Abstract
Arsenic is a ubiquitous metalloid and heavy metal that contributes to the global decline in human fertility. Humans are constantly exposed to arsenic through biotic and abiotic sources, especially ingestion of arsenic-contaminated food and water. Its exposure is associated with several adverse health challenges, including reproductive toxicity. In spite of its reported adverse effects, arsenic exposure remains a global challenge. Hence, this study provides a comprehensive review of the literature on the impact and mechanism of arsenic on male and female reproductive function. Additionally, a review of the potential therapeutic strategies is presented. Evidence from the literature reveals that arsenic upregulates reactive oxygen species (ROS) generation which mediates arsenic-induced suppression of the hypothalamic-pituitary–gonadal axis and inactivation of 3β-HSD and 17β-HSD activities, leading to reduced gonadal steroidogenesis. Through several oxidative stress-dependent signaling, arsenic induces the apoptosis of the germ cells, thus contributing to the development of infertility. At the moment, there is no specific treatment for arsenic-induced reproductive toxicity. However, increasing data form the scientific literature reveals the benefits of antioxidants in ameliorating arsenic-induced reproductive toxicity. These molecules suppress ROS generation and maintain optimal activities of the hypothalamic-pituitary–gonadal axis, leading to optimal steroidogenesis and gametogenesis as well as improved germ cells. Overall, this study revealed the impact and associated mechanism of arsenic-induced reproductive toxicity. It also provides evidence from the literature demonstrating potential therapeutic measures in managing arsenic-induced reproductive toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are constantly exposed to metalloids and heavy metals such as mercury, chromium, cadmium, lead, and arsenic, which are major contaminants of air, water, food, and soil, thus posing a risk to the ecosystem (Balali-Mood et al. 2021; Besong et al. 2023a, b). Out of these heavy metals, arsenic remains the most potent environmental toxicant, exerting its toxicity to animals and plants (Rahman and Singh 2019). It is a natural earth crust element that is ubiquitously distributed in the environment due to natural sources and human activities (Yin et al. 2022). It exists in numerous organic and inorganic states with different toxicity profiles. Commonly, it exists in three valence states: As(0) (metalloid arsenic, 0 oxidation state), As(III) (trivalent state, such as arsenites), and As(V) (pentavalent state, such as arsenates) (ATSDR 2007).
Recent evidence suggests that arsenic exposure causes potent toxicity to the reproductive system (Wirth and Mijal 2010; De Palma et al. 2022). Studies have also affirmed that the male reproductive system is more susceptible to arsenic toxicity because of the direct binding to sulfhydryl groups of proteins such as sperm chromatin and flagellum (De Palma et al., 2022). Conversely, in the female reproductive system, arsenic exposure alters reproductive hormones and biogenic amines that regulate spermatogenesis and oogenesis (Jana et al., 2006). It has been thought that arsenic produces ROS by the oxidation of arsenite to arsenate through arsenic methylation leading to oxidative stress (OS) which can cause damage to physiological functions of the cell leading to various diseases such as cancers, diabetes, atherosclerosis, cardiovascular disease, and infertility which can be concluded that arsenic triggers potential mechanisms to induce OS. Arsenic exposure reduces the number of sperm due to reduced GSH and increased malondialdehyde (MDA) as well as increasing inflammatory factors such as tumor necrosis factor-alpha (TNF-α), cyclo-oxygenase (COX), nuclear factor-kappa B (NF-kB), and caspase 3 (Shao et al. 2018; Im Chang et al. 2007). Moreover, arsenic has been documented to also increase urinary concentration of total arsenic and lowers semen volume, sperm concentration and motility, and serum testosterone levels (Akhigbe et al. 2024).
It also increases ROS levels in the testes and alters hormonal secretion and spermatogenesis via the inhibition of androgen receptor activity (Jana et al. 2006; Rosenblatt and Burnstein 2009; Saberi Sis and Zargari 2017). Arsenic also inhibits the activities of DNA-binding domain (DBD) of steroid receptors and causes alterations in enzymes such as lactate dehydrogenase (LDH), acid phosphatase (ACP), γ-glutamyl transpeptidase (GGT) (Renu et al. 2018; Minatel et al. 2018; Wai et al. 2019; Palma-Lara et al. 2020). It is also noteworthy to know that arsenic also affects the female reproductive system through the alteration of some regulator enzymes in steroidogenesis such as 3β-hydroxysteroid dehydrogenase (3-βHSD) and 17β-hydroxysteroid dehydrogenase (17βHSD) due to low levels of gonadotropin (Ilieva et al. 2021; Shao et al. 2018), alteration in the levels of some neurotransmitters (reduction LH, FSH and estradiol), and reduction in gonadotropin secretion (Ilieva et al. 2021; Bhardwaj et al 2021).
Moreover, mounting evidences have revealed potential therapeutic strategies in the management of arsenic-induced reproductive toxicity with a swift approach of a chelation therapy such as dimercaprol (BAL), meso-2,3-dimercaptosuccinic acid (DMSA), and 2,3-dimercapto-1-propanesulfonic acid (DMPS) to bind and remove arsenic from the body (Kalia & Flora 2005); antioxidant supplementation to combat arsenic-induced reproductive toxicity (Flora et al. 2007); Phytochemicals for arsenic removal and detoxification (Sinha et al. 2007); and gene therapy approaches to enhance arsenic metabolism and excretion (Drobná et al. 2010).
Human anthropogenic and agricultural activities such as mining, smelting metal, burning fossil fuels, and using pesticides for home and agricultural usage can contaminate the environment with arsenic (Chung et al., 2014). Environmental, medicinal, and occupational sources are the main sources of human exposure to arsenic (Wang et al. 2012). However, ingestion of arsenic-contaminated food and water remains the main source of exposure (Chung et al., 2014). In high-endemic areas of arsenic, the exposure level can range from tens to hundreds, and occasionally even thousands, of micrograms per liter (μg/L), which is much higher than the WHO limit of ≤ 10 μg/L (WHO 2018; Shaji et al. 2021). According to estimates, approximately 500 million people worldwide could be exposed to unacceptably high doses of arsenic (Shaji et al. 2021).
Arsenic exposure is associated with deleterious adverse effects such as neurological manifestations, gastroenteritis, metabolic disease, vascular changes, and cancers like lung, bladder, kidney, prostate, and hepatocellular carcinoma (Abernathy et al. 2003; Adegunlola et al. 2012; Bibha et al. 2023). Human and experimental studies have also revealed that arsenic induces reproductive toxicity via its endocrine-disrupting activity (Wang et al. 2016; Gunduzoz et al. 2019; Besong et al. 2023b). Evidences on epidemiological studies show the deleterious impact of arsenic exposure on reproductive functions. In Bangladesh, it was documented that men with high arsenic exposure showed significantly lower sperm count, motility, and morphology compared to men with lower arsenic exposure (Hossain et al. 2021). Several of the highly exposed men were diagnosed with azoospermia (complete absence of sperm in semen). However, the study highlighted the detrimental effects of chronic arsenic toxicity on male fertility in this region (Hossain et al. 2021). More so, some data document the link between arsenic and spontaneous abortion and stillbirths in pregnant women with relative risks ranging from 1.8 to 3.1 cases and a significant correlation between higher arsenic levels in drinking water and an increased incidence of spontaneous abortion (Huynh et al. 2020; Biswas et al. 2021). Sharma et al. (2022) demonstrated that arsenic contamination correlates positively with preterm birth (3.1, 95% CI: 1.8–5.3), low birth weight (2.9, 95% CI: 1.6–4.9), and gestational diabetes (2.4, 95% CI: 1.3–4.2). Recently, Nguyen et al. (2023) reported the negative impact of arsenic exposure on reproductive health.
Despite its reported deleterious effects, arsenic exposure remains a global challenge. The understanding of arsenic exposure, the mechanisms of its reproductive toxicities, and the potential benefits of drug candidates from experimental studies will open novel therapeutic windows in the management of arsenic-induced reproductive toxicity. Therefore, the present study provides a comprehensive review of the literature on the impact and mechanism of arsenic on male and female reproductive function. Additionally, a review of the potential therapeutic strategies is presented.
Methods
This study was based on the data from the scientific literature that were retrieved from a search conducted using these databases: PubMed, EMBASE, Scopus, and Google Scholar. The following keywords were used alone and in combination: “arsenic,” “reproductive function,” “male reproduction,” “female reproduction,” “sperm,” “sperm cells,” “testis,” “germ cell,” “ovary,” “ova,” and “ovum.” Searches were performed without restrictions to the year of publication and country of origin.
Arsenic exposure, metabolism, and mechanism
Sources of arsenic exposure are basically classified as abiotic and biotic (Bibha et al. 2023). Abiotic sources comprise geological elements like minerals, underground water, and geothermal processes, as well as manmade elements like farming, manufacturing, and mining operations. Arsenic used industrially, like in antifungal wood preservatives, has the potential to contaminate soil (Yanitch et al. 2020). Arsenic is also used in pharmaceuticals, optical, and glass industries, in the manufacture of sheep dips, alloy, antifouling paints, arsenic-containing pigments, microelectronics, pesticides, and insecticides (Yang et al. 2018). Through leaching, arsenic-contaminated soil contaminates surface and ground water (Bibha et al. 2023). Erosion and leaching from geological formations or anthropogenic sources, the use of pesticides and fertilizers, and metal processing, industrial, and mining activities contribute to soil contamination (Yang et al. 2018). Cigarette smoking contributes significantly to arsenic bioavailability by inhibiting arsenic methylation (Yang et al. 2018). Inhalation of arsenite and arsenate and the use of arsenic-containing cosmetics also add up to human exposure (Yang et al. 2018).
Another main source of arsenic exposure is arsenic contamination of water bodies through improper disposal of untreated sewage and the use of arsenic-based agrochemicals, thus leading to increased arsenic consumption by aquatic animals (Kumari et al. 2017; Liu et al. 2019), as well as contamination of crops through irrigation (Kaur et al. 2017). Consumption of these arsenic-contaminated aquatic animals and plants contributes to human exposure (Bibha et al. 2023). Following exposure to inorganic arsenic, there is a reduction of As V to As III, which in turn undergoes oxidative biomethylation under the action of methyltransferase to generate monomethylarsonic acid, pentavalent organic arsenicals, and dimethylarsinic acid that are in turn voided through the urine (Afolabi et al. 2016). Photooxidation may also be important in the metabolism of arsenic (Amyot et al. 2021). Arsenic is rapidly distributed to body tissues and accumulates over time. Accumulation of arsenic and its metabolites distorts numerous physiological processes, including reproductive functions. Arsenic crosses the blood-gonadal barrier and accumulates in the testis and ovarian where it alters testicular and ovarian structure and functions (Huang et al. 2016; Akhigbe et al. 2024).
Arsenic-induced reproductive toxicity is mediated by the induction of OS which causes loss of mitochondrial organization, leading to an alteration in the mitochondrial integrity and membrane potential. Moreover, the release of apoptotic proteins (cyt-c and activation of Bax) and decreased expression of Bcl-2 promotes apoptosis. Mitochondria produce ROS through complex I and III (Muller et al. 2004; Mishra et al. 2008). The methylation of arsenic is a detoxification of arsenic that is associated with its methylation in the liver by As3MT, and the production of its methylated metabolites includes MMAV, MMAIII, DMAV, and DMAIII. In this pathway, arsenic needs glutathione (GSH) and other thiols. Depleting GSH and other thiols alters the redox status, producing arsenic methylated metabolites which in turn increase oxidative stress (Thomas et al. 2007; Dopp et al. 2010).
Additionally, changes in certain signaling pathways, including the tyrosine phosphorylation and mitogen-activated protein kinase (MAPK) pathways as well as transcription factors, including NF-kB, AP-1, apoptosis, p53 activation, and Bax expression, all contribute to the production of ROS, which raises OS (Nagesh et al. 2019). Arsenic causes damage to proteins, carbohydrates, lipids, and DNA by producing OH or O2 radicals that leads to the production of carbonyl, aldehydes, and keto compounds. This metalloid also damages some amino acid residues such as cysteine and methionine, which can in turn lead to protein structure alteration, degradation, unfolding, and fragmentation and the inactivation of enzymes (such as antioxidant enzymes, pyruvate dehydrogenase) and also the production of advanced glycation end products (AGEs) (Zargari 2021). Arsenic causes damage to carbohydrates and lipids leading to the production of ketoamines, ketoaldehydes, and fatty acid radicals (ROO) as well as changes in the carbohydrate metabolism (i.e., the inhibition of pyruvate dehydrogenase complex, hyperglycemia, and glucose intolerance) and MDA, HNE, the oxidation of cellular membranes, and the inactivation of membrane-bound receptors (Wirtitsch et al. 2009; Sabir et al. 2019). Arsenic also damages DNA leading to alterations in DNA bases (such as the production of 8-OHdG, in turn, the altered bases can modify the site of binding of transcription factors and change the expression of related genes), alterations in DNA repair enzymes, DNA strand break, and the cross-linkage of DNA–protein (De Vizcaya-Ruiz et al. 2009).
Arsenic and male reproductive function
According to Chakraborti et al. (2002), exposure to arsenic can have both acute and long-term harmful effects, putting people at risk for serious health issues like skin cancer, diabetes, liver, kidney, and CNS illnesses. Also, arsenic exposure results in reproductive toxicity, including testicular damage (Sarkar et al. 2008). Moreover, studies suggest that arsenic exposure causes testicular toxicity by directly affecting the hypothalamic-pituitary–testicular axis by affecting the secretion and function of gonadotropins, primarily gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) (Besong et al. 2023b). Research suggests that arsenic exposure can reduce the sensitivity of gonadotroph cells to GnRH, leading to decreased secretion of LH and FSH (Jana et al. 2006). This reduction in gonadotropins impairs the stimulation of Leydig cells which are responsible for testosterone production, thus lowering testosterone levels via both direct inhibition of steroidogenesis in Leydig cells and reduced sensitivity of these cells to LH (Jana et al. 2006).
It has also been revealed that a high arsenic level may suppress the sensitivity of gonadotroph cells to GnRH as well as gonadotropin secretion by elevating plasma levels of glucocorticoids. This has led ultimately to the development of gonadal toxicity (Sarkar et al. 2008; Pant et al., 2004). Nevertheless, exposure to arsenic poisoning has led to the inhibition of testicular androgenesis and reduction of the testicular weight and accessory sex organs (Sarkar et al., 2003; Pant et al., 2004). Moreover, following exposure to arsenic exhibited severe cellular damage in spermatogenic cells indicating a cellular degeneration in the eosinophilic multinucleated giant cell of the seminiferous tubule (Omura et al. 2000). These results demonstrate that after arsenic exposure, spermatogonia’s meiotic maturation has been significantly disturbed (Omura et al. 2000). The testis of arsenic-exposed rats showed a degeneration of interstitial Leydig cells, which could put a chemical strain on cellular function (Omura et al. 2000). Leydig cell atrophy may develop from cellular exhaustion owing to a persistent stress effect while the initial increase in cell width may be a better indicator to adapt the metal-produced stress (Omura et al. 2000). Sarkar et al. (2008) found that testicular shrinkage and a gradual, dose-dependent decrease in the number of Leydig cells in the interstitium were the outcomes of exposure to sodium meta arsenite (30 or 40 mg L1 for 30, 45, or 60 days).
Furthermore, the drop in serum testosterone may result from direct inhibition of testosterone steroidogenesis or from Leydig cells’ decreased sensitivity to luteinizing hormone (Hinshelwood et al. 1994). Significantly lower levels of steroidogenic enzyme activity in the testes of experimental mice suggest a decrease in steroidogenesis, which may in turn inhibit the male mice’s ability to reproduce (Sarkar et al. 2008). Since both FSH and LH are the regulators of high steroidogenic activities, variations in their levels in plasma may be the cause of this alteration in steroidogenic enzyme activity in experimental mice (Sarkar et al., 2003). Subsequent research has demonstrated that arsenic in drinking water is linked to genotoxicity and oxidative stress in mouse testicular tissue (Biswas et al. 2006; Chang et al. 2007a, b). According to Sarkar et al. (2008), these results were shown to cause steroidogenic dysfunction, which impaired spermatogenesis. Also, low gonadotropin levels in rats treated with arsenic may be the cause of the increase in the luminal parts of the seminiferous tubules linked to decreased spermatozoa mass, as these low levels also result in decreased synthesis of steroidogenic enzymes (Sarkar et al. 2008). It is known that exposure to arsenic results in a reduction in the synthesis of testicular steroidogenic enzymes (Sarkar et al. 2008).
Interestingly, the oxidative stress induced by arsenic is strongly linked with decreased sperm quality, including motility and DNA. The damage to sperm DNA and proteins compromises the integrity and functionality of sperm, reducing their ability to fertilize an egg. Moreover, the oxidative damage to mitochondrial DNA in sperm affects ATP production further impairing sperm motility. This reduction in sperm quality and motility is one of the primary factors contributing to arsenic-induced male infertility (Li et al. 2023).
Additionally, exposure to arsenic alters spermatogenesis, resulting in a decline in sperm quality, which is associated with low sperm count and motility and increases abnormal sperm, as seen in mice exposed to arsenic (Li et al. 2023). Rats exposed to arsenic also had a much increased percentage of defective sperm and dead sperm. Arsenic has a number of detrimental effects on spermatogenesis, many of which have been thoroughly investigated in experimental animals and via epidemiological studies (Renu et al. 2018). In addition to degenerating stage VII germ cells in mice and impairing spermatogenesis, exposure to arsenic also lowers testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels (Renu et al. 2018). Likewise, exposure to arsenic causes an accumulation of the metal in soft tissues, such as rodent testes and epididymis (Dua et al. 2015).
According to studies in rodents, arsenic has been shown to have deleterious effects on testicular tissue, including marked testicular spermatogenic degeneration with decreased epithelial height and tubular diameter and increased luminal diameter by the induction of oxidative stress (Jahan et al. 2015; Uygur et al. 2016). Additionally, testicular structures gradually deteriorate as a result of arsenic exposure. Consequently, there is a gradual but noticeable decline in testosterone levels, which affects the functional characteristics of spermatozoa and suggests that arsenic affects Leydig cells, which in turn impairs spermatogenesis. Similarly, arsenic altered the histomorphometry of the testicular architecture through oxidative stress (Souza et al. 2015). Arsenic causes oxidative stress in the testes and modifies hormone levels that are crucial for spermatogenesis, like luteinizing hormone, follicle-stimulating hormone, and testosterone (Jahan et al. 2015). According to research on exposure to arsenic, sperm motility is reduced because arsenic binds to sulfhydryl or thiol groups on sperm proteins or inhibits sperm motility-related enzymes (Li et al. 2023). Additionally, a significant number of thiol-rich protamines found in sperm nuclear chromatins and flagellum make them more vulnerable to arsenic (Behairy et al. 2024). Nonetheless, it has been speculated that one of the ROS products, H2O2, may permeate the membrane and impact the sperms’ essential enzymes, reducing sperm motility (Yánez-Ortiz et al. 2021).
Arsenic exposure markedly reduced the activities of GST and CAT, although SOD levels progressively increased along with a corresponding rise in lipid peroxidation in the testes, suggesting oxidative stress. Comparable outcomes were noted when exposure to arsenic caused decreased CAT activity and increased SOD activity (Kharroubi et al., 2014). Furthermore, in Swiss albino mice, exposure to arsenic also reduces GST activity (Biswas et al. 2010). It is clear that accumulated arsenic may cause testicular architecture damage. This may occur directly when arsenic crosses the blood-testis barrier and reaches germinal cells, or indirectly through interference with spermatogenesis, which results in oxidative stress in the testicular compartment and reduced male fertility.
Arsenic and female reproductive function
Arsenic as a toxic drug can adversely affect female reproductive capacity via the induction of oxidative stress (Barsøe et al. 2021). Ovarian functions can be affected by increased production and interaction of free radicals since cyclic metabolic events take place in the ovary during the reproductive period of mammals (Erkan et al. 2021). Experimentally, the ovary of a mouse model has been previously reported to show oxidative damage following arsenic exposure (Wang et al. 2012). In that study, arsenic triggered follicular-mitochondrial dysfunction via the stimulation of p66Shc which catalyzes the formation of free radicals from mitochondrial proteins (cytochrome C) (Ommati et al. 2020a, b). Mitochondrial function is very crucial in the development and maturation of follicles, fertilization, and succeeding embryo growth, being the site for energy production (Santulli et al. 2015). An increased level of free radicals forced open transition pores on the follicular-mitochondrial membrane, breaking into the follicle cytosol to induce follicular oxidative damage (Betts et al. 2014). Arsenic caused apoptosis of oocytes via ROS-antioxidant imbalance (Agarwal et al. 2012). During oocyte maturation, surrounding granulosa cells nurse the developing oocyte via the supply of antioxidants to create ROS-antioxidant balance, and secretion of hyaluronic acid needed for fertilization (Huang and Wells 2010). Many studies on animal models and humans have reported anovulation, which weakened the body’s enzymatic antioxidant defense (Budhwar et al. 2017). Arsenic downregulates ovarian glutathione levels to induce oxidative damage on the pre-antral follicle via the increased ROS interaction (Niringiyumukiza et al. 2019). Arsenic has been reported to inhibit the surrounding granulosa cell growth via the alteration of granulosa cell-related genes (PTGS, TNFAIP6, and HAS2). Hyaluronic Acid deficiency and meiosis abruption decrease the chances of fertilization and embryonic growth in natural conception and IVF. The inflow of generated free radicals into the oocyte’s nucleus and interaction with DNA can result in the breakage of the paired strand of DNA, resulting in ovarian toxicity and consequent infertility (Kitchin 2001; Akram et al. 2018).
Arsenic has been reported to abrupt ovarian steroidogenesis via the interruption of the hypothalamo-pituitary-ovarian axis (Chen et al. 2022a, b). Arsenic increases serotonergic neurotransmission in the pre-optic area of the hypothalamus (POA). However, serotonin elevation inhibits the proliferation of GnRH neurons in the hypothalamus causing a negative feedback on the release of gonadotropins (follicle-stimulating hormone and luteinizing hormone). FSH and LH suppressions in turn inhibit ovarian steroidogenic enzymes, 3β-HSD and 17β-HSD (Rosenfield et al. 2021). In addition, arsenic-induced gonadotropin suppression may also be due to excessive secretion of glucocorticoids and catecholamines from the adrenal cortex having established their effects in gonadotropic cell resistance to GnRH (Ghosh et al. 2013). Arsenic has been reported to interrupt the estrogenic signaling pathway via alteration of estrogen-related gene expression resulting in infertility since the expression of estrogen-regulated genes in an ovary/uterus depends on the sensitivity of estrogen receptors to estrogen (Amir et al. 2021). Estrogen receptor resistance in the uterus interrupts the estrogen signaling pathway via inactivation of cell growth proteins responsible for endometrial cell proliferation (Amir et al. 2021). The expression of estradiol-regulated vascular endothelial growth factor (VEGF) genes which initiate cyclical angiogenesis in the uterus can also be downregulated by arsenic, which may be a cause for unconstrained miscarriages (Dickson and Stancel, 2000). Additionally, expression of steroidogenic factor-1 (SF-1) needed for ovarian steroid hormone synthesis could be altered by arsenic methylation instead of DNA, affecting ovarian steroidogenesis and follicular development (Huang et al. 2020; Chen et al. 2022a, 2022b). Furthermore, arsenic exposure has been reported to induce estrogen-dependent diseases like breast and uterine cancer, and spontaneous miscarriages in humans (Amir et al. 2021). Although mechanisms of arsenic-induced hypothalamo-pituitary-ovarian axis interruption remain unclear, it was presumed to exert an opposing action on the ovary which may alter LH and FSH levels and resulting oocyte impairment (Mukherjee and Gopalakrishnan 2023). Female infertility could result from hypothalamo-pituitary-ovarian axis interruption and consequent hormonal imbalance which can be created by arsenic toxicity (Rattan et al. 2017).
In addition, arsenic exposure can significantly disrupt the hypothalamo-pituitary-ovarian (HPO) axis by interfering with the controls and release of gonadotropins (FSH and LH), which are essential for ovarian follicular development, ovulation, and the production of sex hormones like estrogen and progesterone. Arsenic also impacts estrogen signaling by altering the expression of genes involved in this pathway such as the estrogen signaling that is important for various reproductive processes (Mukherjee and Gopalakrishnan 2023). Arsenic has been shown to cause estrogen receptor (ER) resistance, particularly in the uterus by modifying the expression of estrogen-regulated genes. This resistance disrupts the normal estrogen signaling pathway leading to the inactivation of cell growth proteins essential for endometrial cell proliferation and vascularization. Specific genes affected by arsenic include the vascular endothelial growth factor (VEGF) genes which are critical for angiogenesis during the menstrual cycle and pregnancy (Mukherjee and Gopalakrishnan 2023). Additionally, arsenic alters the expression of other estrogen-related genes, such as PTGS2, TNFAIP6, and HAS2, which are involved in granulosa cell function, cumulus expansion, and hyaluronic acid production. These alterations may impair follicular development, ovulation embryo implantation, suppression of gonadotropins and ovarian steroidogenesis, and poor endometrial receptivity further contributing to infertility (Amir et al. 2021). Overall, the impact of arsenic on the HPO axis and estrogen signaling pathways underscores the importance of addressing environmental exposures in reproductive health to improve fertility outcomes and reduce the risk of reproductive disorders.
The hypothalamus is the master controller of gonadal steroid synthesis, which rhythmically releases gonadotropin-releasing hormone (GnRH) into circulation. Arsernic-induced ROS hyper-production can interrupt connections between GnRH-secreting neurons at the arcuate nucleus of the hypothalamus and gonadotropin-secreting cells inhibit pulsatile excitatory impulses for FSH and LH release (Agarwal et al. 2005). Unavailability of excitatory impulses to the gonadotropic cells of the anterior pituitary inhibits the synthesis and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) which regulate ovarian function. Naturally, ovarian steroids (estrogen and progesterone) diminish during folliculogenesis and are constantly replenished via negative feedback on both the hypothalamus and the anterior pituitary to maintain the normal cyclical reproductive event (Spaziani et al. 2021). Also, estrogen acts as a self-stimulating steroid by exerting a positive feedback effect on granulose cells to maintain a reproductive level of estrogen. Interrupted GnRH release, diminished serum LH and FH levels, and consequential estrogen decline are accountable for arsenic-induced alterations in follicular development and maturation (Smith et al. 2003; Sarkar et al. 1991). Decreased ovarian estrogen could be attributed to arsenic-induced low LH concentrations (Kitchin 2001). Furthermore, arsenic exposure excessively triggers the pituitary-adrenocortical axis, which in turn exaggerates ACTH secretion (Liu et al. 2005). Increased level of glucocorticoids in circulation renders gonadotropic cells resistant to GnRH, and in turn, inhibits LH and FSH synthesis and release (Colognato et al. 2007). Downregulation of LH release reduces the oocytes’ quantity and functions and inhibits ovulation and estrus cycle (Biswas et al. 2019). A study reported an extended menstrual cycle and infrequent menstrual flow in women following arsenic exposure (Nurminen 1995).
Oogenesis, as the process of gamete (ovum) formation in females, can be compromised during arsenic exposure. Naturally, oogonia (germ cells) undergo several changes of development before becoming mature oocytes (Gosden 2002). Oogenesis can be inhibited via the interruption of the H-P-O axis, disruption of estrogen signaling pathways by polychlorinated biphenyls, and alteration of estrogen-related genes, which have been previously reported following arsenic exposure (Rattan et al. 2017; Kang et al. 2022). Decreased primordial germ cells following arsenic exposure suggests that exposure during pregnancy can inhibit the formation of primordial germ cells from primitive steak and further interrupt their migration into the developing gonads (Legoff et al. 2019). Arsenic toxicity can impair the development, maturation, and ovulatory function of oocytes which can adversely affect female fertility (Foster and Hughes 2011). Arsenic-induced ROS influx into the follicular cytoplasm results in hypertrophy of the follicular cells and ROS interaction with the cytoplasmic components, which will in turn induce follicular oxidative damage and prevention of ovulation (Chattopadhyay et al. 2001). Arsenic toxicity also induces abnormal methylation of histone H3 lysine 4, a marker for DNA transcription, suggesting that arsenic obstructs meiosis during oocyte maturation after puberty (Ommati et al. 2020a, b). Additionally, a study on arsenic-exposed mice reported impaired meiosis and embryo development, blastocysts’ apoptosis, and compromised blastocyst implantation which result from fertilization of arsenic-induced oxidative stressed oocytes (Navarro et al. 2004).
Potential therapies in the management of arsenic-induced reproductive toxicity
Although there is a dearth of human studies exploring possible therapeutic strategies in the management of arsenic-induced reproductive toxicity, there are compelling data from animal models (Table 1). These experimental studies provide insights into the possible mechanism of actions of various pharmacological agents and nutraceuticals in preventing and/or attenuating arsenic-induced reproductive dysfunction.
Male reproduction
Evidence of pharmacological interventions effective in arsenic-induced male reproductive dysfunction abounds in academic literature. These pharmacological interventions include phytochemicals such as Chlorophytum borivilianum, Pulsatilla nigricans, Withania somnifera, Alchornea cordifolia, and Pistia stratiotes and drugs like N-acetlycysteine, telmisartan, diphenyl diselenide, and melatonin. Chlorophytum borivilianum showed a reduction in Arsenic-induced lipid peroxidation, acid and alkaline phosphatase, and cholesterol in mouse Leydig and Sertoli cells (Sharma and Kumar 2012). It has been demonstrated that arsenic suppresses reproductive function by impairing testicular steroidogenesis and spermatogenesis via oxidative stress, lipid peroxidation, inflammation, autophagal, apoptosis, and ferroptotic mechanisms (Meng et al., 2020; Rachamalla et al., 2022). However, 30 days of co-exposure to borivilianum at a dose of 800 mg/kg in Swiss albino mice led to a decrease in oxidative stress evidenced by a decrease in lipid peroxidation and an increase in glutathione-mediated antioxidant defense. Co-exposure to Chlorophytum borivilianum improved testicular metabolic function with an associated decrease in cholesterol, alkaline phosphatase, and reproductive function with an associated increase in testosterone, sperm count, and motility (Sharma and Kumar 2012). It also hindered the degeneration of spermatogenic germ cells in the seminiferous tubules and associated cell loss (Sharma and Kumar 2012).
Oral co-exposure to extract of Pulsatilla nigricans for 90 days at 35 mg/kg led to an increase in levels of sorbitol dehydrogenase for sperm maturation with an associated increase in glutathione, catalase, and superoxide dismutase and a decrease in y-GT levels and lactate dehydrogenase in the testis of Charles Foster rats (Samadder et al. 2012). Similarly, Withania somnifera exposure at 100 mg/kg improved blood testosterone, luteinizing hormone levels, testicular histoarchitecture, spermatogenesis, sperm morphology, and libido (Kumar et al. 2015). According to Ajibade and Olayemi (2020), there was an increase in testosterone and FSH levels, sperm count, sperm motility, and the expression of anti-apoptotic B-cell-lymphoma 2 and androgen receptor binding protein following 30-day oral administration of Alchornea cordifolia at 100 ug/kg also known as water cabbage. Pistia stratiotes exerts protective effects on the testicular function of rats by restoring sperm count, semen volume, sperm viability, and motility following oral administration for 14 days at a dose of 100 mg/kg (Ola-Davies and Oloye 2019). Laboratory investigation in mice showed that N-acetylcysteine administered intraperitoneally at 75 mg/kg supports testicular function by decreasing oxidative stress and lipid peroxidation while increasing the activity of testicular enzymes 3β- and 17β-dehydrogenase and levels of testicular testosterone (Reddy and Roth 2013). The increased testosterone led to an increase in sperm quality, motility, and the weight of reproductive organs. Further, telmisartan was demonstrated to have ameliorative effects on testicular dysfunction following exposure to arsenic (Fouad et al. 2015). Administration of telmisartan markedly decreased testicular concentrations of malondialdehyde, and nitric oxide while decreasing the activity of myeloperoxidase and expression of the TNF-α, NFKB, COX-2, VEGF, and caspase 3 (Fouad et al. 2015). Therefore, telmisartan improves testicular function in arsenic-exposed rats by decreasing lipid peroxidation, inflammation, and caspase 3–dependent apoptosis.
In mice, exposure to diphenyl diselenide (DPDS) at 2.5 mg kg for 45 days improved deficits in reproductive function by abrogating testicular oxidative stress, inflammation, and caspase 3–dependent apoptosis (Adedara et al. 2019). This is evidenced by improved oxidative status and testosterone and decreased activity of myeloperoxidase, caspase 3, and levels of TnF-α, nitric oxide, and interleukin 1B levels (Adedara et al. 2019). Exposure to DPDS increased sperm parameters and ameliorated arsenic-induced histological lesions (Adedara et al. 2019). Melatonin counteracted arsenic-medicated testicular lipid peroxidation, redox imbalance, germ cell apoptosis, and histological degeneration when administered intraperitoneally and intragastrically in rats (Uygur et al. 2016). Ethanol extract of Chromolaena odorata at 200 mg/kg increased reproductive organ weights, endocrine properties, and sperm parameters in Wistar rats (Ola-Davies and Oloye 2019).
Ascorbic acid has been demonstrated to exert antioxidant effects ameliorating the impairment of testicular functions following arsenic exposure for 5 weeks in mice (Im chang et al. 2007). There was an increase in testicular weights on oral co-administration of ascorbic acid. It also reversed the activities of steroidogenic enzymes 3β-HSD and 17B-HSD, epididymal sperm counts, and testicular oxidative imbalance (Im chang et al. 2007).
Beyond that, this acetate has been shown to modulate metabolic processes to support reproductive function in Wistar rats (Besong et al. 2023b). Acetate inhibits histone deacetylation and oxidative and inflammatory changes to cause an increase in the activities of 3B-HSD and 17B-HSD and levels of GnRH, LH, FSH, and T. The increase in testicular steroidogenesis led to improved sexual behavior (Besong et al. 2023b). Guvvala et al. (2017) investigated the effects of green tea extract on arsenic intoxicated in Swiss albino mice. Acute exposure to epigallocatechin-3-gallate restored deficits in testosterone levels and the following sperm parameters: sperm concentration, motility, structural membrane integrity, and mitochondrial membrane potential. It also restored lipid peroxidation changes and oxidative imbalance by scavenging free radicals with the abundance of hydroxyl bases (Guvvala et al. 2017). It also mediates antioxidant changes by inhibiting the generation of hydroxyl radicals, superoxide, and hydrogen peroxide (Guvvala et al. 2017).
Ogunlade et al. (2021) described the potentiating response of D-ribose-L-cyteine on sodium arsenic-induced endocrine alterations, spermatogenic deficits, and histomorphometric abnormalities in rats exposed to 30 mg/kg for 28 days. D-ribose-L-cysteine ameliorated the distortion of testicular morphology, oxidative stress, and impaired semen quality while increasing hormone concentrations (Ogunlade et al. 2021).
Fisetin is a bioactive flavonoid with strong antioxidant effects on rat testes. Treatment with FIS resulted in significant improvement in an arsenic-induced decrease in enzyme antioxidant defense while decreasing metabolic alterations evidenced by decreased cholesterol, low-density lipoprotein, and triglycerides and increased high-density lipoprotein. These have a stimulatory effect on the expression of steroidogenic enzymes 3B-HSD, 17B-HSD, STAR, CYP11A1, and CY17A1 to cause increased secretion of T, LH, and FSH and associated increase in sperm count, motility, and sperm morphology. This is characterized by attenuation of histoarchitectural degenerative changes and decreased caspase-3, Bax, and Bcl-2 (Ijaz et al. 2023).
Ellagic and ferulic acids exert protective effects on Swiss albino mice at 40 mg/kg after exposure for 40 days causing a decline of lipid peroxidation and protein carbonylation, with an associated increase in sperm kinematics and increased expression of StAR, Ppargc1a, and Nfe212 (Guvvala et al. 2019). Laboratory investigation of the effect of grape seed proanthocyanidin on arsenic-induced reproductive toxicity in male mice showed that administration of the extract improved oxidative stress, Nrf2, and NADPH. This suggests that the extract counteracts arsenic-induced reproductive toxicity by mitigating oxidative damage and inhibiting Nrf2 (Li et al. 2015).
Female reproduction
Vitamin B and folic acid have also been demonstrated to be protective agents against arsenic-induced reproductive toxicity in female rats. At doses 0.07 μg and 4.0 μg respectively/100 g b.wt./day, they mitigated the disorganization of uteroovarian histoarchitecture, disruption of female gonadal function, ROS generation, and DNA fragmentation (Deb et al., 2021). In another study, Peerveen et al. (2024) showed that pectic polysaccharide (CCPS) from Momordica charantia administered for 8 days at varying oral doses of 1.5, 2.0, and 2.5 mg/kg led to a decrease in uterine oxidative stress and lipid peroxidation evidenced by decreased MDA and increased activities of CAT and SOD (Perveen and Chattophadhayy 2024). The study demonstrated that CCPS has chelating properties which ameliorated arsenic-induced ovarian toxicity and damage (Perveen and Chattophadhayy 2024).
Similarly, dietary N-acetyl cysteine (NAC) at 250 mg/kg has also been shown to demonstrate chelation properties in arsenic-induced reproductive dysfunction rat model (Dash 2021). NAC caused a decrease in the structural disintegration of ovarian-uterine tissues while increasing antioxidant enzymatic activities and gonadotropin’s utility (rise in LH, FSH) significantly favoring ovarian steroidogenesis. It also inhibited the inflammatory condition following the arsenication inflammatory reaction to diminish the progression of ovarian-uterine apoptosis (Dash 2021).
Modal et al. (2013) demonstrated that a high protein diet prevents female reproductive toxicity and damage due to arsenic in a 30-day long exposure duration. Arsenic exposure led to a reduction in ovarian and uterine weight, utero-ovarian degeneration, ovarian DNA damage, and a decrease in ovarian activities of steroidogenic enzymes 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase leading to decreased serum estradiol level via an oxidative stress and lipid peroxidation. However, consumption of a high protein diet showed effective protection against these observations with associated optimum antioxidant defense.
Conclusion and future perspectives
Convincing data from the literature demonstrate that arsenic exposure promotes ROS generation and induces oxidative stress. This suppresses the hypothalamic-pituitary–gonadal axis and inactivates 3β-HSD and 17β-HSD, leading to reduced gonadal steroidogenesis and spermatogenesis. This cascade of events triggers the apoptosis of oocytes and sperm cells, thus contributing to the development of infertility (Fig. 1). More so, there are increasing data that reveal the benefits of antioxidants in ameliorating arsenic-induced reproductive toxicity. These molecules suppress ROS generation and maintain optimal activities of the hypothalamic-pituitary–gonadal axis, resulting in adequate steroidogenesis and gametogenesis as well as improved germ cells. However, more experimental studies exploring other possible pathways through which arsenic may mediate its reproductive toxicity is recommended. Also, clinical trials validating the therapeutic values of potential drug candidates in the management of arsenic-induced reproductive toxicity should be conducted.
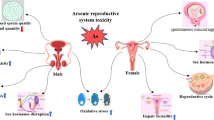
Schematic illustration of the impact of arsenic exposure on male and female reproduction. Arsenic exposure promotes reactive oxygen species (ROS) generation and induces oxidative stress, which suppresses the hypothalamic-pituitary–gonadal axis and inactivates 3β-HSD and 17β-HSD. This leads to reduced gonadal steroidogenesis and apoptosis of oocytes and sperm cells, thus contributing to the development of infertility
Data availability
Data will be made available on request.
References
Abernathy CO, Thomas DJ, Calderon RL (2003) Health effects and risk assessment of arsenic. J Nutr 133:1536S-1538S
Adedara IA, Adebowale AA, Atanda OE, Fabunmi AT, Ayenitaju AC, Rocha JB, Farombi EO (2019) Selenium abates reproductive dysfunction via attenuation of biometal accumulation, oxido-inflammatory stress and caspase-3 activation in male rats exposed to arsenic. Environ Pollut 254:113079
Adegunlola JG, Afolabi OK, Akhigbe RE, Adegunlola GA, Adewumi OM, Oyeyipo IP, Ige SF, Afolabi AO (2012) Lipid peroxidation in brain tissue following administration of low and high doses of arsenite and L-ascorbate in Wistar strain rats. Toxicol Int 19(1):47
Afolabi OK, Wusu AD, Ogunrinola OO, Abam EO, Babayemi DO, Dosumu OA, Onunkwor OB, Balogun EA, Odukoya OO, Ademuyiwa O (2016) Paraoxonase 1 activity in subchronic low-level inorganic arsenic exposure through drinking water. Environ Toxicol 31(2):154–162
Agarwal A, Gupta S, Sharma RK (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:1–31
Ahsan H, Chen Y, Parvez F, Zablotska L, Argos M, Hussain I, Graziano J (2006) Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol 163(12):1138–1148
Ajibade TO, Olayemi FO (2020) Polyphenol-rich fraction of Alchornea cordifolia leaf ameliorates arsenite-induced infertility in male rats. Andrologia 52(10):e13754
Akhigbe RE, Akhigbe TM, Adegbola CA, Oyedokun PA, Adesoye OB, Adeogun AE (2024) Toxic impacts of arsenic bioaccumulation on urinary arsenic metabolites and semen quality: a systematic and meta-analysis. Ecotoxicology and Environmental Safety, 281, 116645.Flora, S. J. S. (2011). Arsenic-induced oxidative stress and its reversibility. Free Radical Biology and Medicine, 51(2), 257–281
Akram Z, Jalali S, Kalsoom O, Batool S, Shami SA (2018) A study on the effects of arsenic toxicity on oviduct histomorphology in the female rat. J Histotechnol 41(4):149–159
Amir S, Shah STA, Mamoulakis C, Docea AO, Kalantzi OI, Zachariou A, Tsatsakis A (2021) Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. Int J Environ Res Public Health 18(4):1464
Amyot M, Bélanger D, Simon DF, Chételat J, Palmer M, Ariya P (2021) Photooxidation of arsenic in pristine and mine-impacted Canadian subarctic freshwater systems. J Hazardous Mater Adv 2:100006
ATSDR (2007) Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services, Atlanta, GA Available at http://www.atsdr.cdc.gov/ToxProfiles/tp2.pdf
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Baltaci BB, Uygur R, Caglar V, Aktas C, Aydin M, Ozen OA (2016) Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia 48(10):1202–1213
Barsøe IM, Ebdrup NH, Clausen HS, Lyngsø J, Schullehner J, Ramlau-Hansen CH, Bay B, Knudsen UB (2021) Drinking water arsenic and adverse reproductive outcomes in men and women: a systematic PRISMA review. Water 13(14):1885
Behairy A, Hashem MM, Abo-EL-Sooud K, El-Metwally AE, Soliman AM, Mouneir SM, Hassan BA, Abd-Elhakim YM (2024) Mitigating effect of gallic acid on zinc oxide nanoparticles and arsenic trioxide-induced spermatogenesis suppression, testicular injury, hormonal imbalance, and immunohistochemical changes in rats. Naunyn Schmiedebergs Arch Pharmacol 1–17. https://doi.org/10.1007/s00210-024-03228-y
Besong EE, Akhigbe TM, Ashonibare PJ, Oladipo AA, Obimma JN, Hamed MA, Adeyemi DH, Akhigbe RE (2023a) Zinc improves sexual performance and erectile function by preventing penile oxidative injury and upregulating circulating testosterone in lead-exposed rats. Redox Rep 28(1):2225675
Besong EE, Ashonibare PJ, Obembe OO, Folawiyo MA, Adeyemi DH, Hamed MA, Akhigbe TM, Akhigbe RE (2023b) Zinc protects against lead-induced testicular damage via modulation of steroidogenic and xanthine oxidase/uric acid/caspase 3-mediated apoptotic signaling in male Wistar rats. Aging Male 26(1):2224428
Betts DH, Bain NT, Madan P (2014) The p66(Shc) adaptor protein controls oxidative stress response in early bovine embryos. PLoS ONE 9:e86978
Bhardwaj JK, Paliwal A, Saraf P (2021) Effects of heavy metals on reproduction owing to infertility. J Biochem Mol Toxicol 35:e22823
Bibha K, Akhigbe TM, Hamed MA, Akhigbe RE (2023) Metabolic derangement by arsenic: a review of the mechanisms. Biol Trace Elem Res 6:1–1
Biswas R, Poddar S, Mukherjee A (2006) Investigation on the genotoxic effects of long-term administration of sodium arsenite in bone marrow and testicular cell in vivo using comet assay. J Environ Pathol Toxicol Oncol 26(1):29–37
Biswas J, Roy S, Mukherjee S, Sinha D, Roy M (2010) Indian spice curcumin may be an effective strategy to combat the genotoxicity of arsenic in Swiss albino mice. Asian Pac J Cancer Prev 11:239–247
Biswas P, Mukhopadhyay A, Kabir SN, Mukhopadhyay PK (2019) High-protein diet ameliorates arsenic-induced oxidative stress and antagonizes uterine apoptosis in rats. Biol Trace Elem Res 192(2):222–233
Biswas D, Sengupta M, Chowdhury A (2021) Arsenic exposure and stillbirth in Bangladesh: a case-control study. Sci Total Environ 754:142045
Budhwar S, Singh V, Verma P, Singh K (2017) Fertilization failure and gamete health: is there a link. Front Biosci (Schol Ed) 9(3):395–419
Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY (2007a) Arsenic induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol 218:196–203
Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY (2007b) Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol 218(2):196–203. https://doi.org/10.1016/j.taap.2006.11.009
Chattopadhyay S, Ghosh S, Debnath J, Ghosh D (2001) Protection of sodium arsenite-induced ovarian toxicity by coadministration of L-ascorbate (vitamin C) in mature Wistar strain rat. Arch Environ Contam Toxicol 41:83–89
Chen Y, Sun Y, Zhao A, Cai X, Yu A, Xu Q, Wang W (2022a) Arsenic exposure diminishes ovarian follicular reserve and induces abnormal steroidogenesis by DNA methylation. EcotoxicolEnviron Safety 241:113816
Chen Y, Sun Y, Zhao A, Cai X, Yu A, Xu Q, Wang P, Yao J, Wang Q, Wang W (2022b) Arsenic exposure diminishes ovarian follicular reserve and induces abnormal steroidogenesis by DNA methylation. Ecotoxicol Environ Saf 241:113816
Colognato R, Coppede F, Ponti J, Sabbioni E, Migliore L (2007) Genotoxicity induced by arsenic compounds in peripheral human lymphocytes analysed by cytokinesis-block micronucleus assay. Mutagen 22(4):255–261
Dash M (2021) Consequence of dietary n-acetyl cysteine on arsenic mediated female reproductive hazards (Doctoral dissertation, Vidyasagar University, Midnapore, West Bengal)
De Palma G, Ortiz A, Apostoli P (2022) Effects of metallic elements on reproduction and development. Handbook on the Toxicology of Metals; Academic Press: Cambridge. MA, USA, pp 565–592
De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME (2009) Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res Genet Toxicol Environ Mutagen 674:85–92
Deb B (2021) Impact of vitamin B12 and folic acid on arsenic induced female reproductive status of albino rats
Dopp E, Kligerman AD, Diaz-Bone RA (2010) Organoarsenicals. Uptake, metabolism, and toxicity. Met Ions Life Sci 7:231–265
Drobná Z, Walton FS, Paul DS, Xing W, Thomas DJ, Stýblo M (2010) Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol 247(2):91–99
Dua TK, Dewanjee S, Gangopadhyay M, Khanra R, Zia-Ul-Haq M, De Feo V (2015) Ameliorative effect of water spinach, Ipomea aquatica (Convolvulaceae), against experimentally induced arsenic toxicity. J Transl Med 13:81. https://doi.org/10.1186/s12967-015-0430-3
Erkan M, Aydin Y, Yilmaz BO, Yildizbayrak N (2021) Arsenic-induced oxidative stress in reproductive systems. Toxicology 145–155
Flora SJS, Bhadauria S, Kannan GM, Singh N (2007) Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28(2):333–347
Foster WG, Hughes CL (2011) Gene expression in oogenesis and implications for transgenerational effects of environmental toxicants. Biol Reprod 84:2–4
Fouad AA, Albuali WH, Al-Mulhim AS, Jresat I (2015) Protective effect of telmisartan treatment against arsenic-induced testicular toxicity in rats. Zeitschrift Für Naturforschung C 70(7–8):175–181
Ghosh SN, De A, Mondal S (2013) Stress hormones and sports performance: a critical analysis. Int j physiol nutr phys educ 3(1):1752–1757
Gosden RG (2002) Oogenesis as a foundation for embryogenesis. Mol Cell Endocrinol 186:149–153
Gunduzoz M, Tutkun L, Iritas SB, Dip A, Deniz S (2019) The evaluation of the effects of occupational arsenic exposure on man reproductive hormones. Medicine 8(2):306–310
Guvvala PR, Ravindra JP, Rajani CV, Sivaram M, Selvaraju S (2017) Protective role of epigallocatechin-3-gallate on arsenic induced testicular toxicity in Swiss albino mice. Biomed Pharmacotherapy 96:685–694
Guvvala PR, Ravindra JP, Selvaraju S, Arangasamy A, Venkata KM (2019) Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 413:1–2. https://doi.org/10.1016/j.tox.2018.11.012
Hinshelwood MM, Demter-Arlotto M, Means GD, Simpson ER (1994) Expression of genes encoding steroidogenic enzymes in the ovary. In: Findlay JK (ed) Molecular biology of the female reproductive system. Academic Press, London, pp 129–145
Hossain MB, Vahter M, Concha G, Broberg K (2021) Environmental arsenic exposure and fertility: a systematic review. Environ Res 195:110805
Huang Z, Wells D (2010) The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod 16(10):715–725
Huang Q, Luo L, Alamdar A, Zhang J, Liu L, Tian M, Eqani SAMAS, Shen H (2016) Integrated proteomics and metabolomics analysis of rat testis: mechanism of arsenic-induced male reproductive toxicity. Sci Rep 6(1):32518
Huang Y et al (2020) Cadmium exposure during prenatal development causes testosterone disruption in multigeneration via SF-1 signaling in rats. Food Chem Toxicol 135:110897
Huynh KT, Nguyen PT, Tran TM (2020) Association between arsenic exposure and spontaneous abortion in Vietnam. Int J Environ Res Public Health 17(11):3961
Ijaz MU, Haider S, Tahir A, Afsar T, Almajwal A, Amor H, Razak S (2023) Mechanistic insight into the protective effects of fisetin against arsenic-induced reproductive toxicity in male rats. Sci Rep 13(1):3080
Ilieva I, Sainova I, Yosifcheva K (2021) Toxic effects of heavy metals (mercury and arsenic) on the male fertility. Acta Morphol Anthropol 28:1–2
Im Chang S, Jin B, Youn P, Park C, Park JD, Ryu DY (2007) Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol 218(2):196–203
Jahan S, Iftikhar N, Ullah H, Rukh G, Hussain I (2015) Alleviative effect of quercetin on rat testis against arsenic: a histological and biochemical study. Syst Biol Reprod Med 61:89–95. https://doi.org/10.3109/19396368.2014.998350
Jana K, Jana S, Samanta PK (2006) Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol 4:1–3
Kalia K, Flora SJS (2005) Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47(1):1–21
Kang H-G, Jeong P-S, Kim MJ, Joo YE, Gwon M-A, Jeon S-B et al (2022) Arsenic exposure during porcine oocyte maturation negatively affects embryonic development by triggering oxidative stress-induced mitochondrial dysfunction and apoptosis. Toxicology 480:153314
Kaur S, Singh D, Singh K (2017) Effect of selenium application on arsenic uptake in rice (Oryza sativa L.). Environ Monit Assessment 189:1–8
Kitchin KT (2001) Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol 172(3):249–261
Kumar A, Kumar R, Rahman MS, Iqubal MA, Anand G, Niraj PK, Ali M (2015) Phytoremedial effect of Withania somnifera against arsenic-induced testicular toxicity in Charles Foster rats. Avicenna J Phytomed 5(4):355
Kumari B, Kumar V, Sinha AK, Ahsan J, Ghosh AK, Wang H, DeBoeck G (2017) Toxicology of arsenic in fish and aquatic systems. Environ Chem Lett 15:43–64
Legoff L, Dali O, D’Cruz SC, Suglia A, Gely-Pernot A, Hémery C et al (2019) Ovarian dysfunction following prenatal exposure to an insecticide, chlordecone, associates with altered epigenetic features. Epigenetics Chromatin 12:29
Li SG, Ding YS, Qiang NIU, Xu SZ, Pang LJ, Lin R, Jing MX, Feng GL, Liu JM, Guo SX (2015) Grape seed proanthocyanidin extract alleviates arsenic-induced oxidative reproductive toxicity in male mice. Biomed Environ Sci 28(4):272–280
Li J, Nan B, Xu Z, Chang H, Xu S, Ren M, Shen H (2023) Arsenic exposure caused male infertility indicated by testis and sperm metabolic dysfunction in SD rats. Sci Total Environ 904:166838
Liu SX, Davidson MM, Tang X, Walker WF, Athar M, Ivanov V, Hei TK (2005) Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res 65(8):3236–3242
Liu Q, Xu X, Zeng J, Shi X, Liao Y, Du P, Tang Y, Huang W, Chen Q, Shou L (2019) Heavy metal concentrations in commercial marine organisms from Xiangshan Bay, China, and the potential health risks. Mar Pollut Bull 141:215–226
Minatel BC, Sage AP, Anderson C, Hubaux R, Marshall EA, Lam WL, Martinez VD (2018) Environmental arsenic exposure: from genetic susceptibility to pathogenesis. Environ Int 112:183–197
Mishra D, Mehta A, Flora SJ (2008) Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: role of glutathione and linked enzymes. Chem Res Toxicol 21:400–407
Mondal S, Mukherjee S, Chaudhuri K, Kabir SN, Kumar Mukhopadhyay P (2013) Prevention of arsenic-mediated reproductive toxicity in adult female rats by high protein diet. Pharm Biol 51(11):1363–1371
Mukherjee AG, Gopalakrishnan AV (2023) The interplay of arsenic, silymarin, and NF-ĸB pathway in male reproductive toxicity: a review. Ecotoxicol Environ Saf 252:114614
Muller FL, Liu Y, Van Remmen H (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279:49064–49073
Nagesh R, Kiran Kumar KM, Naveen Kumar M, Patil RH, Sharma SC (2019) Stress activated p38 MAPK regulates cell cycle via AP-1 factors in areca extract exposed human lung epithelial cells. Cytotechnology 71:507–520
Navarro PA, Liu L, Keefe DL (2004) In vivo effects of arsenite on meiosis, preimplantation development, and apoptosis in the mouse. Biol Reprod 70:980–985
Nguyen LT, Pham HV, Nguyen TH, Le TH (2023) Arsenic exposure and reproductive health in Southeast Asia: a comprehensive review. Environ Health Prev Med 28(1):10
Niringiyumukiza JD, Cai H, Chen L, Li Y, Wang L, Zhang M, Xiang W (2019) Protective properties of glycogen synthase kinase-3 inhibition against doxorubicin-induced oxidative damage to mouse ovarian reserve. Biomed Pharmacotherapy 116:108963
Nurminen T (1995) Maternal pesticide exposure and pregnancy outcome. J Occup Environ Med 37(8):935–940
Ogunlade B, Adelakun SA, Ukwenya VO, Elemoso TT (2021) Potentiating response of D- Ribose-L-Cysteine on Sodium arsenate- induced hormonal imbalance, spermatogenesis impairments and histomorphometric alterations in adult male Wistar rat. JBRA Assisted Reproduction 25(3):358–367. https://doi.org/10.5935/1518-0557.20200109
Ola-Davies OE, Oloye AA (2019) Phytomedical potentials of Chromolaena odorata against arsenic-induced testicular toxicity in Wistar rats. Nigerian J Basic Appl Sci 27(1):67–75
Ommati MM et al (2020a) The footprints of oxidative stress and mitochondrial impairment in arsenic trioxide-induced testosterone release suppression in pubertal and mature F1-male Balb/c mice via the downregulation of 3beta-HSD, 17beta-HSD, and CYP11a expression. Biol Trace Elem Res 195:125–134
Ommati MM, Shi X, Li H, Zamiri MJ, Farshad O, Jamshidzadeh A, Heidari R, Ghaffari H, Zaker L, Sabouri S, Chen Y (2020b) The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: an enduring developmental study in folliculogenesis of mice. Ecotoxicol Environ Saf 204:110973
Omura M, Yamazaki K, Tanaka A, Hirata M, Makita Y, Inoue N (2000) Changes in the testicular damage caused by indium arsenide and indium phosphide in hamsters during two years after intratracheal instillations. J Occup Health 42:196–204
Palma-Lara I, Martínez-Castillo M, Quintana-Pérez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limón OL, Hernández-Zavala A (2020) Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmac 110:104539
Perveen H, Chattopadhyay S (2024) Protection of arsenic induced oxidative stress in reproductive organs and female infertility by pectic polysaccharide of Momordica charantia: an in vivo study in dose dependent manner. In Proceedings of the Zoological Society (pp. 1–11). New Delhi: Springer India.
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191:1–21
Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA (2017) Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 233(3):R109–R129
Reddy KJ, Roth TR (2013) Arsenic removal from natural groundwater using cupric oxide. Groundwater 51(1):83–91
Renu K, Madhyastha H, Madhyastha R, Maruyama M, Vinayagam S, Gopalakrishnan AV (2018) Review on molecular and biochemical insights of arsenic-mediated male reproductive toxicity. Life Sci 212:37–58
Rosenblatt AE, Burnstein KL (2009) Inhibition of androgen receptor transcriptional activity as a novel mechanism of action of arsenic. J Mol Endocrinol 23:412–421
Rosenfield RL, Cooke DW, Radovick S (2021) Puberty in the female and its disorders. In Sperling Pediatric Endocrinology (pp. 528–626). Elsevier.
Saberi Sis F, Zargari F (2017) The effect of aqueous extract of white tea on serum levels of FSH, LH and testosterone in rats exposed to arsenic. J Fasa Univ Med Sci 7:398–405
Sabir S, Akash MSH, Fiayyaz F, Saleem U, Mehmood MH, Rehman K (2019) Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: inserting the association into perspectives. Biomed Pharmacother 114:108802
Samadder A, Das J, Das S, Khuda-Bukhsh AR (2012) Dihydroxy-isosteviol-methyl-ester, an active biological component of Pulsatilla nigricans, reduces arsenic induced cellular dysfunction in testis of male mice. Environ Toxicol Pharmacol 34(3):743–752
Santulli G, Xie W, Reiken SR, Marks AR (2015) Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci 112(36):11389–11394
Sarkar M, Biswas NM, Ghosh D (1991) Effect of sodium arsenite on testicular Δ5-3 β, 17β-HSD activities in albino rats: dose and duration dependent responses. Med Sci Res 19:789–790
Sarkar S, Hazra J, Upadhyay SN, Singh RK, Chowdhury AR (2008) Arsenic induced toxicity on testicular tissue of mice. Indian J Physiol Pharmacol 52:84–90
Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, Divya BV (2021) Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci Front 12(3):101079
Shao Y, Zhao H, Wang Y, Liu J, Li J, Chai H, Xing M (2018) Arsenic and/or copper caused inflammatory response via activation of inducible nitric oxide synthase pathway and triggered heat shock protein responses in testis tissues of chicken. Environ Sci Pollut Res Int 25:7719–7729
Sharma G, Kumar M (2012) Antioxidant and modulatory role of Chlorophytum borivilianum against arsenic induced testicular impairment. J Environ Sci 24(12):2159–2165
Sharma A, Gupta S, Sharma D, Sinha R (2022) Arsenic exposure and adverse pregnancy outcomes: a longitudinal study in India. Environ Health Perspect 130(7):077001
Sinha D, Roy M, Siddiqi M, Bhattacharya RK (2007) Arsenic-induced micronuclei formation in mammalian cells and its counteraction by tea. J Environ Pathol Toxicol Oncol 26(2):177–189
Smith E, Smith J, Smith L, Biswas T, Correll R, Naidu R (2003) Arsenic in Australian environment: an overview. J Environ Sci Health A Tox Hazard Subst Environ Eng 38(1):223–239
Souza AC, Marchesi SC, de Almeida D, Lima G, Ferraz RP, Santos FC, da Matta SL, Machado-Neves M (2015) Effects of sodium arsenite and arsenate in testicular histomorphometry and antioxidants enzymes activities in rats. Biol Trace Elem Res 171:354–362
Spaziani M, Tarantino C, Tahani N, Gianfrilli D, Sbardella E, Lenzi A et al (2021) Hypothalamo-pituitary axis and puberty. Mol Cell Endocrinol 520:111094
Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M (2007) Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med 232:3–13
Uygur R, Aktas C, Caglar V, Uygur E, Erdogan H, Ozen OA (2016) Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol Ind Health 32(5):848–859
Wai KM, Umezaki M, Mar O, Umemura M, Watanabe C (2019) Arsenic exposure through drinking water and oxidative stress status: a cross-sectional study in the Ayeyarwady region. Myanmar J Trace Elem Med Biol 54:103–109
Wang X, Zhang C, Zhang Y, Ru X, Gong Q (2012) Establishment of a mouse model of ovarian oxidative stress (in Chinese). J South Med Univ 32:1643–1645
Wang X, Zhang J, Xu W, Huang Q, Liu L, Tian M, Xia Y, Zhang W, Shen H (2016) Low-level environmental arsenic exposure correlates with unexplained male infertility risk. Sci Total Environ 571:307–13
WHO (2018) World Health Organization Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/arsenic. [Cited on 10/04/2024].
Wirth JJ, Mijal RS (2010) Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 56:147–167
Wirtitsch M, Roth E, Bachleitner-Hofmann T, Wessner B, Sturlan S (2009) Omega-3 and omega-6 polyunsaturated fatty acids enhance arsenic trioxide efficacy in arsenic trioxide-resistant leukemic and solid tumor cells. Oncol Res 18:83–94
Yánez-Ortiz I, Catalán J, Delgado-Bermúdez A, Carluccio A, Miró J, Yeste M (2021) Addition of reduced glutathione (GSH) to freezing medium reduces intracellular ROS levels in donkey sperm. Veterinary Sci 8(12):302
Yang YP, Zhang HM, Yuan HY, Duan GL, Jin DC, Zhao FJ, Zhu YG (2018) Microbe mediated arsenic release from iron minerals and arsenic methylation in rhizosphere controls arsenic fate in soil-rice system after straw incorporation. Environ Pollut 236:598–608
Yanitch A, Kadri H, Frenette-Dussault C, Joly S, Pitre FE, Labrecque M (2020) A four-year phytoremediation trial to decontaminate soil polluted by wood preservatives: phytoextraction of arsenic, chromium, copper, dioxins and furans. Int J Phytorem 22(14):1505–1514
Yin G, Xia L, Hou Y, Li Y, Cao D, Liu Y, Chen J, Liu J, Zhang L, Yang Q, Zhang Q (2022) Transgenerational male reproductive effect of prenatal arsenic exposure: abnormal spermatogenesis with Igf2/H19 epigenetic alteration in CD1 mouse. Int J Environ Health Res 32(6):1248–1260
Zargari F (2021) Arsenic and oxidative stress: an overview. In: Kumar N (ed) Arsenic Toxicity: Challenges and Solutions, 1st edn. Springer, Singapore, pp 27–63
Author information
Authors and Affiliations
Contributions
Conceptualization and design: ATM and ARE. Funding acquisition: ATM and ARE. Investigation: AAE, OOD, ATM, OPA, ACA, and ARE. Methodology: ARE. Project administration: AAE, OOD, ATM, OPA, ACA, SWA, AOA, and ARE. Supervision: ARE. Validation: ATM, SWA, AOA, and ARE. Writing-original draft: AAE, OOD, ATM, OPA, ACA, and ARE. Writing-review and editing and final approval: AAE, OOD, ATM, OPA, ACA, SWA, AOA, and ARE. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Consent to participate
N/A.
Consent for publication
All authors consented to the submission and publication of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adeogun, A.E., Ogunleye, O.D., Akhigbe, T.M. et al. Impact of arsenic on male and female reproductive function: a review of the pathophysiology and potential therapeutic strategies. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03452-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03452-6