Abstract
As an essential factor in the prognosis of Systemic lupus erythematosus (SLE), lupus nephritis (LN) can accelerate the rate at which patients with SLE can transition to chronic kidney disease or even end-stage renal disease (ESRD). Proteinuria due to decreased glomerular filtration rate following podocyte injury is LN’s most common clinical manifestation. Podocyte pyroptosis and related inflammatory factors in its process can promote lupus to involve kidney cells and worsen the occurrence and progression of LN, but its regulatory mechanism remains unknown. Accumulating evidence has shown that upstream stimulatory factor 2 (USF2) plays a vital role in the pathophysiology of kidney diseases. In this research, multiple experiments were performed to investigate the role of USF2 in the process of LN. USF2 was abnormally highly expressed in MRL/lpr mice kidney tissues. Renal function impairment and USF2 mRNA levels were positively correlated. Silencing of USF2 in MRL/lpr serum-stimulated cells significantly reduced serum-induced podocyte pyroptosis. USF2 enhanced NLRP3 expression at the transcriptional level. Silencing of USF2 in vivo attenuated kidney injury in MRL/lpr mice, which suggests that USF2 is important for LN development and occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that produces autoantibodies and immune complexes (ICs) that affect various organs and deposits complement (Aringer et al. 2019). Severe SLE has many fatal complications, such as pericarditis, pleurisy, kidney failure, and brain injury. Lupus nephritis (LN) is one of the most common complications of SLE, and it is characterized by glomerular deposition of ICs, microscopic hematuria, proteinuria, and progressive renal impairment (Susan Allison 2022). The chances of progressing to chronic kidney disease or end-stage renal disease (ESRD) are greater for patients with SLE who have LN (Anadi Mahajan et al. 2020). LN is responsible for a significant proportion of morbidity and mortality in patients with SLE, and it affects 30–60% of adults and 70–80% of children (Amy et al., 2020). The interaction between immune response regulation and the pathological process involving resident renal cells promotes the incidence and development of LN, which often manifests as decreased podocyte density, mesangial cell proliferation, neutrophil infiltration, foot process fusion, and podocyte phenotypic changes (Feng Yu et al. 2017). Podocytes are integral to the glomerular filtration barrier and belong to terminally differentiated cells. Proteinuria may be induced after injury, which is the main clinical manifestation of LN (Yuan yuan Qi et al., 2018). Several changes have been observed in the podocytes of LN patients, which increase proteinuria and damage the kidneys (Carlos Rafael-Vidal et al., 2021). Podocyte injury plays a role in the progression of LN, but the mechanism is not clear.
Various factors cause podocyte injuries, including apoptosis, necrosis, and pyroptosis. The assembly and activation of the (Nod)-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is a key event in the occurrence of pyroptosis (Hélène Duez and Benoit Pourcet, 2021). The NLRP3 inflammasome activates caspase-1 to cleave pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) molecules into bioactive IL-1β and IL-18, respectively (Parimal Samir et al. 2019; Bhesh Raj Sharma and Thirumala-Devi Kanneganti, 2021). Moreover, Caspase-1 cleaves GSDMD into a segmented N-terminal fragment, and caspase-1 forms a pyroptotic pore by attaching to the cell membrane. IL-1β and IL-18 escape through the GSDMD pore and result in a pro-inflammatory response (Mausita Karmakar et al. 2020; Christophe Paget et al. 2022). As a result of excessive pore formation, the plasma membrane becomes compromised and causes pyroptosis (Sannula Kesavardhana et al. 2020). Activation of the NLRP3 inflammasome promotes podocyte injury, which leads to proteinuria and reduces renal function (Geun-Shik Lee et al. 2012; Yang Zhou et al. 2020). The activated caspase-1 level in podocytes from NZM2328 mice with severe proteinuria is elevated, and increased expression of NLRP3 and IL-1β is found in kidney biopsies and podocytes (Fu et al. 2017). Increasing evidence has suggested a role for pyroptosis in the progression of LN (Pragnesh et al., 2017). Notably, Cao et al. observed that combination therapy suppressed pyroptosis and reduced disease progression (Heng Cao et al. 2021). Therefore, the study of podocyte pyroptosis and its molecular regulatory mechanisms is of great interest to improve LN prevention and treatment.
Upstream stimulatory factor 2 (USF2) is a transcription factor that belongs to the Myc family and forms dimers with DNA binding domains (Li Chen et al. 2006). USF2 plays an important role in kidney disease pathophysiology (Shuxia and Wang 2015). High glucose exposure resulted in the accumulation of USF2, which induced thrombospondin1 (THBS1) gene expression and transforming growth factor (TGF)-activity in glomerular mesangial cells that contributed to the development of diabetic nephropathy (Lihua Shi et al. 2009). Knock down of USF2 downregulated THBS1 to inhibit the TGF-β/Smad3 signaling pathway, reduce pyroptosis, and further ameliorate AKI-induced sepsis (Jian Sun et al., 2022). However, the expression of USF2 in LN renal tissues and its function in disease progression have rarely been reported. Therefore, elucidating the role of USF2 in LN pathology is crucial.
The present study found that USF2 was highly expressed in MRL/lpr mouse kidney tissue, and this abnormal expression may be responsible for activating NLRP3. Silencing USF2 effectively inhibited podocyte pyroptosis and ultimately attenuated kidney injury in LN.
Materials and methods
Animal studies
The Jackson Laboratory (Bar Harbor, ME, USA) provided female MRL/lpr mice and C57BL/6 mice for all experiments, which were approved by the Institutional Animal Care and Use Committee of Guizhou Medical University (approval number: 2,200,335). The control (NC) group consisted of same-age female C57BL/6 mice, and the LN groups were the female MRL/lpr mice. Interference fragments of siRNA-USF2 were injected via the tail vein, and the mice were randomly divided into the MRL/lpr + sh-NC and MRL/lpr + sh-USF2 groups. The animals were sacrificed using intraperitoneal overdose injections of 0.6% sodium pentobarbital. Kidney, serum, and urine samples were collected for further research.
Cell lines and reagents
Human conditionally immortalized glomerular podocytes (ATCC, USA) were used in experiments. McCoy’s 5 A Medium (Modified) (Biological Industries, USA) was used to culture cells at 37 °C in an incubator with 5% CO2 and 10% fetal bovine serum (FBS; Gibco, USA). Antibody-related information is shown in Supplementary Table 1. Invitrogen provided Lipofectamine 2000 (Carlsbad, CA, USA). The siRNAs for USF2 and the control were obtained from GenePharma (Shanghai, China), as shown in Supplementary Table 2. ImmunoChemistry Technologies, LLC provided the FAM-FLICA® Caspase-1 (YVAD) Assay Kit (Minnesota, USA).
Western blotting (WB)
Protein concentrations were obtained quantitatively using a BCA kit (Beyotime) after total protein extraction in RIPA lysis buffer. Analysis of protein samples was performed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After incubation of the primary antibodies overnight at 4 °C, the secondary antibodies were detected using HRP-conjugated secondary antibodies. The immunoblots were analyzed using an enhanced chemiluminescence (Smart) WB detection kit, and the signal density was analyzed using an image analysis system (ImageJ, National Institutes of Health, USA). Relative protein levels were calculated based on the density ratio of the target protein to GAPDH.
Analysis of histopathology
The kidney tissues were immersed in a 4% paraformaldehyde solution before wax embedding. Tissues were serially sectioned into 4-µm thick slices. The sections were routinely dewaxed in water and stained with hematoxylin and eosin (HE) following standard procedures (Robert et al., 2014) to assess kidney tissue damage. Masson’s trichrome and Sirius red staining were used to assess collagen deposition. Glomerular basement membrane lesions were observed using periodic acid-Schiff (PAS) staining. Shirong Zheng (Shirong Zheng and Paul N Epstein, 2021) et al. section’s staining method was used for immunohistochemistry (IHC). Sections were probed with appropriate primary antibodies. The USF2 antibody was used at a 1:80 dilution followed by corresponding secondary antibodies. A light microscope was used to examine and image the slides (Olympus Corporation, Tokyo, Japan). Immunofluorescence was performed according to previously described methods (Racetin et al. 2021; Bo Diao et al. 2021). The renal tissue activity index (AI) score of lupus nephritis was calculated using the Austin method (Austin et al., 1984). This index was defined as the sum of individual scores of the following items considered to represent measures of active lupus nephritis: glomerular proliferation, leucocyte exudation, karyorrhexis/fibrinoid necrosis (x2), cellular crescents (x2), hyaline deposits, and interstitial inflammation. The maximum score was 24 points for the Activity Index. The same expert animal pathologist blindly evaluated all pathological sections. We observed the sections using an Olympus confocal microscope (Olympus, Tokyo, Japan). Sections were analyzed using the ImageJ analysis system (National Institutes of Health, USA).
Luciferase reporter assay
Transfection and dual luciferase reporter assay 293T cells were co-transfected with pGL3-Basic NLRP3 promoter vectors, pCMV-USF2 plasmids, vectors and plasmids purchased from Era Biotech (Shanghai, China). The luciferase activity of the extracts was measured using a Dual Luciferase Assay, as directed by the manufacturer. The plasmid pRL-TK, containing the Renilla luciferase gene, was used as an internal control. The PRL-TK plasmid was purchased from Promega (Madison, Wisconsin, USA).
Quantitative real-time PCR (RT-PCR)
TRIzol (Takara, Japan) was used for total RNA extraction. A reverse transcription kit from Takara was used to synthesize cDNA following the manufacturer’s instructions. qPCR was performed using Sybrgreen qPCR Master Mix (Takara, Japan) on a Bio-Rad CFX Connect Real-Time Detection System. The comparative Ct method was used to determine the fold change in expression levels using the formula 2−ΔΔCt with GAPDH as a calibrator. The primer sequences are shown in Supplementary Table 2.
Caspase-1/PI double staining
A flow cytometer was used to measure caspase-1 and PI fluorescence of samples for pyroptosis analysis. A FLICA 660 Caspase-1 Detection Kit was used to detect active caspase-1, and PI staining was used to evaluate membrane integrity. The cell density for podocyte samples was adjusted to 2–5 × 105/ml, as recommended by the manufacturer. We added FLICA 660 working solution to cell suspensions in a 1:30 − 1:60 (v/v) ratio. The cells were incubated at 37 °C, washed with wash buffer and centrifuged. A wash buffer was used to resuspend and gently mix the cells after discarding the supernatants. PI was added to the suspensions five minutes before flow cytometry analysis (NovoCyte, California, USA).
Chromatin immunoprecipitation (ChIP) assay
The Millipore ChIP assay kit (Bedford, Massachusetts, USA) was used to perform the ChIP assays according to the manufacturer’s protocol. Briefly, immunoprecipitation was performed using an antibody against USF2 (Abcam, UK), and irrelevant IgG was used as a control. Using specific primers targeting the predicted USF2 binding site on the conserved region of the NLRP3 promoter, PCR was used to identify the precipitated DNAs.
Statistical analysis
This report presents quantitative data as the means ± standard deviation (SD). The data were analyzed in GraphPad Prism 5.00 (San Diego, CA, USA), and statistical significance was determined using one-way analysis of variance (ANOVA). Differences were considered significant at a P value less than 0.05. Each experiment was performed at least three times.
Results
USF2 expression was increased in MRL/lpr mouse renal tissue
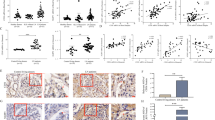
The MRL/lpr and C57BL/6 mice were sacrificed, and their kidneys were analyzed to determine the expression of USF2. HE, Masson’s trichrome, Sirius red, and PAS were used to stain renal tissue sections from MRL/lpr and C57BL/6 mice to investigate the morphological differences between the two groups. IHC was used to examine the expression and localization of USF2. Compared to the C57BL/6 group, the renal tubules in the MRL/lpr group were irregularly arranged, and significant focal atrophy was observed. The glomeruli showed inflammatory cell infiltration, mesangial cell hyperplasia, and protein-like degeneration, with significant damage to the glomerular structure. Masson’s trichrome and Sirius red staining showed that the MRL/lpr group had significantly higher collagen and matrix levels in the renal tissue compared to the C57BL/6 group. PAS staining revealed severe mesangial matrix proliferation in the glomeruli of the MRL/lpr group (Fig. 1A, B). Immunohistochemical results revealed that USF2 was primarily found in the nucleus. USF2 expression in the renal tissue of MRL/lpr mice was significantly higher than in C57BL/6 mice (Fig. 1A, C). In this study, the AI score of lupus nephritis in the MRL/lpr group was significantly higher than that in the C57BL/6 group (Fig. 1D). USF2, caspase-1, cleaved caspase-1, IL-18 and IL-1β protein levels were determined using WB analysis, and the findings confirmed that USF2, caspase-1, cleaved caspase-1, IL-18 and IL-1β expression was significantly higher in MRL/lpr mouse kidney tissue (Fig. 2A, B). The tissue immunofluorescence findings confirmed that USF2 and caspase-1 expression was significantly higher in MRL/lpr mouse kidney tissue (Fig. 2C, D). These findings indicate that MRL/lpr mice had damaged kidneys and increased USF2 expression.
Pathological analysis of renal tissue in mice. Renal tissue sections from MRL/lpr and age-matched C57BL/6 mice were stained with (a) HE, and the results revealed inflammatory cell infiltration (red arrow) and mesangial cell hyperplasia (green arrow). Masson’s trichrome, Sirius red, and PAS showed mesangial matrix proliferation (yellow arrow). (b) % control is the relative positive area. Immunohistochemical analysis of mouse kidneys for USF2 results revealed that it was primarily found in the nucleus (blue arrow). (c) % control is the relative positive area. d. Increased renal pathological scores in the MRL/lpr mouse group
USF2 protein levels in mouse kidneys. a, b. WB was used to detect USF2, caspase-1, cleaved caspase-1, IL-18 and IL-1β protein expression in the kidneys of MRL/lpr and age-matched C57BL/6 mice, and densitometry was used to analyze the protein expression. c, d. USF2 and caspase-1 protein expression was detected using immunofluorescence analysis. DAPI was used for nuclear staining
USF2 expression in MRL/lpr mouse renal tissues positively correlates with biochemical function indicators
Serum creatinine (CRE), blood urea nitrogen (BUN) and urinary protein were evaluated to reflect the degree of kidney damage and functional change. These three indicators were distinctly increased in LN patients. To investigate whether USF2 expression correlated with renal function, these three indicators were assessed in the two groups of mice. We observed a significant increase in biochemical function indicators in the MRL/lpr mice compared to the control group (Fig. 3A, C, E), which increased rapidly as nephritis progressed. The USF2 mRNA levels in renal tissue positively correlated with biochemical function indicator levels (Fig. 3B, D, F). These findings indicate that USF2 expression contributes to renal function impairment in MRL/lpr mice.
Correlation between USF2 mRNA levels in renal tissue and the degree of renal impairment. a. Urinary protein levels in mice were detected for analysis of renal function. b. Spearman’s correlation analysis was used to determine the relationship between USF2 mRNA expression and urinary protein levels. c. BUN levels in mice were detected for analysis of renal function. d. Spearman’s correlation analysis was used to determine the relationship between USF2 mRNA expression and BUN levels. e. Serum CRE levels in mice were detected for analysis of renal function. f. Spearman’s correlation analysis was used to determine the relationship between USF2 mRNA expression and serum CRE levels. The data are presented as the means ± SD of 3 independent experiments
Silencing USF2 significantly delays podocyte pyroptosis
To better understand the role of USF2 in podocyte pyroptosis, siRNA was transfected into podocytes to silence USF2 expression after serum stimulation, and the podocytes were divided into four groups. Immunofluorescence and WB analyses were performed to determine podocyte marker protein, USF2 and pyroptosis protein levels. PI staining was used to identify pyroptosis-induced changes in membrane permeability in the pyroptosis assay. The pyroptosis rate was determined by double-staining for active caspase-1 and PI (Hao et al. 2020). The results showed that stimulating serum from patients with LN increased USF2 protein expression and promoted podocyte pyroptosis.
Silencing of USF2 partially abrogated the LN serum-induced increase in NLRP3, caspase-1, and IL-1β expression (Fig. 4A). High expression of synaptopodin proteins is necessary to preserve podocyte structure, which controls the shape and motility of podocyte foot processes. The podocyte expression of desmin, an established marker of podocyte injury (Huby AC et al. 2009; Lin MH et al. 2018), was monitored using confocal microscopy. The results showed that LN serum stimulation increased desmin expression and decreased synaptopodin expression, which suggests a pathway for podocyte damage (Fig. 4B). Flow cytometry data revealed that the ratio of double-positive podocytes obtained from an MRL/lpr serum-stimulated model was significantly higher than the control group. However, silencing USF2 prevented pyroptosis caused by LN serum in podocytes (Fig. 4C). These data demonstrate that inhibiting USF2 effectively reduces pyroptosis in podocytes induced by LN serum.
Effect of USF2 silencing on pyroptosis progression in podocytes. USF2 siRNA was transfected into podocytes stimulated with serum from MRL/lpr mice, and serum from C57BL/6 mice added to the cells of the NC group at the same dose. a. USF2, NLRP3 and related indicators of pyroptosis levels were analyzed using WB in the 4 indicated groups b. USF2, synaptopodin and desmin levels were detected using immunofluorescence analysis in the 4 indicated groups. c. The rate of pyroptotic cell death was examined using active PI and caspase-1 double staining and flow cytometry (n = 3). USF2 knockdown led to a decreased rate of pyroptotic cell death. The data are presented as the means ± SDs. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was performed. Compared to the C57BL/6 serum group: *P < 0.05, **P < 0.01, ***P < 0.001. Compared to the MRL/lpr serum + siRNA-NC group: #P < 0.05, ##P < 0.01, ###P < 0.001
USF2 transcriptionally regulates NLRP3 expression in lupus nephritis
The mRNA levels of USF2 and NLRP3 were examined using qRT-PCR in mouse kidney tissues of each group to determine whether these levels correlated with LN progression. Compared to the C57BL/6 group, MRL/lpr mice showed significant increases in the mRNA levels of USF2 and NLRP3 (Fig. 5A, B). Spearman’s correlation analysis showed that USF2 expression positively correlated with NLRP3 in mouse kidney tissue (P < 0.001, r = 0.8170. Figure 5D, E), and the upregulation of USF2 expression increased NLRP3 mRNA levels in cells (Fig. 5C). USF2 may act as a novel regulator of inflammasome production as a transcriptional regulator. An investigation of the binding relationship between USF2 and NLRP3 was performed using the JASPAR bioinformatics prediction website, and the binding site was discovered (Supplementary Fig. 1). A luciferase reporter experiment confirmed that the pGL3-Basic and pCMV-USF2 co-transfection had no significant effect on the relative luciferase activity, but it increased the co-transfection with pGL3-Basic-NLRP3 and pCMV-USF2 (Fig. 5F). A ChIP assay using NLRP3-specific primers confirmed the interaction of the USF2 protein with the NLRP3 gene promoter (Fig. 5G). Therefore, USF2 may regulate inflammasome production by targeting the NLRP3 promoter and inducing transcriptional activation of NLRP3.
USF2 promotes NLRP3 expression via transcriptional regulation in lupus nephritis. a. USF2 mRNA expression in MRL/lpr and age-matched C57BL/6 mice. b. NLRP3 mRNA expression in MRL/lpr and C57BL/6 mice. c. After transfection of cells with pCMV-vector or pCMV-USF2, the mRNA levels of USF2 and NLRP3 were measured. d, e. Spearman’s correlation analysis of the relationship between USF2 and NLRP3 mRNA levels. f. The NLRP3 promoter is activated by USF2. In HEK293 cells, the NLRP3 promoter construct was co-transfected with pCMV-USF2, and the relative luciferase activity was determined. g. ChIP‒qPCR confirmed the binding relationship between USF2 and the NLRP3 promoter. Compared to IgG: *P < 0.05, **P < 0.01, ***P < 0.001
USF2 depletion attenuates the progression of kidney injury in MRL/lpr mice
To investigate the function of USF2 in mice, siRNA-USF2 was injected via the tail vein into mice for USF2 knockdown. IHC staining showed that compared to the MRL/lpr + sh-NC group, the expression of USF2 was decreased in the MRL/lpr + sh-USF2 group (Fig. 6A). HE staining of renal sections from the MRL/lpr + sh-NC group showed renal tubular epithelial cells with edema, degeneration, swelling, necrosis and renal tubular cells infiltrated by inflammation with dilated lumens. The glomerular histological structure was damaged, and atrophy was observed (Fig. 6B). Sirius red and PAS staining showed that the MRL/lpr + sh-NC group exhibited clear pathological changes in the kidney, such as proliferation of mesangial cells, destruction of the glomerular structure, and infiltration of inflammatory cells, including cellular enlargement, basement membrane thickening, and increased cellular casts (Fig. 6C, D). As shown in Fig. 6E, MRL/lpr + sh-USF2 mice had significantly reduced renal pathology scores compared to MRL/lpr + sh-NC mice (Fig. 6E). These results suggest that silencing USF2 would improve renal histopathological damage in MRL/lpr mice.
Pathological analysis of renal tissue in mice with silenced USF2. a. Immunohistochemical analysis of mouse kidneys for USF2. The results showed that USF2 was primarily found in the nucleus and that USF2 expression was reduced in the MRL/lpr + sh-USF2 group (blue arrow). b, c, d. Kidney tissue sections from mice were subjected to HE staining, Sirius red staining and PAS staining. The results revealed inflammatory cell infiltration (red arrow), proliferation of mesangial cells (green arrow), mesangial matrix proliferation (yellow arrow), and renal tubular atrophy (light blue arrow). e. Decreased renal pathological scores in the MRL/lpr + sh-USF2 mouse group
Discussion
SLE is an autoimmune disease that is characterized by self-tolerance loss, the formation of nuclear autoantigens and ICs, and inflammation of multiple organs (Romo-Tena J and Kaplan MJ, 2020). There are various clinical presentations of SLE, including skin, kidney, joints, and nervous system involvement, and chronic and relapsing disease patterns (Sullivan et al., 2016). LN is a complex clinical manifestation of SLE associated with significant morbidity and mortality. Despite effective anti-inflammatory and immunosuppressive therapies, most patients develop CKD or ESRD (Ronith Chakraborty et al. 2020; Michela Gasparotto et al. 2020). SLE is primarily caused by antibodies against different nuclear autoantigens, such as DNA and RNA double-stranded chains (May et al. 2020; Bai Yunqiang et al. 2018). The formation of ICs between autoantibodies and autoantigens and their abnormal clearance may cause permanent inflammation and contribute to tissue or organ damage (Joshua et al., 2019). The immune complex is deposited in the glomeruli, which activates complement and infiltration of immune cells that lead to proinflammatory responses and pathological changes in the glomeruli (Desmond Yh Yap et al., 2018). The MRL/MpJ-Faslpr (MRL/lpr) mouse strain was chosen because these mice produce anti-DNA autoantibodies and renal immune deposits containing IgG, IgM, IgA, and C3, which affect the kidney’s systemic autoimmune disease (Hasanain Alaridhee et al. 2020). This process is consistent with the pathological findings of LN mouse kidneys in the present study. When IC is deposited with complement activation, it induces LN, which damages the glomeruli and leads to tissue inflammation (Hamza Sakhi et al. 2019). Evidence suggests that podocytes are directly or indirectly affected by IC deposits in LN (Wang et al. 2014), which has led many studies to emphasize the importance of podocyte injury in this disease (Andrew et al., 2016).
A podocyte is a component of the glomerular filtration machinery that is crucial for renal function (Perico et al. 2016a, b). Podocytes are highly differentiated epithelial cells, and foot process extensions anchor podocytes to the basement membrane, where they form slit diaphragms, which are considered filtration barriers (Reiser J and Altintas MM, 2016). Cellular junctions are found at the slit diaphragm, where podocyte-specific proteins interact with the actin cytoskeleton, including synaptopodin, nephrin, podocin, and desmin (Lili Zhou et al. 2019). The actin cytoskeleton plays an important role in podocyte foot formation and dynamics (Sanja Sever and Mario Schiffer, 2018). Foot process effacement (FPE) with secondary podocyte detachment may result from disruption of the actin cytoskeleton, which is linked to heavy proteinuria (Perico et al. 2016a, b). Podocyte injury is common in LN patients, and it is responsible for the development of proteinuria and glomerular damage (Wang et al. 2014). Pyroptosis is important in the pathogenesis of LN because it causes the release of intact nuclei and serves as a source of autoantigens for potential antinuclear antibodies (M Magna and D S Pisetsky, 2015; Ye Yang et al. 2020; Yaqiu Wang and Thirumala-Devi Kanneganti, 2021). NLRP3 is an intracellular multiprotein complex that activates the pyroptosis signaling pathway, which is a proinflammatory programmed cell death signaling pathway (Amy et al., 2020; Spinello et al. 2019). NLRP3 activation recruits and activates the caspase-1 precursor, and inflammation increases NLRP3 transcription and activity (Juan et al. 2022). Caspase-1 of the inflammasome component is activated with inflammasome activation and assembly, which enhances proteolytic cleavage and the release of proinflammatory cytokines, such as IL-1β and IL-18 (Van Opdenbosch and Lamkanfi 2019). Cleaved caspase-1 activates the pore-forming effector protein GSDMD, which causes pyroptosis by puncturing cell membranes and releasing proinflammatory cytokines (Daigo Nakazawa et al. 2017). The NLRP3 inflammasomes in podocytes are activated by RIP3-dependent pathways in mice and people with LN, and the inhibition of RIP3 slows disease progression (Fu et al. 2019; Guo et al. 2019). Increased expression of NLRP3, caspase-1 and IL-1β in LN is evident in kidney biopsies and podocytes (Fu et al. 2017). Lupus-prone mice had improved proteinuria, renal histological lesions, and podocyte foot process effacement when NLRP3 inflammasomes were suppressed with a caspase-1 inhibitor (Liu et al. 2014). Therefore, the role of podocyte pyroptosis in LN is increasingly important and has been investigated by several researchers (Heng Cao et al. 2021).
USF2 regulates adenovirus major late promoter transcription (Sawadogo M and Roeder RG, 1985). USF2 is ubiquitously expressed in mammals and has a molecular weight of 44 kDa. An essential component of the Myc family of transcription factors is a basic helix loop helix/leucine zipper domain that plays a role in dimerization and binding to DNA. USF2 forms homo- and heterodimers that recognize the CACGTG core sequence (E box) in vitro (Sirito et al. 1994). USF2 regulates numerous genes (Yanzhang Li et al., 2010). Research studies increasingly suggest that diabetes-induced kidney injury may be induced by high glucose-stimulated USF2 expression in HK-2 cells via the activation of CREB (Nishant et al. 2011). Transgenic USF2 mice overexpress TGF-β to modulate kidney disease progression, and increased TGF-β levels are associated with activation of profibrotic pathways triggered by oxidative stress (Shu Liu et al. 2007). USF2 promotes renin gene expression in kidney cells, which results in angiotensin II production. Angiotensin II increases TGF-β levels and extracellular matrix production in kidney cells, which result in renal fibrosis (Matsuda et al. 2013). TGF-β promotes the expression of plasminogen activator inhibitor 1 (PAI-1) in the kidney via the Smad-p53-USF2 pathway, which is upregulated in obstructed kidneys (Samarakoon et al. 2012)., USF2 was upregulated during Th17 cell differentiation from naïve CD4 + cells, and silencing USF2 reduced proinflammatory cytokine TNF-α levels in Th17 cells (Rauhamäki et al. 2018). Inhibition of USF2 gene expression reduced T-bet expression, proinflammatory cytokines, and granulocyte-macrophage colony-stimulating factor (GM-CSF) gene expression in rheumatoid arthritis (RA) pathogenesis. Direct targeting of the USF2 signaling pathway in anti-TNF-refractory RA may be a promising therapeutic strategy (Dan et al. 2020). Tang et al. discovered that pyroptosis-related genes that were highly expressed in one cluster positively correlated with USF2, ZNF623, PSMB5, and other genes (Tang et al. 2022). Notably, Sun et al. (Jian Sun et al., 2022) reported that the knock down of USF2 inhibited the TGF-β/suppressor against the Smad3 signaling pathway in sepsis-induced acute kidney injury (SAKI), and THBS1 expression was downregulated, which reduced pyroptosis and improved renal injury.
The degree of renal function injury significantly and positively correlated with USF2 overexpression in the current study. USF2 silencing effectively inhibited podocyte pyroptosis. SLE is characterized by an immune-mediated inflammatory response that increases USF2 production in renal tissues, which activates the downstream NLRP3/caspase-1 pathways to increase pyroptosis in podocytes and promote LN formation. The current study revealed that USF2 had a protective mechanism against LN by regulating NLRP3 to induce pyroptosis in podocytes. The direct role of USF2 in treating clinical LN patients has not been fully characterized and warrants further study.
Data Availability
All data of this study are available upon request.
References
Amy H, Chan, Kate Schroder (2020) Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217(1):e20190314. https://doi.org/10.1084/jem.20190314
Anadi Mahajan J, Amelio K, Gairy G, Kaur, Roger A, Levy D, Roth, Damon B (2020) Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus 29(9):1011–1020. https://doi.org/10.1177/0961203320932219
Andrew S, Bomback, Glen S, Markowitz (2016) Lupus Podocytopathy: a distinct entity. Clin J Am Soc Nephrology: CJASN 11(4):547–548. https://doi.org/10.2215/CJN.01880216
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, David Wofsy (2019) 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 78(9):1151–1159. https://doi.org/10.1136/annrheumdis-2018-214819
Austin HA 3rd, Lr M, Km J, Balow Je (1984) Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 25(4):689–695. https://doi.org/10.1038/ki.1984.75
Bai Yunqiang T, Yanli L Yi, Hu H (2018) Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin Exp Immunol 191(1):1–10. https://doi.org/10.1111/cei.13041
Bhesh R Sharma, Thirumala-Devi K (2021) NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol 22(5):550–559. https://doi.org/10.1038/s41590-021-00886-5
Bo Diao C, Wang R, Wang Z, Feng J, Zhang H, Yang Y, Tan H, Wang C, Wang L, Liu Y, Liu Y, Liu G, Wang Z, Yuan X, Hou L, Ren Y, Wu, Chen Y (2021) Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 12(1):2506. https://doi.org/10.1038/s41467-021-22781-1
Carlos Rafael-Vidal, Irene Altabás, Nair Pérez, Coral Mourino Rodríguez, Jose M Pego-Reigosa, & Samuel Garcia (2021) Calcineurin and systemic Lupus Erythematosus: the Rationale for using calcineurin inhibitors in the treatment of Lupus Nephritis. Int J Mol Sci 22(3):1263. https://doi.org/10.3390/ijms22031263
Christophe Paget E, Doz-Deblauwe N, Winter, Benoit Briard (2022) Specific NLRP3 Inflammasome Assembling and Regulation in Neutrophils: Relevance in Inflammatory and Infectious Diseases. Cells 11(7):1188. https://doi.org/10.3390/cells11071188
Daigo Nakazawa SV, Kumar J, Marschner J, Desai A, Holderied L, Rath F, Kraft Y, Lei Y, Fukasawa GW, Moeckel ML, Angelotti H, Liapis,Hans-Joachim Anders (2017) Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in ischemic AKI. J Am Soc Nephrology: JASN 28(6):1753–1768. https://doi.org/10.1681/ASN.2016080925
Dan Hu, Tjon EC, Karin M, Andersson GM, Molica MC, Pham B, Healy G, Murugaiyan N, Pochet VK, Kuchroo, Maria I, Bokarewa, Howard LW (2020) Aberrant expression of USF2 in refractory rheumatoid arthritis and its regulation of proinflammatory cytokines in Th17 cells. Proc Natl Acad Sci USA 117(48):30639–30648. https://doi.org/10.1073/pnas.2007935117
Feng Yu M, Haas R Glassock, Ming-Hui Z (2017) Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 13(8):483–495. https://doi.org/10.1038/nrneph.2017.85
Fu R, Guo C, Wang S, Huang Y, Jin Ou, Hu H, Chen J, Xu B, Jijun Zhao (2017) Podocyte activation of NLRP3 inflammasomes contributes to the development of Proteinuria in Lupus Nephritis. Arthritis & rheumatology (Hoboken N J) 69(8):1636–1646. https://doi.org/10.1002/art.40155
Fu R, Xia Y, Li M, Mao R, Guo C, Zhou M, Tan H, Liu M, Wang S, Yang N, Jijun Zhao (2019) Pim-1 as a therapeutic target in Lupus Nephritis. Arthritis & rheumatology (Hoboken N J) 71(8):1308–1318. https://doi.org/10.1002/art.40863
Guo C, Fu R, Zhou M, Wang S, Huang Y, Haoqiang Hu J, Zhao F, Gaskin N, Yang, Shu Man Fu (2019) Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun 103:102286. https://doi.org/10.1016/j.jaut.2019.05.014
Hamza Sakhi A, Moktefi K, Bouachi V, Audard C, Hénique P, Remy M, Ollero, Khalil El Karoui (2019) Podocyte Injury in Lupus Nephritis. J Clin Med 8(9):1340. https://doi.org/10.3390/jcm8091340
Hao K, Jiang W, Zhou M, Li H, Chen Y, Jiang F, Qinghua Hu (2020) Targeting BRD4 prevents acute gouty arthritis by regulating pyroptosis. Int J Biol Sci 16(16):3163–3173. https://doi.org/10.7150/ijbs.46153
Hasanain Alaridhee A, Alharbi Z, Saeed RC, Thomas, Cordula M, Stover (2020) Complement Properdin determines Disease activity in MRL/lpr mice. Med (Kaunas Lithuania) 56(9):430. https://doi.org/10.3390/medicina56090430
Hélène D, Benoit Pourcet (2021) Nuclear Receptors in the control of the NLRP3 inflammasome pathway. Front Endocrinol 12:630536. https://doi.org/10.3389/fendo.2021.630536
Heng Cao J, Liang J, Liu Y, He Y, Ke Y, Sun S Jiang, Jin L (2021) Novel Effects of Combination Therapy through Inhibition of Caspase-1/Gasdermin D Induced-Pyroptosis in Lupus Nephritis. Front Immunol 12:720877. https://doi.org/10.3389/fimmu.2021.720877
Huby Ac R, Mp, Caron K, Smithies O, Dussaule Jc, Chatziantoniou C (2009) Restoration of podocyte structure and improvement of chronic renal disease in transgenic mice overexpressing renin. PLoS ONE 4(8):e6721. https://doi.org/10.1371/journal.pone.0006721
Jian Sun, Xiaoli Ge, Yang Wang, Lei Niu, Lujia Tang, & Shuming Pan (2022) USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol Res 176:105962. https://doi.org/10.1016/j.phrs.2021.105962
Joshua M, Thurman, Roshini Yapa (2019) Complement therapeutics in Autoimmune Disease. Front Immunol 10:672. https://doi.org/10.3389/fimmu.2019.00672
Juan C, Zhu Y, Chen Y, Mao Y, Zhou Y, Zhu W, Wang X, Wang Q (2022) Knocking down ETS proto-oncogene 1 (ETS1) alleviates the pyroptosis of renal tubular epithelial cells in patients with acute kidney injury by regulating the NLR family pyrin domain containing 3 (NLRP3) transcription. Bioengineered 13(5):12927–12940. https://doi.org/10.1080/21655979.2022.2079242
Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Jae Jin Chae (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2 + and cAMP. Nature 492(7427):123–127. https://doi.org/10.1038/nature11588
Li Chen YH, Shen X, Wang J, Wang Y, Gan N, Chen J, Wang SA, Lemaire, Joseph S, Coselli, Xing Li Wang (2006) Human prolyl-4-hydroxylase alpha(I) transcription is mediated by upstream stimulatory factors. J Biol Chem 281(16):10849–10855. https://doi.org/10.1074/jbc.M511237200
Lihua Shi D, Nikolic S, Liu H, Lu, Shuxia, Wang (2009) Activation of renal renin-angiotensin system in upstream stimulatory factor 2 transgenic mice. Am J Physiol Renal Physiol 296(2):F257–265. https://doi.org/10.1152/ajprenal.90493.2008
Lili Zhou X, Chen M, Lu Q, Wu Q, Yuan C, Hu J, Miao Y, Zhang H, Li FF, Hou J, Nie, Liu Y (2019) Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int 95(4):830–845. https://doi.org/10.1016/j.kint.2018.10.032
Lin Mh M, Jb, Kikkawa Y, Suleiman Hy, Tryggvason K, Hodges B, Miner Jh (2018) Laminin-521 protein therapy for glomerular basement membrane and podocyte abnormalities in a model of Pierson Syndrome. J Am Soc Nephrology: JASN 29(5):1426–1436. https://doi.org/10.1681/ASN.2017060690
Liu D, Xu M, Ding L-H, Lv L-L, Liu H, Ma K-L, Zhang A-H, Crowley SD, Bi-Cheng Liu (2014) Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: a novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 57:7–19. https://doi.org/10.1016/j.biocel.2014.09.018
Magna M, Pisetsky DS (2015) The role of cell death in the pathogenesis of SLE: is pyroptosis the Missing Link? Scand J Immunol 82(3):218–224. https://doi.org/10.1111/sji.12335
Matsuda M, Tamura K, Wakui H, Maeda A, Ohsawa M, Kanaoka T, Azushima K, Uneda K, Haku S (2013) Upstream stimulatory factors 1 and 2 mediate the transcription of angiotensin II binding and inhibitory protein. J Biol Chem 288(26):19238–19249. https://doi.org/10.1074/jbc.M113.451054
Mausita Karmakar M, Minns EN, Greenberg J, Diaz-Aponte K, Pestonjamasp, Jennifer L, Johnson (2020) N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun 11(1):2212. https://doi.org/10.1186/s40425-019-0591-3
May Y, Choi RD, Fitzpatrick K, Buhler M, Mahler, Marvin JF (2020) A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun rev 19(3):102463. https://doi.org/10.1016/j.autrev.2020.102463
Michela Gasparotto M, Gatto V, Binda A, Doria, Gabriella Moroni (2020) Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatology (Oxford) v39–v51 59(Supplement_5. https://doi.org/10.1093/rheumatology/keaa381
Nishant P, Visavadiya Y, Li, Shuxia, Wang (2011) High glucose upregulates upstream stimulatory factor 2 in human renal proximal tubular cells through angiotensin II-dependent activation of CREB. Nephron Exp Nephrol 117(3):e62–70. https://doi.org/10.1159/000320593
Parimal Samir S, Kesavardhana DM, Patmore S, Gingras R, Karki, Clifford S, Guy B Briard, David EP (2019) DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature 573(7775):590–594. https://doi.org/10.1038/s41586-019-1551-2
Perico L, Conti S, Benigni A, Remuzzi G (2016a) Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12(11):692–710
Perico L, Conti S, Giuseppe Remuzzi (2016b) Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12(11):692–710. https://doi.org/10.1038/nrneph.2016b.127
Pragnesh M, Mariana J, Kaplan (2017) Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol (Orlando Fla) 185:59–73. https://doi.org/10.1016/j.clim.2016.08.010
Racetin A, Filipović N, Lozić M, Ogata M, Gudelj Ensor L, Kelam N (2021) A homozygous Dab1-/- is a potential Novel cause of autosomal recessive congenital anomalies of the mice kidney and urinary tract. Biomolecules 11(4):609. https://doi.org/10.3390/biom11040609
Rauhamäki S, Postila Pa, Lätti S, Niinivehmas S, Multamäki E, Liedl Kr, Pentikäinen Ot (2018) Discovery of Retinoic acid-related orphan receptor γt Inverse Agonists via Docking and negative image-based screening. ACS Omega 3(6):6259–6266. https://doi.org/10.1021/acsomega.8b00603
Reiser J, Altintas Mm (2016) Podocytes. F1000Res 5:F1000FacultyRev–1114. https://doi.org/10.12688/f1000research.7255.1
Robert D, Cardiff, Claramae H, Miller, Robert JM (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor protocols, 2014(6), 655–658. doi: https://doi.org/10.1101/pdb.prot073411
Romo-Tena J, Kaplan Mj (2020) Immunometabolism in the pathogenesis of systemic lupus erythematosus: an update. Curr Opin Rheumatol 32(6):562–571. https://doi.org/10.1097/BOR.0000000000000738
Ronith Chakraborty A, Mehta N, Nair L, Nemer R, Jain H, Joshi, Rupesh Raina (2020) & ACTH Treatment for Management of Nephrotic Syndrome: A Systematic Review and Reappraisal. International Journal of Nephrology, 2020, 2597079. doi: https://doi.org/10.1155/2020/2597079
Samarakoon R, Overstreet Jm H, Higgins Pj (2012) TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res 347(1):117–128. https://doi.org/10.1007/s00441-011-1181-y
Sanja S, Mario Schiffer (2018) Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases. Kidney Int 93(6):1298–1307. https://doi.org/10.1016/j.kint.2017.12.028
Sannula Kesavardhana RK, Subbarao Malireddi, Thirumala-Devi K (2020) Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol 38:567–595. https://doi.org/10.1146/annurev-immunol-073119-095439
Sawadogo M, Roeder R (1985) Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell 43(1):165–175. https://doi.org/10.1016/0092-8674(85)90021-2
Shirong Zheng, Paul NE (2021) CD45 Immunohistochemistry in Mouse Kidney. Bio-protocol, 11(22), e4230. doi: https://doi.org/10.21769/BioProtoc.4230
Shu Liu L Shi, Shuxia, Wang (2007) Overexpression of upstream stimulatory factor 2 accelerates diabetic kidney injury. Am J Physiol Renal Physiol 293(5):F1727–1735. https://doi.org/10.1152/ajprenal.00316.2007
Shuxia, Wang (2015) Role of upstream stimulatory factor 2 in diabetic nephropathy. Front biology 10(3):221–229. https://doi.org/10.1007/s11515-015-1359-x
Sirito M, Lin Q, Maity T, Sawadogo M (1994) Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res 22(3):427–433. https://doi.org/10.1093/nar/22.3.427
Spinello A, Vecile E, Abbate A, Dobrina A, Magistrato A (2019) How can Interleukin-1 receptor antagonist modulate distinct cell death pathways? J Chem Inf Model 59(1):351–359. https://doi.org/10.1021/acs.jcim.8b00565
Sullivan KEGeorgeC, Tsokos MS, Lo, Patricia Costa Reis, Kathleen E, Sullivan (2016) New insights into the immunopathogenesis of systemic lupus erythematosus. Nature reviews. Rheumatology, 22(12), 716–730. doi: https://doi.org/10.1038/nrrheum.2016.186
Susan Allison (2022) ALCAM-CD6 in lupus nephritis. Nat Rev Nephrol 18(3):137. https://doi.org/10.1038/s41581-022-00548-1
Tang Y, Zhang P, Liu Q, Jingsong Xu (2022) Pyroptotic patterns in blood leukocytes predict Disease Severity and Outcome in COVID-19 patients. Front Immunol 13:888661. https://doi.org/10.3389/fimmu.2022.888661
Van Opdenbosch N, Lamkanfi M (2019) Caspases in cell death, inflammation, and Disease. Immunity 50(6):1352–1364. https://doi.org/10.1016/j.immuni.2019.05.020
Wang Y, Yu F, Song D, Su-Xia Wang, Ming-Hui Z (2014) Podocyte involvement in lupus nephritis based on the 2003 ISN/RPS system: a large cohort study from a single centre. Rheumatology (Oxford) 53(7):1235–1244. https://doi.org/10.1093/rheumatology/ket491
Yang Zhou Z, Tong S, Jiang W, Zheng J, Zhao, Xiangmei Zhou (2020) The roles of endoplasmic reticulum in NLRP3 inflammasome activation. Cells 9(5):1219. https://doi.org/10.3390/cells9051219
Yanzhang Li, Shuxia, Wang (2010) Glycated albumin upregulates upstream stimulatory factor 2 gene transcription in mesangial cells. Am J Physiol Renal Physiol 299(1):F121–127. https://doi.org/10.1152/ajprenal.00074.2010
Yap DY, Tak Mao Chan (2018) Lupus nephritis: an update on treatments and pathogenesis. Nephrol (Carlton Vic) 23:80–83. https://doi.org/10.1111/nep.13469
Yaqiu W, Thirumala-Devi K (2021) From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J 19:4641–4657. https://doi.org/10.1016/j.csbj.2021.07.038
Ye Yang PY, Liu W, Bao SJ, Chen F, Su Wu, Ping YZ (2020) Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 20(1):28. https://doi.org/10.1186/s12885-019-6491-6
Yuan yuan Qi, Zhou X, Cheng F, Hou P, Ren Y, Wang S, Zhao M, Yang L, Martinez J, Hong Zhang (2018) Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-α in lupus nephritis. Ann Rheum Dis 77(12):1799–1809. https://doi.org/10.1136/annrheumdis-2018-213028
Funding
The current work was supported by the Natural Science Foundation of Guizhou Province, China (QianKeHeJiChu-ZK [2022] General 409), the Youth Talent Development Project of Guizhou Education Department (Qian Jiao He [2021]177), and the Science and Technology Foundation of Guizhou Provincial Health Commission (gzwkj2021-378).
Author information
Authors and Affiliations
Contributions
The main experiments were performed by Ying Xie, Xiaoying Li, and Wenli Deng, and the manuscript was written by Ying Xie. Nan Nan, Gong Lei, and Min Chen helped with the cell experiments. Jie Yu, Huimei Zou and Peilei Chen collected animal specimens and performed experiments. Daolin Cui and Fan Zhang revised the article and accept responsibility for its content.
Corresponding authors
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval and consent to participate
All animal experiments were performed according to the principles and procedures approved by Guizhou Medical University’s Animal Care and Use Committee (approval number: 2,200,335, Guiyang, China).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, Y., Li, X., Deng, W. et al. Knockdown of USF2 inhibits pyroptosis of podocytes and attenuates kidney injury in lupus nephritis. J Mol Histol 54, 313–327 (2023). https://doi.org/10.1007/s10735-023-10135-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10135-8