Abstract
Arsenic (As3+), a significant environmental pollutant that has garnered global attention, is widely recognized for its adverse effects on reproductive health. This study assesses the aphrodisiac activity of Dehydrozingerone (DHZ) against As3+ induced sexual dysfunction in male Wistar rats. Male Wistar rats were divided into control, As3+, and As3++DHZ groups. The As3+ group received 5 mg/kg sodium arsenite (NaAsO2) orally while As3++DHZ group received 50 mg/kg synthesized DHZ along with As3+ for 42 days. Following administration, mount and intromission latency, frequency, and average time were measured to assess aphrodisiac and reproductive toxicity in male Wistar rats which had 1:1 coitus with female rats. On days 14th, 28th, and 42nd, sexual behaviour was measured. Further on 43rd day, animals were sacrificed, blood was collected to measure oxidative parameters and LH hormone, and then testes were collected to profile reproductive damage. As3+ treated rats had lower sperm counts, motility, and abnormalities. These alterations reduced sexual hormones. In addition, As3+ toxicity depleted antioxidant indicators including SOD, GSH and elevated ROS. Compared to the As3+ group, As3++DHZ showed a substantial (p < 0.05) increase in sperm count, motility, and reduced abnormalities. DHZ also reversed the rise in luteinizing hormone caused by As3+ therapy, restored oxidative indicators, and improved seminiferous tubule structural damage. 42 days As3+ exposure slightly increased rats’ sexual desire but not sperm quality. However, As3++DHZ lower libido and sperm quality. Thus, DHZ therapy enhanced rat sexual desire and sperm quality compared to As3+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately about 100 million people are believed to be affected by As3+ through potable water. According to the World Health Organization (WHO), 10 ppb (10 µg/L) of As3+ in potable water is considered safe for consumption (Arsenic 2022). As per WHO standards, As3+ lies in the range of parts per billion, yet a large number of individuals either drink uncontrolled water from wells or reside in areas where the usual level of As3+ lies in the range of parts per million (Allan et al. 2015). Most of the As3+ count in drinking water resides in an inorganic form, making it more hazardous than the As3+ found in foods like grains and vegetables (Akter et al. 2005; Kim and Kim 2015). Therefore, As3+ is a ubiquitous and persistent environmental pollutant that has an impact on practically all of the body’s major organ systems like the kidneys, liver and many more. Apart from all these toxicities, As3+−induced toxicity in the reproductive system causes infertility in both (males and females) and thus has received a lot of attention recently (Sarkar et al. 2003; Souza et al. 2016a, b; Zubair et al. 2017). Under preclinical experimental studies, administration with As3+ to male animals results in slow deposition of As3+ within the tissues of the testes, which gradually causes the stiffening of the basement membrane of the testis, degeneration of vessels along with the rise of hemorrhage in interstitial tissues, Leydig cells deformities, all of which combined result in a substantial decrease in sperm count along with its motility which ultimately results in abnormal sperms (Zubair et al. 2014; Bashandy et al. 2016; Souza et al. 2019).
Excessive As3+ exposure imbalances the natural antioxidant defence mechanism and concomitantly abundant reactive oxygen species (ROS) production by inhibiting the synthesis of different antioxidants like glutathione-s-transferase, glutathione peroxidase, superoxide dismutase, catalase and many more, exacerbating oxidative stress within the testis of male animals (Bashandy et al. 2016; de Almeida Lima et al. 2018). Apart from this, increased luminal regions, decreased accumulation of spermatozoa, necrotic alterations, and an unorganized cellular structure were also induced due to As3+ exposure (Reddy et al. 2011; Abdel Aziz et al. 2018; Khair et al. 2021). Besides the fact that numerous experimental methods have been created to explore how exposure towards As3+ results as an outcome of these diverse diseases, the exact molecular processes leading to reproductive as well as developmental toxicity due to As3+ exposure remain obscured, and a direct correlation between the experimental and human exposures have not been established yet.
To ensure restoration of As3+−induced reproductive toxicities, a variety of natural compounds are utilized as aphrodisiacs, including rhinoceros horn, yohimbine, mandrake herb, etc. (Kotta et al. 2013). The development of Viagra (sildenafil) given for the treatment of impotency (primarily erectile dysfunction) in the 1990s marked a paradigm shift in the study of sexual dysfunction. However, due to some of its adverse effects, sildenafil was in the centre of a storm. Therefore, the need to find natural supplements is highlighted. The quality of sexual life is maintained in large part by diet, which is one of the key variables. Understanding the components of the diet and how they affect a man’s ability to reproduce and engage in sexual activity could aid in resolving issues with these aspects of life to some extent (Rai et al. 2018). For the efficient working of the male reproductive system in both animals as well as in humans, a variety of natural compounds offer a set of properties that have therapeutic values. These natural products can either comprise a single bioactive molecule or might be a combination of different bioactive compounds. A review article by Roberta et al. have summarized the overview of various bioactive compounds such as curcumin (Lin et al. 2015), ellagic acid (Hamza and Al-Baqami 2019), vitamin C (Adelakun et al. 2018a, b) and vitamin E (Yuncu et al. 2015), and other natural products that carry bioactive compounds in their composition, such as garlic (Nasr 2017), ginger (Al-Shathly et al. 2020), grape (Hasona 2018), green tea (Mosbah et al. 2015), microalgae, algae (Guner et al. 2020; Wang et al. 2020) and propolis (Abd-Elrazek et al. 2020). Most of these agents have been reported to possess antioxidant as well as anti-inflammatory properties, indicating the potential advantages of these agents against the male reproductive system (Martins et al. 2021).
In the present study, we evaluated the aphrodisiac effect of Dehydrozingerone (DHZ), a chemical ingredient of ginger, i.e. Zingiber officinalis. DHZ naturally exists as a phenolic compound, which has received a lot of interest from medicinal chemists. It has shown a broad spectrum of biological characteristics, including antioxidant (Parihar et al. 2007), anti-platelet (Hampannavar et al. 2016), anti-depressant (Moorkoth et al. 2021), anticancer (Kumar et al. 2017), anti-inflammatory, anti-malarial, and anti-fungal activity (Hampannavar et al. 2016). However, the effect of this compound has not been explored against reproductive toxicity. Considering the reproductive toxicity of As3+ and the benefits of various bioactive herbal ingredients, this study was designed to explore the effect of DHZ against As3+mediated sexual dysfunction in male Wistar rats by evaluating the aphrodisiac and antioxidant assessment along with sperm quality evaluation (both quality and quantity).
Materials and methods
Chemicals required
Sodium arsenite (NaAsO2) and Dulbecco’s Modified Eagle’s medium (DMEM) were procured from Sigma Aldrich. Eosin dye and ethanol were obtained from HiMedia Laboratories Pvt. Ltd. Mumbai, India, while acetonitrile, acetone, vanillin, sodium hydroxide, and HCl were received from SRL Company.
Synthesis of DHZ
5.0 g of vanillin was dissolved in 20.00 ml acetone, and this mixture was placed on an ice bath till the temperature fell below 5 °C, followed by the addition of 20.00 ml distilled water solution of 2.8 g of sodium hydroxide which was added gradually. The solution was mixed at 300 rpm for 1.5 h and then kept overnight at room temperature. On a consecutive day, 600 ml cold water and 200 g ice were added, and later, 10% HCl was added with continuous stirring until a yellow precipitate was formed, which was subsequently filtered and dried. Recrystallization was performed with 40% ethanol. The characterization of the final product was performed by HPLC and LC-HRMS (Pathak et al. 2020; Moorkoth et al. 2021).
HPLC analysis
A solution of 100 µg of DHZ in 1.00 ml acetonitrile (100 µg/ml) was dissolved in 10.00 ml of distilled water to obtain a 10 µg/ml concentration of DHZ. Using column C18 and maintaining the conditions such as temperature at 30 °C, the detector’s wavelength at 340 nm and acetonitrile with 0.1% o-phosphoric acid water at a 50:50 ratio as a mobile phase was used to analyze the DHZ sample by applying run time of 15 min (mins). Further, on the confirmation of purity peak at a particular retention time, calibration curve generation was carried out at 3.125 µg/ml, 6.25 µg/ml, 12.5 µg/ml, 25 µg/ml and 50 µg/ml concentration of DHZ. All these concentrations were prepared using the serial dilution method.
DHZ analysis by LC-HRMS and HPLC
LC-HRMS for mass spectroscopy of DHZ sample of 10 µg/ml concentration was prepared in the same way as prepared for HPLC analysis. Optimized chromatographic conditions are as follows: column-Hypersil gold (c18-100* 2.1, particle size-1.9µ), mobile phase A (100% water + 0.1% Formic acid), mobile phase B (100% methanol + 0.1% formic acid), rate of flow: 0.3 ml/mins, the volume of injection: 10 µl, autosampler temp.− 25 °C, column oven temp. 40 °C 10, UV visible: 220 nm, 240 nm, 254 nm & 340 nm. The run time was kept at 50 min. Gradient programme: 0.0 min (%A-95, %B-5),1 min (%A-95, %B-5), 10 min (%A-50, %B-50), 30 min (%A-5, %B-95), 40 min (%A-95, %B-5) & 50 min (%A-95, %B-5).
Optimized mass spectroscopy conditions: Ion source- HESI (positive and negative mode), spray voltage positive ion: 3500 V, negative ion: 2500 V, sheath gas: 40arb, sweep gas: 0 arb, auxiliary gas: 15 arb, vaporizer temp.: 350 °C, ion transfer tube temp.: 320 °C, application mode: small molecules. Full scan details: orbital resolution-60,000, scan range (m/z): 100–1000 microscans, 1 m/z, RF lens (%)-70, polarity- positive and negative, respectively.
Experimental animals
Prior approval was obtained from IAEC, NIPER Hajipur with IAEC number: NIPER-H/IAEC/06/21 and the experimental procedures were conducted as per the guidelines of CCSEA, Govt of India. Both male and female Wistar rats (n = 21 each) of eight weeks with 80-120gm were obtained from Central Drug Research Institute (CDRI), Lucknow, UP. Animals were acclimated for 7 days in a well-controlled condition of NIPER Hajipur animal house (having temperature, 24–26 ± 20 °C; humidity, 55 ± 5%, 12 h light/dark-cycle) with distilled water ad libitum and food supply. Animals were allowed to mate in the animal house for an aphrodisiac behaviour study. Before mating, animals were divided into respective groups based on the study schedule.
Animals grouping & dosing schedule
The first group was considered as the control group and received 0.25% w/v CMC at a dose volume of 10 ml/kg. The second group received As3+ (NaAsO2−5 mg/kg, 6 days/week for 6 consecutive weeks) by oral gavage. This group was considered as the disease control group (Jana et al. 2006a, b). The third group was considered as the treatment group and received DHZ (50 mg/kg) orally along with NaAsO2 (5 mg/kg for 6 days/week for 6 consecutive weeks) (Tirunavalli et al. 2021). There were total of 7 animals in each group, with an equal number of males and females, and they were housed in separate cages.
Male rats
Male rats received oral doses (by oral gavage) every day at 18:00 p.m. for 42 consecutive days. After two hours of dosing (20:00 p.m.), the test was conducted on the 14th, 28th and 42nd days of the study period in a glass box illuminated by a dim red lamp (10 W). Before the initiation of the test, each male and female rat was individually acclimated for 15 min in the glass chamber.
Female rat selection
Rats (female) were tracked for two successive oestrous cycles in a row. Animals with a normal oestrous cycle were chosen. The oestrous cycle was monitored by examining 3 kinds of cells present in a vaginal smear. These smears were made by aspirating or flushing cells from the lining of the vagina with 0.9% saline/distilled water using a sterile disposable plastic pipette with a 1.5 mm internal tip bore. A light microscope was used to examine one to two drops of the acquired cell solution on the slide. Smear with the appearance of spherical nucleated epithelial cells reflected the pro-oestrous phase. The oestrous phase was characterized by cornified cells lacking a nucleus arranged in an irregular form, while the existence of a majority of round-shaped leucocytes indicated the di-oestrous phase. Female rats lying in their receptive phase of the oestrous cycle were used to study male rats’ aphrodisiac behaviour (pro-oestrus phase)(Das et al. 2016).
Male rat sexual behaviour test
A 1:1 ratio of male rat and female rat (receptive) were placed in a glass tank for an aphrodisiac parameters’ evaluation in a dark, isolated room having a dim red bulb light (10 W) in the evening (between 20:00–23:00 h), for maximum 30 min. Two hours after the treatment, male rats were individually put into respective glass chambers for around 15 min to facilitate acclimatization. The receptive female rats were gently transferred into the glass chamber of previously carrying male rats, and the dim red light was switched ON after 5 min (10 W). The experiment commenced by introducing the female rat inside the glass chamber. After a duration of 15 min, the male and female rats were thereafter separated. The sexual behaviour parameters of rats were observed following the coupling of male and female rats for a duration of 15 min. The female rat’s response was characterized by lordosis, which is a stiff position involving the arching of the back and deflection of the tail to facilitate mounting and intromission by a male rat. The standard factors that define sexual behaviour were measured through manual scoring, using a timer (Das et al. 2016; Rai et al. 2018).
-
Mount latency: Time taken for the initial mounts (either with/without penile penetration) by the commencement of the test;
-
Mount frequency: Mount numbers that occurred apart from intromission till ejaculation;
-
Mount average time: Average time of mounting;
-
Intromission latency: Duration between the commencement of the test and the initial intromission (as measured by pelvic thrusting);
-
Intromission frequency: Number of intromissions till ejaculation;
-
Intromission average time: Average time of intromission.
Evaluation of potential toxicity in the reproductive system of male rats
After the 42nd day of treatment, the male Wistar rats were anaesthetized by diethyl ether. Blood was withdrawn from the retroorbital plexus and animals were sacrificed by euthanasia (cervical dislocation). Testes were isolated and stored in 10% formalin solution. Evaluation of sperm’s motility, count and morphological analysis was performed to assess toxicity profiling of the male reproductive system from the epididymis (Obinna and Agu 2021). Epididymis tissue homogenate was used for estimation of ROS, SOD and GSH.
Sperm count and motility
Sperms were extracted from the cauda epididymis region by fragmenting them at many places and then washing them in DMEM (pre maintained at 37 °C) on a suitable petri plate. The occasional shaking of the petri dish for 10 min allowed sperm to diffuse in the media. The medium’s tissue was separated, and the sperms were appropriately diluted in DMEM media. The diluted sperm solution was used in the Neubauer® chamber for sperm count and motility testing (Das et al. 2016). The countessTM 3 FL automatic cell counter detected the motility and allowed the counting of sperms. Within 5 min of duration, forward movement and flagella activity revealed sperm motility (beating/inertness), which was an indicator of the number of living sperms and represented a total number of sperms in percentage.
Sperm morphological analysis
For staining spermatozoa, 0.5% anionic dye solution of Eosin Y [0.5 g/100 ml] carrying 0.9% [0.9 g/100 ml] aqueous solution of sodium chloride was utilized. For diluting the sperm suspension, a predetermined amount of 0.5 ml DMEM was used, and the drop was settled on a glass slide, spread, and air-dried. Later, the smear (air-dried) was later immersed in a staining solution for approximately 30 min before being air-dried and examined under the microscope. About 100 spermatozoa were examined through a bright field microscope at 40 × magnification. Following that, sperm abnormalities were evaluated and classified as primary or secondary. Any change in the morphology or size of the sperm’s head (headless tail) was presumed to be a primary defect. Alterations in the sperm’s tail (such as bent neck, tailless head, midpiece deformity, and kinked tail) were considered secondary abnormalities (Menon et al. 2011; Filho et al. 2015).
Evaluation of ROS generation
Production of ROS was measured by 2,7 dichloro dihydrofluorescein diacetate (H2DCF-DA), which is a non-fluorescent dye but gets converted into a highly fluorescent-dichlorofluorescein (DCF) form through cellular peroxides whose fluorescence was observed through ELISA plate reader at excitation 504 nm and emission 530 nm (Hedayat et al. 2015).
Evaluation of superoxide dismutase (SOD) level
The activity of SOD in tissue homogenates was assessed by utilizing a SOD kit (Sigma Aldrich- Catalogue no: 19160-1KT-F). The assay was based on SOD´s capacity to prevent phenazine methosulphate, which was meant to be reduced by nitroblue tetrazolium dye. In a 1:10:1 ml ratio, 0.02 ml sample was added to 0.2 ml of working solution (50 mM/L phosphate buffer pH 8.5, 1 mM/L nitroblue tetrazolium, 1 mM/L NADH). The reaction begins by adding 0.02 ml working solution to the mixture followed by plate incubation at 37 °C for 20 min and absorbance was taken at 450 nm. SOD activity, i.e. inhibition rate (%) was calculated by applying the formula:
SOD activity was expressed as per mg of protein (Bolin et al. 2010).
Evaluation of GSH level
The response of glutathione peroxidase in tissue homogenates was estimated using a glutathione peroxidase assay kit. The technique is based on an indirect assessment of glutathione activity, in which glutathione peroxidase reduces an organic peroxide to create oxidized glutathione. Glutathione reductase uses NADPH as a cofactor to recycle this product to its reduced state (Nakamura et al. 2019).
Evaluation of luteinizing hormone (LH) level
Analysis of LH was done through the Enzyme-Linked Immunosorbent Assay (Tsatmali et al. 2006) kits available commercially from Elabscience Biotechnology Co, Ltd., USA. The sensitivity of the kit was 0.91 mIU/ml. The absorbance was detected using a SpectraMax ID5 Multi-Mode Microplate Reader.
Histology of testis
The testes were fixed in 10% formalin, and further embedded in paraffin. Sections were made of 5 μm thickness and stained with haematoxylin–eosin stain. The slides were studied microscopic changes using a microscope.
Statistical analysis
Graph pad prism software (version 5.0) was employed to analyze the data that were obtained statistically. Two-way ANOVA was used for the statistical comparison, followed by Tukey’s multiple comparison tests for mount latency, mount frequency, mount average time, intromission latency, intromission frequency and intromission average time. For the remaining analysis, One-way ANOVA was applied followed by Bonferroni multiple comparison tests. Every value was expressed as Mean ± SEM, with p < 0.05 as statistical significance. Normality of the data assessed by D’Agostino-Pearson omnibus (K2).
Results
Synthesis and characterization of DHZ (C11H12O3)
Vanillin was effectively used as the starting material for the synthesis of DHZ by yielding a yellow product, which was later characterized and its purity was confirmed by LC-HRMS (Figs. 1 and 2) and HPLC analysis (Figs. 2 and 3). The base peak was observed as [M + 1] 193.0859 m/z with a relative abundance of 100% DHZ.
Mount latency (ML)
Sodium arsenite-treated animals did not show any difference after 14 days of dosing, although a slight decrease in mount latency on the 28th day (53 ± 12.01s) in comparison to the normal group (86.67 ± 8.81 s) was observed. On the 28th day, a similar decrease was seen in the As3+ + DHZ treated group (49 ± 5.57s) as compared to the normal group. However, As3+ treated animals after the 42nd day of dosing showed a marked decrease (110 ± 10 s) as compared to the normal control (120 ± 11.54s) group. A significant (p < 0.05) decrease in mount latency was seen in As3+ + DHZ treated animals as compared to the As3+ group (Fig. 4).
Mount latency in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two-way ANOVA followed by Tukey’s multiple comparison test, where $p < 0.05 v/s normal, *p < 0.05 v/s Arsenic group. A-Arsenic group (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Mount frequency (Lucinda et al. 2010)
5.66 ± 0.33, 6.67 ± 0.33 and 8.3 ± 0.67 MF were found in the normal control group after 14, 28 and 42 days of dosing vehicle (0.25% w/v CMC) respectively. Treatment with As3+ showed a significant (p < 0.05) decrease in MF on the 42nd day to 4.67 ± 0.33 as compared to the normal control group, while no significant (p < 0.05) change was seen on the 14th (5.33 ± 0.67) and 28th day (6.0 ± 1.0). As3+ + DHZ treatment resulted in a significant elevation in MF on the 14th (8.3 ± 0.33) and 42nd day (9.00 ± 0.58) as compared to the As3+ alone treated group. However, no significant change was seen in the As3+ + DHZ treatment group on the 28th day as compared to the normal control group (Fig. 5).
Mount frequency in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test, where $p < 0.05 v/s normal and *p < 0.05 v/s Arsenic group, A-Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Mount average time (MAT)
3.33 ± 0.33, 3.3 ± 0.66 and 3.3 ± 0.33 MAT was recorded in normal control group after 14th, 28th and 42nd days of dosing respectively. Treatment with As3+ showed a minor increment in MAT on the 14th testing day (4.0 ± 0.57) but then the same was decreased by 3.3 ± 0.33 and 3.0 ± 0.57 on the 28th and 42nd day respectively as compared to the normal control group. As3+ + DHZ treatment results in a less significant increase in MAT on the 14th and 28th day of testing 4.6 ± 0.33 and 4.3 ± 0.33 respectively, but after the 42nd day of dosing, a significant increase (p < 0.05) was observed in MAT 5.00 ± 0.57 in comparison to As3+ group (Fig. 6).
Mount average time in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test, where *p < 0.05 v/s Arsenic group, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Intromission latency (IL)
As3+ treated animals after the 14th day of dosing, showed a slight decrease in intromission latency as 293.33 ± 58.11s respectively in comparison to the normal control group i.e. 363.33 ± 43.33s. On the 28th day, As3+ showed a slight increase in intromission latency to 425 ± 75s as compared to normal control 373.33 ± 50.42s. But on day 42nd of testing, the As3+ group resulted in more IL as 493 ± 29.63s in comparison to normal control (296.67 ± 26.03s). As3+ + DHZ treated animals showed a significant increase in IL after the 14th day of dosing (373.33 ± 12.01s) as compared to the As3+ group. However, after the 28th day and 42nd of dosing, a slight decrease (316.66 ± 56.07s and 253.33 ± 20. ± 27s) in IL were found as compared to As3+group (p < 0.05) (Fig. 7).
Intromission latency in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test, where **p < 0.01 v/s Arsenic group on day 42, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Intromission frequency (Obinna and Agu 2021)
3.33 ± 0.33, 3.66 ± 0.88 and 2.66 ± 0.33 IF observed in normal control group after 14th, 28th and 42nd days of dosing respectively. Treatment with As3+ showed a significant (p < 0.05) increment in IF after the 14th days of dosing (7.0 ± 1.5) but after the 28th and 42nd days of dosing slight increment of 6.33 ± 0.33 and 2.66 ± 0.66 as compared to the normal control group was reported. As3+ + DHZ treatment resulted in an increase in IF on the 14th and 28th day of testing to 5.33 ± 1.4 and 5.66 ± 1.2 respectively, but after the 42nd day of dosing, a significant increase (p < 0.05) was observed in IF (6.66 ± 0.3) as compared to As3+ group (Fig. 8).
Intromission latency in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test where *p < 0.05 v/s control, $p < 0.05 v/s Arsenic group, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Intromission average time (IAT)
3.33 ± 0.33s, 3.3 ± 0.66s and 3.3 ± 0.33s IAT observed in normal control group after 14th, 28th and 42nd days of dosing respectively. Treatment with As3+ showed a significant increment in IAT on the 14th and 28th testing days with 5.66 ± 0.33s and 6.0 ± 0.57s but on the 42nd day of testing, a decrease was seen (4.3 ± 0.33) as compared to the normal group. As3+ + DHZ treatment resulted in an increase in IAT on the 14th and 28th day of testing at 6.0 ± 0.5s and 7.0 ± 0.57s but after the 42nd day of dosing, a significant increase (p < 0.05) was observed in IAT (6.33 ± 0.88s) as compared to As3+ group (Fig. 9).
Intromission average time in various groups on day 14, day 28 and day 42. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test where *p < 0.05 v/s arsenic on day 42, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Male reproductive system toxicity study
Effect on sperm count
Sperm count reduction was observed in As3+ treated animals (1.05 ± 0.05) with significance (p < 0.05), in comparison to the normal control group (1.53 ± 0.05). As3+ + DHZ administered animals showed a significant increment (p < 0.05) in sperm Count (1.6 ± 0.17) as compared to As3+ treated animals (Fig. 10).
Effect of treatments on Sperm Count. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test where *p < 0.05 v/s control and #p < 0.05 v/s Arsenic group, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Effect on sperm motility (%)
71 ± 3.5% sperms were found to be motile, which was lesser than normal group (87 ± 5.5%). As3+ + DHZ treatment resulted in a significant increase (p < 0.05) in motile sperms (96.5 ± 1.5%) as compared to the As3+ group (Fig. 11).
Effect of treatments on Sperm motility. Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test where *p < 0.05 v/s Arsenic group, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
Sperm abnormality (%)
Primary abnormalities
As3+ treatment showed a significant (p < 0.05) increase (3.6 ± 0.08) in the headless tail as a comparison to the normal control group (3 ± 0.05). As3+ + DHZ treatment resulted in a significant (p < 0.05) decrease to 3.1 ± 0.14 compared to the As3+ group, while in the tailless head, the As3+ group showed a minor increment i.e. 2.2 ± 0.18 v/s 1.9 ± 0.14 as normal control. Also, the As3+ + DHZ treatment resulted in a minor reduction in the tailless head (3.1 ± 0.14) in comparison to the As3+ group (Fig. 12).
Secondary abnormalities
Kinked tail (1.26 ± 0.03), midpiece abnormality (1.13 ± 0.03), and bent neck (1.3 ± 0.05) deformities were observed in sperms of normal group animals. Administration with As3+ showed a minor increase to 1.3 ± 0.05 and 1.23 ± 0.06 in Kinked tail and midpiece abnormality along with a significant increase in Bent neck to 1.5 ± 0.05 in comparison to a normal control group. As3+ + DHZ therapy led to a minor fall in kinked tail 1.2 ± 0.05), midpiece abnormality (1.13 ± 0.03) and a significant (p < 0.05) fall in bent neck deformities 1.2 ± 0.05 in comparison to As3+ group (Fig. 12).
Sperm abnormalities in various groups Data passed the normality of residual test D’Agostino-Pearson omnibus (K2). Data are presented as mean ± SEM and analyzed by Two way ANOVA followed by Tukey’s multiple comparison test where *p < 0.05 v/s control #p < 0.05 v/s Arsenic group, A- Arsenic (Sodium arsenite) and AD- Arsenic (Sodium arsenite) and DHZ
ROS estimation
As3+ control group resulted in a significant (p < 0.01) increase in ROS fold (1.36 ± 0.06) v/s normal control group (1.03 ± 0.015). A significant decrease (p < 0.01) in the ROS fold of As3++ DHZ group animals (0.94 ± 0.034) was observed as compared to the group treated with As3+ (Fig. 13).
Antioxidant SOD estimation
SOD activity (inhibition rate %/ mg of protein) was elevated in the control group (105.1 ± 5.1) while treatment with As3+ depicted a significant decrease (p < 0.01) (67.48 ± 4.08) as compared to the normal control group. Though, in comparison to the As3+ group, the As3+ + DHZ therapy led to a significant (p < 0.01) rise in SOD activity (107.3 ± 7.0) (Fig. 14).
GSH estimation
GSH level (µM/mg of protein) was found to be 41.16 ± 4.3 in the As3+ group which significantly reduced (p < 0.05) in comparison to the normal control group (65.03 ± 5.2). As3+ + DHZ treatment resulted in a significant (p < 0.05) increase in GSH level (66.89 ± 4.1) as compared to the As3+ group (Fig. 15).
Effect on LH hormone levels
The level of LH in normal control was found to be 7.55 ± 1.10 mIU/mL. The As3+ treatment showed a significant (p < 0.05) increase in LH level (3.17 ± 0.50 mIU/mL) as compared to normal control. Treatment with DHZ decreased the LH level (7.69 ± 0.37 mIU/mL) significantly (p < 0.05) as compared to As3+treated animals (Fig. 16).
Histology of testis
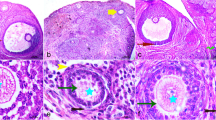
In control group, normal architecture of the seminiferous tubules was observed with mature sperm in the lumen. All stages of germ cells along with sertoli cells and leydig cells were observed. Groups when treated with the As3+, normal architecture of the seminiferous tubules were altered, vacuolations of germ cells were observed with reduction in the germ cells, increased tubular lumen with very few mature sperm. However, when groups were treated with As3+ (Sodium arsenite) and DHZ, significant structural recovery was observed with near normal architecture of the seminiferous tubules and also increases in the number of sperms in the lumen. (Fig. 17)
In the control group normal architecture of the seminiferous tubules were observed. Mature spermatogonia is noted in the seminiferous tubules with luminal spermatid and spermatozoa. No maturation arrest and cytoplasmic vacuoles were detected, Interstitium appears unremarkable. But groups which were treated with the As3+, normal architecture of the seminiferous tubules were altered; however mature spermatogonia were well appreciated. Prominent cytoplasmic vacuolation was observed in the germ cells. The sequence of maturation was altered with a decrease in the mature sperm. Congested capillaries in the interstitium were observed. Further groups which were treated with As3+ (Sodium arsenite) and DHZ, significant structural recovery was observed with various stages of spermatocytes. Sertoli cells appeared normal. Mature sperms were found in the lumen. Interstitium appeared unremarkable. (Fig. 17)
Discussion
As3+ causes several health problems, including the issues associated with the male reproductive system (Sarkar et al. 2003). Intoxication with NaAsO2 not only modifies testicular histopathological characteristics and its weight, but also lowers the activity of many markers such as hormones, enzymes, and the body’s antioxidant defence system (Pant et al. 2004; Morakinyo and Adeniyi 2008). As3+ toxicity leads to damage in the reproductive tissues due to oxidative stress and related pro-apoptotic processes, making this complex system worth to study (Samadder et al. 2012).
Ayurveda has reported numerous herbal medicines and their beneficial effects on human health. These herbal ingredients are generally known to play a prominent role in regulating people’s behaviour and health by maintaining their antioxidant imbalance. The objective of the present experiment was to scientifically assess the impact of DHZ, an active ingredient of Zingiber officinale, a common dietary ingredient and a documented medicinal herb as per Ayurveda, on sexual and reproductive health. An unsaturated derivative of the natural substance zingerone is DHZ, which has a strong anti-inflammatory effect in a variety of models and removes free radicals like superoxide anions and hydroxyl ions from the body (Singh et al. 1987). The DHZ was synthesized and characterized using LCHRMS and HPLC. The graph represents the base peak at 193.089 m/z [M + H] + for the molecular formula C11H12O3 of DHZ (as the actual molecular weight of the compound is being reported as 192.21) along with 99.09% purity with 0.999930 correlation coefficient value. These results successfully validate that the synthesized yellow colour compound was DHZ only.
In the present study, As3+ was administered in the form of NaAsO2 at a 5 mg/kg dose in the animals of two groups, namely As3+ and As3+ and DHZ groups, respectively. The aphrodisiac activity investigation was performed using male Wistar rats with female Wistar rats, which were in the pro-oestrous phase of the oestrous cycle. Sexual arousal measures, such as mount and intromission latencies, and libido (sexual desire) characteristics, which include mount frequency (Lucinda et al. 2010) and intromission frequency (Obinna and Agu 2021), were assessed individually using sexual-behavioural parameters (ML & IL) along with average time required for mount and intromission (Kenjale et al. 2008). In our results, the mounting frequency and latency of As3+ + DHZ treated animals showed a significant decrease in the ML while a slight increment in the MF activity in the same group with a slight increase in mounting time. Therefore, alterations in the ML and MF indicate that DHZ has a modulatory effect on the sexual appetite of As3+ affected animals. DHZ’s beneficial effect in improving sexual dysfunction induced by As3+ can be understood by the fact that DHZ is known to increase dopamine levels in the brain, which is considered to improve sexual function in animals (Moorkoth et al. 2021). Though spermatogenesis requires 52 days, the investigation was carried out over 42 days with continuous dosing of the DHZ (Lucinda et al. 2010) to study sperm maturation, which happens in epididymis transit for fertilization. The study was aimed at a late stage of spermatogenesis and the maturation phase of sperm. Luteinizing hormone is essential for testosterone production from Leydig cells (Oduwole et al. 2021). Testosterone helps in the production and maturation of sperm. A decreased amount of LH indicates a decrease in testosterone production. In the present study, the LH level was found to be reduced by As3+ treatment compared to the normal control group. Similar results have been reported earlier by Jana et al. 2006a, b; where As3+ treatment reduced the production of testosterone (Sarkar et al. 2003; Jana et al. 2006a, b). This indicates As3+ alters testosterone production and causes a decrease in sperm count. Treatment with DHZ showed a reversal in these parameters.
Many researchers have reported that exposure to As3+ has a toxic effect on the male reproductive organ. It has been demonstrated that As3+ hinders steroidogenesis, lowers sperm quality, and damages the development of reproductive organs, all of which can be combined to cause infertility in males. Excess ROS generation creates oxidative stress, which damages the DNA, proteins, and lipid content of the cell and leads to these harmful effects (Ferreira et al. 2012). According to the literature, actively respiring spermatozoa cause electron leakage due to excessive ROS, which is mediated by the redox activities occurring inside the cells. ROS can be produced in spermatozoa through two mechanisms: the first process involves the plasma membrane’s NADPH oxidase system, and the second mechanism involves the mitochondria’s NADH-reliant oxidoreductase reaction, although the second one seems to be the primary origin of the generation of ROS. Spermatozoa content is higher in mitochondria since their motility requires a continuous supply of energy at a constant rate (Henkel 2011). As a result, the coexistence of defective spermatozoa present in the sperm significantly up-regulates ROS generation, which ultimately affects the performance of mitochondria and, as a result, sperm function such as its motility, gets hampered. The bulk section of ROS produced by human spermatozoa is found to be O2− which is an electron-reduced output of O2−. It dismutates with itself to produce H2O2. H2O2 and O2− go through the Haber-Weiss reaction in the presence of transition metals like As3+, resulting in extremely reactive and damaging OH− formation. These OH− radicals are extremely potent initiators of the LPO cascade and can result in sperm function loss due to membrane fluidity disruption (Chen et al. 2013). The result of ROS estimation performed through the DCFDA assay has supported the idea that DHZ reduces the concentration of ROS and represses the oxidative stress within the reproductive cells. According to findings, As3+ triggers the production of ROS, which then interact with spermatozoa that are rich in polyunsaturated fatty acids (PUFAs), particularly the mid-spermatozoa, and causes peroxidation, which ultimately results in spermatozoa destruction that reduces the motility and viability of sperms. Our finding showed an elevation in ROS level in the epididymis of the As3+treated group, which might be reflecting its toxicity by the aforementioned mechanism. However, protection could be observed against this toxicity by the treatment of DHZ.
Our findings also suggested that the level of SOD and GSH were elevated due to As3+ toxicity and the concentration was significantly reduced after the treatment with DHZ. Considering the available literature on SOD, it is reported that by promoting the breakdown of superoxide into oxygen and H2O2, SOD shields sperm from superoxide anions, resisting lipid peroxidation and enhancing motility (Agarwal et al. 2004). ROS that have the potential to harm sperm are removed with the help of SOD. The primary reducing agents found to be in the body are GSH and glutathione peroxidase, which work as scavenging antioxidants in the epididymis and testes (Mora-Esteves and Shin 2013). Their modification in the membrane of spermatozoa provides lipid constituents with protection, preserving sperm viability along with its motility (Lanzafame et al. 2009). GSH has been reported in previous in vitro studies to preserve tail-beat frequency, reduce LPO and improve characteristics of sperm membrane (Griveau and LANNOU 1994). Thus, the present finding on ROS, SOD and GSH showed the protective environment for sperm maturation in Epididymis.
Thus, the present study is important in the assessment of reproductive toxicity of As3+ in male rats. The present results indicated that animals administered alone with As3+ showed no significant changes in sperm motility and viability, but animals treated through As3+ along with DHZ have shown significant improvement in the activities as well as the quality of sperms i.e. its viability, motility and abnormalities. Earlier studies reported loss of spermatogenic cell layers due to As3+ intoxication (Sharma and Kumar 2014). Similar results were seen in As3+ intoxicated tests architecture. DHZ also showed alleviation of the toxicological impact on seminiferous tubule induced by As3+.
Conclusion
The overall study concluded that As3+ exposure for 42 days slightly improved the sexual desire of rats but not the quality of sperm. However, exposure of same along with DHZ reduces the libido and adversely affects sperm quality. Thus, DHZ treatment improved the sexual desire of rats and the quality of sperms as compared to As3+ treated group.
Data availability
No datasets were generated or analysed during the current study.
References
Abd-Elrazek AM, El-Dash HA, Said NI (2020) The role of propolis against paclitaxel-induced oligospermia, sperm abnormality, oxidative stress and DNA damage in testes of male rats. Andrologia 52(1):e13394
Abdel Aziz RL, Abdel-Wahab A, Abo El-Ela FI, Hassan NEY, El-Nahass ES, Ibrahim MA, Khalil AA (2018) Dose- dependent ameliorative effects of quercetin and l-carnitine against atrazine- induced reproductive toxicity in adult male albino rats. Biomed Pharmacother 102:855–864
Adelakun S, Omotoso O, Aniah J, Oyewo O (2018a) Senecio biafrae defeated tetracycline-induced testicular toxicity in adult male sprague dawley rats. JBRA Assist Reprod 22(4):314–322
Adelakun SA, Akinola BK, Akingbade GT (2018b) Fertility enhancing activities of bioactive components of cochlospermum planchonii rhizome on cisplatin induced reproductive dysfunctions in sprague-dawley rats. J Family Reprod Health 12(3):148–159
Agarwal A, Nallella KP, Allamaneni SS, Said TM (2004) Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 8(6):616–627
Akter KF, Owens G, Davey DE, Naidu R (2005) Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 184:97–149
Al-Shathly MR, Ali SS, Ayuob NN (2020) Zingiber officinale preserves testicular structure and the expression of androgen receptors and proliferating cell nuclear antigen in diabetic rats. Andrologia 52(3):e13528
Allan AM, Hafez AK, Labrecque MT, Solomon ER, Shaikh MN, Zheng X, Ali A (2015) Sex-dependent effects of developmental arsenic exposure on methylation capacity and methylation regulation of the glucocorticoid receptor system in the embryonic mouse brain. Toxicol Rep 2:1376–1390
Arsenic. WHOW (2022) World Health Organization (WHO). Arsenic. https://www.who.int/news-room/fact-sheets/detail/arsenic Accessed 10 March 2022
Bashandy SA, El Awdan SA, Ebaid H, Alhazza IM (2016) Antioxidant potential of spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxid Med Cell Longev 2016:7174351
Bolin AP, Macedo RC, Marin DP, Barros MP, Otton R (2010) Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol Toxicol 26(5):457–467
Chen SJ, Allam JP, Duan YG, Haidl G (2013) Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288(1):191–199
Das S, Singhal S, Kumar N, Rao C, Sumalatha S, Dave J, Dave R, Nandakumar KJA (2016) Standardised extract of safed musli (Chlorophytum borivilianum) increases aphrodisiac potential besides being safe in male Wistar rats. Andrologia 48(10):1236–1243
de Almeida Lima GD, Sertorio MN, Souza ACF, Menezes TP, Mouro VGS, Goncalves NM, de Oliveira JM, Henry M, Machado-Neves MJRT (2018) Fertility in male rats: disentangling adverse effects of arsenic compounds. Reprod Toxicol 78:130–140
Ferreira M, Matos RC, Oliveira H, Nunes B, Pereira Mde L (2012) Impairment of mice spermatogenesis by sodium arsenite. Hum Exp Toxicol 31(3):290–302
Filho RM, Beletti ME, de Oliveira F (2015) Ultrastructure of bovine sperm chromatin. Microsc Res Tech 78(12):1117–1120
Griveau J, Lannou DL (1994) Effects of antioxidants on human sperm preparationtechniques. Int J Androl 17(5):225–231
Guner O, Guner A, Yavasoglu A, Karabay Yavasoglu NU, Kavlak O (2020) Ameliorative effect of edible Halopteris scoparia against cadmium-induced reproductive toxicity in male mice: a biochemical and histopathologic study. Andrologia 52(6):e13591
Hampannavar GA, Karpoormath R, Palkar MB, Shaikh MS (2016) An appraisal on recent medicinal perspective of curcumin degradant: dehydrozingerone (DZG). Bioorg Med Chem 24(4):501–520
Hamza RZ, Al-Baqami NM (2019) Testicular protective effects of ellagic acid on monosodium glutamate-induced testicular structural alterations in male rats. Ultrastruct Pathol 43(4–5):170–183
Hasona NA (2018) Grape seed extract attenuates dexamethasone-induced testicular and thyroid dysfunction in male albino rats. Andrologia 50(5):e13002
Hedayat F, Stevanovic S, Miljevic B, Bottle S, Ristovski ZJCI, Quarterly CE (2015) Evaluating mol assays measuring oxidative potential part matter Chem Indus Chem Eng Quart 21(1–2):201–210
Henkel RR (2011) Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl 13(1):43–52
Jana K, Jana S, Samanta PK (2006a) Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol 4:9
Jana K, Jana S, Samanta PK (2006b) Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reproductive Biology Endocrinol 4:1–13
Kenjale R, Shah R, Sathaye S (2008) Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytother Res 22(6):796–801
Khair A, Awal MA, Hoque MN, Talukder AK, Das ZC, Rao DR, Shamsuddin M (2021) Spirulina ameliorates arsenic induced reproductive toxicity in male rats. Anim Reprod 18(3):e20210035
Kim YJ, Kim JM (2015) Arsenic toxicity in male reproduction and development. Dev Reprod 19(4):167–180
Kotta S, Ansari SH, Ali J (2013) Exploring scientifically proven herbal aphrodisiacs. Pharmacogn Rev 7(13):1–10
Kumar C, Rasool RU, Iqra Z, Nalli Y, Dutt P, Satti NK, Sharma N, Gandhi SG, Goswami A, Ali A (2017) Alkyne-azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway. Medchemcomm 8(11):2115–2124
Lanzafame FM, La Vignera S, Vicari E, Calogero AE (2009) Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online 19(5):638–659
Lin C, Shin DG, Park SG, Chu SB, Gwon LW, Lee JG, Yon JM, Baek IJ, Nam SY (2015) Curcumin dose-dependently improves spermatogenic disorders induced by scrotal heat stress in mice. Food Funct 6(12):3770–3777
Lucinda LM, Rocha CB, Reboredo MM, Faria VC, Sá RJADABDC (2010) Assessment of sperm production and reproductive organs of Wistar rats to long-term exposure of Caesalpinia Ferrea. Anais da Academia Brasileira de Ciências 82:907–914
Martins RV, Silva AM, Duarte AP, Socorro S, Correia S, Maia CJ (2021) Natural products as protective agents for male fertility. BioChem 1(3):122–147
Menon AG, Barkema HW, Wilde R, Kastelic JP, Thundathil JCJCJOVR (2011) Associations between sperm abnormalities, breed, age, and scrotal circumference in beef bulls. Can J Vet Res 75(4):241–247
Moorkoth S, Prathyusha NS, Manandhar S, Xue Y, Sankhe R, Pai KSR, Kumar N (2021) Antidepressant-like effect of dehydrozingerone from Zingiber officinale by elevating monoamines in brain: in silico and in vivo studies. Pharmacol Rep 73(5):1273–1286
Mora-Esteves Cesar, Shin David (2013) Nutrient supplementation: improving male fertility fourfold. Semin Reprode Med 31(04):293–300
Morakinyo A, Adeniyi O (2008) Effects of Zingiber officinale on reproductive functions in the male rat. Afr J Biomed Res. https://doi.org/10.4314/ajbr.v11i3.50750
Mosbah R, Yousef MI, Mantovani A (2015) Nicotine-induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Exp Toxicol Pathol 67(3):253–259
Nakamura SI, Wongkaew A, Nakai Y, Rai H, Ohkama-Ohtsu N (2019) Foliar-applied glutathione activates zinc transport from roots to shoots in oilseed rape. Plant Sci 283:424–434
Nasr AY (2017) The impact of aged garlic extract on adriamycin-induced testicular changes in adult male Wistar rats. Acta Histochem 119(6):648–662
Obinna VC, Agu GO (2021) Effect of toluene on male reproductive parameters in wistar rats. J Appl Life Sci Int 24(3):26–32
Oduwole OO, Huhtaniemi IT, Misrahi M (2021) The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int J Mol Sci 22(23):12735
Pant N, Murthy RC, Srivastava SP (2004) Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol 23(8):399–403
Parihar VK, Dhawan J, Kumar S, Manjula SN, Subramanian G, Unnikrishnan MK, Rao CM (2007) Free radical scavenging and radioprotective activity of dehydrozingerone against whole body gamma irradiation in Swiss albino mice. Chem Biol Interact 170(1):49–58
Pathak N, Cheruku SP, Rao V, Vibhavari RJA, Sumalatha S, Gourishetti K, Rao CM, Kumar N (2020) Dehydrozingerone protects temozolomide-induced cognitive impairment in normal and C6 glioma rats besides enhancing its anticancer potential. 3 Biotech 10(10):438
Rai A, Das S, Chamallamudi MR, Nandakumar K, Shetty R, Gill M, Sumalatha S, Devkar R, Gourishetti K, Kumar N (2018) Evaluation of the aphrodisiac potential of a chemically characterized aqueous extract of Tamarindus indica pulp. J Ethnopharmacol 210:118–124
Reddy PS, Rani GP, Sainath SB, Meena R, Supriya C (2011) Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J Trace Elem Med Biol 25(4):247–253
Samadder A, Das J, Das S, Khuda-Bukhsh AR (2012) Dihydroxy-isosteviol-methyl-ester, an active biological component of pulsatilla nigricans, reduces arsenic induced cellular dysfunction in testis of male mice. Environ Toxicol Pharmacol 34(3):743–752
Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM (2003) Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 5(1):27–31
Sharma G, Kumar M (2014) Arsenic induced histological alterations in testis of Swiss albino mice and protection by Chlorophytum borivilianum. Asian Pac J Reprod 3(4):282–287
Singh GB, Leach GD, Atal CK (1987) Antiinflammatory actions of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones. Arzneimittelforschung 37(4):435–440
Souza AC, Marchesi SC, Ferraz RP, Lima GD, de Oliveira JA, Machado-Neves M (2016a) Effects of sodium arsenate and arsenite on male reproductive functions in Wistar rats. J Toxicol Environ Health A 79(6):274–286
Souza ACF, Marchesi SC, Domingues de Almeida G, Lima RP, Ferraz FC, Santos SLP, Machado-Neves M (2016b) Effects of sodium arsenite and arsenate in testicular histomorphometry and antioxidants enzymes activities in rats. Biol Trace Elem Res 171(2):354–362
Souza ACF, Bastos DSS, Sertorio MN, Santos FC, Ervilha LOG, de Oliveira LL, Machado-Neves M (2019) Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 7(5):730–740
Tirunavalli SK, Gourishetti K, Kotipalli RSS, Kuncha M, Kathirvel M, Kaur R, Jerald MK, Sistla R, Andugulapati SB (2021) Dehydrozingerone ameliorates lipopolysaccharide induced acute respiratory distress syndrome by inhibiting cytokine storm, oxidative stress via modulating the MAPK/NF-κB pathway. Phytomedicine 92:153729
Tsatmali M, Walcott EC, Makarenkova H, Crossin KLJM, Neuroscience C (2006) Reactive oxygen species modulate differ neurons clonal cortical cultures. Mole Cellular Neurosci 33(4):345–357
Wang PT, Sudirman S, Hsieh MC, Hu JY, Kong ZL (2020) Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed Pharmacother 125:109992
Yuncu M, Bukucu N, Bayat N, Sencar L, Tarakcioglu M (2015) The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. Turk J Med Sci 45(3):517–525
Zubair M, Ahmad M, Ahmad N, Naveed MR, Idrees M, Sallam MA, Bashir MIJGV (2014) Toxic effects of arsenic on reproductive functions of male rabbit and their amelioration with vitamin E. Natl Lib Med 12(2):213–218
Zubair M, Ahmad M, Qureshi ZI (2017) Review on arsenic-induced toxicity in male reproductive system and its amelioration. Andrologia 49(9):e12791
Acknowledgements
We thank Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Government of India for providing infrastructural support and fellowship to Anuj Choudhary, Ruchi Pandey, Dipak Rathod.Funding Declaration: No funding was received for this work.Competing Interest declaration: We, the authors declare no competing Interests.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Anuj Choudhary: investigation, visualization, data curation, formal analysis, writing—original draft, writing—review & editing; Ruchi Pandey: writing—original draft, writing—review & editing; Deepak Rathod: investigation, formal analysis; Suhani Sumalatha: investigation, formal analysis; Krishna Murti: supervision, formal analysis, visualization; Velayutham. Ravichandiran: supervision, visualization; Nitesh Kumar: project administration, conceptualization, visualization, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choudhary, A., Pandey, R., Rathod, D. et al. Dehydrozingerone ameliorates arsenic-induced reproductive toxicity in male Wistar rats. J Mol Histol (2024). https://doi.org/10.1007/s10735-024-10255-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10735-024-10255-9