Abstract
Research on multi-scale temporal dynamics of lotic algal assemblages remains scarce. In this study, we analyzed epilithic algae sampled monthly from a Chinese subtropical mountain river network from 2004 to 2007, by using a multivariate time series modeling approach. We hypothesized that (1) multi-scale temporal dynamics exist within algal communities; (2) physical and chemical conditions drive algal temporal dynamics; and (3) tributary sites differ in algal temporal changes. This study revealed 2–4 site-specific algal temporal dynamics, contributed by 23–45% component taxa. Among the time-related taxa, percentages of high profile guild taxa were higher than both the low profile and the motile guild taxa. Several algal temporal dynamics were found to be driven by water temperature, conductivity, or current velocity, within which influences of conductivity at two sites resulted in directional changes in algal communities. Furthermore, tributary sites differed in algal temporal changes when compared to the two mainstream sites. Our findings imply that natural fluctuations and agricultural disturbance together shaped algal temporal dynamics in the studied river network. In conclusion, for accurately tracking algal temporal dynamics, we recommend that long-term and high-frequency biomonitoring protocols are developed. Moreover, both the mainstream and tributary sites should be monitored simultaneously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporal dynamics of algal assemblages are frequently addressed in stream ecology (Kennen et al., 2012; Miller et al., 2012) because such studies form an important foundation for answering other complex questions, such as ecological stability mechanisms, succession trends, or disturbance responses of lotic algal assemblages (Angeler, 2009; Angeler et al., 2010; Virtanen et al., 2011; Baho et al., 2012; Schneck & Melo, 2013). Numerous studies have revealed that temporal variability of lotic algal assemblages is the results of interactions among biological traits (e.g., resistance, resilience, reproductivity ability, etc.) and environmental factors (e.g., nutrients, irradiance, hydrodynamic regimes, predatory pressures, etc.) (Rosemond et al., 2000; Soininen & Eloranta, 2004; Yang et al., 2009; Graba et al., 2013). Algal short-term dynamics usually involve two phases: the first, accrual phase, which is driven by immigration/colonization and exponential growth, and the second, loss phase, which is dominated by processes of death, emigration, sloughing, and grazing (Biggs, 1996). During short-term dynamics, algal structures are controlled primarily by nutrients supply (Passy & Larson, 2011). Considering intra-annual and inter-annual dynamics, algal biomass and biodiversity display distinct seasonal cycles, and community composition changes among seasons and years (Miller et al., 2012; Tan et al., 2014). Long-term fluctuation tendencies of algal assemblages are synchronous with temporal nutrient dynamics and hydrological and climatic regimes (Reid et al., 2006; Korhonen et al., 2013).
Hitherto, most studies on temporal dynamics of algal assemblages focus on community metrics such as biomass, richness, or biodiversity indices (Rosemond et al., 2000). Temporal patterns of community composition (represented by taxa abundance data) or temporal turnover (represented by similarity/dissimilarity matrices of community compositions across different sampling times) is also frequently addressed by treating algal community as a single metric (Passy, 2006; Korhonen et al., 2010, 2013). However, algal assemblages are composed of species possessing diverse biological characteristics and distinct environmental optima (Stevenson, 2014). These species differ in body size, attachment ability, growth rate, dispersal ability, population abundance, and sensitivity/tolerance to disturbances (Virtanen et al., 2011). Therefore, it is reasonable to expect that different temporal dynamics among algal species will exist within the whole community. That is, some species will exhibit short temporal fluctuation dynamics, and some species will have relatively long fluctuation periodicities. In contrast, some species within the same community will display stochastic, time-unrelated dynamics. Species-specific temporal dynamics are essential for sustaining a dynamically stable algal community because asynchronously temporal variability among species can buffer environmental stress and fluctuation effectively. Disentangling multiple temporal scales of variability patterns within a community, namely multi-scale temporal dynamics (Legendre & Gauthier, 2014), can provide especially useful information on temporal changes of an algal assemblage. Such process-oriented research can elucidate temporal dynamics of algal communities more comprehensively than previous studies, which is especially important if we are curious about the stability mechanisms of lotic algal communities under changing environments or determining contributions of component species to temporal dynamics of the whole algal community. However, research on multi-scale temporal dynamics within lotic algal assemblages remains scarce.
In the present study, we applied a multivariate time series modeling approach to research multi-scale temporal dynamics within epilithic algal assemblages, which were sampled monthly from five sites of one Chinese subtropical mountain river network from 2004 to 2007. Three hypotheses are to be tested: (1) Multi-scale temporal dynamics exist within algal communities, and contributions of ecological guilds to algal dynamics will differ. (2) Several physical and chemical variables are important driving forces for algal temporal dynamics. (3) Algal assemblages from different tributaries will differ in temporal evolution.
Materials and methods
Study region
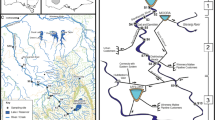
The Xiangxi River is a tributary of the Three Gorges Reservoir in Hubei Province of China. The river is 94 km long with three main tributaries, the Jiuchong, Gufu, and Gaolan River (Wang et al., 1997). This mountain watershed is located in a subtropical climate region characterized by hot and rainy summers and cold and dry winters, with a mean annual air temperature of 16.9°C (range: 5.5–27.7°C) and precipitation of 900–1,200 mm (Tang, 2003). Woodland is the dominant land use in the catchment, with a few towns and farmland distributed along the riverside (Seeber et al., 2010).
Five sites were selected within the whole river network, with two sites located in the mainstream (xx1 and xx2, following the water flow direction) and one site in each of the three tributaries (named jc, gf, and gl, respectively) (Fig. 1). For these sites, there was no visible human disturbance in the stream or riparian zone of the sample reaches during the study duration. However, stream water quality of these sites might be influenced by agricultural activities in upstream catchments, especially in rainy seasons.
Algal data
Epilithic algae were sampled monthly from September 2004 to June 2007. Three to five moveable stones (with a diameter range of 15–60 cm) were randomly selected at each site during each sampling occasion. The sampling area was confined using a circular lid (radius: 2.7 cm). For each stone, the surface within the lid was vigorously scrubbed using a nylon brush and rinsed 3–4 times with distilled water. All subsamples from each site were combined into one composited sample and its volume was recorded. The algal sample was preserved with 4% formalin for further identification and enumeration.
In the laboratory, soft algae were first identified to the lowest possible taxonomic level (most to genus) and counted, using a 0.1-ml counting chamber at 400× magnification under a compound microscope (Olympus CX21: Olympus Optical Co., Japan). All diatoms were grouped into one category during this process. Subsequently, species-level identification and enumeration of diatoms were conducted, after the sample was acid cleaned and slide mounted at 1,000× magnification with an oil immersion objective (Hu & Wei, 2006). At least 500 valves were counted for each sample, and relative abundance was calculated for each taxon. Algal identification was based on Patrick & Reimer (1966, 1975), Jao (1988), Qi (1995), Li & Bi (1998), Shi (2004), and Zhu (2007).
Physical and chemical variables
At each site, conductivity and water temperature (WT) were measured by using an Environmental Monitoring System (HORIBA W23: Horiba Co., Japan). Wetted width and water depth were measured using a tape measure and current velocity with a current flow meter (LJD type, Chongqing hydrological instruments factory, China). A 600 ml stream water sample was collected and preserved in an acid-proof bottle by adding concentrated sulphuric acid to regulate pH <2 in the field. In the laboratory, the acidified water sample was partitioned into two parts. One part was used to measure concentrations of TN, TP, and SiO2 on a Skalar segmented flow analyzer (Skalar Analytical B.V., The Netherlands) following the user manual. The other part was used to measure alkalinity using the titration method following the national water monitoring protocol (Chinese NEPA, 2002). Summary for physical and chemical variables is presented in Table 1.
Statistical analyses
To make the results comparable among the five sites, data collected in November 2006 and April and May 2007 were excluded from analysis because several sites were not sampled in these months. Therefore, the time vector is composed of 31 months (i.e., 34 continuous sampling months from September 2004 to June 2007, in which data for three discrete months were excluded).
All analyses were performed with R software (version 3.0). Each site was analyzed separately. Time variables were firstly produced by using the principal coordinate of neighbor matrices (PCNM) approach, which converts the linear time vector (31 months) into various frequency sine waves representing nonlinear and independent fine to broad temporal scales (Borcard & Legendre, 2002; Borcard et al., 2004; Angeler et al., 2009). Although this method is more frequently used in spatial analysis, it is also competent to disentangle temporal structures within multivariate data (Angeler et al., 2011; Legendre & Gauthier, 2014). Ten PCNM variables were obtained in the case of our study. Redundancy analysis (RDA) was then applied to detect relationships between algal relative abundance and PCNM variables (RDA-PCNM) (Borcard et al., 2011), accompanied by forward selection procedures for selecting important PCNM variables (Blanchet et al., 2008; Tang et al., 2013b). RDA was re-run with selected PCNM variables, and significant RDA axes were identified by the Monte Carlo permutation test (with 9,999 times). The linear combination scores (lc scores) on each significant RDA axis are the coordinates of the algal community on each sampling occasion in the space of selected PCNM variables (Borcard et al., 2011), representing a special temporal trend. Therefore, the number of significant RDA axes represents the number of distinct temporal dynamics within the whole community (Angeler & Johnson, 2012). Since RDA axes are orthogonal to each other, temporal dynamics revealed by RDA-PCNM are independent from each other (Angeler & Johnson, 2012). RDA-PCNM was performed with function ‘quick PCNM’ in PCNM package (Legendre et al., 2010a). Algal relative abundance data were Hellinger transformed prior to analysis to avoid problems caused by Euclidean distance (Legendre & Gallagher, 2001).
Correlation between raw relative abundances of each taxon and modeled temporal trends (lc scores of algal assemblages on each significant RDA axis) was analyzed by using Spearman rank correlation with a test of significance. Taxa that significantly correlated with a specific temporal trend composed of one distinct taxa group.
Generalized additive mixed model (GAMM) was applied to examine the relationship between physical and chemical variables and each temporal dynamic of algal communities for each site. GAMM is competent to model relationships by using smooth function, where the shape of the relationship between response and predictor is not known a priori (Zuur et al., 2009). In addition, GAMM can account for temporal autocorrelation of model residuals, which is evident in our data (detected by function ‘acf’). Water temperature, current velocity, TN, TP, SiO2, conductivity, and alkalinity were predictor variables, with a response variable of lc scores of the algal community on specific significant RDA axis. Cubic regression spline was used to fit fixed effect for each predictor, with cross-validation determining the optimal amount of smoothing. Temporal autocorrelation structure in residuals was fitted with the auto-regressive model of order 1 (Zuur et al., 2009). Because P values for each smoother estimated from GAMM are approximate, only predicators with P < 0.001 were selected as significant ones. Prior to fitting, all the predictor variables were examined for extreme values and collinearity following the steps suggested by Zuur et al. (2010). Variables were log10 (x + 1) transformed when extreme values were detected. GAMM was fitted using function ‘gamm’ in package ‘mgcv’ (Wood, 2014).
We further detect correlations between ecological guilds and physical and chemical variables to confirm algae–environment relationships. That is, algal taxa were assigned to one of the three ecological guilds: high profile guild, low profile guild and motile guild following Passy (2007) and Passy & Larson (2011). Spearman correlations between percentage of each ecological guild and physical and chemical variables that used in GAMM were analyzed for each site, and significance of correlations was tested. Moreover, we compared difference in percentages among ecological guilds for time-related taxa that appeared in the taxa groups. Considering that number of taxa groups differed among sites and many taxa appeared in more than one group at each site, we combined all the time-related taxa for each site and then calculated percentages for each ecological guild. Differences between percentages of the three guilds across all the sites were compared using one-way ANOVA.
Finally, a two-way ANOVA model was applied to compare the differences of time series of algal relative abundance data among the 5 sites. Because there is no replication for individual sites on each sampling occasion, our dataset belongs to an un-replicated (each site) repeated-measures (sampling time) design. Following classical statistical theory, a test of the space–time (S–T) interaction is impossible due to lacking of degree of freedom (Zar, 1999). Legendre et al. (2010b) proposed a new method that codes space or time using PCNM variables to economize on the degree of freedom, which makes tests on the effects of S, T, and S–T interactions all possible. We adopted this method to our data by using PCNM variables representing time factor. This analysis was conducted with TSI models in ‘PCNM’ package, in which ANOVA Model 5 was selected due to its accurate assessment of Type I error and powerful detection of S–T interactions compared with other models (Legendre et al., 2010b). Since this method only provides general results when more than two sites are included, pairwise comparisons were then performed given that significant S–T interactions among all the five sites existed.
Results
A total of 219 algal taxa, comprising 190 Bacillariophyceae, 16 Chlorophyceae, 13 Cyanophyceae, and 1 Xanthophyceae, were identified during the study. The number of taxa ranged from 144 (jc) to 163 (xx1 and gf), with mean value of 157. Rossithidium linearis (Smith) Round & Bukhtiyarova and Cocconeis placentula Ehrenberg were predominant species, occurring in all sites and all months, with mean relative abundance of 43.9 and 14.0%, respectively. The other 8 frequently occurring taxa (with mean relative abundance >1%) included Achnanthes lanceolata (Brébisson ex Kützing) Grunow (6.2%), Oscillatoria sp. (3.9%), Stephanodiscus minutulus (Kützing) Cleve & Möller (3.3%), Achnanthidium minutissimum (Kützing) Czarnecki (2.5%), Eunotia sp. 1 (2.0%), Diatoma vulgaris Bory (1.8%), Navicula cryptocephala Kützing (1.2%), and Synedra ulna (Nitzsch) Ehrenberg (1.1%).
Three to six site-specific PCNM variables were selected as significant time variables in RDA (Table 2). Algal dynamics in most sites were related to PCNM1-5 in which PCNM1 and 5 were important to the all sites. PCNM1 represented a nearly finished fluctuation during the study duration (34 months) (Supplementary Fig. 1). PCNM2 displayed unimodal dynamic with an approximate 24-month periodicity. PCNM3–5 had periodicities of approximately 18, 16, and 12 months, respectively. In contrast, PCNM6 and 7, with fluctuation periodicities of ~10 months, only contributed to algal dynamics in 1–2 sites. These significant PCNM variables totally explained 10% (xx1) to 32% (gl) of variation in algal abundance (Table 2).
RDA-PCNM detected 2–4 significant RDA axes, indicating site-specific number of time-related taxa groups (Fig. 2). The dynamics associated with the first two taxa groups were most important for all the sites, which contributed on average 53 and 21% of explained variation in algal community compositions, respectively. The patterns associated with the third and fourth taxa groups each explained approximately 12 and 10% of the explained variation in the algal community compositions (Table 3). Additional analysis detected significant S–T interactions among study sites (R 2 = 0.21, F = 1.587, P < 0.001). Pairwise comparisons further showed that algal communities differed in temporal changes among tributaries, while the difference between xx1 and xx2 was not significant (Supplementary Table 1).
The linear combination scores (lc scores) of algal communities on the space of significant principal coordinates of neighborhood matrices (PCNM) variables along the temporal axis for each study site of Xiangxi River network (the red dotted line represents the equilibrium line where the lc score = 0)
Among all observed algal taxa, 23% (xx1) to 45% (gl) contributed significantly to temporal dynamics of the whole community (Supplementary Table 2). Each time-related taxon associated with one or more taxa groups, in which more than half of the taxa were associated with the first taxa group (Supplementary Table 3). Water temperature was the unique significant predictor for four temporal dynamics of algal assemblages in site xx2, jc and gl. Conductivity was important to the first temporal dynamic of xx1. The third temporal dynamic of site gf was highly related to both conductivity and current velocity (Table 4).
Similar to GAMM results, temporal dynamics of ecological guilds were also significantly correlated to WT, conductivity, or current velocity (Supplementary Table 4). Considering time-related taxa, the percentages of high profile guild taxa (with the mean value of 46.0% for the all sites) was significantly higher than that of the low profile (mean: 29.3%) and the motile guild taxa (mean: 24.7%) (F 2,12 = 10.38, P = 0.002).
Discussion
Multi-scale temporal dynamics of algal communities and driving forces
Multi-scale temporal dynamics within lotic epilithic algal communities were observed in our study. For xx1 and xx2, algal dynamics were dominated by relatively long temporal trends (with periodicities >12 months); in contrast, relative short temporal trends (with periodicities <12 months) were also important to jc, gf, and gl. This result indicates that temporal trends of algal assemblages differed among mainstream and tributaries sites. Curves displaying lc scores of algal communities along time axis on significant RDA axes fluctuated in amplitudes and frequencies (Fig. 2); therefore, it is difficult to draw distinct fluctuation cycles. Additionally, we did not find directional trends in most curves. It implies that temporal dynamics of algal assemblages in Xiangxi River network were not dominated by any single periodic factor or external process during the study duration. Since PCNM1 was important to all sites, some observed temporal patterns may be parts of long-term periodic dynamics.
Less than 50% of taxa were found to contribute to temporal dynamics of algal assemblages, and the ten dominant taxa (with mean relative abundance >1%) all appeared in time-related taxa groups of more than three sites (Supplementary Table 3). This finding indicates that only small part of taxa within algal assemblages had time-related dynamics, and abundant taxa were highly possible to contribute to such dynamics. Owing to inadequate autecological information, it is difficult to interpret why these taxa displayed time-related dynamics. However, when they were categorized into ecological guild groups, a clearer trend was discovered. We found that nearly half of time-related taxa belonged to the high profile guild, indicating that this ecological guild was an important intrinsic trait in shaping temporal dynamics of algal assemblages. This can be attributed to the fact that the high profile taxa have relatively low growth rates, lower dispersal, and smaller population size due to their tall stature, compared to the low profile guild (Passy, 2008; Virtanen et al., 2011). The high profile taxa consequently are more susceptible to flow-related disturbances and prefer relative nutrient-rich conditions, resulting in more distinct temporal patterns than those of the low profile guild taxa. The motile guild taxa can escape from unfavorable environments owing to their mobility (Stevenson & Bahls, 1999), and therefore had the lowest proportion in time-related taxa groups. Our results imply that ecological guilds are good indicators for algal temporal dynamics. Stenger-Kovács et al. (2013) also drew similar conclusions in research on Hungarian streams (but see Virtanen et al., 2011).
Multi-scale temporal dynamics of algal communities in the present study were mainly determined by WT, conductivity, and current velocity. Water temperature is an essential driving force for most algal autecological traits such as enzymic activities, photosynthetic processes, growth rates, interactions among species, etc. (DeNicola, 1996; Allan & Castillo, 2007). Periodical fluctuations of solar radiation induce temporal variability of stream water temperatures. Correspondingly, taxa-specific temperature optima cause different temporal dynamics within the entire algal community. In addition to WT, conductivity also was another influential environmental factor. Conductivity, indicating total ionic concentrations in river water, influences algal physiologic and biochemical processes. Also, each algal taxon has specific optimum for conductivity (Potapova & Charles, 2003; Philibert et al., 2006). Therefore, algal responses to temporal changes in conductivity concentration of stream water were also taxa specific, resulting in different temporal dynamics among algal communities. Lastly, current velocity also showed significant influence on algal temporal dynamics. Algal taxa with different morphological forms and attaching capabilities prefer or tolerate specific current velocity conditions (Tornés et al., 2015). Temporal changes in hydrodynamic conditions induce different algal temporal dynamics.
These important physical and chemical variables, however, only had an influence on six within the 16 observed algal temporal dynamics. This finding can be explained in two ways. First, we set a conservative P value of 0.001 in GAMM analysis following the suggestion of Zuur et al. (2010) because this method only provides an approximate estimation for the significance of each independent variable. This borderline excluded several variables with margin P values between 0.05 and 0.001. Second, several likely important environmental variables have been omitted due to procedural limitations. For example, climatic and water flow fluctuations may affect temporal patterns of algae (Hering et al., 2010; Tang et al., 2013a). However, such variables are difficult to characterize for each site in this remote mountain river network with only one gauge station and one meteorological station. Simulated values from watershed modeling technique may be an appropriate remedy for these parameters. Moreover, pH is also an important environmental variable for algal pattern (Andrén & Jarlman, 2008; Smucker & Vis, 2011), but this variable was excluded in our analysis due to insufficient data. Obviously, the development of more robust and flexible approaches to analysis that can handle missing values is needed in the future for more accurate modeling of temporal monitoring data.
Potential implications in stream biomonitoring
Our findings have several applied implications. First, variations in lc scores of algal communities on each RDA axis can be used to identify whether the assemblages have been impacted by anthropogenic disturbances when the information on influential environmental variables is also considered. Directional changes of lc scores along a time axis indicate that variations in community composition are mainly driven by human disturbance. Conversely, if there is no directional change of lc scores, the data suggest that environmental effects are minor (Angeler & Johnson, 2012). In our study, most lc scores fluctuated around the equilibrium line (lc score = 0), implying that algal assemblages were mainly influenced by natural variation. However, the difference between the beginning and ending lc scores of xx1 on RDA1 and of gf on RDA3 is relatively large. Subsequent GAMM analysis suggests that conductivity had a significant influence on these lc scores. Because agricultural activities in the watershed often increase conductivity of stream water (Potapova & Charles, 2003), this parameter is commonly used as an indicator of agricultural disturbance. We hence infer that agricultural activities in the watershed had partly changed algal temporal dynamics of xx1 and gf. We can therefore see that RDA-PCNM, accompanied with other statistical techniques (GAMM in our study), can be used to determine which environmental forces have driven directional changes of algal assemblages, which is unobtainable when using other temporal analysis methods, such as time-lag analysis (Collins et al., 2000; Kampichler & van der Jeugd, 2013).
Furthermore, important PCNM variables with distinct fluctuation frequencies can be detected by using RDA-PCNM, which can be used to quantify monitoring duration and frequency (Angeler et al., 2009). For example, PCNM1 indicated that our study duration of 34 months was inadequate for detecting algal temporal dynamics completely, and a longer monitoring period should be employed in future studies. In addition, PCNM6 and 7 suggested the shortest fluctuation frequency (~10 months) of algal assemblages in the three tributary sites, which is informative for setting sampling frequency. To capture the complete process of the shortest fluctuation, the minimum sampling frequency should be every ~3 months (at the beginning, middle and ending time of the frequency). To obtain algal temporal dynamics more accurately, the sampling frequency may less than 3 months. A more frequent sampling schedule, such as monthly in our study, is even better for characterizing fluctuation processes of algal communities with greater accuracy.
Finally, results of RDA-PCNM and S–T interaction tests are helpful for selecting locations of monitoring sites. We found that algal assemblages at the mainstream sites (xx1 and xx2) had similar fluctuation dynamics in the study duration. However, algal assemblages from the three tributary sites changed distinctly. We thereby suggest simultaneous sampling of both mainstream and tributary sites in algae-based stream monitoring.
In conclusion, time series modeling is an effective approach for disentangling multi-scale temporal dynamics within lotic epilithic algal assemblage. The discovered temporal dynamics imply that the algal assemblages of the Xiangxi River network were influenced by both natural fluctuations and directional changes driven by agricultural disturbance. To effectively track variation processes of algal assemblages from fine to broad scales, we recommend a long-term monitoring duration (3 years minimum) with a high monitoring frequency (i.e., monthly). Moreover, both the mainstream and tributaries sites should be monitored simultaneously.
References
Allan, J. & M. Castillo, 2007. Stream Ecology: Structure and Function of Running Waters. Springer, New York.
Andrén, C. & A. Jarlman, 2008. Benthic diatoms as indicators of acidity in streams. Fundamental and Applied Limnology/Archiv für Hydrobiologie 173: 237–253.
Angeler, D. G., 2009. Species-specific and context-dependant disruption of temporal population fluctuations resulting from hypereutrophication events. Environmental Pollution 157: 3174–3182.
Angeler, D. G. & R. K. Johnson, 2012. Temporal scales and patterns of invertebrate biodiversity dynamics in boreal lakes recovering from acidification. Ecological Applications 22: 1172–1186.
Angeler, D. G., O. Viedma & J. M. Moreno, 2009. Statistical performance and information content of time lag analysis and redundancy analysis in time series modeling. Ecology 90: 3245–3257.
Angeler, D. G., C. Trigal, S. Drakare, R. K. Johnson & W. Goedkoop, 2010. Identifying resilience mechanisms to recurrent ecosystem perturbations. Oecologia 164: 231–241.
Angeler, D. G., S. Drakare & R. K. Johnson, 2011. Revealing the organization of complex adaptive systems through multivariate time series modeling. Ecology and Society 16: 5.
Baho, D. L., H. Peter & L. J. Tranvik, 2012. Resistance and resilience of microbial communities – temporal and spatial insurance against perturbations. Environmental Microbiology 14: 2283–2292.
Biggs, B., 1996. Patterns in benthic algae of streams. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego: 31–56.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632.
Borcard, D. & P. Legendre, 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153: 51–68.
Borcard, D., P. Legendre, C. Avois-Jacquet & H. Tuomisto, 2004. Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R. Springer, New York: 227–292.
Chinese National Environmental Protection Agency, 2002. Water and Wastewater Monitoring Methods, 4th ed. Chinese Environmental Science Publishing House, Beijing.
Collins, S. L., F. Micheli & L. Hartt, 2000. A method to determine rates and patterns of variability in ecological communities. Oikos 91: 285–293.
DeNicola, D., 1996. Periphyton responses to temperature at different ecological levels. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego: 149–181.
Graba, M., S. Sauvage, F. Y. Moulin, G. Urrea, S. Sabater & J. M. Sanchez-Pérez, 2013. Interaction between local hydrodynamics and algal community in epilithic biofilm. Water Research 47: 2153–2163.
Hering, D., A. Haidekker, A. Schmidt-Kloiber, T. Barker, L. Buisson, W. Graf, W. Grenouillet, A. Lorenz, L. Sandin & S. Stendera, 2010. Monitoring the responses of freshwater ecosystems to climate change. In Kernan, M. R., R. W. Battarbee & B. Moss (eds), Climate Change Impacts on Freshwater Ecosystems. Wiley-Blackwell, Oxford: 84–118.
Hu, H. & Y. Wei, 2006. The Freshwater Algae of China: Systematics. Science Press, Beijing, Taxonomy and Ecology.
Jao, C., 1988. Flora Algarum Sinigrum Aquae Dulcis (Tomus I): Zygnemataceae. Science Press, Beijing.
Kampichler, C. & H. P. van der Jeugd, 2013. Determining patterns of variability in ecological communities: time lag analysis revisited. Environmental and Ecological Statistics 20: 271–284.
Kennen, J. G., D. J. Sullivan, J. T. May, A. H. Bell, K. M. Beaulieu & D. E. Rice, 2012. Temporal changes in aquatic-invertebrate and fish assemblages in streams of the north-central and northeastern US. Ecological Indicators 18: 312–329.
Korhonen, J. J., J. Soininen & H. Hillebrand, 2010. A quantitative analysis of temporal turnover in aquatic species assemblages across ecosystems. Ecology 91: 508–517.
Korhonen, J. J., P. Köngäs & J. Soininen, 2013. Temporal variation of diatom assemblages in oligotrophic and eutrophic streams. European Journal of Phycology 48: 141–151.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Legendre, P. & O. Gauthier, 2014. Statistical methods for temporal and space–time analysis of community composition data. Proceedings of the Royal Society B: Biological Sciences 281: 20132728.
Legendre, P., D. Borcard, G. Blanchet & S. Dray, 2010a. PCNM: PCNM spatial eigenfunction and principal coordinate analyses. R package version 2.1/r82.
Legendre, P., M. D. Cáceres & D. Borcard, 2010b. Community surveys through space and time: testing the space-time interaction in the absence of replication. Ecology 91: 262–272.
Li, S. & L. Bi, 1998. Flora Algarum Sinicarum Aquae Dulcis (Tomus V): Ulothricales Ulvales Chaetophorales Trentepohliales Sphaeropleales. Science Press, Beijing.
Miller, M. P., J. G. Kennen, J. A. Mabe & S. V. Mize, 2012. Temporal trends in algae, benthic invertebrate, and fish assemblages in streams and rivers draining basins of varying land use in the south-central United States, 1993-2007. Hydrobiologia 684: 15–33.
Passy, S. I., 2006. Diatom community dynamics in streams of chronic and episodic acidification: the roles of environment and time. Journal of Phycology 42: 312–323.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86: 171–178.
Passy, S., 2008. Species size and distribution jointly and differentially determine diatom densities in US streams. Ecology 89: 475–484.
Passy, S. & C. Larson, 2011. Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microbial Ecology 62: 414–424.
Patrick, R. & C. W. Reimer, 1966. The Diatoms of the United States (exclusive of Alaska and Hawaii). Volume One: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculaceae. The Academy of Natural Sciences, Philadelphia.
Patrick, R. & C. W. Reimer, 1975. The Diatoms of the United States (Exclusive of Alaska and Hawaii), Vol. Two. Entomoneidaceae, Cymbellaceae, Gomphonemaceae, Epithemiaceae. The Academy of Natural Sciences, Philadelphia, Part One.
Philibert, A., P. Gell, P. Newall, B. Chessman & N. Bate, 2006. Development of diatom-based tools for assessing stream water quality in south-eastern Australia: assessment of environmental transfer functions. Hydrobiologia 572: 103–114.
Potapova, M. & D. F. Charles, 2003. Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshwater Biology 48: 1311–1328.
Qi, Y., 1995. Flora Algarum Sinicarum Aquae Dulcis (Tomus IV): Bacillariophyta Centreae. Science Press, Beijing.
Reid, M. A., M. C. Thoms & F. J. Dyer, 2006. Effects of spatial and temporal variation in hydraulic conditions on metabolism in cobble biofilm communities in an Australian upland stream. Journal of the North American Benthological Society 25: 756–767.
Rosemond, A. D., P. J. Mulholland & S. H. Brawley, 2000. Seasonally shifting limitation of stream periphyton: response of algal populations and assemblage biomass and productivity to variation in light, nutrients, and herbivores. Canadian Journal of Fisheries and Aquatic Sciences 57: 66–75.
Schneck, F. & A. S. Melo, 2013. High assemblage persistence in heterogeneous habitats: an experimental test with stream benthic algae. Freshwater Biology 58: 365–371.
Seeber, C., H. Hartmann & L. King, 2010. Land use change and causes in the Xiangxi catchment, Three Gorges Area derived from multispectral data. Journal of Earth Science 21: 846–855.
Shi, Z., 2004. Flora Algarum Sinicarum Aquae Dulcis (Tomus XII): Bacillariophyta Gomphonemacea. Science Press, Beijing.
Smucker, N. J. & M. L. Vis, 2011. Acid mine drainage affects the development and function of epilithic biofilms in streams. Journal of the North American Benthological Society 30: 728–738.
Soininen, J. & P. Eloranta, 2004. Seasonal persistence and stability of diatom communities in rivers: are there habitat specific differences? European Journal of Phycology 39: 153–160.
Stenger-Kovács, C., E. Lengyel, L. O. Crossetti, V. Üveges & J. Padisák, 2013. Diatom ecological guilds as indicators of temporally changing stressors and disturbances in the small Torna-stream, Hungary. Ecological Indicators 24: 138–147.
Stevenson, J., 2014. Ecological assessments with algae: a review and synthesis. Journal of Phycology 50: 437–461.
Stevenson, R. J. & L. L. Bahls, 1999. Periphyton protocols. In Barbour, M. T., J. Gerritsen, B. D. Snyder & J. B. Stribling (eds), Rapid Bioassessment Protocols for Use in Streams and Rivers: Periphyton, Benthic Macroinvertebrates, and Fish, 2nd ed. U.S. Environmental Protection Agency; Office of Water, Washington, DC: 1–23.
Tan, X., X. Xia, Q. Zhao & Q. Zhang, 2014. Temporal variations of benthic diatom community and its main influencing factors in a subtropical river, China. Environmental Science and Pollution Research 21: 434–444.
Tang, T., 2003. Studies on Charasteristics of Epilithic Algae and Ecosystem Management of the Xiangxi River, PhD dissertation, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan.
Tang, T., S. Niu & D. Dudgeon, 2013a. Responses of epibenthic algal assemblages to water abstraction in Hong Kong streams. Hydrobiologia 703: 225–237.
Tang, T., N. Wu, F. Li, X. Fu & Q. Cai, 2013b. Disentangling the roles of spatial and environmental variables in shaping benthic algal assemblages in rivers of central and northern China. Aquatic Ecology 47: 453–466.
Tornés, E., V. Acuña, C. N. Dahm & S. Sabater, 2015. Flood disturbance effects on benthic diatom assemblage structure in a semiarid river network. Journal of Phycology 51: 133–143.
Virtanen, L. K., P. Köngäs, S. Aitto-Oja & J. Soininen, 2011. Is temporal occurrence of diatoms related to species traits, local abundance, and regional distribution? Journal of Phycology 47: 1445–1453.
Wang, J., B. Wang & Z. Luo, 1997. Dictionary of the Yangtze River. Wuhan Press, Wuhan.
Wood, S., 2014. Package ‘mgcv’. Online at http://cran.r-project.org/web/packages/mgcv/mgcv.pdf.
Yang, G., T. Tang & D. Dudgeon, 2009. Spatial and seasonal variations in benthic algal assemblages in streams in monsoonal Hong Kong. Hydrobiologia 632: 189–200.
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Prentice Hall Inc., New Jersey.
Zhu, H., 2007. Flora Algarum Sinicarum Aquae Dulcis (Tomus IX): Cyanophyta Hormogonophyceae. Science Press, Beijing.
Zuur, A., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgements
We thank Xiaodong Qu, Naicheng Wu, and Xiaocheng Fu for fieldwork assistance and Ruiqiu Liu for chemical analyses. We are grateful to Yangdong Pan for valuable comments on the early draft of this manuscript and Alissa Cohen for English improvement. This research was funded by the National Natural Science Foundation of China (No. 31470510), Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07104-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, T., Jia, X., Jiang, W. et al. Multi-scale temporal dynamics of epilithic algal assemblages: evidence from a Chinese subtropical mountain river network. Hydrobiologia 770, 289–299 (2016). https://doi.org/10.1007/s10750-015-2603-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2603-8