Abstract
The function of the heart is defined by its ability to deliver adequate cardiac output to meet the requirements of the body both at rest and with exertion. To fill this role, the heart demonstrates an impressive capacity to tightly regulate energy generation and consumption. Energy production and transfer within cardiac myocytes primarily relies on the process of oxidative phosphorylation. In the failing heart, there is an imbalance between the work of the cardiac system and the energy required to generate this work. This presence of this mismatch has given rise to the concept known as the energy starvation theory. This concept encapsulates observations such as perturbed substrate consumption, insufficient energy transfer and ingestion, reduced substrate and oxygen availability, and diminished energy production in the failing heart. Diminished available cellular energy may further result from a reduction in the biosynthesis of mitochondria and their protein synthesis and from global cellular architectural disarray. In essence, the energy starvation theory posits that cardiac pump function declines due to a reduction in oxygen and substrate availability, and thus leads to a total body starvation of systemic energy. This novel cognitive framework has led to encouraging new directions in a “metabolic therapeutic approach” for the failing heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart has the highest energy consumption of any organ in the body. Energy is stored in the form of two molecules: adenosine 5′-triphosphate (ATP) and creatine phosphate (PCr). These high-energy phosphate molecules are rapidly and reversibly converted to creatine and adenosine 5′-diphosphate (ADP) by the creatine kinase (CK) reaction. The rate of ATP consumption by the heart is around 1 mM/s; at this rate, the ATP and PCr content must be renewed approximately every 20 s. Cardiac myocytes generate more than 90% of their energy through oxidative phosphorylation. Therefore, there is a tight coupling between oxygen utilization and cardiac work, e.g., the biochemical processes producing cardiac energetic fuel are tightly tied to energetic demand and able to rapidly adapt to energetic requirements.
Heart failure (HF) is defined as an inability of the heart to pump sufficient blood to meet the metabolic demands of the body at normal filling pressures [1] and is associated with reduced exercise capacity and increased fatigue and dyspnea. The increased fatigue with low levels of exertion is related to biochemical energetic failure of both cardiac and skeletal muscles. This may result from decreased oxygen and substrate delivery due to coronary artery disease, peripheral arterial atherosclerosis, chronic hypoxia, or mitochondrial defects induced by endogenous or exogenous factors. Other causative factors may include a higher workload of the myocardium due to hypertension, acute or chronic anemia, hyperthyroidism, septic shock, and adverse remodeling of cardiac structure due to structural or valvular heart disease leading to an inefficient use of energy. As a consequence of pump dysfunction, oxygen and metabolic substrates are not sufficiently systemically delivered, and the products of metabolism are not efficiently removed. This generates a deleterious energetic state in the organism in general and the heart in particular. In essence, HF reduces the peak oxygen and substrate accessibility for the individual organs and tissues.
In any form, prolonged stress to the cardiac myocytes initiates metabolic, functional, and anatomical adaptations in an attempt to maintain stroke volume and cardiac output. However, when the stress surmounts these adaptations, these initially advantageous adaptations lead to adverse effects such as hypertrophy, ventricular dilatation, contractile dysfunction, and eventually HF. Activation of intracellular signaling cascades, escalating mechanical load, inappropriate neuroendocrine activation, and extracellular remodeling result in the well-described consequences of HF. Many of these causative factors increase cardiac energy utilization and, hence, worsen the mismatch between energy availability and requirement. This cognitive framework has given rise to the energy starvation model of heart failure. There are two possibilities underlying energy starvation: either ATP turnover rate is diminished or total tissue content of ATP is reduced. This is an important distinction since the rate of ATP turnover does not correlate with the total ATP content of the heart. The energy starvation model has been proposed as the fundamental pathophysiologic underpinning of progressive HF and has provided promising new therapeutic directions [1,2,3].
Since recent studies have extensively reviewed the role of mitochondrial dysfunction in the failing heart [4], this review will focus on substrate metabolism, namely that of glucose and fatty acids in normal and failing hearts, mitochondrial ROS in the failing heart, and remodeling in the pentose phosphate pathways and hexosamine biosynthetic pathways (HBP). We will further elaborate on the therapeutic approaches which leverage this novel metabolic model to treat heart failure.
Due to constraints of focus, some contents have not been incorporated in this review such as the effects of the selective CK isoform knockout [5] and cardiac ketone and amino acid metabolism [6, 7].
Substrate metabolism
The heart’s preferred oxidative substrate is fatty acids. However, it is able to oxidize other substrates including carbohydrates, ketone bodies, lactate, and amino acids. This metabolic flexibility allows the heart to adapt readily to alterations in workload, substrate supply, and energy metabolism by switching to the appropriate substrate for the appropriate conditions [8]. Below is a brief summary of this topic to provide a framework of cardiac energetics.
As the heart hypertrophies in response to states of pressure or volume overload, myocardial energy metabolism transitions from mitochondrial oxidation to glycolysis. This metabolic transition depends upon the cause of the physiological mechanism leading to hypertrophy rather than on the state of hypertrophy itself. The mechanism may be either pathological (e.g., hypertension, aortic stenosis), physiological (e.g., exercise), or both. However, while both hypertension and exercise result in ventricular hypertrophy, hypertension reduces cardiac mitochondrial fatty acid oxidation (FAO) and exercise augments FAO capacity [9].
In the human heart, a slightly elevated or near normal rate of FAO is observed in the early stages of HF. In contrast, significant downregulation of FAO is observed in advanced or end-stage HF. FAO downregulation is related to downregulation of the peroxisome proliferator-activated receptor and retinoid X receptor pathway [10, 11]. In the state of hypertrophy, glycolysis and its enzymatic activity increases and these alterations swiftly reduce the rates of oxidation of free fatty acid and its enzymatic expression and activity [11, 12]. In advanced stages, this adaptation causes a deleterious biochemical state. Similar to the anatomical remodeling noted in end-stage HF, this metabolic remodeling becomes increasingly pathologic and causes further reductions in myocardial fatty acid oxidative capacity, ultimately leading to reduced energetic efficiency and a failure of myocardial metabolic reserve [13]. Observations suggest that the anatomic and physiologic cardiac remodeling and contractile dysfunction distinctive of HF is, in fact, caused by this metabolic remodeling. This led to a deeper investigation of the biosynthesis, regulation, and role of the organelle responsible for the majority of myocardial substrate metabolism—the mitochondria.
Biosynthesis of mitochondria
An imbalance between mitochondrial biosynthesis and degeneration may lead to a reduction in oxidative power of the failing heart. Recently, mitochondrial biosynthesis has become of increased interest as it has a broad-ranging impact on the regulation of metabolic processes [14]. Biosynthesis of mitochondria occurs via development and splitting of pre-existing organelles. Mitochondrial biosynthesis is complex and involves the ingress of mitochondrial proteins, biosynthesis of phospholipid, dynamic changes in the mitochondrial network, and mitochondrial protein synthesis and their regulation. The mitochondria carry their own genetic code which translates 13 proteins. However, >98% of the proteins required for mitochondrial function is translated by the nuclear genome. Thus, to assure appropriate biosynthesis of mitochondria, a tight special and temporal relationship is required between the import of nuclear-translated protein, innate protein synthesis, and assemblage of mitochondrial-translated proteins. Moreover, the fusion and fission mechanisms of mitochondria and replication of mitochondrial DNA must be coordinated [15]. Any defect in this complex biosynthetic process may lead to flawed mitochondrial function and a decrement in the contractile function of the heart. For example, aortic-constriction-induced systolic dysfunction causes not only impaired morphology and reduced volumetric measures of mitochondria but also perturbed expression of most proteins involve in the electron transport chain (ETC) [16, 17].
Impairment of mitochondrial DNA
The reduction in mitochondrial DNA-encrypted proteins detected in HF might be due to an impairment of mitochondrial DNA content. Studies of this hypothesis have returned with mixed findings so far. A murine myocardial infarction model exhibited a decline in mitochondrial DNA copy number [18]. In contrast, a subsequent similar study failed to note a reduction in the mitochondrial DNA [19]. Observational studies have noted that patients with either idiopathic or ischemic dilated cardiomyopathy have unimpaired mitochondrial DNA [20, 21]. In contrast, a recent observational study suggested that a defect in mitochondrial biosynthesis occurs through not only reduced mitochondrial DNA replication but also through a reduction of mitochondrial DNA content in human HF [22]. Oxidative stress is elevated in HF, including a sustained increase in oxygen free radical synthesis in the mitochondria. This may result in a loss of mitochondrial DNA, subsequent impairment of expression of these encoded genes, and defective respiratory chain complexes [18, 23, 24]. Mitochondrial-targeted antioxidant therapy may avert the loss of mitochondrial DNA, left ventricular remodeling, and HF after MI [25].
Mitochondrial transcription factor A (TFAM) is necessary for replication and transcription of mitochondrial DNA (mtDNA), and a decline in the expression of TFAM is detected in HF. Observational studies have found that TFAM regulates mitochondrial DNA copy number [26] and that oxidative phosphorylation is regulated by the quantity of mitochondrial DNA [27]. Moreover, impairment in the replication process of the mtDNA may lead to defective mitochondrial biosynthesis and function in failing hearts [22]. Intriguingly, it has been demonstrated that TFAM overexpression can mitigate the reduction in mtDNA copy number in HF [28, 29].
Impairment of mitochondrial protein
Mitochondrial biosynthesis also includes the construction and folding of nuclear-encrypted mitochondrial proteins [30, 31]. It has been observed that either chaperone protein mutation or deficiency leads to a breakdown in this intricate process and ultimately the abnormal trafficking of mitochondrial proteins causing cardiomyopathy [32, 33].
Impairment of phospholipid synthesis of the mitochondria
Phospholipids play an important role in the structure and function of mitochondrial membranes [34]. Aside from simply acting as a cellular or organelle boundary, phospholipids act as a key regulatory component in critical metabolic processes including electron transport, apoptosis, and mitochondrial lipid and protein importation. Cardiolipin in particular plays a central role in the activity of various inner membrane proteins and in the binding of CK to the mitochondria. Impaired cardiolipin and acyl chain content has been shown to cause mitochondrial dysfunction in HF [35, 36]. The causal role of impaired cardiolipin content/synthesis is currently debated. A study suggests that chronic adrenergic stimulation of mitochondrial calcium-independent phospholipase A (2) may play key role in the dysregulation of cardiolipin [35]. The regulation of mitochondrial respiration via cardiolipin is enacted by thyroid hormones and AMPKα2 [34, 37]. Further studies are needed to reveal the exact role that mitochondrial phospholipid biosynthesis regulation plays in the underlying pathology of HF.
Regulation of biosynthesis of mitochondria in the healthy heart through mitochondrial protein expressions
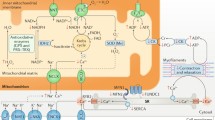
Figure 1 shows the network of co-regulators and DNA-binding transcription factors that control various genes translating mitochondrial proteins serving regulatory or functional roles in the mitochondria. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a key controller of energy metabolism. PGC-1α interacts with estrogen receptor-related receptor (ERRs), peroxisome proliferator-activated receptors (PPARs), and nuclear respiratory factor (NRFs), to augment oxidative phosphorylation and fatty acid oxidation in the mitochondria [15, 38]. Both PPAR α and δ genes, transcripting FAO enzymes, play an important role as transcriptional regulators [39]. PGC-1α also activates the transcription of the aforementioned nuclear-encoded TFAM, which interacts with the mitochondrial RNA polymerase and eventually activates the transcription and replication of mitochondrial DNA [15, 40]. A subset of PGC-1α target genes are upregulated by ERRα and participate in fatty acid oxidation in mitochondria and peroxisomes, mitochondrial respiration, and cellular fatty acid transport [41]. Gene expression of PGC-1α and PGC-1β is tightly correlated with the mitochondrial oxidative capacity. Functional defects in either PGC-1α or PGC-1β in a mouse model lead to no phenotypic changes in the heart, illustrating that the mitochondria can compensate for the loss of these co-activators [39, 42]. However, observations reveal that the PGC-1α content of mitochondria is linked with the protein transformation and mitochondrial metabolic dynamics [43, 44]. Moreover, mitochondrial biosynthesis relies on protein expression regulations, particularly for fusion and fission. The dynamin-related protein 1 (Drp1) and its receptor Fis1 facilitate the fission process. The mitofusin (Mfn1 and 2), optic atrophic type 1 protein (OPA1), and dynamin-related proteins facilitate the fusion process [45]. It has been suggested that stress (such as hypertrophy, ischemia, and HF) interferes with the crucial biosynthetic processes of mitochondrial fusion/fission, perhaps through dysregulation of the expression of these key proteins [46, 47]. This evolving field requires further study in developed cardiomyocytes.
Regulation of biosynthesis of mitochondria in the failing heart through mitochondrial protein expressions
In HF, a reduction in cardiac oxidation power causes a decline in mitochondrial function and PGC-1α expression as well as NRFs and TFAM transcription factor content [19]. Various experimental studies have confirmed the reduced expression of cardiac PGC-1α and mitochondrial proteins in HF [15, 48,49,50]. In contrast, the causal role of PGC-1α in human HF cases is inconsistent in studies [51, 52]. The inconsistency in observations between animal models and humans with HF might be due to the type and/or the severity of the ailment, the paucity of similar age controls, the gender discrepancies, and the varying medical management of patients. However, a reduction in ERRα gene level is consistently and clearly involved in a reduction of mitochondrial metabolic potential in human HF [53]. Dilated cardiomyopathy patients exhibited a reduced expression pattern of PGC-1β [21], and experimental models of HF have demonstrated reduced expression of both PGC-1β and ERRα. This observed simultaneous decline in expression of PGC-1α and PGC-1β genes emphasizes the significance of a mitochondrial biosynthetic impairment in the underlying disease. In summary, a reduced expression of the PGCs/PPARs/ERRs group contributes to a global reduction in myocardial energetics, contributing to HF.
As opposed to the above pathways, the transcriptional regulation of the CK system is not currently well-defined. Observational studies suggest that the activity of muscle CK (M-CK) is highest in tissues with the lowest mitochondrial volume density and lowest oxidative capacity (e.g., fast twitch or “white” muscle). In contrast, higher mitochondrial isoform of CK (mi-CK) activity is noted in tissues with higher oxidative capacity (e.g., slow twitch muscle) [54]. It is speculated that the mitochondrial biosynthesis transcription cascade controls the expression of the mitochondrial protein mi-CK. In PGC-1α knockout mice, both skeletal and cardiac muscles demonstrated a significant reduction in the expression of genes involved in fatty acid oxidation and ATP production, including in mi-CK expression [55, 56]. Moreover, ERRα and γ also help to regulate the expression of mi-CK [57]. All these finding suggest that a deficiency in PGC-1α transcriptional flow and reduction in mitochondrial DNA replication lead to a defect in mitochondrial biosynthesis in HF.
Autophagy and mitochondrial turnover
The mitochondria contain extensive protein quality control processes including autophagy, the selective elimination of decaying mitochondria [58]. Further quality control included mitochondrial turnover through the processes of fusion and fission, which occur simultaneously. Extensive mitochondrial turnover through fusion/fission may lead to derangements in mitochondrial metabolic dynamics and morphology in HF, causing a further imbalance in the autophagytic system, and eventually generating dysfunctional mitochondria [58]. This hypothesis is still underexplored. Evidence for the role of poor mitochondrial quality and their regulatory pathways defined by PINK-PARKIN phenomenon for mitochondrial autophagy in the heart has been well described in recent reviews [59, 60] which suggest that PINK deficiency is causal to LV dysfunction and PARKIN deficiency increases susceptibility to LV failure following MI.
Role of the mitochondria
Role of the mitochondria in the healthy heart
The mitochondria are arranged within the sarcolemma and myofilaments with adequate and appropriate diffusion space in cardiomyocytes. This anatomical association allows for optimal bioenergetic generation for contraction [61]. The myocardium demonstrates impressive energetic efficiency—there is no surplus of energy generation versus energy consumption. The constant interaction between oxygen utilization and cardiac work generates a steady state of global cellular PCr and ATP content [62]. Experimental modeling confirms that the content of metabolites within the myocyte even changes in response to the normal respiratory cycle [63]. The requirement of maintaining overall metabolic homeostasis during pathologic cardiac remodeling enforces considerable energetic pressure on every myocyte. The process of homeostasis induced by metabolic remodeling is still controversial [62, 63]. Hence, robust biochemical signaling pathways maintain the balance between energy utilization and oxygen consumption, but the exact roles of such signals are still ambiguous. In this area, two mechanisms are proposed. First, aerobic metabolism and cardiac work is regulated via calcium as it controls sarcoplasmic reticulum and myosin ATPase activity. Second, an augmentation of cardiac work and oxygen consumption occurs at constant intracellular Ca+2 concentrations due to enlargement in cardiomyocyte length in accordance with the Frank-Starling mechanism [62, 64]. Thus, the speculation that cellular respiration and myocardial contraction are completely controlled by Ca+2 concentration is incomplete. Thus, the mitochondrial dehydrogenases and F0/F1 ATPase system must also be involved in the maintenance of metabolic homeostasis [65].
The second regulatory mechanism is based on the presence of energetic microdomains at both energy production and consumption cellular sites. The cardiomyocyte is not composed of a homogenous cytoplasmic milieu [66], and both ATP synthesis and consumption are not strictly stochastic. Rather, energetic processes are tightly spatially and temporally coordinated within dedicated structural and functional moieties. Phosphotransfer kinases and an elegant cellular framework both facilitate the connections between these microdomains. Glycolytic enzymes are organized in the myofilaments and sarcoplasmic reticulum where they are involved in ATP synthesis using ion pumps [67]. The mitochondrial ADP in situ (in the sarcoplasmic reticulum) is significantly less than that in isolated mitochondria preparations [68]. This reduced sensitivity to high concentrations of ADP permits appropriate channeling of adenine nucleotides and proper control of oxygen utilization by ATPases. In fact, the cytoskeletal protein tubulin regulates respiration by reducing the penetrability of porin [69]. Cytoskeletal impairment has been shown to enhance sensitivity to ADP while inhibiting the precise regulation of mitochondrial oxidation [70]. The differentially organized CK isoenzymes are pooled near adenine nucleotide translocase (ANT) or ATPases allowing the interconnection between ATP synthesis and consumption. By viewing mitochondrial function through a system biological approach, we find that cytosolic metabolites play a key role in the regulatory mechanism of respiration and energy fluxes [63].
Role of the mitochondria in the failing heart
In the failing heart, it is unknown whether peak oxygen consumption is restricted by oxidative ATP generation capability or the capabilities of the ATPases to consume ATP. At rest, intracellular oxygen is not the limiting factor in utilization, but at extreme exertion, intracellular oxygen is the limiting factor [71]. Myocardial peak oxidative capability can be assessed by gauging the activity of enzymes such as cytochrome oxidase or citrate synthase. In experimental and clinical HF, correlations between cardiac function and diminutions in the activity of enzymes involved in the respiratory chain, citric acid cycle, or protein expression of F(0)F(1)-ATPase have been widely published [20, 72,73,74]. Moreover, a proteomic study has validated that remodeling in energy production and functional aberrations of mitochondria are implicated in the HF progression [75]. The genomic pattern reflects a downregulation of metabolic processes in rapid ventricular-pacing-induced HF, supporting the notion that metabolic remodeling is a primary, causative agent in underlying pathology [76, 77]. Furthermore, reduced ADP/O ratio has been detected and is associated with a 14% reduction in efficacy of the failing myocardium [78]. Moreover, the mitochondria within the failing hearts exhibit augmented vulnerability to reactive oxygen species and calcium. Permeability of the inner mitochondrial membrane increases drastically due to the presence of the high-conductance channel permeability transition pore (PTP) [79] and an early augmentation in PTP vulnerability is noted in the hypertrophic heart [80]. Augmented vulnerability of PTP to reactive oxygen species and calcium plays a significant role in accelerated necrosis and apoptosis of the cardiomyocyte. These transitions arise before any demonstrable alterations in mitochondrial respiratory properties demonstrating a phase of preclinical mitochondrial impairment. Isolation of mitochondria permits the study of the oxidative ability of the mitochondria. The isolation approach and the subsequent alterations in mitochondria must be accounted for when using isolated mitochondria in the study. This approach has been used to measure the decline in oxidation power in HF [51, 81,82,83]. The observed reduction in ADP sensitivity [84] seems to be associated with cytoskeletal alterations including augmented tubulin content, although a causal relationship remains murky. Furthermore, creatine expression is reduced leading to reduced creatine sensitivity, and mitochondrial CK activity leads to reduction in oxidative power. In the failing heart, this leads to a reduced capacity for myocardial energy production through oxidative phosphorylation. Both the reduced oxidative power and diminished contribution of mitochondria to contraction-relaxation coupling lead to energy starvation in the failing heart. Because of the tight relationship between exercise and oxygen utilization, the reduced oxidative power of the hypertrophic heart and the dysregulation between previously tightly maintained energy consumption sites both lead to a de facto functional restriction and contribute to cardiac malfunction.
Reservoir of energy
With the use of 31P nuclear magnetic resonance (NMR) spectroscopy and noninvasive measurements of PCr/ATP ratio of the whole heart, we are now able to acquire precise measures of the energetic milieu of the heart [85]. The cardiac PCr/ATP ratio varies from 1.8 to 2.1 in healthy human and animal models. This measurement of the PCr/ATP ratio reveals the rate of ATP production in mitochondria and the efficiency of energy transfer via creatine kinase [85,86,87,88,89,90,91]. This energetic assessment demonstrates that the failing heart is incapable of sustaining its energy reservoir. A reduction in PCr/ATP ratio and concomitant augment in measured ADP is observed in cardiac hypertrophic animal models [74, 92, 93] as well as in humans with HF [94,95,96,97,98,99]. In the early stages, this reduction in PCr/ATP is primarily due to a reduction in [PCr] up to 50 to 70%, while [ATP] is either conserved [89, 94, 95] or only moderately decreased by up to 30% [100]. Intriguingly, measurements are comparable between LVH and HF, indicating that in a pressure overload model, the PCr/ATP ratio begins to fall with the development of hypertrophy prior to the onset of a decrement in systolic function [93, 95]. A loss in total creatine and purine content has also been reported [99]. Importantly, the PCr/ATP ratio is well-correlated with clinical and hemodynamic factors and acts as a prognostic index in HF [101]. Furthermore, reduced high energy phosphate (HEP) concentrations are also seen in cardiac hypertrophy and may play a vital role in the perturbed energetics in hypertrophic cardiomyopathy and HF [99, 102]. The exact causal mechanisms connecting reduced PCr/ATP ratio and impaired myocyte contraction remain unclear. It may be that the observed reduction in PCr/ATP ratio is simply the result of perturbed energy fluxes rather than a casual abnormality. It seems likely that the reduction in PCr/ATP ratio and concomitant increase in ADP concentration both thermodynamically and kinetically affect the ATPases crucial to the systolic-diastolic cycle [103].
HEP transfer system
HEP transfer system in the healthy heart
The HEP transfer system is a vital characteristic of energy metabolism. The CK and adenylate kinase (AK) enzymes act as “energy shuttles” in this system [104] and transfer energy-rich phosphoryl moieties [85, 105,106,107]. CK occurs in different forms in cardiac and skeletal muscle cells and regulates the reversible relocation of an HEP group between ATP and creatine. Cytosolic CK occurs in the dimeric form comprised of two subunits, M and B, and generate three, MM, BB, and MB, isoenzymes. The mi-CK only occurs in the mitochondria and may be found in octameric and dimeric assemblies [108]. Mitochondrial CK composes 20 to 40% of the total CK activity in myocytes. Myofibrillar (MM-CK) is a structural myofilamental protein and is functionally associated with myosin ATPase, hence offering a crucial energy shuttle to allow peak force generation via optimal energy kinetics [109]. MM-CK is firmly attached to the membrane of the sarcoplasmic reticulum (SR) where it is functionally connected to the Ca2+-ATPase (SERCA), and assures a sufficient energy supply for calcium uptake [54]. During oxidative phosphorylation, synthesized ATP is transported by ANT in the intermembrane space leading to the transfer of a phosphoryl group by mi-CK to PCr and ADP through creatine. Consequently, ADP is accessible for the next round of oxidative phosphorylation [106, 108]. Creatine plays an important role in the regulation of cardiac mitochondrial respiration because of the firm coupling between mi-CK and ANT. Creatine augmentation reduces the apparent Km for ADP [110]. This permits the precise coupling between energy consumption and mitochondrial respiration. Mathematical modeling and CK flux measurements employing 31P NMR spectroscopy confirm the presence of confined adenine nucleotide lakes interconnected via intracellular energy transport by CK [111, 112]. Apart from the CK system, other phosphotransfer arrangements (e.g., the myokinase system) are also subdivided into compartments within cells [104]. The regulation of energy production relocation and consumption by phosphotransfer kinases permits the mitochondria to efficiently participate in the systole-diastole cycle.
HEP transfer system in the failing heart
Cumulative CK content is reduced by approximately 50% in HF and is reliably associated with ejection fraction [113]. A reduction in CK content has been implicated as causal in the decrement in contractile reserve in the failing heart [99]. In fact, many studies have detected that both MM-CK and mi-CK are diminished in clinical and experimental HF [1, 3, 114]. Impaired metabolism of cardiac HEPs is associated with reductions in mi-CK and mitochondrial proteins, and these all are associated with LV hypertrophy severity [93]. Furthermore, a reduction in mi-CK precedes hypertrophy and is now commonly accepted as a characteristic metabolic step in the progression of HF.
Energetic fluxes of CK can also be measured using 31P NMR spectroscopy. Impairment of the cumulative CK flux has been defined as an early hallmark of cardiomyopathies [3, 87, 88, 99]. An approximate 30–50% reduction in CK fluxes is observed in LV hypertrophy [93]. Concomitantly, 18O studies demonstrate that the myokinase system, which usually serves a lesser role in the regulation of ATP supply, is considerably augmented in compensation. Both experimental and clinical studies demonstrate that a reduction in CK flux is prevalent in HF [92,93,94,95, 115]. The progression from ventricular hypertrophy to HF is associated with a striking reduction in CK flux without any significant modification in metabolite content [94]. Indeed, the degree of reduction in CK flux is a better gauge of HF than the degree of ventricular hypertrophy. However, NMR measurements of cellular fluxes do not permit the isolation of differences in fluxes between the various isoforms of CK, nor can it identify differences between the cardiac chambers in energy fluxes [116]. No reports are available for fluxes of focused CKs with unidirectional phenomenon [111, 112].
Fatty acid metabolism in healthy hearts
Fatty acid (FA) metabolism is initiated in three primary steps: (i) FAs are engulfed by the cytosol, (ii) they pass through the mitochondrial membrane, and (iii) they reach the mitochondria for oxidation. The FAs’ cytosolic engulfing process is assisted by the plasma membrane FA-binding protein and CD36 (FA translocase protein) transporters [117, 118]. In the cytosol, esterification converts free FAs to fatty acyl-coenzyme A (CoA), and then on to triglycerides or long-chain acylcarnitine using carnitine palmitoyl transferase (CPT) I [117, 119]. In the heart, the turnover of triglyceride pool is usually high and epitomizes a vital source of fatty acyl-CoA [120]. Long-chain acylcarnitine enters into the mitochondrial matrix and again reverts to long-chain acyl-CoA using CPT II. There, β-oxidation can generate acetyl-CoA, FADH2, and NADH+H+ using acyl-CoA, FAD, and NAD+, respectively.
In skeletal muscle, impaired β-oxidation allows acylcarnitine to leak into the cytosol from the mitochondria and thereafter into the systemic circulation. Circulating acylcarnitine is involved in the pathophysiology of insulin resistance [121]. The predominant form of the muscle CPT isoform (CPT Ib) occurs in the normal adult heart [122]. But the augmented expression of liver CPT isoform (CPT la) has been observed in fetal and hypertrophic hearts [123]. The carboxylation of cytosolic acetyl-CoA generates manolyl-CoA, that act as an inhibitor of CPT I [124]. Malonyl-CoA can revert into acetyl-CoA with the help of malonyl-CoA decarboxylase [125]. This pathway has been studied as a treatment strategy for ischemic heart disease [126]. Another study reveals that the activity of CPT 1 can be controlled through malnonyl-CoA-independent processes [127].
Fatty acid metabolism in the failing heart
Diminished FA use has been observed in most studies of the failing heart. High-salt diet-stimulated failing heart and rapid pacing reduces the FA uptake [10, 128] and diminishes messenger RNA (mRNA) expression and translation of FA transporter proteins with contractile dysfunction [10, 128,129,130,131]. Another study reveals a reduction in the rate of β-oxidation and the expression of relevant enzymes during the early stages of contractile dysfunction [129]. Collectively, these observations are in agreement with the outcomes of several other studies observing hypertensive and abdominal aortic constriction models [132,133,134,135] but conflict with myocardial infarction and salt-sensitive models [128, 130]. In general, even prior to the appearance of overt anatomical and functional abnormalities, experimental and human studies prove that β-oxidation is diminished in the early stages of the failing myocardium.
Glucose metabolism in the healthy heart
Myocardium uses glucose primarily via by two approaches: (i) uptake of exogenous glucose and (ii) glucose derived from stored glycogen. Glucose transporters (GLUTs), (GLUT1 and GLUT4), facilitate transport of glucose into the cytosol. GLUT1 predominates in the fetal heart, and GLUT4 predominates in the adult heart. GLUT1 is involved in basic glucose uptake in many cell types, while GLUT4 facilitates glucose uptake primarily in striated muscle, including the myocardium [136, 137]. Glycolysis is the first step in glucose oxidation and produces pyruvate, NADH2, and ATP. This ATP is required for the prerequisite Ca2+ uptake into the sarcoplasmic reticulum required for diastolic relaxation [138, 139]. Pyruvate can be converted into lactate in the cytosol, or it can enter into the mitochondrial matrix where it is either oxidized into acetyl-CoA or converted into oxaloacetate or malate via a carboxylation step. Pyruvate dehydrogenase (PDH) is a multienzyme complex involved in the oxidation of pyruvate. The carboxylation of pyruvate is crucial in its role replenishing TCA cycle intermediate metabolites, a process known as the anaplerotic pathway [140]. Pyruvate’s conversion to malate via carboxylation is an essential anaplerotic process in the myocardium, and impairment in this process leads to contractile dysfunction [141].
Glucose metabolism in the failing heart
With contractile dysfunction, glucose uptake in the myocardium is diminished in a thoracic aorta constriction (TAC) mouse model of heart failure, unaffected in myocardial infarction rats, and augmented in salt-sensitive rats [128, 142, 143]. In the early stages of hypertrophy of an abdominal aortic constriction model, the rate of glycolysis is minimally augmented but glucose oxidation is unaltered [132, 144]. In aortic constriction rats, substrate oxidation appraisal reveals unaltered glucose oxidation compared at controls during the early stage of hypertrophy, with an eventual reduction in glucose oxidation as systolic dysfunction develops [129]. Recent observations suggest that perturbed glucose oxidation may contribute to mitochondrial impairment, reducing the expression of genes involved in glucose oxidation and inducing scarcity of the PDH complex [128, 145]. In contrast, an unaffected rate of glucose oxidation rate was observed in myocardial infracted rats with systolic dysfunction [143]. Interestingly, hypertensive rats during the early stages of cardiac hypertrophy exhibit an augmented level of glucose oxidation and flux of the PDH complex when compared to controls [134, 146]. Further, augmented glucose oxidation rates were observed in canine experimental models of heart failure and in idiopathic dilated cardiomyopathy patients [10, 147]. Many of these contradictory observations regarding the role of glucose metabolism in the failing myocardium are likely due to methodological discrepancies. Thus, while alterations in FA oxidation have been firmly connected to contractile dysfunction in the failing heart, the role of alterations in glucose oxidation remains unclear and may vary due to condition, organism, and unaccounted environment stimuli.
Mitochondrial ROS in the failing heart
Many studies have investigated the metabolic and structural changes in the failing heart, but very few studies have adequately examined the mechanism underlying the remodeling observed in HF. The failing heart exhibits augmented ROS levels, but the fundamental role of ROS in the pathology of HF remains unclear as the outcomes of human studies of antioxidant therapies remain inconsistent [148, 149]. An experimental study exhibited that angiotensin II augmented mitochondrial ROS levels, causing mitochondrial damage, mitogen-activated protein kinase activation, and eventually hypertrophy and fibrosis of cardiac myocytes [150]. Another study demonstrated that in an angiotensin II-induced cardiomyopathy mouse model, mitochondrial protein oxidative damage was increased, and administration of a mitochondrial-targeted antioxidant peptide led to a significant reduction in NADPH oxidase 4 (NOX4) expression and ameliorated myocyte fibrosis and the development of cardiomyopathy [151]. Importantly, in the initial study, the model was comprised of early-stage TAC mouse hearts which exhibited trivial diastolic dysfunction and no alteration in left ventricular ejection fraction [150] representing an early stage of compensated hypertrophy. These observations suggest that augmented mitochondrial ROS levels may be an inciting event to stimulating structural remodeling causing the clinical sequelae of hypertensive heart. Figure 2 illustrates the effects of ROS on myocardial energetics and their contribution to cardiac pathology. Complexes I and III of the ETC play a major role in the production of ROS within the mitochondria. Hence, alterations in the ETC may cause electron outflow leading to ROS production [152]. This augmented level of ROS production may subsequently affect ETC function, leading to a dysregulation cycle (Figs. 2 and 3). One study has linked augmented levels of ROS production and the increased use of FA in myocardial metabolism [153]. Higher levels of free FAs (possibly due to augmented lipolysis) have been observed in the failing heart [154]. Moreover, myocardial lipid accumulation has also been observed in experimental models of HF [155]. Consequently, the metabolic use of FA is detrimental for the failing heart because of higher ROS production [154]. However, experimental models lacking cardiac-specific acetyl-CoA carboxylase-2 demonstrate that augmented FA use does not adversely affect left ventricular remodeling after TAC. Moreover, failing hearts exhibited reduced FA oxidation. Hence, the precise mechanism underlying the increased production of mitochondrial ROS in the evolution of heart failure requires further research.
Outline of metabolic remodeling and suggested possible mechanisms connected to the development of the failing heart. Metabolic pathways are in green color arrows. Augmented/predominant pathways are represented by thick lines. Reduced pathways are represented by thin/dotted lines. The query symbols indicate currently unclear factors and effects
A graphical diagram of the connection between metabolism in myocytes and contractile function, exhibiting the function in the setting of a normal ATP production and b diminished ATP production. Magnetic resonance imaging reveals a low end diastolic volume (EDV) and end systolic volume (ESV), normal left ventricular mass (LVmass), and high ejection fraction (EF) in the healthy heart and b high LVmass, low EF, and high ESV in the failing heart. Dotted thin black lines indicate alternative pathways with minor role in contractile function. Thick continuous black lines indicate the major role of alternative pathways in contractile function. PPP pentose phosphate pathway, HBP hexosamine biosynthetic pathway, ROS reactive oxygen species
Remodeling in the pentose phosphate pathways in the failing heart
The pentose phosphate pathway (PPP) is an obligate source of NADPH, and it is well connected to substrate metabolism and tightly involved in the regulation of the cellular redox environment. Low levels of ROS are necessary for cellular propagation and survival signaling, and NADPH is a requisite in the production of cytosolic ROS. Therefore, the PPP may play a twofold role in the maintenance of redox homeostasis. Experimental animals with pacing-induced HF exhibited augmented superoxide levels and associated G6P dehydrogenase (G6PD) activity [156]. However, G6PD-lacking mice exhibited augmented levels of oxidative stress and diminished contractile function in MI and TAC condition [157]. A recent study reveals that the flux through the PPP gradually increases in the failing heart. Moreover, another study found that dichloroacetate (DCA) therapy augmented this flux and was associated with improved cardiac function [128]. In summation, the PPP is stimulated in the failing heart. Although this likely leads to a concomitant increase in superoxide creation, the augmented flux through the PPP in the failing heart may represent a salutary compensatory metabolic approach, and further augmentation of this pathways may be leveraged in a therapeutic strategy.
Remodeling in the hexosamine biosynthetic pathways in the failing heart
The metabolism of glucose, fatty acids, and amino acids is tightly connected with HBP. Observation of endothelial cells reveals that augmented flux through HBP leads to overproduction of superoxide within the mitochondria [158]. Hence, the generation of mitochondrial ROS may be a required step in the stimulation of the HBP in the cardiomyocytes. The role of O-GlcNAcylation in the failing heart is poorly defined. An experimental model demonstrates augmented flux through the HBP and the uptake of O-GlcNAcylation in the state of hypertrophy [159]. This observation is important as it specifies that stimulation of the HBP is an inciting event that activates remodeling in myocytes. Since this hypertrophy is accompanied by an augmentation in activated T-cell signaling, the stimulation of HBP is potentially harmful [160]. However, experimental models with MI and diminished O-GlcNAcylation demonstrate worsened left ventricular dysfunction [161]. Therefore, although HBP induce signaling pathways that trigger deleterious cardiac remodeling, the subsequent associated signaling pathways may encourage positive changes. Further studies in the clinically failing heart are required to understand the signaling pathways that are controlled by HBP.
Figure 2 illustrates the relationship between metabolic and structural alterations of the cardiomyocytes in the failing heart. This figure demonstrates that the lack of adequate ATP production is simply one facet of metabolic remodeling. This model supports the notion that in the failing heart, alternative non-ATP-generating measures (which possibly play a rather minor role in normal cardiac metabolism) may become increasingly prominent and explicitly influence contractile function, as described in Fig. 3. This illustration offers a model for potential novel therapeutic targets in the failing heart.
Cell architecture and HEP metabolism
Role of the mitochondria to HEP transfer in the healthy heart
Cell architecture plays a major role in cell differentiation and the maintenance of energy homeostasis. The cytoskeletal arrangement differs by muscle type and is subject to rearrangement in response to exertion, underlying diseases, and environmental stresses [162, 163]. In fact, the control of mitochondria by cytosolic ADP varies according to the type of muscle [164], most likely because of the interaction between the mitochondria and the cytoskeleton in oxidative cells [110, 165]. Moreover, alterations in cellular energetics (i.e., CK gene deletion, pharmacological deficiency of creatine, or hypoxia) can cause ultrastructural remodeling [164, 166, 167]. Thus, cell architecture may play an important role in the signaling and regulation of HEP metabolism [168, 169].
Microdomains of energy exist within cellular architecture and at the interfaces of organelle with discretely different ATP/ADP ratios, free and bound CKs, and ATPases. These microdomains play crucial roles in energy governance. ATP synthesized within the mitochondria is directly guided to ATPases and assists in calcium ingestion and myofibrillar contraction as efficiently as ATP provided by CK/PCr and more efficiently than cytosolic ATP, suggesting a nonstop energy shuttle between organelles via adenine nucleotide chambers [166]. Presuming one molecule of ATP synthesis per creatine and an ADP/O ratio of <3, approximately 64% of the ATP used by ATPases is derived from the CK reaction, and direct shuttling from the mitochondria accounts for approximately 36%, exhibiting the higher efficacy of chambered CK in normal situations [166]. Furthermore, the two methods can adjust to alterations in the other, suggesting incomplete redundancy in the confined ATP/ADP-regulating methods of myocytes. One might anticipate the finding that the resting cardiac function of CK-deficient mice is preserved by this compensatory constant energy shuttling [166], and the CK-deficient myocytes rely on SR ATPase for glycolysis [170] causing large-scale cellular remodeling. While these energetic pathways are redundant at rest or even at modest levels of cardiac work, it is likely that at high workloads, they are both simultaneously required to meet the high myocardial energetic demand. Elegant studies of this energy shuttling system have exhibited that the diffusion of ADP generated by cellular ATPases is confined and that it is specifically shuttled to the mitochondria in spite of being discharged into the broader cellular milieu [61, 171]. The chambering and shuttling of ADP augments the efficacy of interplay among myofibrils, mitochondria, the SR and likely auxiliary energy-utilizing schemes.
Role of the mitochondria to HEP transfer in HF
Defects in intracellular architecture contribute to HF [172]. Aberrant protein expression of filaments or microtubules may lead to impaired cardiac function [84, 173, 174]. Interruption in cellular architecture leads to disruption in communication between the mitochondria and the SR, calcium equilibrium, and a reduction in the total number of mitochondria [175]. However, it is necessary to determine if these changes have functional consequences. It is observed that the ATP/ADP ratio is directly proportional to the calcium storage capacity of the SR [45]. This suggests that the energy supply is a key regulating element for SR activity in HF. Myofilaments also exhibit a similar phenomenon. The cardiac system in the absence of HF has two energy shuttle tactics: (i) CK and (ii) straight mitochondrial shuttling. Both these tactics may moderately compensate for each other and are important for providing an energy reservoir when energy requirements and workload is augmented [45]. In states of incompetent cell architecture and mitochondrial content, MM-CK and mi-CK activity declines, causing a reduction in energy production, dissemination, and consumption. This overall leads to a loss of cardiac contractile capacity.
Taking altogether, one finds myocardial substrate utilization either directly or indirectly linked to the mitochondria. Table 1 summarizes, by experimental model or clinical setting, study findings regarding glucose metabolism, fatty acid metabolism, mitochondrial energetics, and morphological alterations. Relevant references are included.
In summary, alterations in cardiac mitochondria including increased number, decreased organelle size and structural integrity, and anatomical dyscrasias indicating mitochondrial linkage disintegration may be causal in the development of heart failure [18, 176]. The presence and degree of mitochondrial deformation (e.g., reduction in organelle matrix, or interruption and convolution of mitochondrial membranes) has been correlated with other well-recognized indicators of HF severity such as LV-EF, LV-EDV, and plasma norepinephrine [177]. An evolving concept is that the systems which regulate the structure of mitochondria, and specifically mitochondrial dynamics, may play an important role in cardiac ailments [178]. However, it is unknown whether a reduction in the expression of structural proteins implicated in mitochondrial synthesis/splitting procedures is intrinsically responsible for the observed energetic impairments, or whether the impairments are simply due to a reduction in the quantity of mitochondria. This needs to be further explored.
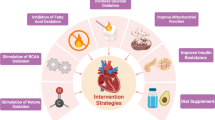
The failing heart and a metabolic therapeutic approach
Despite advances in neurohormonal and device therapy, the mortality rate of HF remains high. The presumed causal role of the imbalance between cardiac work and the production of biochemical energy argues in support of investigating metabolic treatments of HF [8, 15, 179, 180]. Intriguingly, it appears that some of the observed benefits of existing HF therapies may be due to energy-saving properties. Diuretics reduce cardiac energy demands by reducing circulating blood volume. By inhibiting peripheral vascular resistance and RAAS activity, ACE inhibitors reduce the load against which the heart has to work, hence decreasing cardiac energy requirements and neurohormonal upregulation. ACE inhibitor therapy has further been shown to improve the oxidative ability of skeletal muscle [51, 83]. Of note, angiotensin II is also an important regulator of cardiac energy metabolism and function [181]. Angiotensin II damages the mitochondria in the cardiomyocyte by increasing reactive oxygen species production [150] and affects mitochondrial oxidative phosphorylation, including fatty acid oxidation [182, 183]. There is also evidence that angiotensin II regulates glucose oxidation [150] and its inhibition may exert beneficial effects. In addition, angiotensin II can reduce ATP levels by decreasing oxidative metabolism, compromising ATP production [184]. In this context, angiotensin II antagonism represents a very attractive therapeutic approach, and ACE inhibitors and angiotensin receptor antagonists have been shown to improve both left ventricular function and glucose homeostasis [185, 186].
Energy-saving properties have been also observed with β-blockers which decrease adrenergic stimulation of the heart rate and contractility. In addition, β-blockers reduce peripheral lipolysis and FFA availability, thus diminishing FFA utilization in favor of augmented glucose utilization [187]. This change in myocardial energetics could provide an underlying mechanism for the observed decreased myocardial oxygen consumption and improved energy efficiency seen with β-adrenoreceptor blockade in the treatment of ischemic heart disease and heart failure [188].
Moreover, along with the demonstrated reduction in morbidity and mortality for specific patients, cardiac resynchronization therapy (CRT) is associated with improvements in cardiac function, efficiency, and striking improvements in mitochondrial protein expression and function [189,190,191]. Even with these beneficial therapies developed over the past 30 years, HF is still accompanied with excess morbidity and mortality. Circulatory support with a left ventricular assist device (LVAD) is indicated for patients with end-stage heart failure either as a bridge to transplant or destination therapy. Aside from simply providing adequate cardiac output to the systemic circulation, LVAD therapy has also been associated with partial restoration of metabolic dysregulation in the HF state [192].
Novel therapies that target the induction of a switch in cardiac energetic substrate have received much attention. However, the success of these therapies depends on whether the switch from fatty acid to glucose consumption is fundamentally advantageous or disadvantageous, a question which remains unresolved and a therapeutic approach which will still rely on the native mitochondria’s ability to digest the targeted substrates [193]. A recent review comprehensively discussed the effects of direct and indirect modulators of fatty acid oxidation, which are the primary pharmacological agents currently available for metabolic therapy of failing hearts [194]. Studies of the efficacy of a therapeutic approach based on modulation of FA metabolism have been mixed, further indicating that the complexity of downstream effects of pharmacologic metabolic manipulation is underappreciated. A small clinical trial did exhibit advantageous outcomes with perhexiline and trimetazidine molecules indicating that this promising therapeutic tactic deserves further investigation [194] (Fig. 4).
Outline illustrating the action of therapeutic agents comprising fatty acid oxidation inhibition (trimetazidine, etomoxir, perhexiline, PPAR agonists and malonyl CoA decarboxylase (MCD)) and glucose oxidation augmentation (DAC) characters to improve cardiac function. The red arrows illustrate the changes in fatty acid oxidation in the failing heart and green arrows exhibit the role of therapeutic agents to improve cardiac function in failing heart. DCA dicholoroacetate, MCD malonyl coenzyme A decarboxylase. (Modified figure of reference 184)
Since the main regulator of cardiac energetics is PGC-1α, reinstating PGC-1α transcription holds great promise as a novel tactic in the therapeutic regimen of HF. Augmented synthesis of mitochondria by overexpression of PGC-1α is an innovative approach for the metabolic treatment of obesity and the metabolic syndrome. PGC-1α appears to play a major role in the regulation of energy metabolisms in multiple systems. Thus, therapies targeting PGC-1α may be a unique strategy to simultaneously target multiple diseases in which energetic dysregulation is causal [15, 195], including HF. Further study is required to better define the role of PGC-1α and its regulation in the healthy and failing heart.
References
Ingwall JS, Weiss RG (2004) Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95:135–145
Ventura-Clapier R, Garnier A, Veksler V (2004) Energy metabolism in heart failure. J Physiol 555:1–13
Neubauer S (2007) The failing heart—an engine out of fuel. N Engl J Med 356:1140–1151
Ventura-Clapier R, Garnier A, Veksler V, Joubert F (2011) Bioenergetics of the failing heart. Biochim Biophy Acta 1813:1360–1372
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40:1271–1296
Cotter DG, Schugar RC, Crawford PA (2013) Ketone body metabolism and cardiovascular diseases. Am J Physiol Heart Circ Physiol 304:H1060–H1076
Drake KJ, Sidorov VY, McGuinness OP, Wasserman DH, Wikswo JP (2012) Amino acids as metabolic substrates during cardiac ischemia. Exp Bio Med 237:1369–1378
Taegtmeyer H (2002) Switching metabolic genes to build a better heart. Circulation 106:2043–2045
Rimbaud S et al (2009) Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol 46:952–959
Osorio JC et al (2002) Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 106:606–612
Lei B et al (2004) Paradoxical down-regulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36:567–576
Razeghi P et al (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931
Leong HS, Brownsey RW, Kulpa JE, Allard MF (2003) Glycolysis and pyruvate oxidation in cardiac hypertrophy—why so unbalanced? Comp Biochem Physiol Part A Mole Integr Physiol 135:499–513
Benard G et al (2010) Multisite control and regulation of mitochondrial energy production. Biochim Biophys Acta 1797:698–709
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis. The central role of PGC-1α. Cardiovasc Res 79:208–217
Riehle C, Wende AR, Zaha VG et al (2011) PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Cir Res 109:783–793
Bugger H et al (2010) Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res 85:376–384
Ide T et al (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88:529–535
Garnier A et al (2003) Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551:491–501
Scheubel RJ et al (2002) Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J Am Coll Cardiol 40:2174–2181
Sebastiani M et al (2007) Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol 50:1362–1369
Karamanlidis G et al (2010) Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 106:1541–1548
Suematsu N et al (2003) Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107:1418–1423
Tsutsui H, Ide T, Kinugawa S (2006) Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal 8:1737–1744
Matsushima S et al (2006) Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 113:1779–1786
Scarpulla RC (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88:611–638
Rocher C et al (2008) Influence of mitochondrial DNA level on cellular energy metabolism: implications for mitochondrial diseases. J Bioenerg Biomembr 40:59–67
Ikeuchi M et al (2005) Overexpression of mitochondrial transcription factor ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112:683–690
Tsutsui H, Kinugawa S, Matsushima S (2009) Mitochondrial oxidative stress and dysfunction in myocardial remodeling. Cardiovasc Res 81:449–456
Dolezal P, Likic V, Tachezy J, Lithgow T (2006) Evolution of the molecular machines for protein import into mitochondria. Science 313:314–318
Baker MJ, Frazier AE, Gulbis JM, Ryan MT (2007) Mitochondrial protein-import machinery: correlating structure with function. Trends Cell Biol 17:456–464
Mac Kenzie JA, Payne RM (2007) Mitochondrial protein import and human health and disease. Biochim Biophys Acta 1772:509–523
Dabkowski ER et al (2010) Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially-distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299:H529–H540
Hatch GM (2004) Cell biology of cardiac mitochondrial phospholipids. Biochem Cell Biol 82:99–112
Chicco AJ, Sparagna GC (2006) Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292:C33–C44
Houtkooper RH, Vaz FM (2008) Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65:2493–2506
Athea Y et al (2007) AMP-activated protein kinase {alpha} 2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes 56:786–794
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription co-activators. Cell Metab 1:361–370
Wang P et al (2010) Peroxisome proliferator-activated receptor delta is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res 106:911–919
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203
Huss JM, Torra IP, Staels B, Giguere V, Kelly DP (2004) Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091
Lai L et al (2008) Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev 22:1948–1961
Garnier A et al (2005) Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J 19:43–52
Soriano FX et al (2006) Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55:1783–1791
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99
Ong SB et al (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121:2012–2022
Joubert F et al (2008) Local energetic regulation of sarcoplasmic and myosin ATPase is differently impaired in rats with heart failure. J Physiol 586:5181–5192
Witt H et al (2008) Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med 86:1013–1024
Watson PA et al (2007) Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol 293:H246–H259
Faerber G et al (2011) Induction of heart failure by minimally invasive aortic constriction in mice: reduced peroxisome proliferator-activated receptor gamma co-activator levels and mitochondrial dysfunction. J Thorac Cardiovasc Surg 141:492–500
Garnier A et al (2009) Control by circulating factors of mitochondrial function and transcription cascade in heart failure: a role for endothelin-1 and angiotensin II. Circ Heart Fail 2:342–350
Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ (2008) PGC-1alpha and ERRalpha target gene down regulation is a signature of the failing human heart. J Mol Cell Cardiol 46:201–212
Huss JM et al (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6:25–37
Ventura-Clapier R, Kuznetsov A, Veksler V, Boehm E, Anflous K (1998) Functional coupling of creatine kinases in muscles: species and tissue specificity. Mol Cell Biochem 184:231–247
Arany Z et al (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271
Lehman JJ et al (2008) The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295:H185–H196
Dufour CR et al (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5:345–356
Twig G et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446
Saito T, Sadoshima J (2015) Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res 116:1477–1490
Knowlton AA, Liu TT (2016) Mitochondrial dynamics and heart failure. Compr Physiol 6:507–526
Saks VA et al (2001) Intracellular energetic units in red muscle cells. Biochem J 356:643–657
Saks V et al (2006) Cardiac system bioenergetics: metabolic basis of Frank-Starling law. J Physiol 571:253–273
Guzun R, Saks V (2010) Application of the principles of systems biology and Wiener’s cybernetics for analysis of regulation of energy fluxes in muscle cells in vivo. Int J Mol Sci 11:982–1019
Shimizu J, Todaka K, Burkhoff D (2002) Load dependence of ventricular performance explained by model of calcium-myofilament interactions. Am J Physiol Heart Circ Physiol 282:H1081–H1091
Balaban RS (2002) Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34:1259–1271
Weiss JN, Korge P (2001) The cytoplasm: no longer a well-mixed bag. Circ Res 89:108–110
Weiss J, Hiltbrand B (1985) Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest 75:436–447
Saks VA et al (2004) Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem 256–257:185–199
Rostovtseva TK et al (2008) Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A 105:18746–18751
Kay L et al (1997) Study of regulation of mitochondrial respiration in vivo, an analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim Biophys Acta 1322:41–59
Gong G et al (2003) Oxidative capacity in failing hearts. Am J Phys 285:H541–H548
Jarreta D et al (2000) Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res 45:860–865
Marin-Garcia J, Goldenthal MJ, Moe GW (2001) Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res 52:103–110
Liu J et al (2001) Mitochondrial ATPase and high-energy phosphates in failing hearts. Am J Physiol Heart Circ Physiol 281:H1319–H1326
Cieniewski-Bernard C et al (2008) Proteomic analysis of left ventricular remodeling in an experimental model of heart failure. J Proteome Res 7:5004–5016
Gao Z et al (2008) Key pathways associated with heart failure development revealed by gene networks correlated with cardiac remodeling. Physiol Genomics 35:222–230
Gao Z et al (2006) Transcriptomic profiling of the canine tachycardia-induced heart failure model: global comparison to human and murine heart failure. J Mol Cell Cardiol 40:76–86
Murray AJ et al (2008) Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 44:694–700
Lisa FD, Bernardi P (2009) A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol 46:775–780
Marcil M et al (2006) Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. J Mol Cell Cardiol 41:998–1009
Sousa ED et al (2002) Cardiac and skeletal muscle energy metabolism in heart failure: beneficial effects of voluntary activity. Cardiovasc Res 56:260–268
Boudina S et al (2002) Alteration of mitochondrial function in a model of chronic ischemia in vivo in rat heart. Am J Physiol Heart Circ Physiol 282:H821–H831
Zoll J et al (2006) ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol 101:385–391
Belmadani S, Pous C, Ventura-Clapier R, Fischmeister R, Mery PF (2002) Post-translational modifications of cardiac tubulin during chronic heart failure in the rat. Mol Cell Biochem 237:39–46
Akki A, Gupta A, Weiss RG (2013) Magnetic resonance imaging and spectroscopy of the murine cardiovascular system. Am J Physiol Heart Circ Physiol 304(5):H633–H648
Chacko VP, Aresta F, Chacko SM, Weiss RG (2000) MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol 279(5):H2218–H2224
Gupta A, Chacko VP, Schär M, Akki A, Weiss RG (2011) Impaired ATP kinetics in failing in vivo mouse heart. Circ Cardiovasc Imaging 4(1):42–50
Gupta A et al (2012) Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest 122(1):291–302
Gupta A, Chacko VP, Weiss RG (2009) Abnormal energetics and ATP depletion in pressure-overload mouse hearts: in vivo high-energy phosphate concentration measures by noninvasive magnetic resonance. Am J Physiol Heart Circ Physiol 297:H59–H64
Gupta A et al (2013) Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS One 8(10):e74675
Akki A et al (2012) Creatine kinase over-expression improves ATP kinetics and contractile function in post-ischemic myocardium. Am J Physiol Heart Circ Physiol 303:H844–H852
Neubauer S et al (1995) Impairment of energy metabolism inintact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest 95:1092–1100
Ye Y, Gong G, Ochiai K, Liu J, Zhang J (2001) High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation 103:1570–1576
Weiss RG, Gerstenblith G, Bottomley PA (2005) ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A 102(3):808–813
Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG (2006) Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 114(11):1151–1158
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G (1991) Altered myocardial high energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 122:795–801
Conway MA et al (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by P-31 magnetic resonance spectroscopy. Lancet 338:973–976
Neubauer S et al (1992) 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary heart disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86:1810–1818
Ingwall JS (2009) Energy metabolism in heart failure and remodeling. Cardiovasc Res 81:412–419
Beer M et al (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274
Neubauer S et al (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96:2190–2196
Crilley JG et al (2003) Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41:1776–1782
Tian R, Nascimben L, Ingwall JS, Lorell BH (1997) Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation 96:1313–1319
Bessman SP, Geiger PJ (1981) Transport of energy in muscle: the phosphoryl creatine shuttle. Science 211:448–452
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands—the phosphocreatine circuit for cellular energy homeostasis. Biochem J 281:21–40
Dzeja PP, Terzic A (2003) Phosphotransfer networks and cellular energetics. J Exp Biol 206:2039–2047
Akki A et al (2014) Skeletal muscle ATP kinetics are impaired in frail mice. Age 36:21–30
Wyss M, Smeitink J, Wevers RA, Wallimann T (1992) Mitochondrial creatine kinase—a key enzyme of aerobic energy metabolism. Biochim Biophys Acta 1102:119–166
Ventura-Clapier R, Veksler V, Hoerter JA (1994) Myofibrillar creatine kinase and cardiac contraction. Mol Cell Biochem 133:125–144
Saks V et al (2010) Structure-function relationships in feedback regulation of energy fluxes in vivo in health and disease: mitochondrial interactosome. Biochim Biophys Acta 1797:678–697
Joubert F, Hoerter JA, Mazet JL (2002) Modeling the energy transfer pathways. Creatine kinase activities and heterogeneous distribution of ADP in the perfused heart. Mol Biol Rep 29:177–182
Joubert F, Mazet JL, Mateo P, Hoerter JA (2002) 31P NMR detection of subcellular creatine kinase fluxes in the perfused rat heart: contractility modifies energy transfer pathways. J Biol Chem 277:18469–18476
Ingwall JS, Atkinson DE, Clarke K, Fetters JK (1990) Energetic correlates of cardiac failure: changes in the creatine kinase system in the failing myocardium. Eur Heart J 11:108–115
Sylven C, Lin L, Kallner A, Sotonyi P, Somogyi E, Jansson E (1991) Dynamics of creatine kinase shuttle enzymes in the human heart. Eur J Clin Investig 21:350–354
Hove MT, Neubauer S (2007) MR spectroscopy in heart failure—clinical and experimental findings. Heart Fail Rev 12:48–57
Joubert F, Gillet B, Mazet JL, Mateo P, Beloeil J, Hoerter JA (2000) Evidence for myocardial ATP compartmentation from NMR inversion transfer analysis of creatine kinase fluxes. Biophys J 79:1–13
van der Vusse GJ, van Bilsen M, Glatz JF (2000) Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res 45:279–293
Glatz JF, Luiken JJ, Bonen A (2001) Involvement of membrane-associated proteins in the acute regulation of cellular fatty acid uptake. J Mol Neurosci 16:123–132
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258
Banke NH et al (2010) Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ Res 107:233–241
Koves TR et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56
Cook GA et al (2001) Differential regulation of carnitine palmitoyltransferase-I gene isoforms (CPT-I alpha and CPT-I beta) in the rat heart. J Mol Cell Cardiol 33:317–329
Sorokina N et al (2007) Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 115:2033–2041
Zammit VA, Fraser F, Orstorphine CG (1997) Regulation of mitochondrial outermembrane carnitine palmitoyltransferase (CPT I): role of membrane-topology. Adv Enzym Regul 37:295–317
Hamilton C, Saggerson ED (2000) Malonyl-CoA metabolism in cardiac myocytes. Biochem J 350(pt 1):61–67
Dyck JR et al (2006) Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation 114:1721–1728
Zhou L et al (2008) Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol 294:H954–H960
Kato T et al (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3:420–430
Doenst T et al (2010) Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 86:461–470
Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R (2001) Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res 52:407–416
Heather LC et al (2006) Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 72:430–437
Akki A, Smith K, Seymour AM (2008) Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem 311:215–224
Allard MF, Schönekess BO, Henning SL, English DR, Lopaschuk GD (1994) Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Phys 267:H742–H750
Christe ME, Rodgers RL (1994) Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol 26:1371–1375
Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP (1996) Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94:2837–2842
Aerni-Flessner L, Abi-Jaoude M, Koenig A, Payne M, Hruz PW (2012) GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc Diabetol 11:63
Abel ED (2004) Glucose transport in the heart. Front Biosci 9:201–215
Entman ML, Bornet EP, Van Winkle WB, Goldstein MA, Schwartz A (1977) Association of glycogenolysis with cardiac sarcoplasmic reticulum, II: effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: evidence for a phorphorylase kinase membrane complex. J Mol Cell Cardiol 9:515–528
Kusuoka H, Marban E (1994) Mechanism of the diastolic dysfunction induced by glycolytic inhibition. Does adenosine triphosphate derived from glycolysis play a favored role in cellular Ca2+ homeostasis in ferret myocardium? J Clin Invest 93:1216–1223
Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC (2011) Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res 90:210–219
Russell RR 3rd, Taegtmeyer H (1991) Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest 87:384–390
Zhabyeyev P et al (2013) Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res 97:676–685
Amorim PA et al (2010) Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. J Thorac Cardiovasc Surg 140:1160–1167
Degens H et al (2006) Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol 101:17–26
Dai DF et al (2012) Mitochondrial proteome remodeling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovasc Res 93:79–88
Dodd MS et al (2012) In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovasc Res 95:69–76
Dávila-Román VG et al (2002) Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40:271–277
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P (2000) Vitamin E supplementation and cardiovascular events in high-risk patients: the heart outcomes prevention evaluation study investigators. N Engl J Med 342:154–160
Lonn E et al (2005) HOPE and HOPE-TOO trial investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293:1338–1347
Dai DF et al (2011) Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108:837–846
Dai DF et al (2011) Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58:73–82
Burgoyne JR, Mongue-Din H, Eaton P, Shah AM (2012) Redox signaling in cardiac physiology and pathology. Circ Res 111:1091–1106
Schönfeld P, Wojtczak L (2007) Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta 1767:1032–1040
Opie LH, Knuuti J (2009) The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol 54:1637–1646
Krishnan J et al (2009) Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9:512–524
Gupte SA et al (2006) Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol 41:340–349
Hecker PA et al (2013) Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ Heart Fail 6:118–126
Du XL et al (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97:12222–12226
Facundo HT et al (2012) O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol 302:H2122–H2130
Wilkins BJ et al (2004) Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94:110–118
Watson LJ et al (2010) O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A 107:17797–17802
Heling A et al (2000) Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 86:846–853
Schneider AG, Sultan KR, Pette D (1999) Muscle LIM protein: expressed in slow muscle and induced in fast muscle by enhanced contractile activity. Am J Phys 276:C900–C906
Veksler VI et al (1995) Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem 270:19921–19929
Appaix F et al (2003) Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp Physiol 88:175–190
Kaasik A et al (2001) Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res 89:153–159
Mekhfi H et al (1990) Myocardial adaptation to creatine deficiency in rats fed with beta-guanidino propionic acid, a creatine analogue. Am J Phys 258:H1151–H1158
Arber S et al (1997) MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88:393–403
Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol 150:1283–1297
Boehm E, Ventura-Clapier R, Mateo P, Lechene P, Veksler V (2000) Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibers of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol 32:891–902
Saks V et al (2003) Heterogeneity of ADP diffusion and regulation of respiration in cardiac cells. Biophys J 84:3436–3456
Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S (2010) Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Investig 90:520–530
Hein S, Kostin S, Heling A, Maeno Y, Schaper J (2000) The role of the cytoskeleton in heart failure. Cardiovasc Res 45:273–278
Cooper G (2006) Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol 291:H1003–H1014
van den Bosch BJ et al (2005) Regional absence of mitochondria causing energy depletion in the myocardium of muscle LIM protein knockout mice. Cardiovasc Res 65:411–418
Schaper J et al (1991) Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 83:504–514
Sabbah HN et al (1992) Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 24:1333–1347
Chen L, Knowlton AA (2010) Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol 58:213–229
van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ (2008) Metabolic remodeling of the failing heart: beneficial or detrimental? Cardiovasc Res 81:420–428
Huss JM, Kelly DP (2005) Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115:547–555
Mori J et al (2012) Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail 5:493–503
Pellieux C et al (2006) Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol 41:459–466
Pellieux C, Montessuit C, Papageorgiou I, Lerch R (2009) Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-alpha. Cardiovasc Res 82:341–350
Fillmore N, Mori J, Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171:2080–2090
Vermes E et al (2003) Studies of left ventricular dysfunction. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the studies of left ventricular dysfunction (SOLVD). Circulation 107:1291–1296
Yusuf S et al (2005) Candesartan in heart failure-assessment of reduction in mortality and morbidity program investigators. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation 112:48–53 2005. Erratum in: Circulation 112: e292
Wallhaus TR, Taylor M, De Grado TR, Russell DC (2001) Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 103:2441–2446
Spoladore R et al (2013) Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol 27:455–464
Christenson SD et al (2008) Effects of simultaneous and optimized sequential cardiac resynchronization therapy on myocardial oxidative metabolism and efficiency. J Cardiovasc Electrophysiol 19:125–132
Kitaizumi K et al (2008) Positron emission tomographic demonstration of myocardial oxidative metabolism a case of left ventricular restoration after cardiac resynchronization therapy. Circ J 72:1900–1903
Agnetti G et al (2010) Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dys-synchronous failing hearts. Circ Cardiovasc Genet 3:78–87
Sajgalik P et al (2016) Current status of left ventricular assist device therapy. Mayo Clin Proc 91(7):927–940
de Brouwer KF et al (2006) Specific and sustained down-regulation of genes involved in fatty acid metabolism is not a hallmark of progression to cardiac failure in mice. J Mol Cell Cardiol 40:838–845
Lionetti V, Stanley WC, Recchia FA (2011) Modulating fatty acid oxidation in heart failure. Cardiovac Res 90:202–209
Mudd JO, Kass DA (2008) Tackling heart failure in the twenty-first century. Nature 451:919–928
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, A., Houston, B. A comprehensive review of the bioenergetics of fatty acid and glucose metabolism in the healthy and failing heart in nondiabetic condition. Heart Fail Rev 22, 825–842 (2017). https://doi.org/10.1007/s10741-017-9623-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9623-6