel Sinanodonta woodiana (Lea 1834), which, in its native China, produces glochidia larvae that attach to fish hosts (Dudgeon and Morton 1984). Like C. fluminea, it has been introduced into the countries of Western (Austria, Belgium, Croatia, France, Germany, Italy, Portugal, Spain) and Central (Czech Republic, Hungary, Poland, Romania, Serbia, Slovakia, Ukraine) Europe, and has been identified from some locations in Indonesian and Caribbean islands. It is thought that the species has been introduced into such waters from China as glochidia attached to species of carp destined for aquaculture purposes in Europe and elsewhere (Douda et al. 2012).
The mytilid L. fortunei (Dunker 1857) occurs in the rivers of China and East Asia (see Chapter “Distribution and Spread of Limnoperna fortunei in China” in this volume) but aroused little interest until it too was introduced outside its native range into the potable freshwater supply system in Hong Kong around 1968 (Morton 1975) probably from the East River, itself a tributary of the Pearl where the species occurs naturally (Miller and McClure 1931). There is a dearth of early information on this animal, most records being found in old and, often, obscure journals. Early studies led to a plethora of names being erected for the species including Dreissena siamensis Morelet, 1866, Limnoperna depressa Brandt and Temcharoen, 1971, Limnoperna lemeslei Rochebrune, 1882, Limnoperna supoti Brandt, 1974, Modiola cambodjensis Clessin, 1889, Modiola lacustris Martens, 1875, Mytilus martensi Neumayer, 1898 and Volsella fortunei Dunker, 1857. Today, however, all these names of putative Asian species have been subsumed within L. fortunei, an account of the species’ nomenclatural history being provided by Morton and Dinesen (2010).
Limnoperna fortunei is of interest, however, from a number of viewpoints. In the first instance it is, as a representative of the Mytiloidea, living in a unique habitat. Secondly, L. fortunei possesses a striking, albeit superficial, similarity to species of Dreissena. Finally, and most importantly, prior to 1968, L. fortunei was unstudied (other than taxonomically) and largely unknown outside China. The species does appear to be native to mainland China (Morton and Dinesen 2010). Holikoshi (1935), first recorded L. fortunei (as Volsella (Limnoperna) lacustris [v. Martens]) from Taiwan, and this record was subsequently accepted by Kuroda (1941) in his catalogue of the island’s malacofauna. In the absence of reference specimens, however (Huang 2008), the species was not positively (re)discovered and recorded from Taiwan until > 50 years later (Tan et al. 1987). Limnoperna fortunei was first found in Lake Biwa, Japan, in 1992 (Kimura 1994a b) and is, today, known to be widespread in 10 of Japan’s 47 prefectures (Tominaga et al. 2009; see Chapter “Colonization and Spread of Limnoperna fortunei in Japan” in this volume) and is now a pest of potable water supply systems (Magara et al. 2001; Goto 2002). Tominaga et al. (2009) also suggested, based on genetic studies, moreover, that introductions had occurred independently on at least two occasions but that several Japanese populations were established by dispersal of individuals from other non-native ones.
Around 1989, L. fortunei appeared in the Río de la Plata estuary, in Argentina (Pastorino et al. 1993; Darrigran and Pastorino 1995). By the early part of the twenty-first century, the species had spread to Rio Grande do Sul, Brazil (Santos et al. 2005) and then, in 2006, to Uruguay, Paraguay and Bolivia. It is a highly successful invasive species in South America and is expected to spread further (see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume).
The initial appearance of L. fortunei in the water supply complex of Hong Kong was considered by Morton (1975) to be a re-enactment of the earlier patterns of colonisation typical of the species of Dreissena and C. fluminea and, thus, that not only might this mytiloidean be detrimental to water supply systems throughout its initial range expansion into Hong Kong, Taiwan and, especially, Japan, but also South America. Moreover, it has the potential to be introduced elsewhere, notably North America and Europe (Oliveira et al. 2010), where, if successful, would add to the suite of already recognised problems engendered by alien freshwater bivalves.
This study of the biology and the anatomy of L. fortunei complements and adds to that of Morton (1973) and draws on other information published on the species subsequent to its range expansion outside the (natural) borders of China and proliferation in its alien range extensions. It seeks to explain this invasive success in terms of biology and anatomy although, ultimately, a greater understanding of the species will be needed as its spread continues inexorably.
The following glossary, intended for readers without a malacological background, offers brief definitions of the specialised terms used throughout this chapter. Most of the features listed are illustrated in Fig. 5.
-
Adductor muscle: one of normally two muscles that appose the shell valves against the opening force of the ligament; the insertion of these muscles in the shell interior is marked by a conspicuous adductor muscle scar.
-
Branchial chambers: the supra-branchial chamber is the space above the ctenidia, among the folds of the two demibranchs (exhalant chamber); the infra-branchial chamber is the mantle cavity below the gills (inhalant chamber).
-
Byssal groove: the groove along which the byssus passes post secretion and prior to attachment externally.
-
Byssus: fibres produced by the byssal gland in the foot and used to anchor the animal to a substratum.
-
Crystalline style: rod-shaped body in the stomach, produced by secretions within the style sac, with enzymatic properties that are released by rotation and trituration of ingested food particles against the stomach’s gastric shield.
-
Ctenidium: each of the gills, which serve for respiration, potential food collection and particle selection.
-
Demibranch: one of the two folded lamellae on each side of the visceral mass, comprising inner and outer demibranchs which together form a ctenidium.
-
Digestive diverticula: blind tubes opening laterally from the stomach wall and where intracellular digestion occurs.
-
Dissoconch: the final juvenile and adult shell of a bivalve.
-
Dorsal hood: pocket connected dorsally to the stomach.
-
Druse: a clump or aggregation of mussels around either a stone or other hard object, held together by byssal threads.
-
Exhalant siphon: siphon through which water and faeces from the mantle cavity are exhaled and expelled, respectively.
-
Filibranchiate (eleutherorhabdic): a ctenidium characterised by loosely connected filaments held in place by interlocking cilia projecting from ciliated discs.
-
Food-sorting caecum: a blind pouch connected to the stomach where food particles are selected for either consumption or rejection.
-
Gastric shield: chitinous dorsal region of the stomach wall against which the crystalline style´s rotating action fragments food particles. Is probably also enzymatically active.
-
Heteromyarian: with unequal sized adductor muscles. The posterior typically larger than the anterior.
-
Hinge plate: the internally flattened area of the dorsal margin of the shells that bears the ligament.
-
Hinge teeth: calcareous interlocking teeth and sockets that help keep the valves aligned during opening and closing (absent in L. fortunei).
-
Homorhabdic: a ctenidial type characterised by a single type of filament.
-
Inhalant aperture: the aperture for water intake into the mantle cavity. Typically separate from the pedal/byssal gape, but not in mytiloids.
-
Labial palps: paired triangular particle-sorting lamellae situated on either side of the mouth.
-
Ligament: elastic structure that connects the two shell valves at the hinge line. The ligament opens the valves when the adductor muscles relax, maintaining tonus.
-
Mantle: outer layer of tissue lining the internal surface of the shell valves.
-
Mantle margin: Typically three folded margin of the left and right mantle lobes which secrete the periostracum and the shell and may be selectively fused to form, for example, the exhalant siphon.
-
Mesosomal lobes: lobes of the sac-like extension of the visceral mass and mantle containing the paired gonads.
-
Nepioconch: the developmental stage of the shell after the planktonic prodissoconch stages and separated from the dissoconch, by a growth discontinuity.
-
Opisthodetic: the ligament type restricted to the postero-dorsal margin of the shell beyond the umbones.
-
Pallial line: scar on the interior of the shell marking the attachment of the pallial retractor muscles.
-
Pallial sinus: an embayment in the posterior part of the pallial line that marks the attachment of the siphonal retractor muscles and defines the mantle cavity space into which the siphons can retract.
-
Parivincular: dorsal ligament type.
-
Pericardial gland: excretory organs that accumulate waste products and discharge them into the pericardial cavity, from where they are eliminated via the kidneys into the supra-branchial chamber.
-
Pericardium: transparent sac or peritoneum, that contains the heart.
-
Periostracum: outer organic layer of the shell and comprising three layers in the Mytiloidea.
-
Prodissoconch: larval shell at the apex of the umbones, comprising two growth phases (prodissoconch I and II), separated by a growth line. The oldest region of the shell.
-
Siphonal septum: sensory membrane at the apex of the inhalant aperture and beneath the exhalant siphon and restricting the size of the former.
-
Statocyst: capsule-like sense organ conveying information about orientation.
-
Style sac: cylindrical extension of the stomach that secretes the crystalline style.
-
Typhlosole: an infolding along the inner wall of the intestine and stomach and whose function is to transport particles in an intestinal groove for either digestion, rejection or resorting.
-
Umbo: rounded extremity of the bivalve shell that represents its oldest part, characterised by the prodissoconchs.
-
Umbonal keel: prominent, angled or rounded, feature on the external shell surface that begins at the umbo and extends obliquely to the postero-ventral margin of each valve (=or rounded, feat
Biology
When living in relatively slow flowing waters, L. fortunei characteristically occurs in clumps, or nodules (sometimes referred to as druses), of individuals living bound together in thin monolayers (see Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume) by stout byssal threads.
The species prefers the deeper waters of freshwater bodies (Nakano et al. 2010; Brugnoli et al. 2011, and see below), but naturally occupies the shallower banks of colonised habitats because this is where water movements keep substrata free of settling silt. Limnoperna fortunei can also withstand periodic, but brief, natural bouts of emersion (Iwasaki 1997) but which may, however, be longer under experimental conditions (Montalto and Ezcurra de Drago 2003; see Chapter “Control of Limnoperna fortunei Fouling by Desiccation” in this volume). When settling, newly metamorphosed individuals are strongly thigmotaxic with a preference for the angled crevices between vertical walls. They are also attracted, as in many bivalves, by the presence of adult conspecifics (Sardiña et al. 2009) and the tendency to aggregate is reinforced by this behaviour. Juveniles of L. fortunei are also negatively phototaxic and positively geotaxic and Morton (1975) showed in reservoir experiments using three-dimensional settling panels that the greatest recruitment was (1) at depths of 7–10 m, (2) to the undersurfaces of the panels (negative phototaxis) and (3) the vertical crevices of upper surfaces (positive geotaxis; Fig. 4 in Morton 1975), as later demonstrated by Uryu et al. (1996) in the laboratory.
The strong negative phototaxis exhibited by L. fortunei under experimental conditions of strong light (Uryu et al. 1996) is interesting because no photosensory receptors have been identified for this species hitherto. This study will cast light on this problem (see below). The juvenile behaviour, however, helps explain how L. fortunei can survive in the pipelines and culverts of water supply systems. In fast-flowing waters, such as are found in some invaded rivers and potable water pipes, L. fortunei inhabits crevices and pits, although from these foci succeeding generations can spread out to cover more and more of any exposed surfaces. Its byssus makes it extremely difficult to dislodge in such situations. As with the two species of Dreissena and C. fluminea, therefore, the ecological effects of L. fortunei invasions are not only profound but ecosystem-wide (Boltovskoy et al. 2009; Cataldo et al. 2012; Boltovskoy and Correa 2015).
Anatomy
The Shell and Ligament
The shell of L. fortunei has four stages. Prodissoconch I and prodissoconch II are both free-swimming larval stages, the first reaching a length of between 115 and 117 μm (Cataldo et al. 2005). Prodissoconch II, the veliger, is larger (~ 320 μm), highly torsioned (~ 180º), with respect to subsequent stages and coloured rose-violet (Morton and Dinesen 2010). The nepioconch forms subsequently and may reach a shell length of ~ 1300 μm. This stage is sometimes referred to as the plantigrade stage because it possesses a long thin, pipe-like, crawling foot. The final shell stage, the dissoconch, is formed by the juvenile individual and becomes the permanent shell of the adult. This pattern of shell development is shared with some other mytiloidean lineages, including Mytilus and Perna (Ockelmann 1983). In addition to Fig. 2 in Cataldo et al. (2005), other illustrations of the L. fortunei larvae are provided by Choi and Kim (1985, their Figs. 4–8) and Pinheiro dos Santos et al. (2005, their Figs. 2–10; see Chapter “Larval Development of Limnoperna fortunei” in this volume). The nepioconch grows progressively and typically rapidly into the juvenile. With growth, the distances moved by crawling juveniles decrease with increasing shell length (Uryu et al. 1996). Byssal attachment typically occurs when an habitat suitable for adult occupation has been chosen and the dissoconch quickly assumes the adult form.
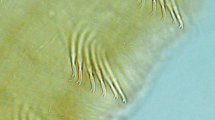
Before it does this, however, Montalto and Rojas Molina (2014) have shown that the dissoconch, bysssally attached, juvenile shell of L. fortunei is characterised by an array of byssal setae. These have been considered to be of periostracal origin, that is, periostracal “hairs”, but are in fact laid down by the foot (Ockelmann and Dinesen 2009). They were first identified for the shells of representatives of the mytiloid Dacrydiinae by Ockelmann (1983) and described in more detail by Ockelmann and Dinesen (2009) for species of Adula and Mytilus (also Mytiloidea). Most recently, they have been described for another mytiloid, Modiolus modiolus (Linnaeus 1758) by Dinesen and Morton (2014). They are the most noticeable features of the juvenile, nepioconch, shell of M. modiolus and, as in L. fortunei, predominantly occur postero-dorsally. These authors also showed that the byssal setae of M. modiolus are homologous to the distal regions of a byssal thread, that is, the “plaque foam” of Silverman and Roberto (2007). This distal region of a thread comprises a primary layer attached to a surface—the plaque foam—and a more proximal region of collagen that, yet further proximally, unites into a bundle (or stem) attached to the byssal retractor muscles (Silverman and Roberto 2007). The byssal setae thus terminate as a tapering thread of collagen, as seen in L. fortunei by Montalto and Rojas Molina (2014). It also seems possible that they are characteristic of the juvenile shells of most, if not all, mytiloids although this survey has never been undertaken. Similarly, the setae have often been considered to be a feature of the juvenile mytiloid shell (Ockelmann and Dinesen 2009; Dinesen and Morton 2014), although Montalto and Rojas Molina (2014) show that adult L. fortunei may possess them too. It may simply be that in many mytiloids, M. modiolus being a good example (Ockelmann and Dinesen 2009; Dinesen and Morton 2014), that when an adult size is reached, the foot can no longer reach the highest levels of the shell. With age too, the setae may be lost through abrasion.
Montalto and Rojas Molina (2014) review the possible functions of the L. fortunei juvenile’s byssal setae and it seems likely that, as suggested for M. modiolus by Dinesen and Morton (2014), the principal function is defense although the threads themselves may camouflage such vulnerable shells but this would also come under the definition of predator avoidance. In a significant experiment, Wright and Francis (1984) scraped off the byssal setae from the shells of M. modiolus and showed that they became more susceptible to the drilling activities of the intertidal whelk Nucella lapillus (Linnaeus 1758). The setae thus either inhibit the drilling activity of the predator or prevent it gaining a good grip on the shell.
Subsequently, adult L. fortunei can grow to a shell length of about 45 mm, but the more usual size ranges from 20 to 30 mm, very few individuals proceeding past their second year of life (in Hong Kong) into a third (elsewhere) to allow this (Morton 1977). The general colour of the shell is golden brown, this being mostly attributable to the thick periostracum that covers the shell, which presumably has a mineralogy and structure typical of the Mytiloidea (Taylor et al. 1969) although this has never been studied. The umbones are close to the end of the valves and there are no hinge teeth. The shell is attached to the substratum by an array of byssal threads which can, in some circumstances, as in detached and subsequently experimentally manipulated Mytilus galloprovincialis Lamarck, 1819 (Morton 2011), be shed and regrown but also when, in nature, an alternative, presumably better, site for adult occupation is either searched for or chosen.
Morton (1973) showed that the overall dimensions of the shell of L. fortunei inhabiting Plover Cove Reservoir in Hong Kong were relatively regular as seen from the ratio of width:height:length, that is: 1:1.18 (± 0.18):2.60 (± 0.50). Similarly the ratios of shell width:shell length and shell height:shell length were calculated as being 0.38 (± 0.06) and 0.45 (± 0.06), respectively. One important feature of the shell of L. fortunei, however, is that it is exceptionally thin and brittle, adult individuals 35 mm in length having a mid-shell thickness of < 200 μm, whereas M. galloprovincialis individuals of the same shell length are > 1400 μm thick. This may of course be a consequence of either a lack of soluble calcium in the occupied freshwaters, as in Hong Kong, for example (see Table 1 in Morton 1975), as compared with seawater, or the low requirements for such salts in L. fortunei (see Fig. 4 in Karatayev et al. 2007b), or, again, both.
Views of a the shell as seen from the right lateral aspect, b the dorsal, c the ventral, d the anterior and e the posterior aspects. x----y maximum shell width. (Modified after Morton and Dinesen 2010)
Figure 1 provides different views of a L. fortunei shell. The adult, dissoconch, shell of L. fortunei is roundly heteromyarian and distinctively inequilateral when seen from the right side (Fig. 1a) That is, the anterior margin is almost round whereas the posterior margin is more pointedly rounded. The umbones are terminal, almost at the apex of the rounded anterior end and the antero-dorsal margin is less steep than the posterior and almost straight. The shell is somewhat keeled, this form being created by the pattern of growth inflating the shell anteriorly and narrowing it posteriorly. The outer, impermeable, proteinaceous, periostracal layer of the shell is smooth and shiny and is thick where it curls inwards at the shell margin. Seen from the dorsal aspect (Fig. 1b), the shell is thus slightly inflated anteriorly and more narrowly pointed posteriorly. As a consequence, the dorsal valve margins are slightly sinusoidal, that is, the shell is somewhat inequivalve. The inconspicuous ligament of L. fortunei is internal and located along the outside of the dorsal ridge of the anterior shell septum. Ventrally (Fig. 1c), there is a narrow byssal notch. From the anterior aspect (Fig. 1d), the shell is diamond-shaped in outline and swollen ventrally with the ligament visible for the virtual height of the shell. From the posterior aspect (Fig. 1e), the shell is less wide, as also seen in Fig. 1b and c, and more pointed ventrally. The shell is widest (Fig. 1d and e) at the lowest one third of the dorso-ventral height of the shell.
An interior view of the left shell valve. (Modified after Morton and Dinesen 2010)
Internally, the shell of L. fortunei (Fig. 2) is thin, transparent and nacreous. The internal shell layer is pearly white, only slightly lustrous, darker postero-dorsally and lighter antero-ventrally. This is caused by the nacre of the shells’ interior being purple above and white below the keel. The ligament of L. fortunei is internal and relatively long. With elastic properties, the ligament acts in opposition to the counteractive forces of the paired adductor muscles, keeping the animal in a state of tonus. With death, the muscles relax and the ligament thus keeps the valves separated. The muscle scars on the shell of L. fortunei comprise a large posterior adductor muscle and, anteriorly to it, two, separate, posterior byssal retractor muscles, labelled (1) and (2) in the figures. Internal to these is a posterior pedal retractor muscle scar but this is so small as to be virtually indistinguishable as a shell scar. The slight pallial sinus is thin and irregular although the pallial line is much thinner mid ventrally. There is a row of some nine ctenidial attachment scars of the ascending lamella of the outer demibranch that lead towards the scar of the anterior byssal retractor muscle, which is located just posterior to the umbo and half hidden by the hinge plate. There is a relatively large, kidney-shaped, anterior adductor muscle scar situated antero-ventrally.
An interior view of the anterior region of the left shell valve. (Modified after Morton and Dinesen 2010)
A more detailed internal view of the anterior shell of L. fortunei shows no evidence of d-series hinge teeth and there are no antero-ventral folds or crenulations (Fig. 3). The shell is thus extremely simple, and this allows it to be separated from that of the only other freshwater mytiloid, Sinomytilus harmandi (Rochebrune 1882) which has an anterior septum upon which is located the scar of the anterior adductor muscle (Morton and Dinesen 2010). Each umbo lies slightly posterior to the anterior shell margin, which is smoothly rounded, that is, not crenulated as in some other mytiloids. A transverse section through the ligament and the dorsal region of the shell is illustrated in Fig. 4. The opisthodetic, paravincular ligament consists of two layers with staining reactions similar to those of Mytilus edulis (Linnaeus 1758; Trueman 1950; Beedham 1958), indeed of all representatives of the Mytiloidea (Yonge and Campbell 1968). The posterior outer ligament layer stains red and the inner ligament layer blue with both Mallory’s triple stain and Masson’s trichrome. Other mytiloids possess a ligament with a similar structure. In all these taxa, and in L. fortunei, the periostracum extends over the ligament, thereby adding another layer. This was termed fused periostracum (Owen et al. 1953) although, since periostracum must cover the entire shell, there is no fusion. The term is nevertheless retained herein simply to emphasise that it is present.
The shell of L. fortunei is illustrated in transverse section in Fig. 5. Its overall form accommodates the organs of the visceral mass dorsally. Ventrally, this leads to a single medial foot the base of which houses the byssal gland. This discharges its secretions into the byssal groove which forms, one-by-one, an array of threads that the foot plants onto the substratum thereby securing the animal, encased within its protective shell securely onto its chosen domicile. The shell is more swollen ventrally, providing (1) a flatter base, which achieves greater stability for it in lotic waters, and (2) allows accommodation for the ctenidia that are responsible for respiration and particle collection resulting from the feeding activities of this suspension feeding bivalve. The aragonitic shell of L. fortunei is encased within a shiny, proteinaceous, periostracum that is secreted by the outer surface of the outer fold of the mantle margin, which tracks the edges of both valves and, dorsally, secretes the ligament. The mantle, surrounding the body tissues in a protective cloak, also, as in many other mytiloids, contains elements of the gonads.
With this general plan of the body form in front of us, we can now proceed to a more detailed description of the anatomy of L. fortunei.
The Musculature and Byssus
The musculature of L. fortunei is seen from the right side in Fig. 6. The anterior adductor muscle is small and located on the anteroventral floor of the shell valves. In this respect L. fortunei is similar to other mytiloideans (White 1937; Wilson 1967). There is also a pair of thin anterior byssal retractor muscles arising from beneath the hinge plate and thus separate from the adductor muscle. In contrast to the anterior adductor, the posterior adductor muscle is large, circular and anteriorly adjoining byssal retractor muscles are divided into two major components. There is a small posterior pedal retractor muscle that has its origin anterior to the posterior byssal retractors. The pallial line, created by the pallial retractor musculature is generally thick especially posteriorly, but becomes thinner mid ventrally. The posterior byssal retractor is also illustrated connecting up with the byssus at the base of the foot within the visceral mass (Fig. 6). The foot has a distinct byssal groove.
Figure 7 is a left-right longitudinal section through the byssal apparatus at the base of the visceral mass of L. fortunei. Also seen in this section are the paired anterior byssal retractor muscles and above them the pedal ganglia with lateral pedal nerves radiating into the foot. Unlike in many other bivalves, for example well-studied representatives of the Anomalodesmata (Morton 1985), no statocysts seem to occur dorsal to the pedal ganglia. As in other hitherto studied mytiloids, for example Modiolus modiolus (Linnaeus 1758; Dinesen and Morton 2014), the byssal groove is trefoil-shaped in section. The proteinaceous byssus is secreted by an eosinophilic byssal gland that comprises cells some 20 μm in length and arranged around the base of the byssal groove. This was termed the “white” or “collagen” gland in the foot of M. edulis by Brown (1952) and Pujol (1967). A second basophilic gland is arrayed sub-epithelially around the borders of the byssal groove and comprises elongate cells some 25 μm in length. These cells, the “purple” gland of brown (1952), probably secrete mucus, although Pujol (1967) thought it might be “enzymatic” and responsible for tanning the collagen produced by the “white” gland. Again, no specific studies of this structure have made for L. fortunei.
a A posterior view of the animal showing the patterning on the mantle and exhalant siphon and inhalant aperture. The inhalant stream (broken arrows), exhalant stream (open arrows) and ciliary rejection currents (solid arrows) of b an actively filtering animal and c a disturbed animal are also shown. (Modified after Morton 1973; Malacologia, 12:270)
The Siphons
Figure 8a is a posterior view of L. fortunei, showing the pattern of pigmentation on the mantle and exhalant siphon and inhalant aperture. Externally, each mantle lobe is patterned with a brown stripe. These fuse dorsally to the exhalant siphon to form a single stripe. A similar brown stripe patterns the internal surfaces of the mantle lobes forming the inhalant aperture, and there is a dorsal median stripe on the inhalant aperture at the point of fusion of the mantle lobes forming the exhalant siphon (Fig. 8a). The exhalant siphon of L. fortunei is formed by fusion between the inner mantle folds only, this being type A (Yonge 1948; 1957). The inhalant aperture is not separated from the pedal/byssal aperture by fusion of the opposite mantle lobes but is separated functionally by their apposition. The inhalant stream (broken arrows), exhalant stream (open arrows) and ciliary rejection currents (solid arrows) of an actively filtering individual (Fig. 8b) and a disturbed one (Fig. 8c) are also shown. In both cases, as will be further described, the expulsion of pseudofaeces from the inhalant aperture is via its dorsal connection with the base of the exhalant siphon. Such a situation is typical of most representatives of the Mytiloidea (White 1937; Yonge 1955; Wilson 1967; Fankboner 1971), but atypical of more advanced siphonate bivalves. Neither the inhalant aperture nor the exhalant siphon bear either tentacles or papillae (Fig. 8a).
Figure 9a is a more detailed posterior view of junction between exhalant siphon and inhalant aperture of L. fortunei and highlights the siphonal septum. The septum is relatively small like that of Modiolus modiolus and unlike the large structure present in M. galloprovincialis (Dinesen and Morton 2014). The siphonal septum connects the ctenidia to the mantle at the point of fusion of the mantle lobes separating the exhalant siphon from the inhalant aperture. This septum effectively separates posteriorly the infra-branchial chamber from the supra-branchial. The septum is thought to act as a valve in other mytiloids (Fankboner 1971), controlling the volume of water entering the infra-branchial chamber. When filtering water, rates and, thus, efficiency of particle capture and retention are legislated by size and temperature (Sylvester et al. 2005) but also other factors including the relative amounts of seston and other potential food particles in the water column. When the animal is actively filtering and the siphons are extended, the septum is held near-vertically regulating the size of the inhalant stream. When the left and right folds of the inhalant aperture are withdrawn, however, the septum folds up left and right. It is, thus, a highly muscular structure in L. fortunei and, unlike other mytilids, bears five small papillae along its margin.
Figure 9b is a section through one of the papillae of the siphonal septum. The thick block of muscle fibres nearly fills the septum except marginally. The distal end of each papilla comprises a spherical head with an epithelium made up of what appear to be sensory cells each lined apically by microvilli. Internally, there is a nerve complex making contact with the sensory cells. The suggested function of the papillae will be discussed below.
The Mantle Margin
Mantle fusions occur dorsally above the exhalant siphon and between the exhalant siphon and inhalant aperture of L. fortunei. Mantle fusions, as with the siphons, are of the inner mantle folds only and thus of type A (Yonge 1957). Figure 10 is a transverse section through the left ventral mantle margin. The mantle margin, as in most bivalves, except for protobranchs and arcoids, comprises three folds: inner, middle and outer. The outer surface of the outer fold secretes the shell valve. The periostracum is secreted by the epithelia delimiting the periostracal groove, that is, the outer surface of the middle fold against the template of the inner surface of the outer fold. The periostracum is composed of three layers as in all mytiloideans studied hitherto and arises from the periostracal groove. The outer layer is thin (4 µm) and stains slightly grey with Heidenhain’s haematoxylin but not at all with either Mallory’s or Masson’s stains. The middle layer is 20–25 μm thick at its greatest depth and stains light blue in Masson’s trichrome. It is probably some kind of mucoid secretion. This layer does not, however, as does its counterpart in M. edulis (Fig. 1 in Beedham 1958), possess vacuoles. An inner laminated layer ultimately achieves a thickness of between 25 μm and 35 μm, and when first secreted stains red with both Mallory’s and Masson’s stains. Towards the margin of the shell valve, the outer laminations of this layer stain blue.
The inner fold of the mantle is ciliated densely, and at its junction with the general mantle surface there is a ventral pallial rejectory tract that transports unwanted particles posteriorly towards the inhalant aperture for eventual expulsion from the infra-branchial chamber. The swollen inner folds are highly glandular, the sub-epithelial cells are probably secreting mucus. The pallial retractor muscle, which attaches the mantle to the shell valves at the pallial line on each one, sends fibres mostly into the point of union of the middle and outer folds, that is, the base of the periostracal groove. There is a large pallial nerve. Distally, the mantle margin contains, as in some other mytiloids, much of the gonadal tissue of the animal, in this case the testes.
The Ciliary Currents of the Mantle
The ciliary currents of the left mantle lobe of L. fortunei are illustrated in Fig. 11. The ciliary currents of the mantle (including the siphons) and, as we shall see, the visceral mass and foot are all rejectory and serve to keep the mantle cavity free of either too large or unwanted particles. To achieve this, particles falling on to the mantle dorsally are passed anteriorly towards the mouth. Such particles, however, are all eventually transported ventrally, in a clockwise direction as seen from the right (counterclockwise when seen from the left) and, from the region around the mouth and labial palps are then passed posteriorly within the ventral pallial rejectory tract towards the inhalant aperture. Pseudofaeces are not concentrated at the base of the inhalant aperture to be expelled by the rapid adduction of the shell valves as, typically, in eulamellibranchs possessing a distinct siphon. Instead, the lobes of the inhalant aperture are highly mobile in L. fortunei and bear on their inner surfaces strong ciliary tracts, which pass the pseudofaeces dorsally towards the exhalant aperture as illustrated in Fig. 8b. When actively filtering, with the inhalant lobes fully expanded, water passes into the mantle cavity. The ciliary currents of the mantle take pseudofaeces towards the inhalant aperture against this incoming stream. Rapid closure of the shell valves forces water out of both apertures but, particularly, the exhalant siphon, thereby ejecting the pseudofaeces and the faeces, as we shall see. When the animal is disturbed, the shell valves only open partially (Fig. 8c), but sufficiently to allow pseudofaeces to be similarly removed from the mantle cavity via a reduced inhalant aperture.
The Ctenidia and Labial Palps and Their Ciliary Currents
The organs of the mantle cavity of L. fortunei and their ciliary currents after removal of the right shell valve and mantle lobe are illustrated in Fig. 12. A comparable illustration of those of M. edulis were provided by Kellogg (1915, Fig. 18). The first detailed study of the bivalve ctenidia were undertaken by Ridewood (1903), but elaborated on in a series of classical studies by Daphne Atkins (died 1961) some of which are referred to herein. The ctenidia, which fulfill the dual roles of respiration and particle capture and transport, comprise two sub-equal demibranchs of which the outer is the longer dorso-ventrally. The upper, dorsal, margins of the ascending lamellae of the outer and inner demibranchs are attached to the mantle and the visceral mass, respectively, by ciliary fusions (Atkins 1937a). The ventral margin of the outer demibranch always lies tucked behind the incurving mantle margin with the associated, here thickened, periostracum. Like many other mytiloids also (Fankboner 1971), the outer demibranchs of L. fortunei are some five or six filaments shorter at their anterior ends than the inner ones. Fankboner (1971) states that “a functional advantage for this anatomical reduction is unclear”. For L. fortunei, however, the advantage of this arrangement is clear in that it enables the ventral marginal food grooves of both demibranchs to be in contact with the sorting and, hence either acceptance or rejection functions of the inner surfaces of both inner and outer labial palps thereby greatly increasing the efficiency of particle selection by these structures. The ctenidial-labial palp junction of L. fortunei thus falls into Category I elucidated by Stasek (1963), and is typical of the Mytiloidea in general.
The ctenidia of L. fortunei are flat, homorhabdic and filibranchiate (eleutherorhabdic). The ascending and descending lamellae of both demibranchs are cross-connected by inter-lamellar junctions, or unions (Fig. 13), Similarly, the dorso-ventrally aligned filaments which make up each lamella are connected laterally to each other by inter-filamentary junctions that maintain ctenidial cohesion. The junctions that cross-connect the individual filaments comprise ciliary discs as in other mytiloideans and as such are weak so that the filaments readily separate one from another when damaged. In lamellibranch bivalves, inter-filamentary union is achieved by tissue junctions that are much more robust.
The apices of the ctenidial filaments comprise a number of ciliary types that collectively fulfill the roles of filtration, particle entrapment and transportation. The currents through the ctenidia, that is, from the infra- to the supra-branchial chambers, are created by lateral cilia that are, thus, largely responsible for the forceful inhalant and exhalant streams into and out of the mantle cavity. The filtering apparatus itself is the responsibility of eulaterofrontal cirri, the fine structure of which have been illustrated and described for M. edulis by Owen (1974). Such a structure is typical of all studied mytiloideans, including L. fortunei, and all those bivalves that Atkins (1938) classified as the Macrociliobranchia, and which were refined histologically by Owen (1978). Other ciliary tracts on each filament head, principally the apical frontal cilia, are concerned with the transport either up or down of those particles flicked on to them by the eulaterofrontal cirri. It is the activities of these cilia that feed particles into the various ctenidial food grooves for onward transmission to and sorting by the labial palps.
The various ciliary currents of the labial palps and anterior end of the ctenidium of the right side. (Modified after Morton 1973; Malacologia, 12:271)
The ciliation of the ctenidial surfaces of L. fortunei is of type B(I) (Atkins 1937b; Fig. 12b, 13a, b). Acceptance tracts are situated within the ventral marginal food grooves of both demibranchs, in the ctenidial axis and in the junctions of the ascending lamella of the inner and outer demibranchs with the visceral mass and mantle, respectively. Only those particles arriving on the labial palps inside the ventral marginal food groove of the inner demibranchs, however, pass into the proximal oral groove and directly to the mouth. The ctenidial-labial palp junction of L. fortunei, as seen from the right side, is illustrated in Fig. 14. Particles arriving at the anterior end of the ctenidium via (1) the crests of the ventral marginal food grooves of both inner and outer demibranchs, (2) inside the ventral food groove of the outer demibranch and (3) in all three dorsal food grooves are subjected, before ingestion, to the ciliary selection currents of the labial palps. The abrupt termination of the outer demibranch, creating this unusually complicated sorting process, has not been observed in other bivalve taxa other than those of the Mytiloidea.
Particles are removed from the anterior ctenidial termini by the unridged portion of the labial palps, the ciliary currents of which subsequently pass the particles onto their ridged sorting region. This function is the attribute of the system of parallel ridges and grooves, which pass selected particles of a suitable nature and size over the crests of the ridges towards the proximal oral groove for ultimate ingestion. Too large and/or unwanted particles are passed laterally towards the opposite free edge of the palp for rejection. Recirculatory currents also exist. Details of the labial palp ciliation need not be elaborated upon since they are essentially the same as those described by Fankboner (1971) and are typical of mytiloids in general. The ciliary currents of the lips of the mouth are rejectory, passing unwanted material back to the labial palps for rejection along the prescribed course.
The anatomy and ciliary currents of the foot and visceral mass after removal of the right ctenidium. The ciliary currents of the ascending lamella of the inner demibranch of the left ctenidium are also shown. (Modified after Morton 1973; Malacologia, 12:270)
The Ciliary Currents of the Visceral Mass and Foot
The ciliary currents of the surface of the visceral mass (Fig. 15) of L. fortunei, which near exclusively houses the gonads and byssal gland, pass particles downwards dorsally and then postero-ventrally to be concentrated at the postero-ventral tip of the visceral mass. From this point, they are presumably removed by the ventrally directed ciliary tracts of the ascending lamellae of the inner demibranchs. Being too large to enter the ventral marginal food grooves of these demibranchs, such material ultimately passes into the ventral, posteriorly directed, rejectory tracts of the mantle (Fig. 10; 11). The crawling and byssal thread-planting foot possesses ciliary currents that pass particles dorsally to join the posterior stream on the visceral mass and in this way these are also ultimately expelled from the mantle cavity. In Fig. 15, the ciliary currents of the ascending lamella of the inner demibranch of the left ctenidium, behind the visceral mass and foot, are also shown.
The Alimentary Canal
Figure 15 gives a general impression of the course of the intestine in L. fortunei, not within, but above and between the byssal retractor muscles and below the organs of the pericardium. The course of the intestine is illustrated more precisely in Fig. 16. Here, all other tissues except for the ventricle of the heart have been ignored and the right byssal retractor muscle blocks have been pulled to the right thereby exposing the intestine. The course of the intestine in L. fortunei is similar to that seen in other mytiloideans. The oesophagus passes upwards from the mouth, which lies between the anterior byssal retractor muscles and is closely applied to the dorsal surface of the anterior adductor muscle. The ciliated oesophagus opens into the stomach, which is located under the anterodorsal margin of the shell and is surrounded by the dark digestive diverticulae (Fig. 15). From the posterior end of the stomach arises the combined style sac and mid-gut, which passes backwards between both blocks of the posterior byssal retractor muscles. Just anterior to the posterior adductor muscle, the style sac terminates but the mid-gut, now separated from it, loops forwards alone to pass back between the posterior byssal retractors. The mid-gut loops again on the left side of the stomach (not the right as originally thought by Morton 1973), and turns posteriorly again to penetrate the ventricle of the heart, pass between the posterior pair of byssal retractor muscles, over the posterior adductor muscle, to terminate in an anus on the posterior face of this structure facing the exhalant siphon (Fig. 9). The histological structures of the style sac and intestine of L. fortunei are essentially the same as those described for M. edulis by Giusti (1971) and for all other mytiloideans hitherto studied.
The structure and ciliary currents of the interior of the stomach after opening by a horizontal incision in the right side. (Modified after Morton 1973; Malacologia, 12:274)
The Stomach
The structure and ciliary currents of the interior of the stomach of L. fortunei, after opening by a horizontal incision in the right side, are illustrated in Fig. 17. The stomach is elongate and bears a close similarity to the stomachs of other mytiloideans (Graham 1949; Purchon 1957; Reid 1965; Dinamani 1967; Fankboner 1971) and thus belongs to type III and Section I of the stomach types elucidated by Purchon (1957) and Dinamani (1967), respectively. An attempt has been made in this description of the stomach of L. fortunei to combine the nomenclatorial systems of Purchon (1957), Reid (1965) and Dinamani (1967).
In L. fortunei, as in all bivalves, the floor of the stomach is dominated by the major typhlosole and associated intestinal groove, which arise in the style sac and pass forwards to penetrate the food-sorting caecum. The crystalline style is secreted in the style sac and, protruding into the stomach, rotates against the typically saddle-shaped gastric shield covering the left dorso-lateral wall of the stomach. The gastric shield sends a flare into the left pouch. The major typhlosole does not divide, as reported for Adula falcata Gould, 1851 by Fankboner (1971). The minor typhlosole also arises in the style sac and passes, for a short distance, along the right side of the stomach. On this side of the stomach too and associated with the right duct tract, are two groups of openings (1 and 2) into the ducts that lead to the digestive diverticula. Purchon (1957) considered the right duct tract to be a sorting area and termed it Sorting Area 3. A further sorting area (2) can be recognised dorsal to the entrance of the food-sorting caecum and separating this opening from the entrance to the left pouch. A sorting area of the left duct tract would appear to be the floor and walls of the left pouch.
The structure and ciliary currents of the food-sorting caecum of the stomach. (Modified after Morton 1973; Malacologia, 12:275)
The structure and ciliary currents on the left side of the stomach of L. fortunei constitutes the origin of what Reid (1965) has termed the left duct tract, which passes particles of food into the capacious food-sorting caecum (Fig. 18). The right duct tract also passes into the food-sorting caecum. This structure is a comparatively long finger-shaped pocket penetrated to its apex by a tongue of the major typhlosole. At the caecum’s apex is a sorting area, which is of type B (Reid 1965), and found only in those bivalves that Purchon (1960; 1963) has grouped together as the Gastrotriteia and which is characteristic of the Mytiloidea (Reid 1965). The caecum also has a cluster of four ducts leading into the digestive diverticulae.
The structure and ciliary currents of the left pouch of the stomach. (Modified after Morton 1973; Malacologia, 12:275)
The structure and ciliary currents of the left pouch of the stomach of L. fortunei are illustrated in Fig. 19. This structure, which the gastric shield sends a flare into dorsally, has two further groupings of ducts leading to the digestive diverticula (3 and 4). Each of the sorting areas in the left pouch is a system of ridges and grooves, which Reid (1965) has identified as type A and which are found in all bivalves.
The stomach of L. fortunei is a site of continuous food particle selection, although this species too, like D. polymorpha, may possess a diurnal rhythm of feeding and digestion (Morton 1969a), after primary sorting by the labial palps, and extra-cellular digestion by the slow dissolution of and release of enzymes from the slowly rotating crystalline style against the gastric shield which is probably also enzymatically productive (Halton and Owen 1968). Cilia on the crests of the major typhlosole and inner folds of the left and right duct tracts pass potential food material entering the stomach into the food-sorting caecum. Ciliary currents in the grooves of the inner folds of the left and right duct tracts and the incurrent fold of the intestinal groove also pass particles into the food-sorting caecum. At the apex of the caecum the B type sorting area (Reid 1965) sends acceptable particles of a suitable size into the outer folds of the left and right duct tracts which pass this material to the ducts of the digestive diverticula of the left pouch and right duct tract. Rejected particles pass out of the food-sorting caecum in the excurrent intestinal groove of the major typhlosole and pass into the mid-gut for ultimate defecation. Particles of intermediate size are probably recirculated by the dorsal hood tract passing them back to the dorsal hood and gastric shield. The minor typhlosole assists the major typhlosole in clearing the stomach of unwanted food into the mid-gut.
No appendix, as has been reported for other mytiloids by Reid (1965) and Fankboner (1971), could be identified in the stomach of L. fortunei. The basic structure of the ducts and the digestive tubules comprising the digestive diverticulae of L. fortunei bear a close similarity to those described by Owen (1955) for M. edulis. A review of the structures and processes involved in the feeding and digestion of the Bivalvia has been provided by Morton (1983).
The Organs of the Pericardium
The course of the rectum through the medial ventricle of the heart and between the two elements of the posterior byssal retractor and over the posterior adductor muscles has been described above. The organs of the pericardium of L. fortunei, as seen from the right side, are illustrated in Fig. 20. In this figure, the course of the intestine, except for the rectum within the ventricle of the heart, has been ignored. Posterior and ventral to the heart are the paired, pale-brown, kidneys. The reno-pericardial apertures of the kidneys are situated on the postero-ventral floor of the pericardium adjacent to the edge of the anterior-most blocks of the posterior byssal retractor muscles. The epithelia of the left and right auricles of the heart contain the similarly light brown pericardial gland.
The renal apertures, which are shared with the gonadal apertures, open onto the visceral mass to left and right beneath the posterior edge of the anterior-most blocks of the posterior byssal retractor muscles. The ascending lamella of the outer demibranch of the right ctenidium attaches to the mantle/shell at the junction between the visceral mass and mantle but is not illustrated here. The ctenidial axis and the point of union of the ascending lamella of the inner demibranch of the right ctenidium with the visceral mass are, however, illustrated in Fig. 20, and it can be seen that the renal/gonadal aperture opens into the supra-branchial chamber between these two attachment points. That is, excretory products and gametes are discharged into that component of the supra-branchial chamber situated between the ctenidial axis and the inner ctenidial demibranch.
Reproduction
Limnoperna fortunei is generally dioecious (Morton 1991), although there is one report of low-level hermaphroditism (Darrigran et al. 1998; see Chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume). The large paired gonads are located in mesosomal lobes in, primarily, postero-dorsal region of the visceral mass. As in M. edulis (White 1937), the gonadal tissues of L. fortunei also invade the mantle, everywhere, as illustrated in Figs. 4, 5 and 10.
Discussion
Limnoperna fortunei is a highly opportunistic species that has been introduced into many locations outside its mainland Chinese borders, notably, Taiwan, Japan and, most recently, South America. Morton and Dinesen (2010) also raised the intriguing possibility that L. fortunei might have been introduced into tropical Indochina (Cambodia, Laos, Thailand, Vietnam), hitherto considered part of its natural range, from China. Its present range in this region might thus reflect the pattern of past human migrations to and from China and the countries of South-east Asia.
Limnoperna fortunei first came to scientific, as opposed to conchological, attention in the late 1960s when it colonised the pipes, conduits and channels of part of the water supply system of Hong Kong (Morton 1975). Limnoperna fortunei is not alone in this respect. It is but the last of a string of freshwater (and to a lesser extent brackish water) invasions into non-native regions by bivalves most notably including D. polymorpha in Europe and latterly with D. bugensis into North America, and C. fluminea into North America and subsequently Europe and South America—all processes facilitated by transoceanic transport in the ballast water of container and other ships and the construction of interconnecting potable water supply systems (see Chapter “Distribution and Colonization of Limnoperna fortunei: Special Traits of an Odd Mussel” in this volume).
But, there is more to these successful invasions than human-mediated introductions. Just as with D. polymorpha, it would seem that L. fortunei is ideally adapted to a life in fast flowing (but not torrents) waters in the possession of a stout byssus and an heteromyarian, or mytiloid, shell form. Both can, however, also thrive in the relatively static waters of lakes and reservoirs (Morton 1969a) and in this habitat can cause problems of sedimentation resulting from their sheer numbers, pseudofaeces and faeces production and the accumulation of dead shells. It would seem that both of these species, despite their superficially specialised form, are liberal in their choice of habitat and are, hence, potentially detrimental at all stages of the water supply process and the industrial and potable resources arising from it.
The close similarity in choice of habitat and form existing between L. fortunei and D. polymorpha (especially, but also D. bugensis) was suggested to be related to some degree of phylogenetic affinity between the parent Mytiloidea and Dreissenoidea (Purchon and Brown 1969), respectively. Yonge and Campbell (1968) showed, however, that the similarities which exist between species of Dreissena and the similarly anteriorly septate mytiloid species of Septifer were due to convergence. Morton (1973, 1992) and Taylor et al. (1972) agreed with this view and further suggested that from both palaeontological and anatomical standpoints, the two taxa are wholly unrelated. It is envisaged, both for Dreissena and Limnoperna, however, that the neotenous retention of the larval byssus (Yonge 1962) in their respective ancestors resulted in the evolution of the heteromyarian form (Yonge and Campbell 1968) in both. Both too have subsequently exploited this condition, with the development of osmoregulatory powers, in the colonisation of freshwater systems.
Scarlato and Starobogatov (1979), placed the monospecific and monogeneric Limnoperna in its own sub-family—the Limnoperninae, a classification followed by Bieler et al. (2010). Although such a taxonomy seems highly dubious to this author, the search for an ancestor to L. fortunei must, nevertheless, be focussed on the Mytiloidea and Morton and Dinesen (2010) suggested that the genus is superficially similar to species of the brackish water Xenostrobus, a taxon formerly identified as Modiolus Lamarck, 1799. Such a superficial similarity prompted Beu (2006) to subsume Xenostrobus into junior synonomy with Limnoperna. Both have been considered to be representative of the Modiolinae, although the phylogeny of neither has been studied properly and there are anatomical differences between the two (see below), which are sufficiently significant to refute Beu’s synonomy. Nevertheless, in some other respects, the two taxa are similar with regard to the possession of some comparable anatomical characters. In particular, both have simple thin modioliform shells lacking any anterior teeth and crenulations with sub-terminal umbones and no external sculpturing. The heteromyarian shells of both have an umbonal keel that is dark brown above and a paler yellow-brown below. The anterior byssal retractor muscle has its origin on the antero-dorsal roof of the shell in L. fortunei as in Xenostrobus securis and X. pulex (Wilson 1967). Similarly, the posterior byssal retractor muscle is divided into two blocks in both L. fortunei and X. inconstans Wilson, 1967 (but not in X. securis and X. pulex also first described by this author). Furthermore, and perhaps most significantly, both X. securis and X. inconstans live at the head of estuaries in Australia whereas other species, for example, X. pulex are marine (Wilson 1967). Ockelmann (1983) described two other species of Xenostrobus, that is X. mangle and X. balani, from estuarine habitats in Thailand.
It would thus seem, at least intuitively, possible that Limnoperna evolved from forms essentially similar to Xenostrobus. Thiele (1934) considered Limnoperna to have a modioline sensu lato ancestry. Conversely, the other freshwater, and also monospecific, Asian mytiloid, S. harmandi, was thought to be of mytiline sensu lato ancestry. If true, the freshwater environments of Indochina have been colonised twice at different times by two, monospecific, mytiloidean genera with different temporal origins. If true, the Limnoperna modioline/musculine lineage of the Mytiloidea dates back to the Devonian (~ 345–395 million years before present—mybp; Morton 1992). Conversely, the Sinomytilus mytiline line shares characters with several of the marine and estuarine species of the Mytilinae sensu lato, of which more modern taxa first appeared in the Permo-Triassic (~ 265–225 mybp; Morton 1992). Limnoperna and Xenostrobus are, thus, it is suggested, both representative of extremely ancient lineages. Hence, attractive as the scenario of a common ancestry for the above two genera might be (albeit both being very distantly related), there are anatomical differences between the two as noted above. For example, the heart of L. fortunei is located between the two blocks of the posterior byssal retractor muscles, whereas in all species of Xenostrobus, it is situated anterior to these muscles. In L. fortunei too, the mid gut loops over the left side of the stomach but, barely, over the right in all three species of Xenostrobus studied anatomically by Wilson (1967). Adding fuel to the ancestry debate, L. fortunei is, in other respects, reminiscent of the much larger and coastal species of Perna, notably with regard to the structure of the posterior byssal retractor muscle-pericardium complex and overall characteristics save for the absence of an anterior adductor muscle in Perna viridis (Linnaeus 1758; Morton 1987; Ockelmann 1995). The question of Limnoperna’s ancestry is, hence, unresolved although it undoubtedly resides in the modioline/musculine sensu lato lineage of the Mytiloidea. This subject is of ongoing personal research.
As if to highlight the above-hypothesised similarity between Limnoperna and Xenostrobus, however, Habe (1981) identified a subspecies of L. fortunei, that is, L. fortunei kikuchii (Fig. 4c in Morton 1997), first recorded from Japan sometime between 1974 and 1979 (Nishimura and Habe 1987) when it was introduced (supposedly) alongside consignments of Corbicula from China. It subsequently colonised the estuarine Shonai Inlet to Lake Hamana and excluded the similarly estuarine Musculista senhausia (Benson 1842; Abdel-Razek et al. 1993a, b). The ability of this sub-species to tolerate salinities of between 0 and 30 ‰ (Kimura et al. 1995), however, cast doubts about its stated identity and, subsequently Morton (1997) and Kimura and Sekiguchi (2009) confirmed it to be X. securis which has a near identical salinity tolerance of 1–31 ‰ (Wilson 1968). Conversely, L. fortunei tolerates only low salinities (Deaton et al. 1989) although, in the regularly fluctuating salinities of experimentally mimicked estuaries, it can survive periodic exposure to 14 ‰ (Sylvester et al. 2013), as must be the case occasionally in its natural habitat of the Pearl River in China for example (Miller and McClure 1931). Xenostrobus securis has now too been introduced widely into, for example, Korea (Shirafuji and Sato 2003) and is now present throughout much the Mediterranean being first recorded in 1991 from the Venice lagoon as Xenostrobus sp. (Sabelli and Speranza 1994; Mizzan 1999). Since its initial discovery, the species has spread throughout the Mediterranean and has most recently been recorded (as Limnoperna securis) from the Bay of Biscay marking its spread into the North Atlantic (Adarraga and Martínez 2011), probably in ballast water.
Hence, although no ancestor to Limnoperna can be identified with certainty in modern, let alone fossil, Mytiloidea, it seems reasonable that such a form should be sought among brackish-water relatives and their ancestors in turn. It has been established, for example, and it is perhaps significant, that species of Dreissena are closely related to the confamilial estuarine species of Mytilopsis, one of which, M. sallei (Récluz 1849) has, itself, become an invasive species albeit of brackish waters (Morton 1981). It would, thus, seem that Dreissena and Limnoperna, convergent as they are, represent the apices of two phyletic streams that have both adapted to life in freshwaters. Significantly, the hard surfaces found in freshwater systems in many parts of the world are not normally colonised by bivalves, most species being endobenthic, for example, representatives of the Unionoidea and Corbiculoidea. The hard surfaces niche was therefore a suitably vacant target for the ancestors of both Dreissena and Limnoperna. Significantly, within their own spheres of influence, both species would appear to be colonising this habitat as fast as it is artificially created for them.
But what features, specifically, not only adapt L. fortunei to life in freshwaters but have also facilitated its colonisation of waters external to its natural range? Limnoperna fortunei possesses ciliary tracts on the internal surfaces of the inhalant aperture, which carry pseudofaeces towards the exhalant siphon. The intermittent, posterior adductor muscle generated, rapid expulsion of water from the exhalant siphon blows these away together with the faeces. Living as it can do in fast-flowing waters, its siphons invariably facing the current, this process is a significant aspect of the morphology of L. fortunei since it enables the animals to feed and remove pseudofaeces at the same time but, more importantly, blows this waste material over the top of the animal and not straight out in front of it. This prevents the pseudofaeces and faeces from being taken back into the mantle cavity. In large colonies occupying more static waters with, for example, low Reynold’s numbers, however, have illustrated how the mass of L. fortunei becomes covered with faeces and pseudofaeces (see Fig. 1 in Boltovskoy et al. 2009).
The outer demibranchs of the ctenidia of L. fortunei are unusually long dorso-ventrally. This adaptation gives a greater surface area for filtration and also places rejected particles travelling anteriorly on the crests of the ventral marginal food grooves of both demibranchs in much closer proximity to the rejection tracts of the mantle. The outer demibranch being longer dorso-ventrally but abruptly shorter antero-posteriorly relative to the inner demibranch also enables the labial palps to exert their selective influence upon all four of the gill lamellae. For an animal living, as L. fortunei can do, in a wide range of lentic and lotic waters experiencing large differences in suspended sediment loads, this ctenidial-labial palp relationship ensures that all particles reaching the anterior end of the ctenidia are potentially made available as food. Potentially because the animal has to balance its particle-collection and -sorting abilities for food against, in waters with high sediment loads, the opposite capability of removing inhaled but unwanted material surplus to requirements. And, thereby, avoiding the clogging of the mantle cavity and its organs. Paolucci et al. (2014) have shown that across 24 South American sites experiencing variations in suspended solid loads, L. fortunei showed phenotypic variation not just in shell morphology but also in relative ctenidial surface area. Also identified was a reduction in mean ctenidial ciliation with increasing suspended sediment loads suggesting a mechanism for balancing the aforementioned dietary and cleansing requirements (see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume). Although, more likely, but unmeasured, these functions are more particularly the role of the labial palps.
The alimentary system of L. fortunei is typical of the Mytiloidea in general, although the relatively large food-sorting caecum in this species, when compared with the short caeca of species described by Dinamani (1967), may indicate a greater selective facility in L. fortunei and, thus, ensure that all potential food material is utilised. This would be especially pertinent for such populations living in more oligotrophic waters. Conversely, in large silt-laden continental waters, the sorting and selection currents in the mantle cavity and stomach facilitate the removal of large amounts of unwanted material. The food-sorting caecum of L. fortunei also possesses ducts to the digestive diverticulae. These ducts, by increasing the total number of apertures to the digestive diverticulae, increase the capabilities of L. fortunei for channelling a greater number of particles into the digestive diverticulae and making its intracellular digestion much more efficient facilitating, in turn, the rapid growth characteristic of this species.
This second structural study of L. fortunei (that is, the present chapter) has, however, identified another aspect of its anatomy hitherto un-noticed. The siphonal septum, unlike other mytiloids studied previously, possesses five sensory papillae. These are innervated, but not ciliary-based as one would expect if they were mechanoreceptors. Rather, the microvilli-fringed sensory cells are reminiscent of simple photoreceptors. In a preliminary experiment to determine if L. fortunei possesses a shadow reflex once acclimated to an electric light, a passing hand shadow did result in the siphons being partially retracted and valves shut slightly in some (< 10 %) of individuals. In a second experiment under conditions of direct, bright, sunlight, of 38 individuals tested for a shadow reflex, 8 (21 %) closed their valves, 16 (42 %) retracted their siphons and 14 (37 %) gave no reaction (Romina Tokumon and Daniela Duchini, pers. comm.). Since the exposed general mantle edges of L. fortunei possess no papillae of any kind, it is possible that those on the siphonal septum are the source of these reactions. It is, moreover, unknown if L. fortunei possesses an endogenous diurnal rhythm of feeding and digestion as demonstrated for D. polymorpha by Morton (1969b). If so, and it would seem logical that is the case, then some kind of optical capability would be necessary (simple photosensory cells in the case of D. polymorpha) to modulate this rhythm. If the structures identified for L. fortunei are photosensory organs (in contrast to simple cells) this would represent an advance on, for example, even the more modern eulamellibranch D. polymorpha, a specialisation not hitherto identified in any other mytiloidean (Morton 2008) and an occurrence seen in only a few, more advanced, bivalve lineages.
Limnoperna fortunei is, thus, a relatively unspecialised mytilid in terms of its shell architecture, that is a lack of external ornamentation and internal teeth and crenulations, but those specialisations that do exist are concerned with greater efficiency in food collection and utilisation. There are quite obviously physiological specialisations, especially with regard to evolved osmoregulatory processes. In essence, however, Limnoperna is a typical representative of the Mytiloidea. Dreissena polymorpha is, similarly, anatomically unspecialised and, it has been suggested before (Morton 1982), that it is the retention of either primitive or, at least, simplified characters in a habitat where there has been a trend in other lamellibranchs towards greater and greater specialisation, for example, representatives of the Unionoidea, that makes the attribute of simplicity so successful. Such characters include, for example, a thin, plain, shell, a byssus, high fecundity and free-swimming larvae.
It is these anatomical features, combined with an r-expressed reproductive strategy (Morton 1991; see Chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume) and life history trait, which makes L. fortunei characteristic of opportunistic species. In such species, minimal energy is spent on shell thickness (particularly advantageous when competing for the colonisation of freshwaters poor in calcium, see above), the life cycle is short, just two, rarely three, years in the case of L. fortunei, but where all activity is focussed on food collection and digestion to maximise energy gain for reproduction. Such characteristics epitomise the success achieved by L. fortunei as a highly invasive species. The sexual strategy and life history trait of L. fortunei have previously been compared with those of D. polymorpha to explain the invasive successes of both species (Karatayev et al. 2007a; see Chapter “Parallels and Contrasts Between Limnoperna fortunei and Species of Dreissena” in this volume). Just as remarkable, however, is the fact that the two families have wholly separate phylogenetic origins and, thus, that such similarities are not just convergent but highlight the success of, ultimately, the byssally attached heteromyarian form in freshwaters in them both.
References
Abdel-Razek FA, Chiba K, Kurokura H, Okamoto K, Hirano R (1993a) Distribution of Limnoperna fortunei kikuchii in Shonai Inlet, Lake Hamana. Suisan Zoshoku 41:89–95
Abdel-Razek FA, Chiba K, Kurokura H, Okamoto K, Hirano R (1993b) Life history of Limnoperna fortunei kikuchii in Shonai Inlet, Lake Hamana. Suisan Zoshoku 41:97–104
Adarraga I, Martínez J (2011) First record of the invasive brackish water mytilid Limnoperna securis (Lamarck, 1819) in the Bay of Biscay. Aquat Invasions 7:171–180
Atkins D (1937a) On the ciliary mechanisms and interrelationships of lamellibranchs. Part III. Types of lamellibranch gills and their food currents. Q J Microscop Sci 79:375–421
Atkins D (1937b) On the ciliary mechanisms and interrelationships of lamellibranchs. Part IV. Cuticular fusion, with special reference to the fourth aperture in certain lamellibranchs. Q J Microscop Sci 79:423–445
Atkins D (1938) On the ciliary mechanisms and interrelationships of lamellibranchs. Part VII. Laterofrontal cilia of the gill filaments and their phylogenetic value. Q J Microscop Sci 80:346–430
Beasley CR, Tagliaro CH, Figueiredo WB (2003) The occurrence of the asian clam Corbicula fluminea in the lower Amazon basin. Acta Amazonica 33:317–324
Beedham DE (1958) Observations on the non-calcareous component of the shell of the Lamellibranchia. Q J Microscop Sci 99:341–357
Beran L (2000) First record of Corbicula fluminea (Mollusca: Bivalvia) in the Czech Republic. Acta Soc Zool Bohem 64:1–2
Beran L (2006) Spreading expansion of Corbicula fluminea (Mollusca: Bivalvia) in the Czech Republic. Heldia 6:187–192
Beu AG (2006) Marine Mollusca of oxygen isotope stages of the last 2 million years in New Zealand. Part 2. Biostratigraphically useful and new Pliocene to recent bivalves. J R Soc New Zeal 36:151–338
Bieler R, Carter JG, Coan EV (2010) Part 2. Classification of bivalve families. Malacologia 52:113–184
Boltovskoy D, Correa N (2015) Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia 746:81–95
Boltovskoy D, Karatayev A, Burlakova L, Cataldo D, Karatayev V, Sylvester F, Mariñelarena A (2009) Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636:271–284
Brown CH (1952) Some structural proteins of Mytilus edulis. Q J Microscop Sci 93:487–502
Brugnoli E, Dabezies MJ, Clemente JM, Muniz P (2011) Limnoperna fortunei (Dunker 1857) en el sistema de embalses del Rio Negro, Uruguay. Oecol Australis 15:576–592
Burch JQ (1944) Checklist of west American mollusks. Minutes of the Conchological Club of Southern California 38:18
Cataldo D, Boltovskoy D, Hermosa JL, Canzi C (2005) Temperature-dependent larval development rates of Limnoperna fortunei (Bivalvia: Mytilidae). J Mollus Stud 71:41–46
Cataldo D, Vinocur A, O´Farrell I, Paolucci E, Leites V, Boltovskoy D (2012) The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande Reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680:25–38
Choi SS, Kim JS (1985) Studies on the metamorphosis and the growth of larva in Limnoperna fortunei. Korean J Malacol 1:13–18 [In Korean]
Darrigran GA, Pastorino G (1995) The recent introduction of a freshwater Asiatic bivalve, Limnoperna fortunei (Mytilidae) into South America. The Veliger 38:171–175
Darrigran GA, Damborenea C, Penchaszadeh PE (1998) A case of hermaphroditism in the freshwater invading bivalve Limnoperna fortunei (Dunker, 1857) (Mytilidae) from Río de la Plata, Argentina. Iberus 16:99–104
Deaton LE, Derby JGS, Subhedar N, Greenberg MJ (1989) Osmoregulation and salinity tolerance in two species of bivalve mollusc: Limnoperna fortunei and Mytilopsis leucophaeta. J Exp Mar Biol Ecol 133:67–79
Dinamani P (1967) Variation in the stomach structure of the Bivalvia. Malacologia 5:225–268
Dinesen GE, Morton B (2014) Review of the functional morphology, biology and perturbation impacts on the boreal, habitat-forming horse mussel Modiolus modiolus (Bivalvia: Mytilidae: Modiolinae). Mar Biol Res 10:845–870
Douda K, Vrtílek M, Slavík O, Reichard M (2012) The role of host specificity in explaining the invasion success of the freshwater mussel Anodonta woodiana in Europe. Biol Invasions 14:127–137
Dudgeon D, Morton B (1984) Site selection and attachment duration of Anodonta woodiana (Bivalvia: Unionacea) glochidia on fish hosts. J Zool (Lond) 204:355–362
Fankboner PV (1971) The ciliary currents associated with feeding, digestion and sediment removal in Adula (Botula ) falcata Gould 1851. Biol Bull 140:28–45
Giusti F (1971) The fine structure of the style sac and intestine in Mytilus galloprovincialis Lam. Proc Malacol Soc Lond 39:95–104
Goto Y (2002) Behavior of nuisance mussel, Limnoperna fortunei, in water supply facilities. Water Sci Technol 46:45–50
Graham A (1949) The molluscan stomach. T R Soc Edin 61:737–776
Habe T (1981) A catalogue of mollusca of Wakayama prefecture. The Province of Kii. I. Bivalvia, scaphopoda and cephalopoda. Pub Seto Mar Biol Lab 7:1–301
Halton DW, Owen G (1968) The fine structure and histochemistry of the gastric cuticle of the protobranchiate bivalve, Nucula sulcata Bronn. Proc Malacol Soc Lond 38:71–81
Hayden B, Caffrey JM (2013) First recording of the Asian clam (Corbicula fluminea (Müller, 1774)) from the River Shannon, with preliminary notes on population size and class distribution. Irish Nat J 32:29–31
Holikoshi Y (1935) Ecology of freshwater mollusca in Taiwan. T Nat Hist Soc Formosa 25:226–231 [In Japanese]
Howlett D, Baker R (1999) Corbicula fluminea (Müller); new to UK. J Conchol 36:83
Huang CW (2008) Population genetic structure of Limnoperna fortunei (Dunker, 1857) in Taiwan based on sequences of mitochondrial cytochrome C oxidase subunit I. MSc Thesis, National Changhua University of Education (Taiwan), pp 1–60
Ituarte CF (1994) Corbicula and Neocorbicula (Bivalvia: Corbiculidae) in the Paraná, Uruguay and Río de la Plata basins. The Nautilus 107:129–135
Iwasaki K (1997) Climbing behaviour and tolerance to aerial exposure of a freshwater mussel, Limnoperna fortunei. Venus (Jpn J Malacol) 56:15–25
Karatayev AY, Boltovskoy D, Padilla DK, Burlakova LE (2007a) The invasive bivalves Dreissena polymorpha and Limnoperna fortunei : parallels, contrasts, potential spread and invasion impacts. J Shellfish Res 26:205–213
Karatayev AY, Padilla DK, Minchin D, Boltovskoy D, Burlakova LE (2007b) Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biol Invasions 9:161–180
Kellogg JL (1915) Ciliary mechanisms of lamellibranches with descriptions of anatomy. J Morphol 26:625–701
Kerney MP, Morton B (1970) The distribution of Dreissena polymorpha (Pallas) in Britain. J Conchol 27:97–100
Kimura T (1994a) Morphological identification of Limnoperna fortunei (Dunker) and Limnoperna fortunei kikuchii Habe. Chiribotan (J Malacol Soc Jpn) 25:36–40 [In Japanese]
Kimura T (1994b) The earliest record of Limnoperna fortunei (Dunker) from Japan. Chiribotan (J Malacol Soc Jpn) 25:34–35 [In Japanese]
Kimura T, Sekiguchi H (2009) Spatial and temporal patterns of abundance of the exotic mytilid Xenostrobus securis and the native mytilid Musculista senhousia in the Lake Hamana, Japan. Biodivers Rec 2 e89:1–7
Kimura T, Kakuta I, Kurokura H (1995) Salinity tolerance and osmoregulation in freshwater and brackish water mytilids (Mytilidae, genus Limnoperna ). Bull Soc Sea Water Sci, Jpn 49:148–152 [In Japanese]
Kinzelbach R (1991) Die Körbchen muscheln Corbicula fluminalis, Corbicula fluminea und Corbicula fluviatilis in Europe (Bivalvia: Corbiculidae). Mainzer Naturwissenschaftliches Archiv 29:215–228
Kuroda T (1941) A catalogue of molluscan shells from Taiwan (Formosa), with descriptions of new species. Memoirs of the faculty of science and agriculture. Taihoku Imperial University 22:65–216
Magara Y, Matsui Y, Goto Y, Yuasa A (2001) Invasion of the non-indigenous nuisance mussel, Limnoperna fortunei, into water supply facilities in Japan. J Water Supply: Res Technol—Aqua 50:113–124
Martínez ER (1987) Corbicula manilensis molusco introducido en Venezuela. Acta Científica Venezolana 38:384–385
Mathews J, van der Velde G, bij de Vaate A, Collas FPL, Koopman KR, Leuven RSEW (2014) Rapid range expansion of the invasive quagga mussel in relation to zebra mussel presence in The Netherlands and Western Europe. Biol Invasions 16:23–42
McMahon RF (1982) The ocurrence and spread of the introduced Asiatic freshwater clam, Corbicula fluminea (Muller) in North America: 1924–1982. The Nautilus 96:134–141
Miller RC, McClure FA (1931) The fresh-water clam industry of the Pearl River. Lingnan Sci J 10:307–322
Mills EL, Rosenberg G, Spidle AP, Ludyanskiy M, Pligin Y (1996) A review of the biology of the quagga mussel (Dreissena bugensis ), a second species of freshwater dreissenid introduced to North America. Am Zool 36:271–286
Mizzan L (1999) Le specie alloctone del macrozoobenthos della Laguna di Venezia: il punto della situazione. Bollettino del Museo Civico di Storia Naturale di Venezia 49:145–177
Montalto L, Ezcurra de Drago I (2003) Tolerance to desiccation of an invasive mussel, Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae), under experimental conditions. Hydrobiologia 498:161–167
Montalto L, Rojas Molina F (2014) Byssal hairs in the invasive Asian freshwater bivalve Limnoperna fortunei (Mytilidae) in the Paraná River system with comments on this species in South America. Molluscan Res. 34:127–138
Morton B (1969a) Studies on the biology of Dreissena polymorpha Pall. (I). General anatomy and morphology. Proc Malacol Soc Lond 38:301–321
Morton B (1969b) Studies on the biology of Dreissena polymorpha Pall. (II). Correlation of the rhythms of adductor activity, feeding, digestion and excretion. Proc Malacol Soc Lond 38:401–414
Morton B (1973) Some aspects of the biology and functional morphology of the organs of feeding and digestion of Limnoperna fortunei (Dunker) (Bivalvia: Mytilacea). Malacologia 12:265–281
Morton B (1975) The colonization of Hong Kong’s raw water supply system by Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) from China. Malacol Rev 8:91–105
Morton B (1977) The population dynamics of Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) in Plover Cove Reservoir, Hong Kong. Malacologia 16:165–182
Morton B (1981) The biology and functional morphology of Mytilopsis sallei (Recluz) (Bivalvia: Dreissenacea) fouling Visakhapatnam harbour, Andhra Pradesh, India. J Mollus Stud 47:25–42
Morton B (1982) Some aspects of the population structure and sexual strategy of Corbicula cf. fluminalis (Bivalvia: Corbiculacea) from the Pearl River, People’s Republic of China. J Mollus Stud 48:1–23
Morton B (1983) Feeding and digestion in Bivalvia. In: Wilbur KM, Saleuddin ASM (eds) The Mollusca. Vol 5: Physiology, Part 2. Academic Press, New York, pp 65–147
Morton B (1985) Statocyst structure in the Anomalodesmata (Bivalvia). J Zool (Lond) 206:23–34
Morton B (1987) The functional morphology of the organs of the mantle cavity of Perna viridis (Bivalvia: Mytilidae). Am Malacol Bull 5:161–166
Morton B (1991) Do the Bivalvia demonstrate environment-specific sexual strategies? A Hong Kong model. J Zool (Lond) 223:131–142
Morton B (1992) The evolution and success of the heteromyarian form in the Mytiloida. In: Gosling EM (ed) The mussel Mytilus : Ecology, physiology, genetics and culture. Developments in aquaculture and fisheries. Elsevier, Amsterdam, pp 21–52
Morton B (1997) The aquatic nuisance species problem: a global perspective and review. In: D’Itri F (ed) Zebra mussels and aquatic nuisance species. Lewis Publishers, Boca Raton, pp 1–54
Morton B (2008) The evolution of eyes in the Bivalvia: new insights. Am Malacol Bull 26:35–45
Morton B (2011) Predator-prey-scavenging interactions between Nucella lapillus (Linnaeus, 1758), Carcinus maenas and Eulalia viridis all exploiting Mytilus galloprovincialis on a rocky shore recovering from tributyl-tin (TBT) pollution. J Nat Hist 45:2397–2417
Morton B, Dinesen G (2010) Colonization of Asian freshwaters by the Mytilidae (Bivalvia): a comparison of Sinomytilus harmandi from the Tonle-Sap River, Phnom Penh, Cambodia, with Limnoperna fortunei. Molluscan Res 30:57–72
Nakano D, Kobayashi T, Sakaguchi I (2010) Predation and depth effects on abundance and size distribution of an invasive bivalve, the golden mussel Limnoperna fortunei, in a dam reservoir. Limnology 11:259–266
Nishimura T, Habe T (1987) Chinese freshwater mussels mixed in the imported Corbicula clam. Chiribotan (J Malacol Soc Japan) 18:110–111
Ockelmann KW (1983) Descriptions of mytilid species and definition of the Dacrydiinae n. subfam. (Mytilacea-Bivalvia). Ophelia 22:81–123
Ockelmann KW (1995) Ontogenetic characters of mytilaceans. Phuket Mar Biol Centre Special Pub 15:85–88
Ockelmann KW, Dinesen GE (2009) Systematic relationship of the genus Adula and its descent from a Mytilus -like ancestor (Bivalvia, Mytilidae, Mytilinae). Steenstrupia 30:141–152
Oliveira MD, Hamilton SK, Jacobi CM (2010) Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Aquatic Invasions 5:59–73
Owen G (1955) Observations on the stomach and digestive diverticula of the Lamellibranchia. Part I. The Anisomyaria and Eulamellibranchia. Q J Microscop Sci 96:517–537
Owen G (1974) Studies on the gill of Mytilus edulis : the eu-latero-frontal cirri. Proc R Society of Lond. Series B, 187:83–91
Owen G (1978) Classification and the bivalve gill. Philos T R Soc Lond, Series B, 284:377–386
Owen G, Trueman ER, Yonge CM (1953) The ligament in the Lamellibranchia. Nature 171:1–6
Paolucci EM, Sardiña P, Sylvester F, Perepelizin PV, Zhan A, Ghabooli S, Cristescu ME, Oliveira MD, MacIsaac HJ (2014) Morphological and genetic variability in an alien invasive mussel across an environmental gradient in South America. Limnol Oceanograph 59:400–412
Pastorino G, Darrigran GA, Martín SM, Lunaschi L (1993) Limnoperna fortunei (Dunker, 1857) (Mytilidae), nuevo bivalvo invasor en aguas del Río de la Plata. Neotropica 39:101–102
Pujol JP (1967) Formation of the byssus in the common mussel (Mytilus edulis L.). Nature 214:204–205
Purchon RD (1957) The stomach in the Filibranchia and the Pseudolamellibranchia. Proc Zool Soc Lond 129:27–60
Purchon RD (1960) The stomach in the Eulamellibranchia: stomach Types IV and V. Proc Zool Soc Lond 135:431–489
Purchon RD (1963) Phylogenetic classification of the Bivalvia with special reference to the Septibranchia. Proc Malacol Soc Lond 35:71–80
Purchon RD, Brown D (1969) Phylogenetic interrelationships among families of bivalve molluscs. Malacologia 9:163–171
Reid RGB (1965) The structure and function of the stomach in bivalved molluscs. J Zool (Lond) 147:156–184
Ridewood WG (1903) On the structure of the gills of the Lamellibranchia. Philos T R Soc, Series B, 211:147–284
Sabelli B, Speranza S (1994) Rinvenimento di Xenostrobus sp. (Bivalvia, Mytilidae) nella laguna di Venecia. Boll Malacol 29:311–318
Santos CP, Würdig Nl, Mansur MC (2005) Fases larvais do mexilhão dourado Limnoperna fortunei (Dunker) (Mollusca, Bivalvia, Mytilidae) na bacia do Guaíba, Rio Grande do Sul, Brasil. Revista Brasileira de Zoologia 22:702–708
Sardiña P, Cataldo D, Boltovskoy D (2009) Effects of conspecifics on settling juveniles of the invasive golden mussel, Limnoperna fortunei. Aquatic Sci 71:479–486
Scarlato OA, Starobogatov YI (1979) General evolutionary patterns and the system of the class Bivalvia. Trudy Zoologicheskogo Instituta, Akademiya Nauk SSSR, 80:5–38 [In Russian]
Shirafuji J, Sato S (2003) Benthic communities of tidal flats in Sacheon and Masan, Gyeongsangnamdo, Korea. Investigation report of tidal flat in cooperation with Japan and Korea. pp 20–25
Silverman HG, Roberto FF (2007) Understanding marine mussel adhesion. Mar Biotechnol 9:661–681
Stasek CR (1963) Synopsis and discussion of the association of ctenidia and labial palps in the bivalved Mollusca. The Veliger 6:91–97
Sylvester F, Dorado J, Boltovskoy D, Juárez A, Cataldo D (2005) Filtration rates of the invasive pest bivalve Limnoperna fortunei as a function of size and temperature. Hydrobiologia 534:71–80
Sylvester F, Cataldo D, Notaro C, Boltovskoy D (2013) Fluctuating salinity improves survival of the invasive freshwater golden mussel at high salinity: implications for the introduction of aquatic species through estuarine ports. Biol Invasions 15:1355–1366
Tan T-H, Pai J-Y, Hsha KC (1987) The recovery of fouling clam, Limnoperna fortunei from Taiwan. Bull Malacol, Republic of China, 13:97–100 [In Chinese]
Taylor JD, Kennedy WJ, Hall A (1969) The shell structure and mineralogy of the Bivalvia, introduction. Nuculacea-Trigonacea. Bull British Museum Nat Hist (Zool), Supplement 3:1–125
Taylor JD, Kennedy WJ, Hall A (1972) The shell structure and mineralogy of the Bivalvia. II. Lucinacea—Clavagellacea Conclusions. Bull British Museum Nat Hist (Zool) 22:255–294
Thiele J (1934) Handbuch der systematischen Weichtierkunde, Part III (Scaphopoda, Bivalvia, Cephalopoda). Familien Mytilidae. Verlag von Gustav Fisher, Jena, pp 797–801
Tominaga A, Goka K, Kimura T, Ito K (2009) Genetic structure of Japanese introduced populations of the golden mussel, Limnoperna fortunei, and the estimation of their range expansion process. Biodiversity 10:61–66
Trueman ER (1950) Observations on the ligament of Mytilus edulis. Q J Microscop Sci 91:225–235
Uryu K, Iwasaki K, Hinoue M (1996) Laboratory experiments on behaviour and movement of a freshwater mussel, Limnoperna fortunei (Dunker). J Mollus Stud 62:327–341
Veitenheimer-Mendes I (1981) Corbicula manilensis, (Philippi, 1844) molusco asiático, na bacia do Jacuí do Guaíba, Rio Grande do Sul, Brasil (Bivalvia, Corbiculidae). Iheringia (Série Zoologia) 60:63–74
White KM (1937) Mytilus. Liverpool Marine Biology Committee Memoirs 31:1–117
Wilson BR (1967) A new generic name for three recent and one fossil species of Mytilidae (Mollusca: Bivalvia) in Southern Australasia, with redescriptions of the species. Proc Malacol Soc Lond 37:279–295
Wilson BR (1968) Survival and reproduction of the mussel Xenostrobus securis (Lam.) (Mollusca-Bivalvia-Mytilidae) in a Western Australian estuary. I. Salinity tolerance. J Nat Hist 2:307–328
Wright MM, Francis L (1984) Predator deterrence by flexible shell extensions of the horse mussel Modiolus modiolus. The Veliger 27:140–142
Yonge CM (1948) Formation of siphons in lamellibranchs. Nature 161:198
Yonge CM (1955) Adaptation of rockboring in Botula and Lithophaga (Lamellibranchia, Mytilidae), with a discussion on the evolution of this habit. Q J Microscop Sci 96:383–410
Yonge CM (1957) Mantle fusion in the Lamellibranchia. Pubblicazione della Stazione Zoologica di Napoli 29:151–171
Yonge CM (1962) On the primitive significance of the byssus in the Bivalvia and its effects in evolution. J Mar Biol Assoc UK 42:113–125
Yonge CM, Campbell JI (1968) On the heteromyarian condition in the Bivalvia with special reference to Dreissena polymorpha and certain Mytilacea. T R Soc Edin 68:21–43
Acknowledgements
I am grateful to the editors of Molluscan Research and Malacologia for permissions to reproduce herein redrawn and relabelled figures originally published in these journals (for details see figure legends).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Morton, B. (2015). The Biology and Anatomy of Limnoperna fortunei, a Significant Freshwater Bioinvader: Blueprints for Success. In: Boltovskoy, D. (eds) Limnoperna Fortunei. Invading Nature - Springer Series in Invasion Ecology, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-319-13494-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-13494-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13493-2
Online ISBN: 978-3-319-13494-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)