Abstract
Amphibian larvae are highly susceptible to contamination, which can lead to lethal and sublethal effects. This impact can be measured by fluctuating asymmetry (FA), which is based on differences between the sides of organisms with bilateral symmetry. We evaluated the effect of acute and chronic exposure to Roundup Original® on Physalaemus cuvieri tadpoles. We measured tadpole survival and estimated the LC5096h. We also evaluated whether a sublethal concentration increases the FA. In acute exposure, survival was reduced and the LC50 was 2.13 mg a.i./l. In chronic exposure, nostril–snout distance and eye width had a significantly higher FA in contaminated tadpoles. The chronic exposure to contaminants could lead to several sublethal effects, which would be used in biomonitoring surveys. Morphological traits affected by contaminants, such as malformations or FA, would be relatively more easily measured from field samples. Because it is cost effective, easy to measure, and can be obtained without tagging or housing field-caught animals, we suggest that FA is a promising marker for monitoring the environmental impacts of contaminants like Roundup. However, additional studies are necessary to understand what additional environmental stressors might impact FA, and how this might alter its utility for use in biomonitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive agriculture plantations demand high levels of agrochemicals use, resulting in large-scale environmental contamination. This contamination can potentially accelerate population declines of native species, which are, in general, non-target organisms of the use of agrochemicals (Davidson et al., 2001, 2002; Sparling et al., 2001; Clay, 2004; Relyea, 2005a; Schiesari et al., 2007; Relyea & Jones, 2009; Schiesari & Grillitsch, 2011). Glyphosate (the active ingredient of Roundup®, Vision® and others) is a non-selective herbicide that is highly effective against weeds and is used on the majority of crops in Brazil (Amarante Jr. et al., 2002). Its commercial formula also includes a surfactant (e.g., polyethoxylated tallow amine—POEA), which increases its toxicity (Giesy et al., 2000). Glyphosate is designed for terrestrial use and is considered to be inactive when absorbed by the soil, although this process is not completely understood (Amarante Jr. et al., 2002). However, several surveys have detected glyphosate, including formulations with POEA, in aquatic environments and associated with riparian vegetation, which probably originated from overspray in aerial application, lixiviation, and overland flow (e.g., Newton et al., 1984; Goldsborough & Beck, 1989; Feng et al., 1990; Giesy et al., 2000; Davidson et al., 2001, 2002; Thompson et al., 2004; Queiroz et al., 2011). According to Giesy et al. (2000), glyphosate and POEA can remain active in water from between 7 and 70 days, depending on the environmental conditions.
Fluctuating asymmetry (FA) can be used to evaluate the effect of stressful external factors (e.g., contamination, environmental disturbance) on the developmental stability of individuals (Palmer & Strobeck, 1986; Clarke, 1993; Sanseverino & Nessimian, 2008). This method is based on the observation of random and non-directional deviations in the theoretical model of perfect symmetry of morphological traits in bilateral organisms (Van Valen, 1962; Palmer & Strobeck, 1986; Sanseverino & Nessimian, 2008). High levels of environmental stress can significantly increase deviations in the symmetry of traits (Clarke, 1993; Hogg et al., 2001), making FA a useful tool for biomonitoring (Clarke, 1993; Johnson et al., 1993; Sanseverino & Nessimian, 2008). Although some studies have found no relationship between an increase in FA and environmental stress (Forbes et al., 1997; Stige et al., 2004; Reis et al., 2011), Beasley et al. (2013) demonstrated that FA is a sensitive biomarker of environmental stress. Furthermore, many studies have confirmed this relationship in different taxa, such as dragonflies (Hardersen & Frampton, 1999; Chang et al., 2007), mammals (Badyaev et al., 2000), and amphibians (Söderman et al., 2007; Delgado-Acevedo & Restrepo, 2008).
Amphibians are the most threatened vertebrate group and have the highest rates of population decline in the world (Stuart et al., 2004). Numerous studies have suggested that contaminants can impact amphibians at multiple levels of biological organization (Boone et al., 2007; Schiesari et al., 2007; Egea-Serrano et al., 2012), although the mechanisms are diverse and sometimes difficult to ascertain. Characteristics such as permeable skin and water dependency increase the susceptibility to pollutants (Schiesari et al., 2007; Allentoft & O’Brien, 2010), especially for species with indirect development (Altig & McDiarmid, 1999a, b). Tadpoles have high phenotypic plasticity and can rapidly respond to environmental changes (Alford, 1999), such as contaminant exposure (e.g., Bridges, 1999; Griffis-Kyle, 2005, 2007; Relyea, 2005a, b, c; Snodgrass et al., 2008; Jones et al., 2010; Relyea, 2012; Lajmanovich et al., 2013). According to Relyea (2012), the majority of studies that have investigated contaminant effects on tadpoles were based on experiments with a single species in a short time period (e.g., 1–4 days), resulting in a lack of empirical evidence of contaminant impact over a long exposure time. This chronic exposure can occur at different levels of disturbance, leading to sublethal effects, such as changes in behavior and ontogenetic traits (e.g., Bridges, 1997, 1999, 2000; Griffis-Kyle, 2007; Shin et al., 2008; Snodgrass et al., 2008; Relyea, 2012).
The geographic distribution of ecotoxicological studies with amphibians is far from uniform. As observed by Schiesari et al. (2007), the Neotropical region contains the largest number of amphibian species and has the highest rates of population decline, but it is the region with the lowest number of species considered in ecotoxicological studies. This observation is quite realistic, especially in Brazil, where information concerning the effect of glyphosate and other contaminants on amphibians is lacking. Different anuran species have different levels of contaminant sensitivity (Boone et al., 2007) and can respond differently to glyphosate concentrations (Relyea & Jones, 2009). Thus, the evaluation of lethal and sublethal effects of contaminants is highly necessary for native species in Brazil, especially in the Cerrado biome, which is considered a biodiversity hotspot (Myers et al., 2000) and has the highest potential for degradation and agricultural expansion (Diniz-filho et al., 2007; Klink & Machado, 2007; Schiesari & Grillitsch, 2011).
Here, we tested the effects of acute and chronic exposure to a commercial formulation of glyphosate (Roundup Original®) on tadpoles of Physalaemus cuvieri (Fitzinger, 1826). We evaluated the survival rates at increasing concentrations of the active ingredient (i.e., acute exposure) and estimated the LC5096h. We also evaluated whether chronic exposure to glyphosate can result in a higher FA in five morphological traits of P. cuvieri tadpoles.
Materials and methods
Sample and study system
Physalaemus cuvieri is a very common species found throughout Brazil, and occurs in landscapes with different degrees of agriculture disturbance (IUCN, 2014). On 22 March, 2013, we collected four egg masses from two different ponds (pond 1, three egg masses: 16°34′40.59″S, 48°56′00.82″W; pond 2, one egg mass: 16°39′42.25″S, 48°49′11.40″W) from Bonfinópolis municipality, Goiás, Brazil. These ponds are very similar in physical structure, with a high percentage of vegetation on their margins, and a low anthropogenic disturbance in the surrounding matrix. The egg masses were transported in plastic bags with water to a laboratory in the Federal University of Goiás, in the state of Goiás, Brazil, where all egg masses were combined in a glass tank (60 cm × 40 cm × 40 cm) with 8 l of dechlorinated water until the tadpoles hatched. All tadpoles used in the experiments described below were randomly selected from this glass tank.
The experiments were carried out in laboratory conditions, with a controlled air temperature (28 ± 2°C) and photoperiod (12 h light/12 h dark). For both experiments, we used a commercial glyphosate formula (Roundup Original®) with 48% glyphosate. In the majority of ecotoxicological studies, the glyphosate concentrations are shown in mg a.i./l (a.i. = active ingredient) or mg a.e./l (a.e. = acid equivalent), where 1 mg a.i./l is equivalent to 0.75 mg a.e./l (Relyea, 2006). We used the notation mg a.i./l and determined the concentrations based on CONAMA 357 (2005), which permits a mean glyphosate concentration of 280 µg/l (i.e., 0.28 mg a.i./l) in class III freshwater types in Brazil. To determine higher concentrations for experimental treatments, we increased the glyphosate concentrations by uniform increments based on recent studies of toxicity in tadpoles (Relyea, 2012; Lajmanovich et al., 2013; Simioni et al., 2013). All glyphosate concentrations were calculated using the informed quantity of glyphosate included in the Roundup Original® formulation, as presented in the leaflet of the product.
As experimental units, we used glass aquaria with 2 l of dechlorinated water, without substrates and with aquarium air compressors. In both experiments, the tadpoles were acclimatized in these aquaria for 24 h before exposure to herbicide. During the experimental trials, the tadpoles were fed ad libitum every 2 days with ornamental fish food and tadpoles found to be dead were removed from the aquaria every 24 h. Surviving tadpoles were sacrificed with benzocaine solution (300 mg/l) and all tadpoles were preserved in 10% formalin. The specimens were deposited in the Herpetological Collection of the Federal University of Goiás/ZUFG (ZUFG 1509: tadpoles of acute exposure/ZUFG 1756 and ZUFG 1757: tadpoles of chronic exposure).
Acute exposure experiment
We maintained the tadpoles in the storage tank until they reached developmental stage 25 (sensu Gosner, 1960). Subsequently, we randomly collected 10 tadpoles for each experimental unit. We prepared treatment-specific solutions with five nominal concentrations (Control = 0 mg a.i./l; T1 = 0.38 mg a.i./l; T2 = 2 mg a.i./l; T3 = 4 mg a.i./l; T4 = 6 mg a.i./l) that were applied in a single pulsed dose in each experimental unit. Considering that each experimental unit contains 2 L of water, we added 25, 16.66, 8.32, and 1.55 µl of Roundup Original® to treatments to represent the nominal concentrations 6, 4, 2, and 0.38 mg a.i./l, respectively; in the control, we added 25 µl of water. We replicated each treatment nine times, totaling 45 experimental units and 450 tadpoles. The tadpoles were exposed to the herbicide for 96 h (4 days) and, during this period, we removed any dead tadpoles every 24 h and recorded the survival rates for each treatment. There was a little variation in water temperature (range = 22.9–23.7°C) and pH (range = 7.0–7.2) between treatments.
Chronic exposure experiment
The tadpoles were maintained in the storage tank for 22 days for growth. After this period, we randomly collected five tadpoles to assign to each experimental unit and applied a Control (0 mg a.i./l) and a Chronic (2 mg. a.i./l) treatment. These treatments were established by adding 8.32 µl of water to the Control and 8.32 µl of Roundup to the Chronic. Solutions were applied by a single pulsed dose without the renewal or replacement of water in aquaria. The lower concentration of glyphosate in the Chronic treatment (i.e., lower than LC50) ensured a reduced mortality rate and allowed an increased exposure time to the herbicide, allowing us to evaluate the herbicide effect on morphology. The tadpoles remained exposed to the experimental treatments for 15 days. We replicated each treatment 10 times, totaling 20 experimental units and 50 tadpoles were exposed to each treatment. There was no difference in the pH of the water (Chronic = 7.175 ± 0.259; Control = 7.135 ± 0.218, range = 6.4–7.5; t = −0.181, d.f. = 18, P = 0.858) and water temperature (Chronic = 23.875 ± 0.267°C; Control = 23.830 ± 0.267°C, range = 23.2–24.3°C; t = −0.408, d.f. = 18, P = 0.687) between treatments.
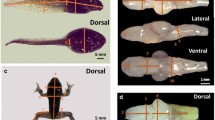
After 15 days, we randomly collected 30 surviving tadpoles from the control (Control) and 30 surviving tadpoles from the treatment (Chronic). Each tadpole was positioned against a millimeter ruler in a Petri-dish using ultrasound gel, and submerged in water. We then obtained images in a dorsal view with a Sony α230, 10.2 megapixel camera, equipped with an ocular macro Sigma Zoom 24–70 mm lens, supported on a tripod at a height of 30 cm. We measured five bilateral morphological traits for each tadpole to calculate the FA indices (Fig. 1), with ImageJ 1.46r software. We also measured the total length (TL) and development stage of tadpoles (Gosner, 1960).
Morphological traits used to calculate the fluctuating asymmetry indices (solid lines). NSD nostril–snout distance, ESD eye–snout distance, EL eye length, EW eye width; the variable RPN (relative position of the nostrils) is obtained by the equation: PRN = ESD/NSD. Dashed lines demonstrate an example of asymmetric (right) and symmetric (left) individuals for NSD
Statistical analyses
In the acute exposure experiment, we performed an ANOVA using the survival rate of tadpoles as a response variable and the concentration level of glyphosate as an experimental factor, followed by an a posteriori Tukey test, to verify the prediction that a higher glyphosate concentration increases tadpole mortality. To estimate the LC5096h value, we used Probit regression analyses (Bliss, 1935; Fisher, 1935).
We assessed the glyphosate effect in the chronic exposure experiment by measuring FA of P. cuvieri tadpoles. We opted to use FA as a measure of environmental stress (Beasley et al., 2013) because FA can be calculated as an instant measure, i.e., an investigator can collect a tadpole in the field and estimate the FA value without housing the specimens. We used the index FA = (R − L) (Palmer & Strobeck, 1986), where R and L represent the right and left sides, respectively. The outlier values, either negative or positive, were kept, because these are expected in FA studies and can have biological significance (Palmer & Strobeck, 1986; Leung & Forbes, 1997; Hardersen, 2000). Following the approach suggested by Palmer & Strobeck (1986), we repeated all measurements of morphological traits three times, separated by at least 1 month between measurement sessions and applied a general linear mixed model, using each morphological trait as a response variable, with side as a fixed factor and individuals as a random factor to estimate human measurement errors. We estimated the variation of random factors (variance component of the model) according to the ANOVA method (Searle et al., 1992), which provides an estimate for the variance of random factors, for the variance in the dependent variable affected by random factors, and also to test whether variance components were different from zero. This analysis was applied to control and measure the potential contribution of confounding factors (measurement errors, directional asymmetry, and anti-symmetry) on the variation between the left and right sides of the studied organism (Palmer & Strobeck, 1986), increasing the reliability of the AF index to hypothesis test (Palmer & Strobeck, 1986). If our general mixed model did not show any significant error in measurements, we tested which type of FA each morphological variable presents using a single sample Student’s t test to verify whether the means differed significantly from zero (Palmer & Strobeck, 1986). A Kolmogorov–Smirnov test (K–S) was used to evaluate whether the FA indices were normally distributed and a Spearman Correlation test to verify whether FA indices were independent of the TL and developmental stage of the tadpoles.
Our hypothesis that the chronic exposure to glyphosate increases the FA was tested using the module of FA index [FA = (|R − L|)], to include only the absolute values of the differences between right and left sides in each trait. Finally, a Student’s t test with separate variance estimates was used to test the prediction that developmental deviations (i.e., the module of FA index) for each trait were higher in tadpoles submitted to chronic exposure to glyphosate (Chronic) than in those tadpoles not exposed to glyphosate (Control). These analyses were performed according to Zar (1999).
Results
The effect on survival
When exposed to acute concentrations of glyphosate, the survival rates of P. cuvieri tadpoles decreased [F(4) = 3.945, P = 0.008, d.f.error = 38], mainly in the highest concentration treatment (T4), where survival was reduced to approximately 25% when compared to the control (P Tukey HSD = 0.004) (Fig. 2). The estimated LC5096h for P. cuvieri was 2.13 mg a.i./l.
The effect on Fluctuating Asymmetry
In this experiment, no mortality was observed in the control. However, seven tadpoles died in the chronic treatment during the 15 days of exposure. Tadpoles did not differ in total length [TLChronic = 23.388 mm (range: 29.631–15.815), TLControl = 24.015 mm (range: 28.731–19.302), t = 0.912, d.f. = 57, P = 0.365] or in developmental stage [DevChronic = 35.76 (range: 39–33), Devcont = 35.89 (range: 39–30), t = 0.266, d.f. = 57, P = 0.791] between treatments.
The FA results are shown in Table 1. For all traits, the means were not significantly different from zero and we assumed that all traits displayed FA, because they showed a small, random and non-directional variation between symmetry planes. The FA indices were normally distributed. No correlation was observed between the FA indices and TL, indicating that the measurements are independent of tadpole size. Similarly, no correlation was observed between FA indices and the development stage of tadpoles. The absence of correlation invalidated the need to calculate FA indices relative to body size and developmental stage. We observed that the measurement error was significantly smaller than the FA (Table 2), which increases the reliability of the measurements. We observed no directional asymmetry (i.e., no difference between sides) and no anti-symmetry (i.e., FA is independent of the individual and the deviations are random). Tadpoles exposed to the herbicide had a higher FA in the nostril–snout distance (NSD) and eye width (EW) than control tadpoles (NSD: t separate variances = −3.365, d.f. = 57, P = 0.001, Fig. 3; EW: t separate variances = −2.233, d.f. = 57, P = 0.029, Fig. 4).
Discussion
We observed lethal and sublethal effects of glyphosate contamination (i.e., Roundup Original®) on P. cuvieri tadpoles. The survival rate following acute exposure was reduced in tadpoles in stage 25, but only showed statistical significance at a high glyphosate concentration (T4 = 6 mg a.i./l). The reduction in survival was observed in two congeneric species of P. cuvieri. Simioni et al. (2013) exposed tadpoles of P. albonotatus to sublethal concentrations of Gliz 480 SL (25, 50, and 75% of LC50) and observed a reduction in survivorship at higher glyphosate concentrations. Figueiredo & Rodrigues (2014) observed a similar effect for P. centralis on mortality in chronic glyphosate concentrations (25, 50, and 75% of LC50), although the mortality was always significantly higher than that of the control treatment. Worldwide, records of contamination levels in freshwater environments, including streams, lakes and wetlands, range from 1.4 to 7.6 mg a.e./l (Edwards et al., 1980; Mann & Bidwell, 1999; Giesy et al., 2000; Solomon & Thompson, 2003; Thompson et al., 2004). Brazil is the largest pesticide consumer in the world, but few studies have evaluated the presence of pesticides in water carried because of inappropriate application, resulting in a lack of official records regarding glyphosate use (ABRASCO, 2012). For different contaminants, such as Atrazine and Monocrotophos, concentrations ranging from 0.01 to 75.43 µg/l have been reported in rivers, lakes, artesian wells, and rainwater (Bortoluzzi et al., 2006, Silva et al., 2009, Moreira et al., 2012) as a consequence of the inappropriate use of agrochemicals. In some Brazilian regions, this amount of contamination can be related to a pesticide and herbicide use that is up to 3.2 times higher than the global mean (Pignati & Machado, 2007). Thus, it is not unrealistic for tadpoles to be exposed to glyphosate contamination levels comparable to the concentrations used in our experiments and in toxicity bioassays conducted with amphibian eggs and tadpoles in the wild (e.g., Mann & Bidwell, 1999; Lajmanovich et al., 2003; Relyea, 2005b, c; Relyea & Jones, 2009; Jones et al., 2010, 2011; Relyea, 2012; Simioni et al., 2013; Figueiredo & Rodrigues, 2014).
The LC5096h for P. cuvieri was 2.13 mg a.i./l, which is considered moderately toxic according to glyphosate toxicity classification on aquatic organisms (Giesy et al., 2000; U.S.EPA., 2008) and was lower than that recorded for other congeneric species (P. albonotatus, LC5096h = 5.38 mg a.i./l, moderately toxic, Simioni et al., 2013; P. centralis, LC5096h = 19.7 mg a.i./l, slightly toxic, Figueiredo & Rodrigues, 2014). These differences in LC50 values might highlight interspecific differences in the tolerance to contamination levels (Bridges & Semlitsch, 2001; Simioni et al., 2013), but one component of the variation might be due to methodological variation and differences in the formula of the herbicide used in the bioassay, complicating the designation of which species are more tolerant to contamination (Mann et al., 2009; Simioni et al., 2013). For example, different commercial formulations of glyphosate (such as Roundup Ultra Max®, Gliz 480 SL and Glyphosate 480 Agripec®), used in bioassay studies (e.g., Lajmanovich et al., 2011; Simioni et al., 2013; Figueiredo & Rodrigues, 2014) with different concentrations of surfactant substances, could interact with glyphosate, affecting its toxicity (Relyea, 2006). Thus, we were able to find studies with different formulations of POEA that reported glyphosate effects varying from highly toxic (0.1–1 mg a.i./l) to slightly toxic (10–100 mg a.i./l) for amphibian larvae (e.g., Mann & Bidweel, 1999; Relyea, 2005a; Relyea & Jones, 2009; Relyea, 2012; Lajmanovich et al., 2013). Differences in the number of individuals per treatment unit, together with variations in laboratory conditions and nominal concentrations of glyphosate applied to each treatment (e.g., Mann & Bidweel, 1999; Relyea, 2005a; Relyea & Jones, 2009; Relyea, 2012; Lajmanovich et al., 2011, 2013) are also confounding factors that limit comparisons among studies and increase the difficulty of delineating general implications of glyphosate impacts on non-target species, such as tadpoles.
Most analyses of water contamination reflect the dynamic chemical and physical conditions of water bodies, and exclude the temporal and biological responses of organisms. The non-selectivity and frequency of agrochemical application can increase the persistence of toxic substances in the aquatic environment, submitting amphibian species to chronic exposure throughout larval development (Bridges, 2000; Jones et al., 2010). This chronic exposure can cause a reduction in growth (Jones et al., 2010), external malformations (Lajmanovich et al., 2003), reduce hatching success and delay metamorphosis (Griffis-Kyle, 2005, 2007). However, many of these sublethal effects of chronic exposure to contaminants, such as reduction in growth, reduced hatchling success, and delayed metamorphosis, would require tagged individuals in the field to be used as environmental evaluation tools, because they are rate measurements or require more than one measurement during an arbitrary time frame. Conversely, morphological traits, especially those that were demonstrate to be affected by contaminants, such as malformations and FA, would be relatively more easily measured from field samples. Here, we observed an increase in deviations in ontogenetic development in the bilateral characteristics of P. cuvieri tadpoles as a consequence of chronic exposure to Roundup Original®, especially in NSD and EW. These morphological traits are associated with the sensory capabilities of tadpoles and, as suggested by Bosch & Márquez (2000), FA in a sensory structure can affect an individual’s fitness and reduce survival or reproduction rates. In a theoretical population, developmental stability can be used as an indirect estimation of fitness (Clarke, 1995), despite the difficulty in measuring how developmental homeostasis contributes to population fitness (Clarke, 1995; Møller, 1997). We suggest that the increase in developmental deviations of sensory traits affects tadpole fitness, which leads to a reduction in their competitive potential and increased predation risk, although further empirical studies are required to confirm this hypothesis.
Characteristics such as high abundance, a wide distribution, a resolved taxonomy, and low dispersal ability increase the potential of an organism as a bioindicator (Hellawell, 1986; Rainio & Niemelä, 2003). This potential is greater in species that respond to environmental stress via changes in morphological attributes (Johnson et al., 1993). Amphibians are good bioindicators of environmental stress, but their effectiveness can vary among species and with the type of stress (Blaustein, 1994; Blaustein & Wake, 1995). Also, the response variable to be measured from the bioindicator organism should be carefully selected. Ideally, we can assume that a target variable should be relatively easy to be measured and to be taught how to measure, have low cost and be a direct consequence of the impact being evaluated. Physalaemus cuvieri is widely distributed in South America, within stable and abundant populations (Frost, 2014; IUCN, 2014), and is commonly found in ponds that are directly affected by crops and pastures. We observed that long-term exposure to glyphosate contamination can result in an increase in FA values of P. cuvieri within a relatively short time period. This effect of contamination on tadpoles of P. cuvieri, associated with the facility and low operating costs of measuring FA (Clarke, 1993), make these tadpoles a useful and economic approach compared to physiological and genetic approaches, to evaluate the impacts of anthropogenic disturbance on aquatic environments. Also, FA can represent a reliable measurement of environmental impact that does not have to tag individuals during biomonitoring surveys, which simplifies specimen manipulation. However, additional studies are required to understand how multiple stressors could affect FA and, for that, we highlight the need for the standardization of an experimental protocol and the expansion of ecotoxicological studies with FA (Schiesari et al., 2013; Simioni et al., 2013) to increase the capacity of interspecific comparisons and to provide a scientific foundation for new aquatic environment protection laws.
References
ABRASCO, 2012. Um alerta sobre os impactos dos agrotóxicos na saúde. Parte 1 – Agrotóxicos, Segurança Alimentar e Nutricional e Saúde. Dossiê ABRASCO, Associação Brasileira de Saúde Coletiva, Rio de Janeiro, RJ.
Alford, R. A., 1999. Ecology: resource use, competition, and predation. In McDiarmid, R. W. & R. Altig (eds), Tadpoles. The Biology of Anuran Larvae. University of Chicago Press, Chicago: 240–278.
Allentoft, M. E. & J. O’Brien, 2010. Global amphibian declines, loss of genetic diversity and fitness: a review. Diversity 2: 47–71.
Altig, R. & R. W. McDiarmid, 1999a. Diversity: familial and generic characterizations. In McDiarmid, R. W. & R. Altig (eds), Tadpoles. The Biology of Anuran Larvae. University of Chicago Press, Chicago: 295–337.
Altig, R. & R. W. McDiarmid, 1999b. Body plan: development and morphology. In McDiarmid, R. W. & R. Altig (eds), Tadpoles. The Biology of Anuran Larvae. University of Chicago Press, Chicago: 24–51.
Amarante Jr, O. P., T. C. R. Santos, N. M. Brito & M. L. Ribeiro, 2002. Glifosato: propriedades, usos e legislação. Química Nova 25(4): 589–593.
Badyaev, A. V., K. R. Foresman & M. V. Fernandes, 2000. Stress and developmental stability: vegetation removal causes increased fluctuating asymmetry in shrews. Ecology 81: 336–345.
Beasley, D. A. E., A. Bonisoli-Alquati & T. A. Mousseau, 2013. The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: a meta-analysis. Ecological Indicators 30: 218–226.
Blaustein, A. R., 1994. Chicken Little or Nero’s Fiddle? A perspective on declining amphibian populations. Herpetologica 50(1): 85–97.
Blaustein, A. R. & D. B. Wake, 1995. The puzzle of declining amphibian populations. Scientific American 272: 52–57.
Bliss, C. I., 1935. The calculator of the dosage-mortality curve. Annals of Applied Biology 22: 134–167.
Boone, M. D., D. Cownan, C. Davidson, T. B. Hayes, W. A. Hopkins, R. A. Relyea, L. Schiesari & R. Semlistch, 2007. Evaluating the role of environmental contamination in amphibian population decline. In Gascon, C., J. P. Collins, R. D. Moore, D. R. Church, J. E. McKay & J. R. Mendelson III (eds), Amphibian Conservation Action Plan. Proceedings: IUCN/SSC Amphibian Conservation Summit 2005. The World Conservation Union (IUCN), Gland: 32–35.
Bortoluzzi, E. C., D. S. Rheinheimer, C. S. Gonçalves, J. B. R. Pellegrini, R. Zanella & A. C. C. Copetti, 2006. Contaminação de águas superficiais por agrotóxicos em função do uso do solo numa microbacia hidrográfica de Agudo, RS. Revista Brasileira de Engenharia Agrícola e Ambiental 10(4): 881–887.
Bosch, J. & R. Márquez, 2000. Tympanum fluctuating asymmetry, body size and mate choice in female Midwife Toads (Alytes obstreticans). Behaviour 137: 1211–1222.
Bridges, C. M., 1997. Tadpole swimming performance and activity affected by acute exposure to sublethal levels of carbaryl. Environmental Toxicology and Chemistry 19: 1935–1939.
Bridges, C. M., 1999. Effects of a pesticide on tadpole activity and predator avoidance behavior. Journal of Herpetology 33: 303–306.
Bridges, C. M., 2000. Long-term effects of pesticide exposure at various life stages of the Southern Leopard frog (Rana sphenocephala). Archives of Environmental Contamination and Toxicology 39: 91–96.
Bridges, C. M. & R. D. Semlitsch, 2001. Genetic variation in insecticide tolerance in a population of Southern Leopard Frogs (Rana sphenocephala): implication for amphibian conservation. Copeia 1: 7–13.
Chang, X., B. Zhai, M. Wang & B. Wang, 2007. Relationship between exposure to an insecticide and fluctuating asymmetry in a damselfly (Odonata, Coenagriidae). Hydrobiologia 586: 213–220.
Clarke, G. M., 1993. Fluctuating asymmetry of invertebrate populations as a biological indicator of environmental quality. Environmental Pollution 82: 207–211.
Clarke, G. M., 1995. Relationships between developmental stability and fitness: application for conservation biology. Conservation Biology 9(1): 18–24.
Clay, J., 2004. World Agriculture and the Environment. A Commodity-by-commodity Guide to Impacts and Practices. Island Press, Washington, DC.
CONAMA 357, 2005. Conselho Nacional do Meio Ambiente. Resolução no. 357, de 17 de Março de 2005. Publicada no DOU no. 053, de 18/03/2005: 58–63.
Davidson, C., H. B. Shaffer & M. R. Jennings, 2001. Declines of the California red-legged frog: climate, UV-B, habitat, and pesticides hypotheses. Ecological Applications 14: 464–479.
Davidson, C., H. B. Shaffer & M. R. Jennings, 2002. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conservation Biology 16: 1588–1601.
Delgado-Acevedo, J. & C. Restrepo, 2008. The contribution of habitat loss to changes in body size, allometry, and bilateral asymmetry in two Eleutherodactylus frogs from Puerto Rico. Conservation Biology 22(3): 773–782.
Diniz-Filho, J. A. F., L. M. Bini, M. P. Pinto, T. F. L. V. B. Rangel, P. Carvalho, S. L. Vieira & R. P. Bastos, 2007. Conservation biogeography of anurans in Brazilian Cerrado. Biodiversity and Conservation 16: 997–1008.
Edwards, W. M., G. B. Triplett Jr & R. M. Kramer, 1980. A watershed study of glyphosate transport in runoff. Journal of Environmental Quality 9(4): 661–665.
Egea-Serrano, A., R. A. Relyea, M. Tejedo & M. Torralva, 2012. Understanding of the impact of chemicals on amphibians: a meta-analytic review. Ecology and Evolution 2(7): 1382–1397.
Feng, J. C., D. G. Thompson & P. E. Reynolds, 1990. Fate of glyphosate in a Canadian forest watershed. 1. Aquatic residues and off-target deposit assessment. Journal of Agriculture and Food Chemistry 38: 1110–1118.
Figueiredo, J. & D. J. Rodrigues, 2014. Effects of four types of pesticides on survival, time and size to metamorphosis of two species of tadpoles (Rhinella marina and Physalaemus centralis) from the southern Amazon, Brazil. Herpetological Journal 24: 1–9.
Fisher, R. A., 1935. Appendix to bliss (1935): the case of zero survivors. Annals of Applied Biology 22: 164–165.
Forbes, M., B. Leung & G. Schalk, 1997. Fluctuating asymmetry in Coenagrion Resolutum (Hagen) in relation to age and male pairing success (Zygoptera: Coenagrionidae). Odonatologica 26: 9–16.
Frost, D. R., 2014. Amphibian Species of the World: An Online Reference, Version 5.6. Accessible at http://research.amnh.org/herpetology/amphibian. Downloaded on 15 January 2014.
Giesy, J. P., S. Dobson & K. R. Solomon, 2000. Ecotoxicological risk assessment for Roundup® herbicide. Reviews of Environmental Contamination and Toxicology 167: 35–120.
Goldsborough, L. G. & A. E. Beck, 1989. Rapid dissipation of glyphosate in small forest ponds. Archives of Environmental Contamination and Toxicology 18: 537–544.
Gosner, K. L., 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190.
Griffis-Kyle, K. L., 2005. Ontogenetic delays in effects of nitrite exposure on tiger salamanders (Ambystoma tigrinum tigrinum) and wood frogs (Rana sylvatica). Environmental Toxicology and Chemistry 24(6): 1523–1527.
Griffis-Kyle, K. L., 2007. Sublethal effects of nitrite on eastern tiger salamander (Ambystoma tigrinum tigrinum) and wood frog (Rana sylvatica) embryos and larvae: implications for filed populations. Aquatic Ecology 41: 119–127.
Hardersen, S., 2000. Effects of carbaryl exposure on the last larval instar of Xanthocnemis zealandica. fluctuating asymmetry and adult emergence. Entomologia Experimentalis et Applicata 96: 221–230.
Hardersen, S. & C. M. Frampton, 1999. Effects of short term pollution on the level of fluctuating asymmetry – a case study using damselflies. Entomologia Experimentalis et Applicata 92: 1–7.
Hellawell, J. M., 1986. Biological Indicators of Freshwater Pollution and Environmental Management, Vol 44. Elsevier, New York: p. 546.
Hogg, I. D., J. M. Eadie, D. D. Willians & D. Turner, 2001. Evaluating fluctuating asymmetry in a stream-dwelling insect as an indicator of low-level thermal stress: a large-scale field experiment. The Journal of Applied Ecology 38(6): 1326–1339.
IUCN, 2014. The IUCN Red List of Threatened Species, Version 2014.3. Accessible at http://www.iucnredlist.org. Downloaded on 26 January 2014.
Johnson, R. K., T. Weiderholm & D. M. Rosenberg, 1993. Freshwater biomonitoring using individual organisms, populations and species assemblages of benthic macroinvertebrates. In Rosenberg, D. M. & V. H. Resh (eds), Freshwater Biomonitoring and Benthic Macroinvertebrates. Chapman and Hall, New York: 40–105.
Jones, D. K., J. I. Hammond & R. A. Relyea, 2010. Roundup® and amphibians: the importance of concentration, application time, and stratification. Environmental Toxicology and Chemistry 29(9): 2016–2025.
Jones, D. K., J. I. Hammond & R. A. Relyea, 2011. Competitive stress can make the herbicide Roundup® more deadly to larval amphibians. Environmental Toxicology and Chemistry 30(2): 446–454.
Lajmanovich, R. C., M. T. Sandoval & P. M. Peltzer, 2003. Induction of mortality and malformation in Scinax nasicus tadpoles exposed by glyphosate formulations. Bulletin of Environmental Contamination and Toxicology 70: 612–618.
Lajmanovich, R. C., A. M. Attademo, P. M. Peltzer, C. M. Junges & M. Cabagna, 2011. Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: B-esterases and glutathione S-transferase inhibitors. Archives of Environmental Contamination and Toxicology 60: 681–689.
Lajmanovich, R. C., C. M. Junges, A. M. Attademo, P. M. Peltzer, M. C. Cabagna-Zenklusen & A. Basso, 2013. Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water, Air and Soil Pollution 224: 1–13.
Leung, B. & M. R. Forbes, 1997. Fluctuating asymmetry in relation to stress and fitness: effects of trait type as revealed by meta-analysis. Ecoscience 3: 400–413.
Mann, R. M. & J. R. Bidweel, 1999. The toxicity of glyphosate and several glyphosate formulations to four species of Southwestern Australian frogs. Archives of Environmental Contamination and Toxicology 36: 193–199.
Mann, M. R., R. V. Hyne, C. B. Choung & S. P. Wilson, 2009. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environmental Pollution 157: 2903–2927.
Møller, A. P., 1997. Developmental stability and fitness: a review. American Naturalist 149: 916–932.
Moreira, J. C., F. Peres, A. C. Simões, W. A. Pignati, E. C. Dores, S. N. Vieira, C. Strüssmann & T. Mott, 2012. Groundwater and rainwater contamination by pesticides in an agricultural Region of Mato Grosso State in Central Brazil. Ciência & Saúde Coletiva 17(6): 1557–1568.
Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. Fonseca & J. Kent, 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
Newton, M., K. M. Howard, B. R. Kelpsas, R. Danhaus, C. M. Lottman & S. Dubelman, 1984. Fate of glyphosate in an Oregon Forest Ecosystem. Journal of Agriculture and Food Chemistry 32: 1144–1151.
Palmer, A. R. & C. Strobeck, 1986. Fluctuating asymmetry: measurement, analysis, patterns. Annual Review of Ecology and Systematics 17: 391–421.
Pignati, W. A. & J. M. H. Machado, 2007. O agronegócio e seus impactos na saúde dos trabalhadores e da população do estado de Mato Grosso. Fiocruz/Ens, Rio de Janeiro: 81–105.
Queiroz, G. M. P., M. R. Silva, R. J. F. Bianco, A. Pinheiro & V. Kaufman, 2011. Transporte de glifosato pelo escoamento superficial e por lixiviação em um solo agrícola. Química Nova 34(2): 190–195.
Rainio, J. & J. Niemelä, 2003. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodiversity and Conservation 12: 487–506.
Reis, E. F., N. S. Pinto, F. G. Carvalho & L. Juen, 2011. Efeito da Integridade Ambiental Sobre a Assimetria Flutuante em Erythtodiplax basalis (Libellulidae: Odonata) (Kirby). Entomobrasilis 4(3): 103–107.
Relyea, R. A., 2005a. The lethal impacts of Roundup® and predatory stress on six species of North American tadpoles. Archives of Environmental Contamination and Toxicology 48: 351–357.
Relyea, R. A., 2005b. The impact of insecticides and Herbicides on the biodiversity and productivity of aquatic communities. Ecological Applications 15(2): 618–627.
Relyea, R. A., 2005c. The lethal impact of Roundup® on aquatic and terrestrial amphibians. Ecological Applications 15(4): 1118–1124.
Relyea, R. A., 2006. The effects of pesticides, pH, and predatory stress on amphibians under mesocosm conditions. Ecotoxicology 15(6): 503–511.
Relyea, R. A., 2012. New effects of Roundup® on amphibians: predators reduce herbicide mortality; herbicides induce antipredators morphology. Ecological Applications 22(2): 634–647.
Relyea, R. A. & D. K. Jones, 2009. The toxicity of Roundup® Max to 13 species of larval amphibians. Environmental Toxicology and Chemistry 28(9): 2004–2008.
Sanseverino, A. M. & J. L. Nessimian, 2008. Assimetria flutuante em organismos aquáticos e sua aplicação para avaliação de impactos ambientais. Oecologia Brasiliensis 12: 382–405.
Schiesari, L. & B. Grillitsch, 2011. Pesticides meet megadiversity in the expansion of biofuel crops. Frontiers in Ecology and Environment 9: 215–221.
Schiesari, L., B. Grillitsch & H. Grillitsch, 2007. Biogeographic biases in research and their consequences for linking amphibian declines to pollution. Conservation Biology 21(2): 465–471.
Schiesari, L., A. Waichman, T. Brock, C. Adams & B. Grillitsch, 2013. Pesticide use and biodiversity conservation in the Amazonian agriculture frontier. Philosophical Transactions of the Royal Society B 368: 1–9.
Searle, S. R., G. Casella & C. E. McCulloch, 1992. Variance Components. Wiley, Hoboken, NJ: 501 pp.
Shin, C., A. Marco & L. Serrano, 2008. Inter- and intra-specific variation on sensitivity of larval amphibians to nitrite. Chemosphere 71: 507–514.
Silva, D. R. O., L. A. Avila, D. Agostinetto, T. D. Magro, E. Oliveira, R. Zanella & J. A. Noldin, 2009. Pesticides monitoring in surface water of rice production areas in southern Brazil. Ciência Rural 39(9): 2383–2389.
Simioni, F., D. F. N. da Silva & T. Mott, 2013. Toxicity of glyphosate on Physalaemus albonotatus (Steindachner, 1864) from Western Brazil. Ecotoxicology and Environmental Contamination 8(1): 55–58.
Snodgrass, J. W., R. E. Casey, D. Joseph & J. A. Simon, 2008. Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environmental Pollution 154: 291–297.
Söderman, F., S. V. Dongen, S. Pakkasmaa & J. Merilä, 2007. Environmental stress increases skeletal fluctuating asymmetry in the moor frog Rana arvalis. Oecologia 151: 593–604.
Solomon, K. R. & D. G. Thompson, 2003. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. Journal of Toxicology and Environmental Health, Part B: Critical Reviews 6(3): 289–324.
Sparling, D. W., G. M. Fellers & L. L. McConnell, 2001. Pesticides and amphibian population declines in California, USA. Environmental Toxicology and Chemistry 20(7): 1591–1595.
Stige, L. C., D. O. Hessen & L. A. Vøllestad, 2004. Severe food stress has no detectable impact on developmental instability in Daphnia magna. Oikos 107: 519–530.
Stuart, S. N., J. S. Chanson, N. A. Cox, B. E. Young, A. S. L. Rodrigues, D. L. Fischman & R. W. Waller, 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786.
Thompson, D. G., B. F. Wojtaszek, B. Staznik, D. T. Chartrand & G. R. Stephenson, 2004. Chemical and biomonitoring to assess potential acute effects of Vision Herbicide on native amphibian larvae in forest wetlands. Environmental Toxicology and Chemistry 23(4): 843–849.
U.S.EPA. (United States Environmental Protection Agency), 2008. Risks of glyphosate use to federally threatened California Red-legged frog (Rana aurora draytonii). Pesticide effects determination. Washington, D.C. Accessible at http://www.epa.gov/espp/litstatus/effects/redleg-frog/#glyphosate. Downloaded on 30 November 2013.
Van Valen, L., 1962. A study of fluctuating asymmetry. Evolution 16: 125–142.
Zar, J. H., 1999. Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, NJ: 663 pp.
Acknowledgements
We are grateful to Girinos do Brasil (SISBIOTA: grants CNPq 563075/2010-4 and FAPESP 2010/52321-7) for the financial support provided to carry out experiments and field sampling. We thank Arthur Bauer, Marcelo Junqueira, and Fernanda Fava for their help in field sampling. We are also grateful to Mirco Solé for the English review and Simone Morais for the laboratory support. Finally, we thank Arthur Bauer and Wanderson de Souza for the logistic support during experimentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Rights and permissions
About this article
Cite this article
Costa, R.N., Nomura, F. Measuring the impacts of Roundup Original® on fluctuating asymmetry and mortality in a Neotropical tadpole. Hydrobiologia 765, 85–96 (2016). https://doi.org/10.1007/s10750-015-2404-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2404-0