Abstract
Background
Conventional nuclear imaging with bone-seeking radiopharmaceuticals has been shown to be a sensitive test for the detection of transthyretin cardiac amyloidosis (ATTR); however, to date, few data exist on the utility of 18F-sodium fluoride (NaF) positron emission tomography (PET) in subjects with cardiac amyloidosis (CA).
Methods
Myocardial perfusion imaging and cardiac 18F-NaF PET/CT of 7 subjects with ATTR, four with light-chain CA (AL), and four controls were retrospectively reviewed. Qualitative interpretation and quantitative analyses with average left ventricular standardized uptake values (SUVmean) and target-to-background ratios (TBRmean) were performed.
Results
Average TBRmean was significantly increased in subjects with ATTR (0.98 ± 0.09) compared to AL (0.85 ± 0.08, P = .026) and CTL (0.82 ± 0.07, P = .020), while SUVmean was not (P = .14). Receiver-operator characteristic (ROC) analysis yielded an area under the curve (AUC) of 0.91, with a sensitivity/specificity of 75%/100% for TBRmean using a cutoff value of 0.89 for the diagnosis of ATTR. Qualitative interpretation resulted in a sensitivity/specificity of 57%/100% for ATTR.
Conclusions
While 18F-NaF PET/CT demonstrates good diagnostic accuracy for ATTR, particularly when using quantitative analysis, the low TBRmean values observed in ATTR indicate poor myocardial signal. 18F-NaF PET/CT is not yet ready for clinical use in CA until further comparison studies are performed with 99mTc-DPD/PYP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis (CA) consists of a group of diseases, each characterized by the deposition of insoluble, misfolded proteins in the myocardium.1 CA is classified based on the type of protein accumulating in the heart with two main recognized subtypes: light-chain (AL) CA and transthyretin CA (ATTR).2 Classically, CA presents with diastolic dysfunction and left ventricular hypertrophy (LVH) secondary to accumulation of proteins in the myocardium.3 It has been proposed that CA is responsible for a significant proportion of patients presenting with heart failure with preserved ejection fraction or with LVH.4,5 Increased recognition of CA and a growing interest in developing new diagnostic tools have been observed over the past few years, in part due to the development of new treatments such as tafamidis (Vyndaquel) and patisiran (Onpattro) for ATTR.6,7
Although definitive diagnosis of CA has traditionally been established through endomyocardial biopsy (EMB), a large multicentric study has recently demonstrated that EMB can be avoided in a significant number of patients with the use of bone scintigraphy with 99mTc-PYP or 99mTc-DPD.8 Indeed, molecular imaging with these bone agents has a near-perfect sensitivity (> 99%) for the detection of ATTR.8 Furthermore, it was shown that scintigraphy could reliably distinguish between AL and ATTR, as uptake is highly selective for ATTR.8,9,10,11 This distinction is crucial as the management and outcome of patients AL and ATTR differ significantly.
Recently, there has been growing interest in using 18F-sodium fluoride (18F-NaF) for the diagnosis of ATTR.12,13,14,15 Unlike the other bone-seeking agents traditionally used for ATTR, 18F-NaF is a positron emission tomography (PET) tracer, which offers improved imaging properties compared to 99mTc-based tracers, as well as allowing for accurate quantification. These features could potentially lead to enhanced diagnostic performance, especially at the early stages of the disease; however, it has been established that the diagnostic performance of the various bone-seeking radiopharmaceuticals are not all equivalent thus necessitating an assessment of the diagnostic performance of 18F-NaF for this application.16 The purpose of this study is to examine the role of 18F-NaF imaging in the diagnosis of ATTR.
Methods
Patient Cohort
This retrospective study was approved by our institutional review board, and the requirement to obtain informed consent was waived. Medical records of subjects who underwent a 18F-NaF PET/CT study for assessment of CA between October 2017 and September 2018 at the Institut de Cardiologie de Montréal were reviewed. All subjects also underwent same day rest perfusion imaging with either 82Rb-PET/CT or 99mTc-tetrofosmin SPECT/CT. Rest perfusion imaging was systematically performed in all subjects undergoing 18F-NaF PET/CT study for assessment of CA. These images were used to provide myocardial contours for quantitative analyses, as described below. Subjects who underwent 18F-NaF PET/CT along with a perfusion study within 30 days for reasons other than CA evaluation were also included as controls. Subjects with incomplete studies were excluded.
PET/CT Acquisitions
PET/CT scans were performed on hybrid 3D PET/CT scanner (Siemens Biograph mCT Flow 40 with TrueV or Siemens Biograph mCT Flow Edge with TrueV, Knoxville, TN, USA). For 18F-NaF imaging, dynamic cardiac acquisition of 30 minutes was performed, 60 minutes following injection of approximately 370 MBq of 18F-NaF. A low-dose CT for attenuation correction was acquired following NaF acquisition. For subjects who underwent resting imaging with 82Rb PET/CT, imaging was performed on the same scanners with a 6 min PET acquisition using a 30 seconds infusion of 10 MBq/kg (RUBY-FILL, Jubilant DRAXIMAGE Inc., Kirkland, Québec, Canada). Another low-dose CT for attenuation correction was acquired following rest perfusion imaging. Global rest myocardial blood flow (MBF) values were quantitated using Corridor4DM (INVIA Medical Imaging Solutions, Ann Arbor, Michigan, USA), which relies on one-tissue-compartment model with flow-dependent extraction correction.17
18F-NaF PET/CT Interpretation
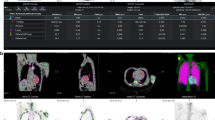
Images were reviewed independently by two certified nuclear medicine specialists (FH and MPG), blinded to all clinical information, using syngo.via VB30 software (Siemens Healthcare, Germany). Disagreements were resolved by consensus. To perform quantitative analyses, LV segmentation was first performed on the rest perfusion images in order to define the LV contours. After registering the individual CTs performed on the corresponding perfusion and 18F-NaF studies, the segmentation performed on the rest perfusion image was applied on the 18F-NaF PET/CT study in order to define matching LV contours. Regions were manually repositioned to exclude uptake from the sternum or from valve calcifications (Figure 1). This method allowed for accurate segmentation of the LV on the 18F-NaF PET/CT studies as segmentation based on the 18F-NaF uptake proved to be inadequate in most cases due to the low myocardial uptake. A second region of interest was positioned in the LV cavity to estimate blood pool activity. Average standard uptake values (SUVmean) and target-to-background ratio (TBRmean) were extracted for the whole LV and for each myocardial segment. TBRmean was calculated by dividing myocardial SUVmean by blood SUVmean.
Example of the algorithm used to segment the left ventricle (LV) on the 18F-NaF PET/CT images. The LV contours drawn on the rest perfusion study were transferred to the 18F-NaF PET/CT studies using the CTs of both acquisitions as the registration reference. The region of interest was then manually repositioned to exclude areas of nonmyocardial uptake. In this example, the 18F-NaF PET/CT images demonstrate increased uptake in the basal segments of the LV as well as in the atria and right ventricular free wall
Gold Standard
The gold standard consisted of biopsy results or, when unavailable, final clinical diagnosis of ATTR was obtained by following the nonbiopsy algorithm which relies on the results of bone scintigraphy and serum protein electrophoresis (SPEP).8
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as frequencies. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared with Student’s two sample t test. When comparing multiple means, a one-way ANOVA was performed followed by the Holm-Sidak test. All statistical analyses were two-tailed, and a P value of < .05 was considered significant. All analyses were performed using GraphPad Prism (GraphPad Prism version 7.0 for Windows, GraphPad Software, California, USA).
Results
Study Cohort
The demographic, clinical, and imaging characteristics of the subjects with CA and control cases are presented in Table 1. The study cohort consisted of 7 subjects with ATTR, four with AL, and four controls. Genetic testing was performed in three subjects with ATTR and no TTR mutations were identified. The diagnosis of CA was confirmed by biopsy in all four AL subjects. For subjects with ATTR, the diagnosis was confirmed by EMB in three cases, while, for the remaining four subjects, the diagnosis was confirmed by positive bone scintigraphy with the absence of light-chain level abnormalities on SPEP. Two control subjects had a hypertrophic cardiomyopathy and two had an ischemic cardiomyopathy.
Subjective Interpretation
Consensus qualitative, gestalt interpretation resulted in four true-positive and three false-negative studies in those subjects with ATTR. Characteristics of subjects with false-negative studies are presented in Table 1. All four studies of the subjects with AL as well as the four controls were correctly interpreted as negative. Qualitative interpretation yielded a sensitivity of 57% (95% CI 18-90%) and a specificity of 100% (95% CI 63-100%) for the detection of ATTR. For the three false-negative studies in subjects with ATTR, the final diagnosis was established by EMB. In one subject with false-negative 18F-NaF PET/CT (ATTR subject #7), the study was interpreted as negative by both readers; however, an EMB confirmed the diagnosis of ATTR and a PYP study showed greater intense uptake than that surrounding bone structures corresponding to grade-3 uptake 10 on 3-hour planar and SPECT images, with a heart-to-background ratio of 1.9 on planar 1-hour images (Figure 2).
18F-NaF PET/CT study (top) of a patient with biopsy-confirmed ATTR. The 18F-NaF PET/CT study was interpreted as negative by both readers. Myocardial uptake is below surrounding blood pool activity with a TBRmean of 0.93. A PYP-SPECT study revealed Grade 3 uptake on the 3-hour planar and SPECT images (bottom)
The interreader agreement was very good with a κ = 0.84 (95%CI: 0.55-1.00). In one case only did the readers’ interpretation differed. After consensus interpretation, the study (of a subject with biopsy-proven AL) was correctly interpreted as negative for ATTR.
Quantitative Interpretation
The average SUV over the entire LV (SUVmean) did no differ significantly (Figure 3, P = .14) in ATTR subjects (1.31 ± 0.30) when compared to AL subjects (0.84 ± 0.55) or controls (1.29 ± 0.21). On the other hand, average LV TBRmean was significantly greater (Figure 3) in ATTR subjects (0.98 ± 0.09) compared to both AL (0.85 ± 0.08, P = .026) and control (0.82 ± 0.07, P = .020) subjects. In four (57%) ATTR subjects, TBRmean was inferior to 1.0, indicating LV uptake inferior to blood pool activity. TBRmean remained essentially stable between 60 and 90 min post tracer injection in all the three groups (Figure 4). The average TBRmean of the 17 LV segments is presented in Figure 5. In ATTR subjects, the basal segments showed increased TBRmean compared to the mid and apical segments, while uptake values in the subjects with AL and the controls were more homogeneous.
ROC analysis of TBRmean yielded an AUC of 0.91 ± 0.08 (P = .0078) with optimal sensitivity and specificity of 75% (95%CI: 35-97%) and 100% (95%CI: 59-100%) using a cutoff TBRmean value of 0.89 for the diagnosis of ATTR. ROC analysis of SUVmean yielded an AUC of 0.66 ± 0.14 (P = .30). The difference between TBRmean and SUVmean AUC did not reach statistical significance (P = .12).
Rest Perfusion Imaging
Rest perfusion imaging was performed with 82Rb PET/CT in 6 (86%) ATTR subjects, 4 (100%) AL subjects, and 3 (75%) control subjects, and 99mTc-tetrofosmin SPECT/CT was performed in the remaining subjects. For those who underwent 82Rb PET/CT rest perfusion imaging, rest MBF values, in mL/min/g, were not significantly different (P = .11) between ATTR (0.57 ± 0.05), AL (0.68 ± 0.13), and control subjects (0.80 ± 0.26).
Discussion
The results of this study suggest that, while visual analysis of 18F-NaF PET/CT shows limited sensitivity in cases of ATTR, quantitative analysis results in moderate sensitivity. In comparison, our results show that gestalt interpretation of 18F-NaF PET/CT yielded a very low sensitivity but a very good interreader agreement. Furthermore, we found that TBRmean was superior to SUVmean for distinguishing ATTR from AL and controls.
Overall, the literature examining the use of 18F-NaF PET in subjects with CA is quite limited (Table 2). Although a case report described negative 18F-NaF PET in two subjects with ATTR,18 two small prospective studies showed promising results. In 12 subjects, Morgenstern et al. showed that uptake in ATTR was significantly greater than in AL and controls.12 Triviery et al. obtained similar results in 14 subjects.13 Of note, myocardial uptake values in both studies were relatively mild, highlighting the importance of accurate quantitative assessment. In addition, the imaging protocols used in these studies varied, with acquisitions performed between 15 and 90 min post-IV injection (PIV).
Although the degree of myocardial 18F-NaF uptake is significantly greater in ATTR compared to AL and control subjects, the degree of uptake was quite modest and, in a majority of subjects (57%), inferior to that of blood pool. This is in striking contrast with PYP which has been shown to demonstrate intense myocardial uptake in ATTR, resulting in near-perfect sensitivity with high interreader agreement.8 Moreover, there was significant overlap between the TBRmean of subjects with ATTR and those with AL (Figure 3), limiting its ability to distinguish between the two subtypes of CA.
Similar to other studies using 18F-NaF PET/CT for the evaluation of CA,12,13 the present study relied on SUVmean and TBRmean rather than SUVmax and TBRmax. We chose to perform quantitative analyses on mean values to avoid issues related to the intense uptake that can be encountered in the presence of valvular calcification, contamination from sternal uptake, and coronary calcifications. When these are present, 18F-NaF uptake can be very intense with uptake blooming into adjacent myocardium, leading to spurious quantification, and can result in an overestimation of the true myocardial uptake. Relying on mean values instead of maximum values can help minimize the impact of such contamination. This is especially critical given the very faint uptake observed in some ATTR subjects.
When using a quantitative approach, an optimal TBRmean cutoff of 0.89 was obtained for the diagnosis of ATTR. This value is comparable with the TBRmax of 0.84 obtained in another recent study by Trivieri et al..13 In both cases, the threshold value is clearly below nearby blood pool activity, emphasizing the importance of quantitative analysis. Indeed, when relying on quantitative analyses, the sensitivity of 18F-NaF PET/CT improved from 57%, for visual interpretation alone, to a sensitivity of 75%. The very faint uptake seen in some ATTR subjects confirms the necessity of using quantitative analysis to support the interpretation of 18F-NaF PET/CT studies as the distinction between degrees of uptake inferior to blood pool is exceedingly challenging without proper quantitative tools. Figure 2 illustrates an example for which both readers interpreted the study as negative for ATTR, while the quantitative analysis correctly classified the subject as positive for ATTR-with a TBRmean of 0.93.
Interestingly, the distribution of 18F-NaF uptake observed in ATTR subjects (Figure 5) shows a basal predominance with relative sparing of the apical and peri-apical segments. This distribution corresponds to a pattern also described on echocardiography where impaired LV longitudinal strain with apical sparing, the so-called cherry-on-sundae pattern, can be observed in patients with CA.19,20
In this study, SUVmean, as opposed to TBRmean, could not be used to discriminate between ATTR versus AL and control. This can likely be explained by the very low uptake seen in ATTR, which translated to low SUV values. Indeed, several technical and biological factors not related to the disease state are affecting SUV values, and when not correcting for blood pool activity, the amplitude of those variations is greater than the actual uptake related to CA.
In light of the results of this study, molecular imaging with PYP or DPD remains the imaging modality of choice to use in the investigation of ATTR, and in order to differentiate between ATTR and AL. Diagnostic performance is such that when using PYP and DPD imaging, diagnosis of CA can be confirmed without biopsy in significant proportion of subjects.8 It is well known that the diagnostic performances of SPECT bone-seeking radiopharmaceuticals are not all equal. For example, it has been shown that methylene diphosphonate (MDP) is less sensitive for the detection of ATTR compared to PYP.16 Although different hypotheses have been proposed to explain the relatively low uptake of 18F-NaF in ATTR compared to other bone tracers, the reasons for these differences in uptake between the various bone-seeking radiopharmaceuticals remain unclear. For example, it has been proposed that variable uptake intensity could be linked to the etiology of CA (mutant vs wild-type), to the stage of the disease (early vs late), or to the composition of the calcium matrix.21 The results of this study suggest that 18F-NaF PET/CT does not share the high sensitivity of PYP or DPD imaging and therefore may be of limited utility in the assessment of ATTR. Furthermore, whether a clearly positive 18F-NaF PET/CT study in the absence of monoclonal gammapathy can be used to confirm the presence of ATTR has not been proven, and more studies are required to evaluate the diagnostic utility of this tracer, as well as to directly compare its accuracy with PYP or DPD.
The rest MBF values of ATTR subjects were on average inferior to the resting MBF values of AL and control subjects, but the difference did not reach statistical significance, likely related to the small sample size. This finding is in keeping with the results of another study demonstrating lower resting MBF in patients suffering from CA.22
This study suffered from several limitations. This was a retrospective study and was subject to the limitations of this study design, the overall number of subjects was also limited but is generally on par with other studies on CA imaging. An additional limitation was the fact that four ATTR subjects lacked biopsy confirmation of CA. For those subjects, the final diagnosis was established by the validated, noninvasive approach methods which rely on the presence of abnormal uptake on bone scintigraphy.8 This introduces a bias which can lead to overestimation of the specificity and therefore the accuracy. Nonetheless, the main finding of this study is that the modest myocardial accumulation of 18F-NaF in subjects with ATTR limits sensitivity.
New Knowledge Gained
The results of this pilot study suggest that the diagnostic sensitivity of 18F-NaF for ATTR may be improved by making use of quantitative analysis; however, the overall sensitivity of the test remains inferior to the reported sensitivities of PYP and DPD SPECT.
Conclusion
While 18F-NaF PET/CT can discriminate between ATTR vs AL and controls, particularly when using quantitative analysis, it appears to be less sensitive than that established in nuclear medicine studies with PYP or DPD. Although 18F-NaF PET/CT shows statistically higher TBR in ATTR compared to AL, the low TBR values observed in ATTR subjects (< 1.0) indicate poor myocardial signal, below that of blood pool activity. As such, 18F-NaF PET is not yet ready for clinical use in CA, and further studies are needed to confirm its utility for the assessment of ATTR.
Abbreviations
- AL:
-
Light-chain amyloidosis
- ATTR:
-
Transthyretin cardiac amyloidosis
- CA:
-
Cardiac amyloidosis
- cMRI:
-
Contrast enhanced magnetic resonance imaging
- DPD:
-
Diphosphonopropanodicarboxylic acid
- EMB:
-
Endomyocardial biopsy
- LVH:
-
Left ventricular hypertrophy
- MDP:
-
Methylene diphosphonate
- NaF:
-
Sodium fluoride
- PYP:
-
Pyrophosphate
- SUV:
-
Standard uptake value
- TBR:
-
Target-to-background ratio
References
Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol 2015;24:343-50. https://doi.org/10.1016/j.carpath.2015.07.008.
Fikrle M, Paleček T, Kuchynka P, et al. Cardiac amyloidosis: A comprehensive review. Cor et Vasa 2013;55:e60-75. https://doi.org/10.1016/j.crvasa.2012.11.018.
Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging 2014;7:552-62. https://doi.org/10.1161/CIRCIMAGING.113.001396.
Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc 2012;60:765-74. https://doi.org/10.1111/j.1532-5415.2011.03868.x.
Bhogal S, Ladia V, Sitwala P, et al. Cardiac Amyloidosis: An Updated Review With Emphasis on Diagnosis and Future Directions. Curr Probl Cardiol 2018;43:10-34. https://doi.org/10.1016/j.cpcardiol.2017.04.003.
Maurer MS, Schwartz JH, Gundapaneni B, et al (2018) Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med https://doi.org/10.1056/NEJMoa1805689.
Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 2018;379:11-21. https://doi.org/10.1056/NEJMoa1716153.
Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016;133:2404-12. https://doi.org/10.1161/CIRCULATIONAHA.116.021612.
Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J Am Coll Cardiol 2005;46:1076-84. https://doi.org/10.1016/j.jacc.2005.05.073.
Hutt DF, Quigley A-M, Page J, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging 2014;15:1289-98. https://doi.org/10.1093/ehjci/jeu107.
Bokhari S, Castaño A, Pozniakoff T, et al (2013) 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidosesclinical perspective. Circulation 6:195-201. https://doi.org/10.1161/CIRCIMAGING.112.000132.
Morgenstern R, Yeh R, Castano A, et al. 18 Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol 2017;25:1-9.
Trivieri MG, Dweck MR, Abgral R, et al. 18F-sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 2016;68:2712-4.
Van Der Gucht A, Galat A, Rosso J, et al. [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol 2016;23:846-9.
Gallegos C, Miller EJ. Use of fluorine-18 sodium fluoride for the diagnosis of transthyretin cardiac amyloidosis: The birth of a new technique. J Nucl Cardiol 2018;25:1568-70. https://doi.org/10.1007/s12350-017-0847-6.
Rapezzi C, Gagliardi C, Milandri A (2018) Analogies and disparities among scintigraphic bone tracers in the diagnosis of cardiac and non-cardiac ATTR amyloidosis. J Nucl Cardiol https://doi.org/10.1007/s12350-018-1235-6.
Lortie M, Beanlands RSB, Yoshinaga K, et al. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765-74. https://doi.org/10.1007/s00259-007-0478-2.
Gagliardi C, Tabacchi E, Bonfiglioli R, et al. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol 2017;24:746-9.
Falk RH, Quarta CC. Echocardiography in cardiac amyloidosis. Heart Fail Rev 2015;20:125-31.
Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442-8.
Ng QKT, Sethi P, Saunders TA, et al. Discordant Findings on 18F-NaF and 99mTc-HDP Bone Scans in a Patient With ATTR Cardiac Amyloidosis. Clin Nucl Med 2018;43:e89-92. https://doi.org/10.1097/RLU.0000000000001933.
Dorbala S, Vangala D, Bruyere J, et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC 2014;2:358-67. https://doi.org/10.1016/j.jchf.2014.03.009.
Disclosures
Patrick Martineau, Vincent Finnerty, Geneviève Giraldeau, Sébastien Authier, Francois Harel, and Matthieu Pelletier-Galarneau have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martineau, P., Finnerty, V., Giraldeau, G. et al. Examining the sensitivity of 18F-NaF PET for the imaging of cardiac amyloidosis. J. Nucl. Cardiol. 28, 209–218 (2021). https://doi.org/10.1007/s12350-019-01675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01675-2