Abstract
Cardiac amyloidosis, characterized by progressive restrictive cardiomyopathy, presents unusual diagnostic challenges. Conventional cardiac scintigraphy has shown limited utility in the quantification of disease burden and serial follow-up of cardiac amyloidosis. The advent of specialized positron emission tomography with specific amyloid-binding radiotracers has the potential to change currently employed diagnostic algorithms for the imaging of cardiac amyloidosis. This review aims to discuss the diagnostic utility of amyloid-binding radiotracers, including Pittsburg compound B, florbetapir, florbetapan, and sodium fluoride. These tracers have promising potential for the early detection of the particular type of cardiac amyloidosis, pursuing relevant medical intervention, assessing amyloid burden, monitoring treatment response, and overall prognostication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cardiac amyloidosis (CA) is characterized by extracellular deposition of misfolded filamentous proteins, derived primarily from circulating immunoglobulin light chains (AL) or transthyretin protein (ATTR) which clinically manifests as restrictive cardiomyopathy [1].
Imaging modalities like echocardiography and cardiac magnetic resonance (CMR) provide objective evidence of cardiac amyloidosis, which include the characteristic findings of increased ventricular wall thickness, diastolic dysfunction, delayed left ventricular gadolinium enhancement, and T-1 weighted images. These myocardial parameters help estimate the location and extent of amyloid deposition, but lack specificity for amyloidosis [2, 3]. Endo-myocardial biopsy (EMB) should have presumably filled this void in specificity, but along with its invasive nature, inability to predict disease burden and the uneven pattern of the amyloid deposition itself implies the limited utility of this modality [4]. Amyloid-binding radiopharmaceuticals in positron emission tomography may answer the diagnostic challenges presented by the progressive nature of heart failure associated with cardiac amyloidosis [5]. Because of their high sensitivity and specificity in early studies, these radiotracers have promising utility for early and accurate detection of CA, estimating disease burden, monitoring the treatment response, and prognostication [6].
The three main cardiac amyloidosis types include hereditary transthyretin amyloidosis (ATTRv-CA), wild-type ATTR amyloidosis (ATTRwt-CA), or immunoglobulin light chain amyloidosis (AL-CA)[7]. Nowadays, the management of CA has seen the development of targeted pharmaceutical therapy for ATTR-CA, including TTR stabilizers/silencers, and chemotherapy regimens for AL-CA, including anti-plasma cell therapies and anti-amyloid antibodies. These therapies are associated with slowing or halting cardiac amyloidosis progression, as such early recognition of cardiac amyloidosis remains of paramount priority so tailored therapy can be initiated accordingly [8]. In recent years, the giant leap forward was with non-invasive, nuclear scintigraphy imaging using bone-avid radiotracers, capable of binding micro-calcifications more commonly seen in ATTR-CA than AL amyloidosis [9]. This non-biopsy approach helped accurately diagnose ATTR-CA and differentiate the type of cardiac amyloidosis, with imaging modalities, discussed earlier and prompted a wide change in clinical approach and proposed diagnostic algorithm [10].
Limitations of cardiac scintigraphy
Currently, cardiac scintigraphy has left several clinical unmet needs in the management of cardiac amyloidosis. These include limitations in detecting the presence of early disease and the ability to monitor disease progression and/or response to therapy. These limitations have recently witnessed immense interest following the introduction of new disease-modifying therapies against cardiac amyloidosis [11].
High-grade tracer uptake in nuclear scintigraphy promised 100% specificity for ATTR-CA detection only in patients with established heart failure and in the presence of typical echocardiographic and CMR findings of advanced cardiac amyloidosis, implying limited sensitivity for the diagnosis of earlier stages of ATTR-CA [12]. Moreover, cardiac scintigraphy has primarily revolved around ATTR-CA diagnosis while neglecting other forms of CA such as AL amyloidosis [12, 13]. Using targeted amyloid-binding radiotracers, PET imaging provides the ability to detect all amyloid deposits regardless of original protein precursor. Early pilot studies using 11C-PiB and 18F-florbetapir have shown that PET may be able to detect AL-CA even before an increase in LV wall thickening or alterations in cardiac biomarkers [14, 15]. This would expand previously limited avenues in the diagnosis, monitoring, and management of AL cardiac amyloidosis.
Additionally, serial cardiac scintigraphy did not provide meaningful documentation of disease progression. Castano et al. thought to investigate the clinical feasibility of serial 99mTc-PYP cardiac scintigraphy in an attempt to quantify disease burden over time. This study included 20 ATTR-CA patients who underwent a second cardiac scintigraphy scan following an average of 1.5 years. The images were assessed and reported with both visual/semiquantitative and quantitative scores (H/CL). Albeit a clear disease progression based on clinical outcomes (progressive in NYHA class and/or death) and biomarkers (troponin and BNP), serial 99mTc-PYP cardiac scintigraphy failed to capture any differences over time [16].
This would suggest that cardiac scintigraphy would not mirror clinical course or increased disease burden and would be relegated for just the diagnosis of ATTR cardiac amyloidosis. As such, PET imaging using amyloid-binding radiotracer holds great potential in this field owing to the PET’s ability to introduce improved quantification measures which might provide a clearer picture of disease burden and subsequently form the basis for monitoring of its progression or response to therapy (Fig. 1).
Amyloid-binding PET radiotracers
11C-labeled Pittsburg compound-B (11C-PiB)
11C-PiB was one of the pioneering amyloid-fibril–binding radiotracers, initially developed to visualize and quantify β-amyloid plaques in Alzheimer’s disease [17]. It was not long before 11C-PiB found application for in vivo and in vitro visualization of cardiac amyloidosis were significantly increased. 11C-PiB retention index (RI) was observed in both major types of CA, as compared to controls subjects [18, 19]. A subsequent study using measures of maximal and mean myocardium-to-blood cavity standard uptake value (SUV) ratios also found significant margins for differentiating between chemotherapy naïve AL-CA patients, compared to AL-CA patients with prior chemotherapy. This warranted recognition for 11C-PiB as a surrogate indicator for detecting active ongoing light chain deposition in the myocardium. The study also served to propose possible prognostic utility of myocardial amyloid uptake [20].
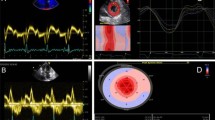
Cardiac amyloid load is a prognostic marker in AL-CA, especially for chemotherapy-responsive patients with amyloid light chain (AL) load of less than 20% having better overall survival [21]. This non-invasive modality may help assess the dynamics of amyloid turnover in response to directed therapy. Furthermore, the degree of 11C-PiB uptake may serve as an independent predictor and prognostic indicator for clinical events like all-cause mortality or acute decompensated heart failure on subsequent follow-up (Fig. 2) [6].
The degree of myocardial 11C-Pittsburgh B compound positron emission tomography uptake reflects the degree of myocardial amyloid deposit on invasive endomyocardial biopsy. Noninvasive evaluation of the degree of myocardial amyloid load can independently predict clinical outcome in AL cardiac amyloidosis patients. (Reuse permission obtained from [6])
Of further interest was using 11C-PiB among patients with genetically and histopathologically diagnosed neuropathic ATTRv-CA with ATTR V30M variant but without characteristic morphological features on ATTRv-CA on echocardiography [22]. The V30M variant of ATTRv-CA has two major phenotypes: early-onset disease (type B fibrils, with mainly have neuropathic manifestations) and late-onset disease (type A fibrils, with both cardiac and neurological manifestations) [23]. Although all patients with either fibrils type had significantly increased 11C-PiB uptake compared to healthy controls, the measured 11C-PiB RI was three times higher in type B fibrils than type A fibrils, implying that there is limited utility in measuring the magnitude of 11C-PiB RI in ATTR patients. The increased 11C-PiB RI in ATTR patients may be due to 11C-PiB high binding affinity for type B fibril, rather than associated with true amyloid burden or predicting risk of cardiomyopathic development [22].
In contrast, cardiac scintigraphy showed increased DPD uptake in type A variant V30M ATTRv-CA and non-V30M ATTRv-CA, and absent uptake in type B variant V30M-ATTRv-CA further implying that positive DPD scintigraphy in ATTRv is associated with the future development of amyloid cardiomyopathy [22, 24]. If used together with TTR gene testing, the level and pattern of 11C-PiB and DPD retention may help in the accurate subclassification of the ATTRv-CA fibril type and differentiate AL-CA from ATTRwt-CA, since 11C-PIB showed uptake in all ATTR-CA and AL-CA cases while DPD is negative in certain ATTRv-CA [24].
A follow-up study found that 11C-PiB uptake, analyzed by visual inspection and quantitative measures of SUV and RI, had significant diagnostic accuracy in discriminating main cardiac amyloidosis subtypes in biopsy-confirmed patients from healthy controls [25]. 11C-PiB uptake was significantly higher in AL-CA than ATTR-CA, suggesting that the fibril type in ATTR-CA was affecting the 11C-PiB binding to amyloid and subsequent visual and quantitative assessments [25]. Cardiac amyloidosis patients without cardiac wall hypertrophy had significantly increased 11C-PIB uptake compared to controls, indicating that 11C-PiB can detect early stages of cardiac amyloidosis before any overt morphological change, the basis of echocardiographic and CMR imaging [25].
However, 11C-PiB binding to cardiac amyloids is variable with some degrees of reversibility in the retention index, thus requiring early imaging for accurate results [18]. In addition, the much shorter half-life of the 11C-PIB radiotracer, compared to 18F-labeled PET tracers, requires an on-site cyclotron for its continuous production and diagnostic utilization, which may be beyond the scope except in specialized centers [25].
18F-Florbetapir
Preliminary studies established the promising potential for 18F-florbetapir to serve as a molecular imaging biomarker for cardiac amyloidosis. 18F-Florbetapir is hypothesized to directly bind to amyloid fibrils and thus causing longer tracer transit time—both features hinting at its diagnostic and quantitative abilities in assessing disease burden [26]. 18F-Florbetapir belongs to the stilbene class of PET tracers. Earlier studies identified at least six surface- and core-binding sites in fibrils of neuritic amyloid-β plaques in autopsied Alzheimer’s disease brain tissue, and a further three-binding-site model boasted a nanomolar affinity for a primary high-affinity site [27]. This characterization encouraged extrapolation of its application to cardiac amyloidosis as the beta-pleated motif of amyloid protein remains the same, irrespective of the precursor protein [28].
Earlier autoradiography studies on autopsy-confirmed AL-CA, ATTR-CA, and non-amyloid control samples demonstrated significantly increased 18F-florbetapir–specific uptake in AL and ATTR samples as compared to controls [29]. Furthermore, significantly increased 18F-florbetapir–specific uptake was seen in AL-CA samples compared to ATTR-CA (2.48 ± 0.40 versus 1.52 ± 0.22 DPM/mm2; p < 0.001), despite overall significantly lower amyloid deposition burden in AL-CA samples as compared to ATTR-CA samples on histology [29]. Unlike the left ventricle myocardial standardized uptake values (SUV), target-to-blood pool ratio (TBR), and LV myocardium-to-liver SUV ratio, 18F-florbetapir myocardial retention index (RI) and liver and cardiac time-activity curves (TACs) are kinetic parameters shown to effectively stratify AL-CA from ATTR-CA [30, 31]. Of significance is the semi-quantitative and incremental value of increased 18F-florbetapir binding in samples with focal and limited amyloid deposits (in the absence of characteristic increased left ventricular wall thickness and increased left ventricular mass on echocardiogram) as compared to controls, signifying its utility in early diagnosis of cardiac amyloidosis [29]. Another study found a strong correlation between cardiac amyloidosis structural parameters (left ventricular wall thickness and left ventricular mass on echocardiography and extracellular volume on CMR), cardiac functional parameters (e.g., LVEF, global longitudinal strain, and relative apical sparing on echocardiogram), and myocardial 18F-florbetapir retention index (RI) as a direct measure of cardiac amyloid burden in three groups of active AL-CA, remission AL-CA, and active systemic AL amyloidosis in the absence of CA (active-non-CA). The study also established 18F-florbetapir RI’s ability to discern the small degree of myocardial amyloid burden in the active-non-CA group without established structural and functional markers of amyloidosis [15].
These studies potentiated the utility of 18F-florbetapir as the litmus test to diagnose, differentiate, and assess the burden of cardiac amyloidosis. It may be worthwhile to consider dual-imaging with bone avid radiotracers and 18F-florbetapir to diagnose cardiac amyloidosis and identify the type because the former modality has a higher affinity for ATTR-CA and the latter for AL-CA [29]. However, interval imaging with 18F-florbetapir in AL-CA treatment-naïve patient before starting therapy and after chemotherapy completion with complete hematological response did not show a significant difference in RI or SUVR, making it a less reliable marker to assess treatment response [32].
18F-Florbetapan
Similar to 18F-florbetapir, PET imaging with 18F-florbetapan had demonstrated utility in accurate detection of b-amyloid neuritic plaques in Alzheimer’s disease before feasibility studies were pursued to diagnose cardiac amyloidosis [33]. Qualitative and quantitative measures, including mean standardized uptake value (SUV) of LV myocardium, TBR-SUV ratio, and 18F-florbetapan retention (percentage mean myocardial SUV change on scheduled time intervals) were significantly higher in CA patients as compared to control subjects. Initial studies identified the potential for 18F-florbetapan to differentiate between non-CA, AL-CA, and ATTR-CA since AL-CA had a higher myocardial SUV variability spherical volume of interest (VOI) and higher myocardial tracer retention (MT–) cut-off [34,35,36]. Expectantly, 18F-florbetapan retention in cardiac amyloidosis was an independent determinant of cardiac dysfunction on echocardiogram and cardiac magnetic resonance imaging (CMR), for functional and morphological parameters, like global LV longitudinal strain and ventricular wall thickness, respectively [34, 35]. Myocardial tracer retention (MTR) and changes in MTR over time (ΔMTR) were noted to correlate with disease progression and amyloid burden quantification, a promising potential for use in treatment response measurement [35].
A recent study investigated the utility of 18F-florbetapan dynamic PET acquisition technique, whereby four 10-min static scans were obtained at pre-determined intervals after radiotracer injection [37]. A significant difference was found in the retention index among patients with AL-CA compared to ATTR-CA patients and non-CA individuals, with AL-CA patients having persistent, high cardiac uptake in all static scans, in contrast, while ATTR-CA patients and non-CA individuals showed a temporal decline in radiotracer uptake after early static scans (Fig. 3) [37].
(Upper panel) Fluorine 18 [18F]-florbetaben cardiac positron emission tomography myocardial time–activity curves in patients with immunoglobulin light-chain amyloidosis (AL) (blue), transthyretin-related amyloidosis (ATTR) (red), and non-cardiac amyloidosis (non-CA) (gray). The 95% confidence interval is represented as a shaded area for each curve. (Lower panel) Early (5 to 15 min), intermediate (30 to 40 min), and late (50 to 60 min) [18F]-florbetaben cardiac positron emission tomography scans in patients with AL and ATTR CA and those with non-CA. SUVmean ¼ mean standardized uptake value. (Reuse permission obtained from [37])
18F-Sodium fluoride (18F-NaF)
As outlined previously, positron emission tomography (PET) has amassed a wide array of novel radiotracers (18Fluorine-labeled florbetapor/florbetapir and 11Chlorine-labeled Pittsburg B) capable of imaging and quantifying cardiac amyloidosis (CA). However, currently, these radiotracers are yet to be approved for clinical use and are only available in limited specialized centers.
18F-Sodium fluoride (18F-NaF), on the other hand, is a readily available and FDA-approved tracer used primarily for the imaging of cancer metastasis especially prostate cancer. 18F-NaF, a PET radiotracer, should in theory offer higher resolution imaging compared to Tc-labeled cardiac scintigraphy and more importantly the ability to obtain quantification of amyloid burden. Thus, similar to other quantitative measurements, this would allow for the earlier detection of cardiac amyloidosis, a more accurate assessment of amyloid disease burden, and response to therapies.
The use of 18F-NaF PET for the imaging of cardiac amyloidosis was reported in the form of a case report by Van Der Gucht et al. which revealed this modality’s potential to identify and differentiate ATTRv (Val122Ile) vs AL amyloidosis. More importantly, the authors highlighted that this technique was more efficient than comparable HMDP imaging citing faster kinetics and overall faster imaging protocols (20- and 60-min imaging acquisitions post-injections) [38]. However, another case report by Gagliardi et al. reported no 18F-NaF uptake in 2 patients with ATTRwt and ATTRv (Ile68Leu). The authors hypothesized that 18F-NaF uptake might not only be subject to differences in amyloid subtype (ATTR vs AL) but also specific TTR mutations [39]. It should be noted that the former case report was done prospectively compared to the latter which was done retrospectively in patients primarily evaluated for prostate cancer [38, 39].
A pilot study conducted by Morgenstern et al. confirmed the finding reported by Van Der Gucht et al. The study consisted of a small cohort of 5 ATTR (3 ATTRwt and 2 Val122Ile), 2 AL, and 5 control patients (prostate cancer). PET along with CT-based attenuation images were acquired at 10- and 60-min (one study at 90 min) post-injection of 10 mCi of 18F-NaF. Next, the scans were assessed both visually and quantitatively with standard uptake values in the entire myocardium (SUVm). Specifically, SUVs were obtained for the entire myocardium and in the 17-segment cardiac model. 18F-NaF uptake was absent in patients with AL and controls compared to ATTR-CA patients who showed a noticeable uptake, particularly in ATTRwt patients. Moreover, based on quantitative assessment of ATTR patients reported higher SUVm vs AL and control patients. 18F-NaF was noted to be diffuse with varied uptake in different cardiac segments. SUVm for ATTR patients was around 1.5*SUVm of controls (p = 0.012) and AL patients (p = 0.078). Although the difference between ATTR and AL SUVm approached but did not reach significance, this can be attributed to the small sample size used in the study [40].
These findings echoed a study by Trivieri et al. which used a hybrid PET/MR imaging approach. Diffuse 18F-NaF uptake was observed in patients with ATTR (4) but not in AL (3) or control (7) patients. 18F-NaF uptake colocalized with regions of LGE and correlated well with native T1 mapping [41]. A larger 18F-NaF PET/MRI study (16 ATTR and 7 AL patients) showed that myocardial-to-blood pool (M/B) ratios were significantly higher in ATTR patients compared to AL patients (p = 0.001) [42].
Although semiquantitative measures were able to distinguish ATTR from AL, the same was not true for visual qualitative assessments [40,41,42,43]. A study by Martineau et al. revealed that visual assessment of 18F-NaF uptake in ATTR patients was limited due to its low sensitivity of 57% (specificity of 100%). 18F-NaF uptake was significantly higher in ATTR compared to AL or controls; nevertheless, uptake was quite mild and inferior to that of blood pool in 57% of the studies which will undoubtedly complicate visual assessments [43].
Faint 18F-NaF uptake reinforces the need for semiquantitative measures to differentiate ATTR from AL. Albeit low uptake values, target-to-background ratio (TBRmean) recorded sensitivity and specificity of 75% and 100% respectively for a cutoff threshold of 0.89 for detection of ATTR (SUVmean failed to show a significant difference between ATTR, AL, and controls consistent with the noted low visual uptakes) [43]. This is comparable to previously published semiquantitative M/B (TBRmax) ≥ 0.90 and 0.84 [41, 42].
As a bone-avid tracer, the hypothesized binding mechanism behind 18F-NaF uptake in cardiac amyloidosis resembles more conventional bone-avid radiotracers compared to the previously discussed novel amyloid-binding tracers. 18F-NaF can detect microcalcifications which form the basis of its use in cancer imaging. TTR amyloid fibrils are thought to bind more calcium compared to AL fibrils which might explain the differentiating capabilities of both 18F-NaF and bone-avid radiotracers [11, 44].
Previous studies have shown that not all bone-seeking tracers are created equal with varied accuracy and clinical utility. Currently, available data on 18F-NaF PET have shown inconsistent findings, weak visual uptake, and low TBG. A recent meta-analysis reported high specificity (100%) for 18F-NaF PET but relatively low sensitivity (63%) [45]. However, the adequate judgment of its current clinical utility remains uncertain due to the limited available data regarding 18F-NaF compared to its counterparts. Current literature (Table 1) has shown mixed methodology which could have contributed to the heterogeneity of the observed accuracy of 18F-NaF. As such, larger comparative studies (against PYP or DPD) are needed to truly elucidate the role of 18F-NaF PET in the workup of cardiac amyloidosis (Tables 2, 3, 4, and 5).
Coronary microvascular dysfunction (CMD)
The coronary microvasculature is comprised of coronary pre-arterioles and arterioles measuring < 500 μm in diameter and hold 90% of the total myocardial blood flow [46]. Although direct imaging of the coronary microvasculature is challenging, PET is at present the most accurate modality in clinical practice for the evaluation of coronary microvasculature function [47, 48].
Coronary microvascular dysfunction (CMD) is defined as ischemic cardiac chest pain with impairment in coronary microvascular blood flow in the absence of epicardial coronary obstruction. Although prevalence is unknown, several studies have shown how microvascular angina is not an uncommon initial presentation for patients with cardiac amyloidosis [49,50,51].
Autopsy studies have shown that amyloidosis causes microvascular dysfunction through three overlapping mechanisms: (1) deposition within vessel wall causing intimal thickening, (2) interstitial deposition causing perivascular compression, and (3) autonomic and/or endothelial dysfunction [52,53,54,55]. These contribute to ischemia and impaired systolic dysfunction seen in some patients with cardiac amyloidosis.
Only one study has prospectively assessed microvascular dysfunction using PET MPI. Dorbala et al. prospectively enrolled 21 patients with cardiac amyloidosis (15 with light chain and 6 with transthyretin) and 10 patients with hypertensive left ventricular hypertrophy [56]. Amyloidosis was diagnosed with either endomyocardial biopsy (n = 10) or extracardiac biopsy with echocardiography cardiac involvement (n = 11). All patients underwent N-13 ammonia PET/CT imaging using standard imaging protocols. Microvascular dysfunction was defined as the presence of reduced peak stress myocardial blood flow (MBF), coronary flow reserve (CFR), or minima coronary vascular resistance.
Ischemic defect and transient ischemic dilatation were seen in 57% and 76% of the patients with amyloidosis. More importantly, stress MBF and MFR were significantly lower in those with amyloid compared to the LVH group (rest MBF 0.59 vs 0.88 ml/g/min p = 0.0004; stress MBF 0.85 vs 1.85 ml/g/min p < 0.0001; CFR 1.19 vs 2.23 p < 0.0001 for amyloid vs LVH group respectively), and microvascular resistance was significantly higher (147 vs 117 ml/g/min/mm Hg p = 0.004). Differences between the two groups were the same even after accounting for age, subclinical myocardial dysfunction, and lower left ventricular mass in the LVH group. Importantly, limitations of note were the small sample size, and how invasive angiography to rule out epicardial stenosis was done at a median of 164 from PET imaging.
Data availability
Not applicable.
Code availability
Not applicable.
References
Merlini G, Bellotti V (2003) Molecular mechanisms of amyloidosis. N Engl J Med 349(6):583–596
Phelan D et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448
Syed IS et al (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3(2):155–164
Kristen AV, Dengler TJ, Katus HA (2007) Suspected cardiac amyloidosis: endomyocardial biopsy remains the diagnostic gold-standard. Am J Hematol 82(4):328
Falk RH, Quarta CC, Dorbala S (2014) How to image cardiac amyloidosis. Circ Cardiovasc Imaging 7(3):552–562
Lee SP et al (2020) Pittsburgh B compound positron emission tomography in patients with AL cardiac amyloidosis. J Am Coll Cardiol 75(4):380–390
Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet 387(10038):2641–2654
Alexander KM, Singh A, Falk RH (2017) Novel pharmacotherapies for cardiac amyloidosis. Pharmacol Ther 180:129–138
Perugini E et al (2005) Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 46(6):1076–1084
Ruberg FL et al (2019) Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 73(22):2872–2891
Castaño A, Bokhari S, Maurer MS (2015) Unveiling wild-type transthyretin cardiac amyloidosis as a significant and potentially modifiable cause of heart failure with preserved ejection fraction. Eur Heart J 36(38):2595–2597
Gillmore JD et al (2016) Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 133(24):2404–2412
Dorbala S et al (2019) ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2—evidence base and standardized methods of imaging. J Nucl Cardiol 26(6):2065–2123
Rosengren S et al (2020) Diagnostic accuracy of [11C]PIB positron emission tomography for detection of cardiac amyloidosis. JACC Cardiovasc Imaging 13(6):1337–1347
Cuddy SAM et al (2020) Improved quantification of cardiac amyloid burden in systemic light chain amyloidosis: redefining early disease? JACC Cardiovasc Imaging 13(6):1325–1336
Castaño A et al (2016) Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology 23(6):1355–1363
Klunk WE et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol 55(3):306–319
Antoni G et al (2013) In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 54(2):213–220
Ezawa N et al (2018) Visualization of multiple organ amyloid involvement in systemic amyloidosis using (11)C-PiB PET imaging. Eur J Nucl Med Mol Imaging 45(3):452–461
Lee SP et al (2015) 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging 8(1):50–59
Kristen AV et al (2016) Cardiac amyloid load: a prognostic and predictive biomarker in patients with light-chain amyloidosis. J Am Coll Cardiol 68(1):13–24
Pilebro B et al (2018) Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J Nucl Cardiol 25(1):240–248
Koike H et al (2004) Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 63(1):129–138
Takasone K et al (2020) Non-invasive detection and differentiation of cardiac amyloidosis using (99m)Tc-pyrophosphate scintigraphy and (11)C-Pittsburgh compound B PET imaging. Amyloid 27(4):266–274
Rosengren S et al (2020) Diagnostic accuracy of [(11)C]PIB positron emission tomography for detection of cardiac amyloidosis. JACC Cardiovasc Imaging 13(6):1337–1347
Choi SR et al (2012) Correlation of amyloid PET ligand florbetapir F 18 binding with Aβ aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Disord 26(1):8–16
Ni R et al (2013) Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain 136(7):2217–2227
Clark CM et al (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 11(8):669–678
Park MA et al (2015) 18F-Florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: autoradiography study. Circ Cardiovasc Imag 8(8)
Dorbala S et al (2014) Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 41(9):1652-1662
Osborne DR et al (2015) A routine PET/CT protocol with streamlined calculations for assessing cardiac amyloidosis using (18)F-florbetapir. Front Cardiovasc Med 2:23
Manwani R et al (2018) A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. Amyloid 25(4):247–252
Baratto L et al (2018) (18)F-Florbetaben whole-body PET/MRI for evaluation of systemic amyloid deposition. EJNMMI Res 8(1):66
Law WP et al (2016) Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J Nucl Med 57(11):1733–1739
Kircher M et al (2019) Detection of cardiac amyloidosis with (18)F-florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur J Nucl Med Mol Imaging 46(7):1407–1416
Seo M et al (2019) Clinical utility of 18F-florbetaben PET for detecting amyloidosis associated with multiple myeloma: a prospective case-control study. Clin Nucl Med 44(9):e503–e509
Genovesi D et al (2021) [18F]-Florbetaben PET/CT for differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging 14(1):246–255
Van Der Gucht A et al (2016) [18F]-NaF PET/CT imaging in cardiac amyloidosis. Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology 23(4):846–849
Gagliardi C et al (2017) Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology 24(2):746–749
Morgenstern R et al (2018) 18Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology 25(5):1559–1567
Trivieri MG et al (2016) (18)F-Sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 68(24):2712–2714
Abulizi M et al (2019) 18F-Sodium fluoride PET/MRI myocardial imaging in patients with suspected cardiac amyloidosis. J Nuc Cardiol: Official Pub Am Soc Nuclear Cardiol
Martineau P et al (2021) Examining the sensitivity of 18F-NaF PET for the imaging of cardiac amyloidosis. Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology 28(1):209–218
Masri A et al (2020) Molecular imaging of cardiac amyloidosis. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine 61(7):965–970
Kim SH, Kim YS, Kim S-J (2020) Diagnostic performance of PET for detection of cardiac amyloidosis: a systematic review and meta-analysis. J Cardiol 76(6):618–625
Gutterman DD et al (2016) The human microcirculation: regulation of flow and beyond. Circ Res 118(1):157–172
Bravo PE, Di Carli MF, Dorbala S (2017) Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev 22(4):455–464
Camici PG, d’Amati G, Rimoldi O (2015) Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 12(1):48–62
Tsai SB et al (2011) Myocardial infarction with “clean coronaries” caused by amyloid light-chain AL amyloidosis: a case report and literature review. Amyloid: The Int J Exp Clinical Investigation: The Official J Int Soc Amyloidosis 18(3):160–164
Al Suwaidi J et al (1999) Systemic amyloidosis presenting with angina pectoris. Ann Intern Med 131(11):838–841
Ogawa H et al (2001) Cardiac amyloidosis presenting as microvascular angina–a case report. Angiology 52(4):273–278
Smith RR, Hutchins GM (1979) Ischemic heart disease secondary to amyloidosis of intramyocardial arteries. Am J Cardiol 44(3):413–417
Hongo M et al (2000) Comparison of electrocardiographic findings in patients with AL (primary) amyloidosis and in familial amyloid polyneuropathy and anginal pain and their relation to histopathologic findings. Am J Cardiol 85(7):849–853
Modesto KM et al (2007) Vascular abnormalities in primary amyloidosis. Eur Heart J 28(8):1019–1024
Buja LM, Khoi NB, Roberts WC (1970) Clinically significant cardiac amyloidosis. Clinicopathologic findings in 15 patients. The Am J Cardiol 26(4):94–405
Dorbala S et al (2014) Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart failure 2(4):358–367
Takasone K et al (2020) Non-invasive detection and differentiation of cardiac amyloidosis using 99mTc-pyrophosphate scintigraphy and 11C-Pittsburgh compound B PET imaging. Amyloid 27(4):266–274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare no competing interests.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saeed, S., Saad, J.M., Ahmed, A.I. et al. The utility of positron emission tomography in cardiac amyloidosis. Heart Fail Rev 27, 1531–1541 (2022). https://doi.org/10.1007/s10741-021-10183-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10183-w