Abstract
Magnetic resonance spectroscopy (MRS) allows for the non-invasive detection of a wide variety of metabolites in the heart. To study the metabolic changes that occur in heart failure, 31P- and 1H-MRS have been applied in both patients and experimental animal studies. 31P-MRS allows for the detection of phosphocreatine (PCr), ATP, inorganic phosphate (Pi) and intracellular pH, while 1H-MRS allows for the detection of total creatine. All these compounds are involved in the regulation of the available energy from ATP hydrolysis via the creatine kinase (CK) reaction. Using cardiac MRS, it has been found that the PCr/CK system is impaired in the failing heart. In both, patients and experimental models, PCr levels as well as total creatine levels are reduced, and in severe heart failure ATP is also reduced. PCr/ATP ratios correlate with the clinical severity of heart failure and, importantly, are a prognostic indicator of mortality in patients. In addition, the chemical flux through the CK reaction, measured with 31P saturation transfer MRS, is reduced more than the steady-state levels of high-energy phosphates in failing myocardium in both experimental models and in patients. Experimental studies suggest that these changes can result in increased free ADP levels when the failing heart is stressed. Increased free ADP levels, in turn, result in a reduction in the available free energy of ATP hydrolysis, which may directly contribute to contractile dysfunction. Data from transgenic mouse models also suggest that an intact creatine/CK system is critical for situations of cardiac stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac magnetic resonance imaging (MRI) has recently been developed with great success and has found its way into mainstream cardiology. However, although cardiac MRI can provide excellent anatomical and functional information, it does not provide biochemical information about the myocardium. In contrast, cardiac MR spectroscopy (MRS) allows for the non-invasive study of cardiac metabolism without the need to give external radioactive tracers, as is required, for example, in positron emission tomography (PET). MRS provides chemical information about compounds that contain nuclei with a nuclear spin. Table 1 summarises information on nuclei of interest for cardiac MRS: These include 1H (protons from metabolites other than water and fat), 13C, 23Na and 31P (which is the most widely studied nucleus). The biochemical information provided by non-invasive imaging of cardiac metabolism would allow us to answer many clinical questions. The main reason why MRS has not yet fulfilled its promise in clinical cardiology is the relatively low resolution that can currently be achieved. Most nuclei studied with MRS have a much lower MR sensitivity than 1H, and the metabolites of interest are present in concentrations that are several orders of magnitude lower than that of water (Table 1). The means that, while MR spectra can be obtained from the human heart, the temporal and spatial resolution of MRS is many times lower than that of MRI, and therefore, high-resolution metabolic imaging by MRS is not yet a clinical reality. In contrast, MRS has been a standard method in experimental cardiology, ever since the first 31P-MR spectrum from an isolated heart was obtained by Radda’s group in 1977 [1]. Since then, MRS has been used for the study of many aspects of cardiac metabolism. Other pioneering groups in this field were those of Ingwall [2] and Jacobus et al. [3]. 31P-MR spectra of the human heart were first obtained in the 1980s [4, 5], while more recently, 1H-MR spectra of the human heart have also been acquired [6].
So far the application of MRS to study heart failure has been limited to studies on cardiac high-energy phosphate metabolism using 31P- and 1H-MRS. ATP is the only substrate for all energy-consuming reactions in the cell as its hydrolysis provides free energy:
Phosphocreatine (PCr), the other major high-energy phosphate compound, acts as an energy buffer and has at least two additional roles: First, PCr serves as an energy transport molecule in the “creatine kinase (CK)/PCr energy shuttle” [7] (Fig. 1). The high-energy phosphate bond is transferred from ATP to creatine at the site of ATP production (i.e., the mitochondria). This reaction is catalysed by the mitochondrial CK isoenzyme (mito-CK):
PCr, which is a smaller molecule than ATP, then diffuses through the cytosol to the myofibrils or to other sites of ATP utilization, where the back reaction occurs, ATP is reformed and is used for contraction. This reaction is catalysed by the myofibrillar-bound MM-CK isoenzyme (M-CK). Free creatine (Crfree) then diffuses back to the mitochondria. This “energy shuttle” is required, because free ADP is present in the cytosol in concentrations (40–80 μM) that are too low to provide the necessary capacity for back diffusion to the mitochondria [7]. Crfree, however, is present at high concentrations. The second crucial function of the PCr/CK system is to maintain the free cytosolic ADP concentrations at a low level. Free ADP cannot directly be measured but is calculated from the CK equilibrium assumption:
where K eq is the equilibrium constant of the CK reaction.
Schematic representation of the creatine kinase (CK)/PCr energy shuttle. The high-energy phosphate bond is transferred from ATP to creatine to form PCr and ADP at the mitochondria. This reaction is catalysed by CK. PCr diffuses to the site of energy utilization, i.e. the myofibrils, where the reverse reaction takes place, to maintain adequately high ATP and low ADP levels
A low free cytosolic ADP concentration is required for normal cardiac function, since ADP largely determines the free energy change of ATP hydrolysis (ΔG ATP; kJ/mol), a measure of the amount of energy released from ATP hydrolysis:
where ΔG o is the standard free energy change at 37°C, [Mg2+] = 1 mM; R = universal gas constant; T = temperature [K]; Pi = inorganic phosphate.
In the normal heart, ΔG ATP is ∼ −58 kJ/mol. Many intracellular enzymes such as SR-Ca2+-ATPase and others will stop functioning properly below a threshold value for ΔG of about −52 kJ/mol.
31P-MRS allows in principle for the examination of PCr, ATP and Pi levels, as well as for the quantification of intracellular pH (pHi), from the chemical shift difference between PCr and Pi, which is pH-sensitive. With 31P saturation transfer techniques the flux through the CK reaction can be measured in the intact heart [8]. 1H-MRS can be used to examine a wide variety of compounds, including creatine and deoxymyoglobin.
MRS of the human heart—methodological aspects
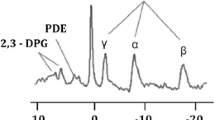
MRS of the human heart comes with substantial technical challenges: Time for signal acquisition is limited by the fact that total examination time should not be more than one hour, or even shorter for patients with severe cardiac disease. Since the heart is rapidly moving, acquisition needs to be gated to the heartbeat, and, possibly, to respiration as well. Finally, signal from skeletal muscle in the chest wall must be suppressed. Therefore, localization techniques are required, which results in further signal loss. However, a new localization method termed SLOOP (spectral localization with optimum pointspread function) [9] allows selection of voxel sizes of not only rectangular, but any shape; this should improve signal to noise by improved matching of voxel and heart shape. For most spectroscopic techniques, scout images are first obtained with MRI, which are used to select the spectroscopic volume(s). The relatively low sensitivity of MRS requires large voxel sizes, usually >25 cm3. Figure 2 (bottom panel) shows a typical 31P-MR spectrum of a healthy volunteer. The three 31P-atoms of ATP and PCr are clearly visible. Compared to 31P spectra from isolated hearts (see below), two additional resonances appear: 2,3-diphosphoglycerate (2,3-DPG), arising from the presence of blood (erythrocytes) in the selected voxel, and phosphodiesters (PDE), a signal assigned to membrane as well as serum phospholipids. The 2,3-DPG resonances appear at about the same frequency as Pi. Therefore, Pi, and as a result pHi, cannot be detected in blood-contaminated spectra. Further technical improvements are required to allow for voxel sizes that are small enough to avoid major blood contamination of 31P-spectra, which should make measurements of Pi and pHi possible. The most common way of quantifying 31P-spectra from the human heart is in relative terms, i.e., the PCr/ATP and phosphodiester/ATP peak area ratios are calculated. PCr/ATP is considered an index of the energetic state of the heart: The CK reaction equilibrium favours ATP synthesis over PCr synthesis by a factor of ∼100. Therefore, ATP will only decrease when PCr is substantially depleted. In chronic heart failure, however, the total creatine pool is depleted [11], further decreasing the PCr/ATP ratio. The meaning of the phosphodiester/ATP ratio is poorly understood; it was suggested that an increase of phosphodiester/ATP indicates membrane damage [12], but phosphodiester/ATP ratios do not seem to change with cardiac disease. Measuring PCr/ATP ratios has the disadvantage that this underestimates the change in PCr when ATP levels are reduced, while changes in ATP levels are not detected at all. Therefore, absolute quantification of PCr and ATP is preferable, although technically demanding. Absolute quantification of 31P-metabolite levels has been performed in two different ways: (i) By obtaining simultaneous signal from a 31P-standard as well as estimates of myocardial mass based on MR imaging [13] or (ii) by simultaneous acquisition of a 1H spectrum that is used to calibrate the 31P-signal to the tissue water proton content [14]. Finally, human 31P-spectra also need to be corrected for the effects of partial saturation [15, 16] and for the amount of blood contamination, since blood contributes signals from ATP, 2,3-diphosphoglycerate and phosphodiesters to the 31P-heart spectrum.
Human cardiac 31P-MR spectra. From bottom to top: spectra from volunteer, patient with dilated cardiomyopathy (DCM) with normal PCr/ATP ratio, DCM patient with reduced PCr/ATP ratio, and DCM patient with severely reduced PCr/ATP ratio; this patient died one week after the MR examination. PDE = phosphodiesters; PCr = phosphocreatine; γ-, α-, and β-P atom of ATP. From: Neubauer et al. [10] Printed with permission, American Heart Association
Total creatine (PCr + Crfree) in the human heart can be measured with 1H-MRS [6]. 1H-MRS has the advantage that the relative MR sensitivity is high, but is complicated due to the dominant signal from water protons. This signal is so much higher than that of any other compound (except fat) that its active suppression is required. By using water-suppressed spectra to examine creatine content and unsuppressed spectra to examine water content absolute metabolite concentrations can be calculated.
A limitation of 31P-MRS has been that the method cannot interrogate the entire heart. Therefore, in most studies only the anterior portion of the heart has been measured. However, a recent study reports on measurements on the inferior wall using acquisition weighted MRS [17]. 31P-MRS is most suited to study myocardium that shows spatially homogenous pathology, such as in dilated cardiomyopathy or in valve disease. However, large voxel sizes make conditions with regional heterogeneity of metabolism, for example heart failure due to ischemic heart disease, difficult to examine.
Normal human myocardium
31P-MR spectroscopy studies of normal human heart have found relatively large variations in PCr/ATP ratios (1.23–2.46). This probably does not reflect biological variation, but instead is most likely due to differences in methodologies used. If spectra are appropriately corrected for partial saturation and blood contamination, the consensus is that the PCr/ATP ratio is close to 1.8 [18]. Absolute concentrations of PCr and ATP, measured by 31P-MR spectroscopy, are in the range of ∼11 and ∼6 μmol/g wet weight, respectively. Some 1H-MRS studies [6, 19] report on measurement of total creatine content. Using wet chemistry techniques, total CK activity (indicating the amount of protein) is ∼12 IU/mg protein, comprised of ∼1% MB-, 89% MM- and 10% mito-CK isoenzyme [20].
CK reaction velocity in the human heart has also been measured with 31P-MRS [21, 22], which was found to be between 3.3 and 5.6 (μmol/g wet weight × s), i.e., similar to CK reaction velocities reported for rat heart. Although feasible in principle, values for free ADP and ΔG ATP, based on simultaneous measurement of PCr, ATP, Pi, pHi and creatine by 31P- and 1H-MRS, have not yet been reported.
Heart failure due to ischemic heart disease
As discussed above, heart failure due to ischemic heart disease is difficult to study with MRS. Due to the heterogeneous nature of the disease, studies will unavoidably show large regional variation; normally perfused, ischemic or scarred regions will all have a different metabolic state, and large voxels will inevitably examine an ill-defined mix of energetic changes due to ischemia and to chronic heart failure. To date, few studies have examined such patients [17, 23], but all have found a significant reduction of PCr/ATP ratios.
Dilated cardiomyopathy
In contrast to ischemic heart disease, dilated cardiomyopathy is ideally suited for the application of MRS to study cardiac energetics in heart failure, since the disease affects the entire heart in a mostly uniform manner.
Using wet chemistry, it was found that in dilated cardiomyopathy, creatine levels were reduced by 51% and total CK activity by 34% compared to controls. Compared to donor hearts, dilated cardiomyopathy hearts showed 50% and 71% reductions of MM- and mito-CK isoenzyme activities, respectively, while CK-MB activity remained unchanged [11].
Figure 2 shows typical 31P-MR spectra from patients with dilated cardiomyopathy of varying severity. Initial studies on 31P-MRS measurements in dilated cardiomyopathy patients did not detect significant reductions of PCr/ATP ratios [12, 24–26]. However, in these early studies patients were not categorized according to the severity of heart failure and patients in all stages of disease were analysed as one group. Hardy et al. were first to show that the myocardial PCr/ATP ratio was significantly reduced (from 1.80 ± 0.06 to 1.46 ± 0.07) in symptomatic patients with dilated cardiomyopathy [23], a finding confirmed by other studies. This decrease of PCr/ATP ratios in dilated cardiomyopathy was found to correlate with the clinical severity of heart failure according to the New York Heart Association class [27], as well as with left ventricular ejection fraction [28]. Thus, in dilated cardiomyopathy, PCr/ATP ratios are reduced in the highly symptomatic stages of heart failure but remain normal during initial stages. In another study, we treated a small group of patients with dilated cardiomyopathy with standard medical therapy including ACE inhibitors, digitalis, diuretics and beta-blockers. This lead to clinical improvement and normalisation of PCr/ATP ratios from 1.51 ± 0.32 to 2.15 ± 0.27 [27]. We also showed that PCr/ATP ratios hold prognostic information on survival of patients with dilated cardiomyopathy. The myocardial PCr/ATP ratio was a significant predictor of long-term survival, with stronger predictive power than either left ventricular ejection fraction or New York Heart Association class [10] (Fig. 3).
Kaplan–Meier life-table analysis for total mortality (%; left) and mortality from cardiovascular causes (%; right) of patients with dilated cardiomyopathy divided into two groups split by PCr/ATP ratio (<1.60 vs. >1.60; top), NYHA class (III vs. I and II; middle), and LVEF (<30% vs. >30%; bottom). EF indicates ejection fraction. PCr/ATP ratio was the strongest predictor of mortality. From: Neubauer et al. [10] Printed with permission, American Heart Association
Recently, absolute concentrations of PCr and ATP were reported in patients with dilated cardiomyopathy [29]. Both PCr and ATP were significantly reduced, PCr by 51%, ATP by 35% and PCr/ATP by 25%. This result underlines the importance of measuring absolute concentrations rather than ratios, as the PCr/ATP ratio clearly underestimates the severity of the changes and the decrease in ATP levels would not be detected at all if only ratios are examined.
In a recent study, Nakae et al. [19] measured myocardial creatine content in patients with dilated cardiomyopathy with 1H-MRS. They reported that myocardial creatine is significantly reduced in patients with dilated cardiomyopathy. Myocardial creatine contents were 28 μmol/g wet weight in control subjects and 16 μmol/g wet weight in patients with dilated cardiomyopathy, which is in line with data from biopsy studies.
Using saturation transfer techniques, Weiss et al. [22] were able to measure flux through the CK reaction in the human heart, both in healthy subjects as well as in patients with mild to moderate heart failure. They showed that in healthy volunteers flux through the CK reaction is faster than that through oxidative phosphorylation (ATP synthesis) and that it does not increase significantly during dobutamine infusion, despite of a doubling of the rate-pressure product. In patients with mild to moderate heart failure, they reported a reduced PCr content but unaltered ATP levels. Flux through the CK reaction, however, was reduced more than PCr levels, and it was suggested that in severe heart failure, where ATP levels decline and PCr levels are further reduced, flux through the CK reaction should be even lower, possibly limiting contractile function.
Experimental studies
The first cardiac MRS study was reported by Garlick et al. [1] using the isolated perfused heart. When this model is placed into an MR tube and the effluent is aspirated, the heart can be positioned into conventional high-resolution MR spectrometers. Ever since, hearts of various species have been used to study myocardial biochemistry. A typical example of a mouse heart 31P spectrum is shown in Fig. 4A. This technique is still the most widely used to study the heart with MRS. In larger animal models, in vivo cardiac MRS has been applied since the 1980s [30–32]. Recently, in vivo 31P- and 1H-MRS in mice have been shown to be feasible [33, 34]. This, however, is technically very demanding because of the miniature size of the mouse heart, and only a few laboratories in the world are currently able to perform such studies.
31P-MR spectra of isolated, perfused mouse hearts. Panel A: heart from a wild-type mouse. Panel B: heart from a mouse overexpressing the cardiac creatine transporter (CrT OE). PCr, the three phosphate groups of ATP (γ-, α-, and β-ATP) and inorganic phosphate (Pi) can be clearly identified. Note the substantially increased PCr content in the CrT OE heart. This figure is an original figure and these spectra have not published before
In various models of heart failure cardiac energetics have been studied using 31P-MRS. For example, high-energy phosphate metabolism in the failing heart was studied with 31P-MRS in rat hearts subjected to chronic myocardial infarction (MI) [35]. Eight weeks after MI induced by ligation of the left anterior descending coronary artery (LAD), hearts were harvested and perfused in the Langendorff mode. We found that PCr in residual intact myocardium was significantly reduced by 31%, but ATP levels were unchanged. Flux through the CK reaction was reduced by 50% and total CK activity was reduced by 17%, while total creatine content decreased by 35%. Calculated [ADP] was unchanged. Thus, energy reserve was substantially reduced in these hearts and when hearts were subjected to acute stress (hypoxia), recovery during reoxygenation was impaired. Similar results were found in pigs that were subjected to MI, which resulted in congestive heart failure [36]. Using in vivo 31P-MRS, it was shown that PCr/ATP ratios were reduced in myocardium remote from the infarct scar in failing hearts. In the same animals, in vivo cardiac 1H-MRS was performed to measure deoxymyoglobin. Infusion of dobutamine resulted in a comparable decrease of PCr/ATP in both groups, but the increase in rate-pressure product was blunted in failing hearts. This attenuated response was not associated with detectable deoxymyoglobin, indicating that insufficient myocardial oxygenation was not a limiting factor. The effect of ACE inhibition or β-receptor blockade after MI was tested in rats [37]. Treatment with either drug partially prevented LV contractile dysfunction, and also largely prevented the reduction in Crtotal, PCr and flux through the CK reaction that occurred in untreated rats.
Reduced PCr/ATP ratios have also been found in other models for heart failure. Dogs with volume-overload hypertrophy due to severe mitral regurgitation showed reduced PCr/ATP, despite normal perfusion [38]. In Syrian cardiomyopathic hamster with advanced heart failure it was shown that ATP, PCr, Crfree, ADP, CK activity and flux through the CK reaction were all significantly reduced [39].
All these studies show a reduction in PCr/ATP ratio, in Crtotal content and a reduced flux through the CK reaction. [ADP] and phosphorylation potential, however, are unaltered in some of these models. This raises the question whether the decreases in PCr and Crtotal are causal to heart failure, a consequence or an escape mechanism to maintain low [ADP].
Several approaches have been made to answer this question. Unchanged [ADP] and phosphorylation potential are found under low-workload conditions, mostly in the isolated heart model where workload conditions are generally lower than in vivo [35]. To address this, several studies have examined [ADP] under high-workload conditions. In post-infarct failing pig hearts it was shown that β-adrenergic stimulation with dobutamine resulted not only in a reduction in PCr/ATP, similar to sham operated hearts, but also in a disproportionate increase of [ADP], which did not occur in control hearts [40]. This study shows that, while [ADP] and phosphorylation potential in failing hearts may be normal under resting conditions, the changes in high-energy phosphates result in an impaired energy state under high-workload conditions. Thus, under such conditions the available free energy might be inadequate and this could contribute to the development of heart failure, and to limited contractile reserve.
Another approach to test whether an impaired CK/PCr system can contribute towards the development of heart failure has been to study models in which the CK/PCr system is impaired, independently of heart failure/hypertrophy. This has been done by targeting the enzyme, CK, or the substrate, creatine.
CK was pharmacologically inhibited using iodoacetamide. It was shown that contractile reserve decreased in line with decreasing CK activity and an inverse linear relationship between contractile performance and ΔG ATP was demonstrated [41]. Another approach was made by knocking out the gene for one or more isoforms of CK in mice [42, 43]. In the heart the main isoforms of CK are cytosolic muscle type CK (M-CK) and mitochondrial CK (mito-CK). Hearts from M-CK knockout mice showed normal contractile characteristics and normal PCr and ATP concentrations, in spite of a 38% reduction in flux through the CK reaction as shown by 31P saturation transfer techniques [44]. Mice lacking mito-CK or both isoforms, in contrast, showed reduced PCr content [45, 46]. Mice lacking both isoenzymes also exhibited increased susceptibility to ischemic injury [47]. Finally, it has been shown that mice lacking mito-CK or both isoforms develop left ventricular hypertrophy and dilatation [48]. These data suggest that it is mito-CK that is most important, as disruption of this isoform results in functional and metabolic derangement.
Impairing the CK/PCr system by targeting the substrate creatine has also been achieved by two different approaches. The heart does not synthesize creatine itself; instead, creatine is made in the liver and the kidneys and taken up via food. Cardiomyocytes depend on the uptake of creatine from blood via a specific creatine transporter protein. To prevent uptake of creatine via this route, β-Guanidinopropionate (β-GP) has been fed to rats [49]. β-GP is a creatine analogue and competes with creatine at the creatine transporter. β-GP can be used in the CK reaction, but its utilization is at least 3 orders of magnitude lower than that of creatine. Feeding 1% β-GP largely depleted the heart of creatine. Using 31P-MRS on isolated hearts it was shown that PCr was also largely depleted but ATP levels remained intact. Flux through the CK reaction was reduced by 90%. These isolated hearts showed left ventricular dilatation and impaired contractile performance, but in vivo, only contractile reserve was impaired. In another study the effect of β-GP feeding was tested in a model of chronic LAD ligation [50]. β-GP feeding after MI did not further impair contractile function but the additional PCr depletion resulted in the inability of hearts to maintain ATP homeostasis. In a separate group rats were fed with β-GP before MI. In this group 24-h mortality was 100%, showing that severe depletion of myocardial creatine was incompatible with survival after a myocardial infarction. A second approach was made by deleting the gene for guanidinoacetate-N-methyltransferase (GAMT) in mice [51]. GAMT is the enzyme that catalyses the second essential step in the synthesis of creatine. By feeding these animals a creatine free diet, hearts were depleted of creatine. Hearts, however, did accumulate guanidinoacetate, the precursor of creatine, normally catalysed by GAMT into creatine. Guanidinoacetate can be used in the CK reaction, but only at a very low rate (<1% of creatine) and phosphorylated guanidinoacetate was found instead of PCr in these hearts with 31P-MRS. These mice did not develop cardiac hypertrophy, but showed reduced contractile reserve during β-adrenergic stimulation and increased susceptibility to ischemia and reperfusion injury. Thus, these models show that, when the heart is stressed, either due to increased workload or ischemia, a high capacity creatine/CK system is critical.
All published studies consistently show that the PCr/ATP as well as the CK reaction velocity is reduced in failing myocardium. PCr content and total creatine content are also consistently reported to be reduced. Since both, total creatine and PCr, are reduced in the failing myocardium, this raises the question whether increasing the myocardial creatine content would be beneficial and could prevent the development of contractile dysfunction. In order to investigate this, rats subjected to MI were fed with a high dose of creatine in their food [52]. Creatine feeding, however, did not increase myocardial (phospho-)creatine in sham operated or infarcted hearts and did not result in improved contractile function in post-MI hearts. Why did the myocardial creatine content not increase in these hearts? As mentioned, creatine content of the heart depends on uptake of creatine via the creatine transporter. When rats are fed with creatine, their plasma creatine content increases but the creatine uptake capacity of the myocardium via the creatine transporter decreases [53]. This downregulation of the creatine transporter also occurs in the failing hearts of post-MI rats and contributes to the reduced creatine content in the failing heart [54]. Therefore, creatine feeding does not increase myocardial creatine content. In order to circumvent this downregulating mechanism, we recently created a mouse with cardiac-specific overexpression of the creatine transporter [55]. These mice indeed show myocardial creatine content and PCr levels increased to supranormal levels, as measured by 31P-MRS (Fig. 4). Surprisingly, however, these mice develop heart failure. The most likely explanation for this is the fact that they fail to keep the increased creatine pool adequately phosphorylated. Therefore, the PCr/Crfree ratio decreases, and as follows from Eq. (2), so does the ATP/ADP ratio. Therefore, the free energy change of ATP hydrolysis is reduced, likely contributing to the development of heart failure. These findings show that in the healthy heart, disturbance of the tightly regulated creatine levels is deleterious. However, the question whether increasing creatine levels in heart failure, a situation where creatine levels are reduced, is beneficial remains to be answered.
Summary and future perspective
Taken together, MRS techniques have clearly shown that total creatine and PCr are substantially reduced in patients with severe heart failure due to dilated cardiomyopathy and that ATP levels are reduced only in advanced dilated cardiomyopathy. Flux through the CK reaction is reduced, to a greater extent than steady-state levels of PCr and ATP, even in mild to moderate heart failure. Further reduction of voxel size for 31P-MRS will be needed to allow for the reduction of blood contamination, and thereby the examination of Pi and pHi. The simultaneous measurement of myocardial creatine, PCr, ATP, Pi and pHi levels in patients with severe heart failure should elucidate whether free ADP levels are increased and free energy change of ATP hydrolysis is decreased.
In experimental studies, PCr and ATP levels as well as flux through the CK reaction are reduced and it has been shown that increased workload or stress can result in an increase of free ADP in failing hearts. Also, increased free ADP levels have been found to correlate with reduced contractile reserve. However, further characterization of existing experimental models as well as development of new models will be required to clarify whether increased ADP levels contribute directly to contractile impairment in heart failure.
So far only 1H- and 31P-MRS have been applied to study heart failure. MRS of other nuclei might in the future also contribute to the study and diagnosis of heart failure. 23Na-MR spectroscopic imaging has recently been shown to be an indicator of myocardial viability [56]. From experiments using fluorescent techniques in isolated cardiomyocytes it is also known that intracellular Na+ levels are increased in the failing myocardium [57]. Thus, 23Na-MRS could potentially be used to measure this in whole hearts. To discriminate between intra- and extracellular Na+, however, a shift reagent is required, and the currently available shift reagents are all toxic and can only be used to study isolated hearts or in terminal in vivo studies. There are many additional adaptations or derangements in myocardial metabolism in the failing heart (Recently reviewed by Stanley et al. [58]). 13C-MRS can be used to study various metabolic pathways in isolated hearts [59] and to measure myocardial substrate selection in vivo [60]. Due to the very low MR sensitivity and the low natural abundance of 13C, however, such studies can currently only be done using (expensive) 13C-labelled compounds. Recently, however, hyperpolarized 13C-MRI/S has been reported [61]. With this technique, the signal to noise is enhanced by a factor of 105 and metabolic 13C imaging may become a possibility. Further development of this technique could lead to the analysis of many complex metabolic pathways and their role in human heart failure.
References
Garlick PB, Radda GK, Seeley PJ (1977) Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun 74:1256–1262
Ingwall JS (1982) Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol 242:H729–744
Jacobus WE, Taylor GJt, Hollis DP, Nunnally RL (1977) Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature 265:756–758
Bottomley PA (1985) Noninvasive study of high-energy phosphate metabolism in human heart by depth-resolved 31P NMR spectroscopy. Science 229:769–772
Blackledge MJ, Rajagopalan B, Oberhaensli RD, Bolas NM, Styles P, Radda GK (1987) Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci USA 84:4283–4287
Bottomley PA, Weiss RG (1998) Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet 351:714–718
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281:21–40
Bittl JA, Ingwall JS (1985) Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem 260:3512–3517
Meininger M, Landschutz W, Beer M, Seyfarth T, Horn M, Pabst T, Haase A, Hahn D, Neubauer S, von Kienlin M (1999) Concentrations of human cardiac phosphorus metabolites determined by SLOOP 31P NMR spectroscopy. Magn Reson Med 41:657–663
Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96:2190–2196
Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD (1996) Creatine kinase system in failing and nonfailing human myocardium. Circulation 94:1894–1901
Auffermann W, Chew WM, Wolfe CL, Tavares NJ, Parmley WW, Semelka RC, Donnelly T, Chatterjee K, Higgins CB (1991) Normal and diffusely abnormal myocardium in humans: functional and metabolic characterization with P-31 MR spectroscopy and cine MR imaging. Radiology 179:253–259
Bottomley PA, Hardy CJ, Roemer PB (1990) Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med 14:425–434
Bottomley PA, Atalar E, Weiss RG (1996) Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med 35:664–670
Neubauer S, Krahe T, Schindler R, Hillenbrand H, Entzeroth C, Horn M, Bauer WR, Stephan T, Lackner K, Haase A et al (1992) Direct measurement of spin-lattice relaxation times of phosphorus metabolites in human myocardium. Magn Reson Med 26:300–307
Van Dobbenburgh JO, Lekkerkerk C, Van Echteld CJA, De Beer R (1994) Saturation correction in human cardiac 31P MR spectroscopy at 1.5 T. NMR Biomed 7:218–224
Beer M, Spindler M, Sandstede JJ, Remmert H, Beer S, Kostler H, Hahn D (2004) Detection of myocardial infarctions by acquisition-weighted 31P-MR spectroscopy in humans. J Magn Reson Imaging 20:798–802
Bottomley PA (1994) MR spectroscopy of the human heart: the status and the challenges. Radiology 191:593–612
Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M (2003) Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol 42:1587–1593
Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD (1985) The creatine kinase system in normal and diseased human myocardium. N Engl J Med 313:1050–1054
Bottomley PA, Hardy C (1992) Mapping creatine kinase reaction rates in human brain and heart with 4 tesla saturation transfer 31P NMR. J Magn Reson 99:443–448
Weiss RG, Gerstenblith G, Bottomley PA (2005) ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 102:808–813
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G (1991) Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 122:795–801
Masuda Y, Tateno Y, Ikehira H, Hashimoto T, Shishido F, Sekiya M, Imazeki Y, Imai H, Watanabe S, Inagaki Y (1992) High-energy phosphate metabolism of the myocardium in normal subjects and patients with various cardiomyopathies – the study using ECG gated MR spectroscopy with a localization technique. Jpn Circ J 56:620–626
de Roos A, Doornbos J, Luyten PR, Oosterwaal LJ, van der Wall EE, den Hollander JA (1992) Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: assessment with proton-decoupled P-31 MR spectroscopy. J Magn Reson Imaging 2:711–719
Schaefer S, Gober JR, Schwartz GG, Twieg DB, Weiner MW, Massie B (1990) In vivo phosphorus-31 spectroscopic imaging in patients with global myocardial disease. Am J Cardiol 65:1154–1161
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K et al (1992) 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86:1810–1818
Neubauer S, Horn M, Pabst T, Godde M, Lubke D, Jilling B, Hahn D, Ertl G (1995) Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 16(Suppl O):115–118
Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274
Grove TH, Ackerman JJ, Radda GK, Bore PJ (1980) Analysis of rat heart in vivo by phosphorus nuclear magnetic resonance. Proc Natl Acad Sci USA 77:299–302
Bottomley PA, Herfkens RJ, Smith LS, Brazzamano S, Blinder R, Hedlund LW, Swain JL, Redington RW (1985) Noninvasive detection and monitoring of regional myocardial ischemia in situ using depth-resolved 31P NMR spectroscopy. Proc Natl Acad Sci USA 82:8747–8751
Balaban RS, Kantor HL, Katz LA, Briggs RW (1986) Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 232:1121–1123
Chacko VP, Aresta F, Chacko SM, Weiss RG (2000) MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol 279:H2218–2224
Schneider JE, Tyler DJ, Ten Hove M, Sang AE, Cassidy PJ, Fischer A, Wallis J, Sebag-Montefiore LM, Watkins H, Isbrandt D, Clarke K, Neubauer S (2004) In vivo cardiac 1H-MRS in the mouse. Magn Reson Med 52:1029–1035
Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, Friedrich J, Gaudron P, Schnackerz K, Ingwall JS et al (1995) Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest 95:1092–1100
Murakami Y, Zhang Y, Cho YK, Mansoor AM, Chung JK, Chu C, Francis G, Ugurbil K, Bache RJ, From AH, Jerosch-Herold M, Wilke N, Zhang J (1999) Myocardial oxygenation during high work states in hearts with postinfarction remodeling. Circulation 99:942–948
Hugel S, Horn M, Remkes H, Dienesch C, Neubauer S (2001) Preservation of cardiac function and energy reserve by the angiotensin-converting enzyme inhibitor quinapril during postmyocardial infarction remodeling in the rat. J Cardiovasc Magn Reson 3:215–225
Zhang JY, Toher C, Erhard M, Zhang Y, Ugurbil K, Bache RJ, Lange T, Homans DC (1997) Relationships between myocardial bioenergetic and left ventricular function in hearts with volume-overload hypertrophy. Circulation 96:334–343
Tian R, Nascimben L, Kaddurah Daouk R, Ingwall JS (1996) Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. J Mol Cell Cardiol 28:755–765
Liu J, Wang C, Murakami Y, Gong G, Ishibashi Y, Prody C, Ochiai K, Bache RJ, Godinot C, Zhang J (2001) Mitochondrial ATPase and high-energy phosphates in failing hearts. Am J Physiol Heart Circ Physiol 281:H1319–1326
Tian R, Ingwall JS (1996) Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol 270:H1207–1216
van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, ter Laak H, Wieringa B (1993) Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74:621–631
Steeghs K, Benders A, Oerlemans F, deHaan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B (1997) Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell 89:93–103
Van Dorsten FA, Nederhoff MGJ, Nicolay K, Van Echteld CJA (1998) 31P NMR studies of creatine kinase flux in M-creatine kinase-deficient mouse heart. Am J Physiol 275:H1191–1199
Saupe KW, Spindler M, Tian R, Ingwall JS (1998) Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res 82:898–907
Spindler M, Niebler R, Remkes H, Horn M, Lanz T, Neubauer S (2002) Mitochondrial creatine kinase is critically necessary for normal myocardial high-energy phosphate metabolism. Am J Physiol Heart Circ Physiol 283:H680–687
Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, Neubauer S (2004) Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol 287:H1039–1045
Nahrendorf M, Spindler M, Hu K, Bauer L, Ritter O, Nordbeck P, Quaschning T, Hiller KH, Wallis J, Ertl G, Bauer WR, Neubauer S (2005) Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res 65:419–427
Neubauer S, Hu K, Horn M, Remkes H, Hoffmann KD, Schmidt C, Schmidt TJ, Schnackerz K, Ertl G (1999) Functional and energetic consequences of chronic myocardial creatine depletion by beta-guanidinopropionate in perfused hearts and in intact rats. J Mol Cell Cardiol 31:1845–1855
Horn M, Remkes H, Stromer H, Dienesch C, Neubauer S (2001) Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation 104:1844–1849
Ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Sebag-Montefiore L, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S (2005) Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation 111:2477–2485
Horn M, Remkes H, Dienesch C, Hu K, Ertl G, Neubauer S (1999) Chronic high-dose creatine feeding does not attenuate left ventricular remodeling in rat hearts post-myocardial infarction. Cardiovasc Res 43:117–124
Boehm E, Chan S, Monfared M, Wallimann T, Clarke K, Neubauer S (2003) Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am J Physiol Endocrinol Metab 284:E399–406
Ten Hove M, Chan S, Lygate C, Monfared M, Boehm E, Hulbert K, Watkins H, Clarke K, Neubauer S (2005) Mechanisms of creatine depletion in chronically failing rat heart. J Mol Cell Cardiol 38:309–313
Wallis J, Lygate CA, Fischer A, Ten Hove M, Schneider JE, Sebag-Montefiore L, Dawson D, Hulbert K, Zhang W, Zhang MH, Watkins H, Clarke K, Neubauer S (2005) Supra-normal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure – insights from creatine transporter over-expression transgenic mice. Circulation 112:3131–3139
Jansen MA, Van Emous JG, Nederhoff MG, Van Echteld CJ (2004) Assessment of myocardial viability by intracellular 23Na magnetic resonance imaging. Circulation 110:3457–3464
Pieske B, Maier LS, Piacentino V 3rd, Weisser J, Hasenfuss G, Houser S (2002) Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 106:447–453
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129
Lewandowski ED, Yu X, LaNoue KF, White LT, Doumen C, O’Donnell JM (1997) Altered metabolite exchange between subcellular compartments in intact postischemic rabbit hearts. Circ Res 81:165–175
Ziegler A, Zaugg CE, Buser PT, Seelig J, Kunnecke B (2002) Non-invasive measurements of myocardial carbon metabolism using in vivo 13C NMR spectroscopy. NMR Biomed 15:222–234
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci USA 100:10158–10163
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the British Heart Foundation and the Medical Research Council, London, England.
Rights and permissions
About this article
Cite this article
ten Hove, M., Neubauer, S. MR spectroscopy in heart failure—clinical and experimental findings. Heart Fail Rev 12, 48–57 (2007). https://doi.org/10.1007/s10741-007-9003-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-007-9003-8