Abstract
To explore the role of Notch1 pathway in the pathogenesis of podocyte injury, and to provide novel strategy for podocyte repair in lupus nephritis (LN). Bioinformatics analysis and immunofluorescence assay were applied to determine the expression and localization of Notch1 intracellular domain1 (NICD1) in kidneys of LN patients and MRL/lpr mice. The stable podocyte injury model in vitro was established by puromycin aminonucleoside (PAN) treatment. Expression of inflammasome activation related gene was detected by qPCR. The podocytes with PAN treatment were cultured with or without N-S-phenyl-glycine-t-butylester (DAPT), an inhibitor of Notch1 pathway. NICD1, Wilm’stumor1 (WT1), nucleotide-binding oligomerization domain-like receptors 3 (NLRP3), and absent in melanoma-like receptors 2 (AIM2) were detected by western blot. In vivo, MRL/lpr mice were administrated with DAPT or vehicle. The LN symptoms were assessed. The podocyte injury was evaluated, and the NLRP3 in podocytes of mice was detected. Notch1 pathway was overactivated in glomeruli of LN patients. NICD1 was colocalized with podocytes of LN patients and MRL/lpr mice. The inflammasome-related genes were significantly increased in podocytes with PAN treatment. NICD1 and NLRP3 were significantly decreased, while WT1 was significantly increased in injured podocytes treated with DAPT in vitro. In vivo, lupus-like symptoms were alleviated in DAPT treatment group. Notch1 pathway was inhibited in kidneys of mice treated with DAPT. The renal inflammation was reduced and the podocyte injury was mitigated in DAPT treatment group. The NLRP3 was decreased in podocytes of mice treated with DAPT. Notch1 pathway was overactivated in podocytes of LN patients and MRL/lpr mice. Blockade of Notch1 pathway reduced renal inflammation and alleviated podocyte injury via inhibition of NLRP3 inflammasome activation in LN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Systemic lupus erythematosus (SLE) was characterized by multiple organ involvement and generation of autoantibodies [1]. Lupus nephritis (LN) was one of the most common complications and also a major cause of mortality of SLE, mainly characterized by proteinuria and progressive decline of renal function [2]. Due to the high heterogeneity of LN, there were still many patients progressed to end-stage renal disease.
Podocytes were terminally differentiated epithelial cells which wrap around the basement membrane of glomeruli, and part of the glomerular filtration barrier. Foot process effacement of podocytes was closely related to the generation of proteinuria. It was generally thought that podocyte injury participated in the pathogenesis of glomerular damage and glomerulosclerosis [3, 4]. Previous studies have shown that extensive fusion of podocytes was observed in LN [5]. Therefore, podocyte injury was involved in the production of albuminuria and loss of renal function in LN patients. Expressions of functional podocyte markers (Synaptopodin, Cd2ap, Nephrin, Podocin, Podocalyxin) were regarded as indicators of podocyte injury [6]. WT1 was regarded as a podocyte-specific nuclear protein, which could regulate the expressions of a variety of podocyte-specific genes to maintain the differentiation of podocytes [7]. However, the mechanisms of podocyte injury in LN remained unclear.
In recent years, podocytes have also been considered as immune cells. The role of podocytes as participants in the immune response should be taken seriously. The inflammasome was thought to be an important part of the innate immune system, which was composed of cytoplasmic sensor (NLRs or ALRs), adaptor protein (apoptosis-associated speck-like protein, ASC) and effector (cysteinyl aspartate specific proteinase, Caspase). It has been demonstrated that, NLRs, ALRs, ASC and caspases were found to be expressed in podocytes [8]. Canonical activation of the inflammasome could promote caspase-1-dependent cleavage of IL-1β and IL-18 precursors to generate mature proinflammatory cytokines [9]. The abnormal activation of inflammasomes, which may induce the pathological inflammation, was believed to be associated with podocyte dysfunction in patients with glomerular diseases [10]. However, studies on whether other inflammasomes besides NLRP3 were involved in podocyte injury were still limited.
Notch pathway was highly conserved in evolution, and played an essential role in embryonic development and maintenance of homeostasis. Canonical Notch pathway was activated through the binding of its receptors (Notch1-4) to ligands (Delta-like 1 (DLL1), DLL3, DLL4, jagged1 (JAG1) and JAG2). After that, Notch receptors underwent a series of proteolytic events and conformational change. Then, its intracellular domain (NICD) was released and transferred to the nucleus to activate downstream target genes. The gene families of Notch target included Hairy/Enhancer of Split (HES), Hairy/Enhancer of Split related to YRPW motif (HEY) and so on [11, 12]. Therefore, the activation of Notch pathway was conducted with a group of genes, including ligands, receptors, transcription factors, and different target gene families. NICD was the activated form of Notch1 protein and significantly, it was also the part in which the Notch1 protein really functioned when the pathway was activated.
The activation of Notch1 pathway was necessary for formation of podocytes and proximal tubules [13]. Once the kidney development was completed, Notch1 signaling activity was downregulated to maintain stable internal environment of the kidney [14]. It was worth mentioning that attenuation of the signal was crucial for the terminal differentiation of podocytes [15]. Abnormal activation of Notch1 pathway has been widely concerned in renal diseases most recently, especially podocyte injury-associated glomerular diseases. Notch1 pathway was generally regarded as the downstream pathway leading to podocyte injury in diabetic nephropathy [16] and focal segmental glomerulosclerosis [17]. LN was a typical glomerular disease involving podocyte injury [18]. However, whether Notch1 pathway was involved in the pathogenesis of podocyte injury in LN was not clarified yet. The crosstalk between Notch1 pathway and inflammatory responses has been confirmed [19]. In addition, it has been shown that Notch1 pathway could regulate activation of inflammasomes [20]. Due to the potential impact of Notch1 pathway on inflammasomes, more and more studies linked these two pathways in multiple fields [21, 22]. However, the relationship between Notch1 pathway and inflammasome activation in podocyte injury of LN has not been reported.
In this study, bioinformatics analysis and experiments were combined to determine the expression and localization of Notch1 pathway in kidneys of LN patients and MRL/lpr mice, and to further explore the role of inflammasome activation on podocyte injury in LN.
MATERIALS AND METHODS
Patients
Five LN patients from Nanjing Drum Tower Hospital were enrolled in this study. The diagnosis of LN was based on the American College of Rheumatology revised classification criteria for SLE and renal involvement [23]. The International Society of Neurology/Renal Pathology Society (ISN/RPS) 2003 classification of LN [24] provided a reference for recognition of histological damage in renal biopsies from LN patients. Three normal kidney tissue samples adjacent to renal carcinoma were used as controls. Demographics of LN patients were provided in the Supplemental Table 1.
Mice
All mice were purchased from Huachuang Sino Co. Ltd. (Jiangsu, China). Female MRL/lpr mice (15 weeks old) were used as murine models of LN, and C57BL/6 (B6) mice of the same sex and age served as control [25].
Bioinformatics Analysis
We downloaded GSE32591 from gene expression database (GEO) (https://www.ncbi.nlm.nih.gov/gds), since this data set had the largest human sample size and met the research requirements. Ninety-three samples were included in GSE32591, consisting of 32 glomerular samples and 32 tubulointerstitial samples of patients with LN, 14 glomerular samples and 15 tubulointerstitial samples of healthy donors. “Limma” R package was applied to find out differentially expressed genes in kidney tissues between LN patients and healthy donors in GSE32591.The corrected p value was identified to be less than 0.05 and | logFC | was identified to be more than 0. Finally, the results of differential gene analysis were presented in the form of volcano map.
Gene Set Enrichment Analysis (GSEA)
GSEA was conducted to investigate the enrichment of Notch1 pathway in kidney tissues of LN patients. The results of GSEA were presented in the form of curve.
DAPT Treatment
DAPT, a γ-secretase inhibitor, was proved to be a potent inhibitor of Notch1 cleavage (not other Notch substrates) [26]. Eight female MRL/lpr mice were randomly divided into two groups at the age of 15 weeks and given either DAPT (5 mg/Kg) or DMSO (vehicle; 0 mg/kg DAPT) by intraperitoneal injection (i.p.) once every other day for 4 weeks [27,28,29]. The mode of DAPT treatment in this study has been proved to be capable of avoiding the influence and toxicities on MRL/lpr mice [27]. Additionally, it has been demonstrated that chronic low-concentration DMSO injections as vehicle had no effect on MRL/lpr mice [30,31,32]. The 19-week-old mice were sacrificed. Peripheral blood and kidneys were collected.
Cell Culture and Treatment
Mouse podocytes were purchased from Jennio Biotech (Guangzhou, Guangdong, China). As mentioned before [33], podocytes were cultured in RPMI 1640 containing 10% FBS and 1% penicillin/streptomycin at 37 °C. Podocytes treated with PAN was a recognized and effective model of podocyte injury in vitro [34]. After the well-differentiated podocytes growing to about 60% confluent, as previously described [35], DAPT of different concentrations (0, 2.5, 5,10 μM) was added to medium 1 h before PAN treatment.
qPCR
Total RNA of podocytes or kidney tissues from mice was extracted by Trizol reagent (Vazyme Biotech, Nanjing, China). cDNA was synthesized using HiScript II Q RT SuperMix (Vazyme Biotech). PCR analysis was conducted using ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech) on PCR systems (Applied Biosystems, Foster City, CA). The relative expression of genes was normalized to β-actin and calculated by the 2−ΔΔCt method. Primer sequences (Genscript Biotech, Nanjing, China) were listed in Supplemental Table 2.
Western Blot
Proteins of podocytes were lysed in RIPA buffer containing 1% phosphatase and protease inhibitors (NCM Biotech, Suzhou, China). Then, proteins were separated and transferred onto PVDF membranes. The following antibodies were applied in our study: anti-Cleaved Notch1(1:1000; Cell Signaling Technology), anti-WT1(1:500; Santa Cruz Biotechnology), anti-NLRP3(1:1000; Abcam), anti-AIM2 (1:1000; Proteintech) and anti-β-actin (1:3000; Servicebio). After that, corresponding secondary antibodies were used to incubate membranes. Target proteins were developed by ECL Enhanced Kit (ABclonal Technology, Wuhan, China) and detected with the chemiluminescence image analysis system (Tanon, Shanghai, China).
Assessment of Renal Pathology
Paraffin-embedded kidney samples were sectioned (5 μm), followed by hematoxylin and eosin (H&E) staining. Pathology of sections (glomerular, tubulointerstitial and vascular lesions) were graded as mentioned previously [36].
Immunofluorescence
Paraffin sections were dewaxed to water and antigen were retrieved using sodium citrate solution. After blocking, sections were stained with Cy3-conjugated Goat Anti-mouse IgG (1:200; Servicebio) or AF488-conjugated anti-C3 antibody (1:100; Santa Cruz Biotechnology), respectively. Other sections were stained with anti-NICD1(1:200), anti-WT1(1:100) and anti-NLRP3 (1:200; ABclonal Technology) antibodies, followed by incubation with fluorescent secondary antibodies. After staining, the sections were photographed using Olympus BX53 microscope and Olympus FV3000 microscope (Tokyo, Japan). The quantification of staining was conducted as mentioned previously [37].
ELISA Assays for IgG and Anti-dsDNA Antibody
The ELISA kit for detection of mouse IgG and anti-dsDNA antibody was purchased from Multisciences (Hangzhou, China) and FUJIFILM Wako Pure Chemical Corporation (Japan), respectively. The serum IgG and anti-dsDNA antibody was detected according to the manufacturer’s instructions.
Measurement of Creatinine and Proteinuria
The serum creatinine was measured by the Creatinine Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China). The urine protein was detected by BCA protein quantification assay kit (Thermo Fisher Scientific).
Assessment of Podocyte Injury
The kidney tissues from mice were fixed in Special Fixative for Immunoelectron Microscopy (Servicebio) at room temperature for 2 h, then kept at 4 °C for 24 h. Samples for transmission electron microscopy were produced and photographed to assess podocyte injury.
Statistical Analysis
Bioinformatics analysis was performed in R software. The data were all expressed as mean ± SD. We analyzed the data with unpaired Student’s t test to determine the difference between two groups, or one-way ANOVA for more than two groups using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). A P value less than 0.05 was regarded as statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.0001).
RESULTS
Notch1 Pathway Was Overactivated in Kidneys of LN Patients and MRL/Lpr Mice
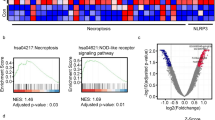
All samples in GSE32591 were firstly analyzed to investigate the expression of Notch1 pathway in kidneys of LN patients. The results of combing volcano map and GSEA revealed that, compared with healthy donors, Notch1 pathway was overactivated in kidneys of LN patients (Supplemental Fig. 1A-B).
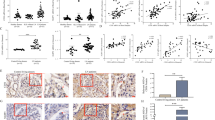
Then, expression data from glomerular samples and tubulointerstitial samples in GSE32591 were analyzed respectively to further localize the activation of Notch1 pathway in kidneys. Combining the results of the volcano map (Fig. 1a) and the results of GSEA (Fig. 1b), showed that we found that Notch1 pathway was overactivated and expression of inflammasome-related genes was significantly up-regulated (NLRP3, IL18, etc.) in glomeruli of LN patients. However, differentially expressed genes between two groups could not be enriched for Notch1 pathway in renal tubules of LN patients (Supplemental Fig. 2A-B).
NICD1 expression was increased in podocytes of LN patients and MRL/lpr mice. a Volcanic map of differentially expressed genes in glomerular samples between LN patients and healthy controls in GSE32591. Red represents up-regulated expression, blue represents down-regulated expression, and gray represents no statistically significant differences. b GSEA results of expression data of glomerular samples in GSE32591. c Representative immunofluorescence staining (×40 magnification) for NICD1 and WT1 in kidney sections of LN patients and the control group, LN patients: N = 5, control group: N = 3. d Representative immunofluorescence staining (×40 magnification) for NICD1 and WT1 in kidneys of MRL/lpr mice and B6 mice, n = 4/group. The scale bar was 10 μm. The double immunofluorescence staining for NICD1 and WT1 was marked with red arrows.
Moreover, results of immunofluorescence assay showed that, compared with the control group, the level of WT1 expression was decreased, while NICD1 expression was increased in the glomeruli of LN patients. Double immunofluorescence staining of NICD1 and WT1 indicated that NICD1 was co-localized with podocytes in glomeruli (Fig. 1c). Similar to human samples, the level of NICD1 expression was also increased and co-localized with podocytes in MRL/lpr mice (Fig. 1d). These findings implied that the overactivation of Notch1 pathway in podocytes may play a potential role in pathogenesis of podocyte injury in LN.
Notch1 Pathway Mediated NLRP3 Inflammasome Activation and Promoted Podocyte Injury In Vitro
To establish the model of podocyte injury in vitro, podocytes were treated with PAN of different doses for different hours. Gene expression of Notch1 was dose-dependently increased in podocytes treated with PAN (0, 10, 50, 100 μg/ml) for 24 h (Fig. 2a). Gene expressions of Hes1 and Synaptopodin were significantly decreased in podocytes treated with PAN at the doses of 50 μg/ml and 100 μg/ml (Fig. 2a). Then, podocytes were treated with PAN at the concentration of 50 μg/ml for different hours (0, 24, 48, 72 h). A large number of podocytes were dead when treated with PAN for 72 h. Thus, 72 h was not taken into account in this study. We found that the increased expression of Notch1 gene and decreased expression of Synaptopodin gene in podocytes treated with PAN was time-dependent. Gene expression of Hes1 was significantly increased in podocytes treated with PAN for 24 h and 48 h (Fig. 2b). According to these results, we selected podocytes treated with 50 μg/ml PAN for 48 h as a model of podocyte injury in vitro.
Notch1 mediated NLRP3 inflammasome activation promoted podocyte injury in vitro. a Gene expressions of Notch1, Hes1 and Synaptopodin of podocytes treated with PAN at different concentrations for 24 h. n = 3 b gene expressions of Notch1, Hes1 and Synaptopodin of podocytes treated with PAN at the concentration of 50 μg/ml for different hours. N = 3 c-d expressions of inflammasome-related genes in injured podocytes. N = 3 e representative images of western blot and relative quantification of protein levels. N = 3. Data were shown as mean ± SD. ns: no significant, *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether inflammasome activation was involved in podocyte injury, mRNA expression of inflammasome-related genes was analyzed. Compared with normal podocytes, expressions of inflammasome-related genes (Asc, Il-1β, Caspase1) (Fig. 2c), and several inflammasomes (Nlrp1, Nlrp3, Nlrc4, Aim2) were significantly increased in injured podocytes (Fig. 2d). NLRP3 and AIM2 were the two most relevant inflammasomes to podocyte injury.
Then, podocytes with PAN treatment were given DAPT of different doses to investigate the regulatory effect of Notch1 pathway on activation of inflammasomes. NICD1 and NLRP3 was down-regulated in a dose-dependent manner in injured podocytes treated with DAPT (0, 2.5, 5, 10 μM) for 48 h, while WT1 was significantly up-regulated with 5 μM DAPT treatment. However, there was no significant difference in AIM2 among the four groups (Fig. 2e). Together, these data indicated that the blockade of Notch1 pathway improved podocyte injury and inhibited the activation of NLRP3 inflammasome in injured podocytes in vitro.
Blockade of Notch1 Pathway Alleviated Symptoms and Decreased Autoimmunity in MRL/Lpr Mice
To further clarify the role of Notch1 pathway in pathogenesis of LN in vivo, DAPT were applied as treatment for MRL/lpr mice. We found that splenomegaly and lymph node enlargement were alleviated (Fig. 3a-b), serum creatinine (Fig. 3c) and urine protein (Fig. 3d) were reduced in DAPT-treated mice. As shown in the Fig. 3e-f, the titer of serum IgG and anti-dsDNA antibodies of treatment group were significantly lower than that of the DMSO group. Gene expressions of Notch1 and Hes1 were decreased in kidney tissues of MRL/lpr mice after DAPT treatment (Fig. 3g-h). These results implied that inhibition of Notch1 pathway could ameliorate lupus-like symptoms of mice.
Blockade of Notch1 pathway alleviated symptoms and decreased autoimmunity in MRL/lpr mice. a-b representative pictures of spleen and left peri-renal lymph node from DMSO-treated and DAPT-treated mice, n = 4. c-d serum creatinine (c) and urine protein (d) were quantified in both groups, n = 4. e-f Total IgG (e) and anti-dsDNA antibody (f) in serum of two groups, n = 4. g-h Gene expressions of Notch1 (g) and Hes1 (h) of kidneys in DMSO-treated and DAPT-treated MRL/lpr mice, n = 4. Data were shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Blockade of Notch1 Pathway Attenuated Renal Injury in MRL/Lpr Mice
To investigate the therapeutic effect of DAPT on kidneys in LN, the extent of renal injury was next assessed. Mesangial proliferation, subendothelial immune complex deposition and lymphocyte infiltration could be seen in the glomeruli of DMSO-treated mice (Fig. 4a). Loss of epithelial cells, interstitial inflammatory infiltration and dilation of renal tubules could be seen in the tubules (Fig. 4b) of DMSO-treated mice, while the tubules of DAPT-treated mice showed only mild pathological changes. Around the blood vessels, less infiltration of inflammatory cells was shown in DAPT treatment group (Fig. 4c). The histological score of the kidneys was decreased with the application of DAPT. DAPT treatment could also reduce the deposition of immune complexes and the complement 3 in kidneys of MRL/lpr mice (Fig. 4d-e). Compared with DMSO-treated mice, expressions of renal injury-related genes (Tgfβ, Kim-1, Fn, Mindin and Col-1) were found to be significantly decreased in kidneys of DAPT-treated mice, while Itgb1 expression showed an upregulating tendency (P = 0.1045) (Fig. 4f). Therefore, our results indicated the pathogenic effects of overactivated Notch1 pathway in kidneys of LN.
Blockade of Notch1 pathway attenuated renal injury in MRL/lpr mice. a-b representative images of H&E staining for glomeruli (a) and renal tubules (b) in kidneys from mice, n = 4. The scale bar was 30 μm. The pictures below (20×) were representative areas framed in the pictures above at higher magnification (40×). Proliferation of mesangial cells (black arrows), subendothelial immune complex deposition (white arrows) and lymphocyte infiltration (yellow arrows) were marked in glomeruli. Loss of epithelial cells (black arrows) and dilation of renal tubules (red arrows) were marked in renal tubules. c Representative images of H&E staining for perivascular areas (×20 magnification), n = 4. The scale bar was 30 μm. Lymphocyte infiltration was marked with black arrows. d-e representative images of immunofluorescence staining (×20 magnification) for mouse IgG (d) and C3 e in kidneys from mice, n = 4. The scale bar was 50 μm. f Expressions of renal injury-related genes in kidney tissues of mice, n = 4. Data were shown as mean ± SD. ns: no significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Blockade of Notch1 Pathway Mitigated Podocyte Injury in MRL/Lpr Mice
Subsequently, severity of podocyte injury was compared between two groups to study the role of Notch1 pathway in podocyte injury in LN. Compared with DMSO-treated mice, gene expressions of markers of podocytes (Synaptopodin, Cd2ap, Nephrin, Podocin, Podocalyxin) were significantly increased in kidneys of DAPT-treated mice, while Wt1 expression showed an increasing tendency (P = 0.1725) (Fig. 5a). Moreover, the results of transmission electron microscopy showed that podocyte injury was markedly improved in DAPT-treated mice (Fig. 5b). Taken together, blockade of Notch1 pathway was proved to have a therapeutic effect on podocyte injury in LN in vivo, which indicating overactivation of Notch1 pathway was involved in the pathogenesis of podocyte injury in LN.
Blockade of Notch1 pathway attenuated podocyte injury in MRL/lpr mice. a Gene expressions of markers of podocytes in kidney tissues of mice, n = 4. b Representative images taken by transmission electron microscopy, n = 4. The pictures below (20×) were representative areas framed in the pictures above at higher magnification (60×). Foot process effacement of podocytes was marked with red arrows. Data were shown as mean ± SD. ns: no significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Blockade of Notch1 Pathway Reduced Renal Inflammation and Inhibited NLRP3 Inflammasome Activation of Podocytes
To explore the underlying pathogenic mechanisms of Notch1 pathway in podocyte injury of LN, gene expressions of proinflammatory cytokines in kidney were detected to evaluate the degree of renal inflammation. Compared with DMSO-treated mice, interleukin (Il-6) and tumor necrosis factor (Tnf)-α expressions were significantly decreased in kidneys of DAPT-treated mice, while chemokine ligand (Ccl)2 expression showed a decreasing tendency (P = 0.0893) and Il-2 expression was significantly increased (Fig. 6a).
Blockade of Notch1 pathway reduced renal inflammation and inhibited NLRP3 inflammasome activation of podocytes in MRL/lpr mice. a Gene expressions of proinflammatory cytokines in kidneys of mice, n = 4. b Gene expressions of inflammasome-related genes in kidneys of mice, n = 4. c Representative immunofluorescence staining (×40 magnification) for NLRP3 and WT1 in kidney sections of mice, n = 4. The averages of podocytes and NLRP3-positive podocytes per glomerulus were calculated in eight randomly chosen glomeruli per mouse. The ratio of NLRP3-positive podocytes to podocytes per glomerulus was analyzed. The double immunofluorescence staining for NLRP3 and WT1 was marked with white arrows. The scale bar was 10 μm. Data were shown as mean ± SD. ns: no significant, *P < 0.05, **P < 0.01, ***P < 0.001.
To verify whether Notch1 pathway could regulate the activation of inflammasomes in kidneys, expressions of inflammasome-related genes were detected. Compared with DMSO-treated mice, Caspase1, Il-1β and Nlrp3 expressions were significantly decreased in kidneys of DAPT-treated mice, while Asc expression showed a downregulating tendency (P = 0.0841). There was no significant difference in Aim2 expression of kidneys between two groups (Fig. 6b). Furthermore, immunofluorescence assay was conducted to assess the degree of NLRP3 inflammasome activation in podocytes of MRL/lpr mice. Our results showed that NLRP3 could be co-localized with podocytes (WT1). The podocyte loss showed a decreasing tendency (P = 0.2392) in DAPT-treated mice, and the NLRP3 was significantly down-regulated in podocytes of DAPT-treated mice (Fig. 6c). Together, these findings suggested that, Notch1 pathway could regulate renal inflammation and mediate the activation of NLRP3 inflammasome in podocytes of LN.
DISCUSSION
In this study, we took advantage of the large sample size in the public database. We applied GSEA to clarify the overall expression level of Notch1 pathway in different parts of the kidneys of LN patient instead of simply analyzing several gene expressions. Our results of bioinformatics analysis emphasized the pathogenic effect of continuous activation of Notch1 pathway on glomeruli in LN. Combined with experimental results, Notch1 pathway was confirmed to be overactivated in podocytes of LN patients. In addition, we also confirmed that Notch1 pathway was similarly overactivated in podocytes of MRL/lpr mice as in that of LN patients.
However, we also observed a high degree of immunofluorescence staining for NICD1 in renal tubules of LN patients. We considered that the heterogeneity of LN, the interaction between Notch receptors[38], and the disease activity of LN patients may contribute to that. The role of Notch pathway in the pathogenesis of renal interstitial fibrosis has been confirmed[39], while the specific Notch receptors and ligands involved remained to be further identified.
Then, overactivation of Notch1 pathway in podocytes was demonstrated to participate in the development of podocyte injury, which was consistent with previous studies[40]. However, the role of podocytes as immune cells seemed to be neglected when exploring the role of Notch1 pathway in the pathogenesis of podocyte injury. In this study, the increased expressions of several inflammasomes (Nlrp1, Nlrp3, Nlrc4, Aim2) in injured podocytes provided additional evidences on the role of inflammasomes in promoting podocyte injury. Considering the pathogenesis of LN and specific stimuli of different inflammasomes, we finally chose NLRP3 and AIM2 as the focus of further research. We found that the activation of Notch1 pathway could promote podocyte injury via the activation of NLRP3 inflammasome rather than AIM2 inflammasome in vitro. This was similar to previous studies reported in several diseases[22]. Apart from that, the non-canonical function of AIM2 in LN was relatively complex and mysterious[41], which required further investigation. Therefore, it was necessary to focus on its non-canonical role when exploring the function of AIM2 in podocyte injury in LN.
Transforming growth factor-β (TGF-β), fibronectin (FN) and collagen type I (COL-1) were generally considered to be the regulatory factors of renal fibrosis[42]. Increased gene expressions of Mindin and kidney injury molecule 1(Kim-1) usually reflected kidney injury at an early stage[43, 44]. With the application of DAPT, expressions of these genes in kidneys of MRL/lpr mice were significantly decreased, indicating that DAPT could alleviate renal injury in LN in vivo. However, in terms of lupus-like symptoms and renal pathology, the DAPT treatment could not recover the injured kidney back to normal (the data did not display). The reasons for that may be related to the non-targeted administration of DAPT and complexity of LN pathogenesis. β1-Integrin (ITGB1) was observed to be present in glomeruli to mediate adhesion between podocytes and the glomerular basement membranes[45]. Hence, increased gene expression of Itgb1 in kidneys of DAPT-treated MRL/lpr mice suggested that blockade of Notch1 pathway might have a potential effect on reducing loss of podocytes. Moreover, decreased gene expressions of markers of podocytes affirmed the protective effect of DAPT on podocyte injury in LN. Therefore, the sustained activation of Notch1 pathway might play an important role in the pathogenesis of podocyte injury in LN. This was consistent with the findings in other podocyte injury-associated glomerular diseases[46, 47].
Notch1 pathway was reported to have a functional role in the development of inflammation[19]. Consistently, decreased gene expressions of proinflammatory cytokines in kidneys of DAPT-treated mice hinted that, blockade of Notch1 pathway could mitigate podocyte injury through reducing renal inflammation in LN. Interestingly, since IL-2 deficiency has been reported to be associated with renal impairment in LN patients[48, 49], it was of possibility that blockade of Notch1 pathway could improve podocyte injury through regulating the production of IL-2 in LN. Nevertheless, more evidence was needed. Furthermore, the regulatory effect of Notch1 pathway on inflammasome activation (NLRP3, but not AIM2) in podocytes was also confirmed in vivo, which was consistent with the results of experiments in vitro. Given the complex crosstalk of signaling pathways in vivo, blocking the upstream pathways of inflammasome activation may a better choice of treatment for podocyte injury in LN. As demonstrated in our study, blockade of Notch1 pathway could also mediate the production of several cytokines to regulate pathological inflammation broadly.
There were still limitations in this study. Podocyte-specific conditional knockout mice as the direct evidence were lacked, and this is what we need to do in the future. Secondly, beneficial effects of DAPT on improving podocyte injury in LN could not rule out the role of DAPT on other renal inherent cells and immune cells. Additionally, although we have conducted several batches of experiments with trend-consistent results, the numbers of MRL/lpr mice in each group of every batch was not large. And it would be better if there were blank controls.
In conclusion, our study provided evidence that blockade of Notch1 pathway could regulate renal inflammation and alleviate podocyte injury via inhibiting NLRP3 inflammasome activation in LN, which confirmed the pathogenic effect of Notch1 pathway on podocytes in LN. Therefore, this study further expanded the understanding of the pathogenesis of LN, and may provide a novel idea for podocyte repair in LN.
References
Tsokos, G.C. 2011. Systemic lupus erythematosus. New England Journal of Medicine 365: 2110–2121. https://doi.org/10.1056/NEJMra1100359.
Yu, C., P. Li, X. Dang, et al. 2022. Lupus nephritis: New progress in diagnosis and treatment. Journal of Autoimmunity 132: 102871. https://doi.org/10.1016/j.jaut.2022.102871.
Blaine, J., and J. Dylewski. 2020. Regulation of the actin cytoskeleton in Podocytes. Cells 9. https://doi.org/10.3390/cells9071700.
Kriz, W. 2002. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microscopy Research and Technique 57: 189–195. https://doi.org/10.1002/jemt.10072.
Rezende, G.M., V.S. Viana, D.M.A.C. Malheiros, et al. 2014. Podocyte injury in pure membranous and proliferative lupus nephritis: Distinct underlying mechanisms of proteinuria? Lupus 23: 255–262. https://doi.org/10.1177/0961203313517152.
Kimura, J., O. Ichii, S. Otsuka, et al. 2013. Close relations between podocyte injuries and membranous proliferative glomerulonephritis in autoimmune murine models. American Journal of Nephrology 38: 27–38. https://doi.org/10.1159/000353093.
Zhou, L., Y. Li, W. He, et al. 2015. Mutual antagonism of Wilms' tumor 1 and β-catenin dictates podocyte health and disease. Journal of the American Society of Nephrology : JASN 26: 677–691. https://doi.org/10.1681/asn.2013101067.
Zhang, C., K.M. Boini, M. Xia, et al. 2012. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension (Dallas, Tex. : 1979) 60: 154–162. https://doi.org/10.1161/HYPERTENSIONAHA.111.189688.
Rathinam, V.A., and K.A. Fitzgerald. 2016. Inflammasome complexes: Emerging mechanisms and effector functions. Cell 165: 792–800. https://doi.org/10.1016/j.cell.2016.03.046.
Cellesi, F., M. Li, and M.P. Rastaldi. 2015. Podocyte injury and repair mechanisms. Current Opinion in Nephrology and Hypertension 24: 239–244. https://doi.org/10.1097/mnh.0000000000000124.
Shang, Y., S. Smith, and X. Hu. 2016. Role of notch signaling in regulating innate immunity and inflammation in health and disease. Protein & Cell 7: 159–174. https://doi.org/10.1007/s13238-016-0250-0.
Zhou, B., W. Lin, Y. Long, et al. 2022. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduction and Targeted Therapy 7: 95. https://doi.org/10.1038/s41392-022-00934-y.
Cheng, H.-T., and R. Kopan. 2005. The role of notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney International 68: 1951–1952.
Vooijs, M., C.-T. Ong, B. Hadland, et al. 2007. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 134: 535–544. https://doi.org/10.1242/dev.02733.
Waters, A.M., M.Y.J. Wu, T. Onay, et al. 2008. Ectopic notch activation in developing podocytes causes glomerulosclerosis. Journal of the American Society of Nephrology : JASN 19: 1139–1157. https://doi.org/10.1681/ASN.2007050596.
Liu, M., K. Liang, J. Zhen, et al. 2017. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting notch signaling. Nature Communications 8: 413. https://doi.org/10.1038/s41467-017-00498-4.
Guo, A., Y. Sun, X. Xu, et al. 2022. MicroRNA-30a targets Notch1 to alleviate Podocyte injury in lupus nephritis. Immunological Investigations 51: 1694–1706. https://doi.org/10.1080/08820139.2022.2027440.
Li, L.S., and Z.H. Liu. 2004. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney International 66: 920–923. https://doi.org/10.1111/j.1523-1755.2004.00837.x.
Quillard, T., and B. Charreau. 2013. Impact of notch signaling on inflammatory responses in cardiovascular disorders. International Journal of Molecular Sciences 14: 6863–6888. https://doi.org/10.3390/ijms14046863.
Outtz, H.H., J.K. Wu, X. Wang, et al. 2010. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. Journal of Immunology 185: 4363–4373. https://doi.org/10.4049/jimmunol.1000720.
Jin, Y., C. Li, D. Xu, et al. 2020. Jagged1-mediated myeloid Notch1 signaling activates HSF1/snail and controls NLRP3 inflammasome activation in liver inflammatory injury. Cellular & Molecular Immunology 17: 1245–1256. https://doi.org/10.1038/s41423-019-0318-x.
Lee, S., S.K. Kim, H. Park, et al. 2020. Contribution of autophagy-Notch1-mediated NLRP3 Inflammasome activation to chronic inflammation and fibrosis in keloid fibroblasts. International Journal of Molecular Sciences 21. https://doi.org/10.3390/ijms21218050.
Hochberg, M.C. 1997. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism 40: 1725. https://doi.org/10.1002/art.1780400928.
Bajema, I.M., S. Wilhelmus, C.E. Alpers, et al. 2018. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney International 93: 789–796. https://doi.org/10.1016/j.kint.2017.11.023.
Andrews, B.S., R.A. Eisenberg, A.N. Theofilopoulos, et al. 1978. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. The Journal of Experimental Medicine 148: 1198–1215. https://doi.org/10.1084/jem.148.5.1198.
Ran, Y., F. Hossain, A. Pannuti, et al. 2017. γ-Secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Molecular Medicine 9: 950–966. https://doi.org/10.15252/emmm.201607265.
Teachey, D.T., A.E. Seif, V.I. Brown, et al. 2008. Targeting notch signaling in autoimmune and lymphoproliferative disease. Blood 111: 705–714. https://doi.org/10.1182/blood-2007-05-087353.
Zhang, W., W. Xu, and S. Xiong. 2010. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. Journal of Immunology 184: 6465–6478. https://doi.org/10.4049/jimmunol.0904016.
Jiao, Z., W. Wang, S. Hua, et al. 2014. Blockade of notch signaling ameliorates murine collagen-induced arthritis via suppressing Th1 and Th17 cell responses. The American Journal of Pathology 184: 1085–1093. https://doi.org/10.1016/j.ajpath.2013.12.010.
Galvao, J., B. Davis, M. Tilley, et al. 2014. Unexpected low-dose toxicity of the universal solvent DMSO. The FASEB Journal 28: 1317–1330. https://doi.org/10.1096/fj.13-235440.
Caren, L.D., H.M. Oven, and A.D. Mandel. 1985. Dimethyl sulfoxide: Lack of suppression of the humoral immune response in mice. Toxicology Letters 26: 193–197. https://doi.org/10.1016/0378-4274(85)90166-3.
Morton, J.I., B.V. Siegel, W.J. Weaver, et al. 1983. The effects of chronic DMSO administration on the spontaneous development of autoimmune disease in NZB, BXSB, and MRL/lpr strain mice. Annals of the New York Academy of Sciences 411: 344–346. https://doi.org/10.1111/j.1749-6632.1983.tb47321.x.
Zhang, Z., L. Niu, X. Tang, et al. 2019. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrology, Dialysis, Transplantation 34: 597–605. https://doi.org/10.1093/ndt/gfy195.
Inokuchi, S., I. Shirato, N. Kobayashi, et al. 1996. Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney International 50: 1278–1287. https://doi.org/10.1038/ki.1996.439.
Nishad, R., D. Mukhi, S.V. Tahaseen, et al. 2019. Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. The Journal of Biological Chemistry 294: 16109–16122. https://doi.org/10.1074/jbc.RA119.008966.
Tao, X., F. Fan, V. Hoffmann, et al. 2006. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii hook F on nephritis in NZB/W F1 mice. Arthritis Research & Therapy 8: R24. https://doi.org/10.1186/ar1879.
Fu, Y., Y. Sun, M. Wang, et al. 2020. Elevation of JAML promotes diabetic kidney disease by modulating Podocyte lipid metabolism. Cell Metabolism 32: 1052–1062.e1058. https://doi.org/10.1016/j.cmet.2020.10.019.
Djudjaj, S., C. Chatziantoniou, U. Raffetseder, et al. 2012. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. The Journal of Pathology 228: 286–299. https://doi.org/10.1002/path.4076.
Bielesz, B., Y. Sirin, H. Si, et al. 2010. Epithelial notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. The Journal of Clinical Investigation 120: 4040–4054. https://doi.org/10.1172/jci43025.
Niranjan, T., B. Bielesz, A. Gruenwald, et al. 2008. The notch pathway in podocytes plays a role in the development of glomerular disease. Nature Medicine 14: 290–298. https://doi.org/10.1038/nm1731.
Yang, M., D. Long, L. Hu, et al. 2021. AIM2 deficiency in B cells ameliorates systemic lupus erythematosus by regulating Blimp-1-Bcl-6 axis-mediated B-cell differentiation. Signal Transduction and Targeted Therapy 6: 341. https://doi.org/10.1038/s41392-021-00725-x.
Wu, Y., J. Luan, C. Jiao, et al. 2021. circHIPK3 exacerbates folic acid-induced renal Tubulointerstitial fibrosis by sponging miR-30a. Frontiers in Physiology 12: 715567. https://doi.org/10.3389/fphys.2021.715567.
Bai, T., X. Wang, C. Qin, et al. 2022. Deficiency of mindin reduces renal injury after ischemia reperfusion. Molecular Medicine (Cambridge, Mass.) 28: 152. https://doi.org/10.1186/s10020-022-00578-2.
Tanase, D.M., E.M. Gosav, S. Radu, et al. 2019. The predictive role of the biomarker kidney Molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. International Journal of Molecular Sciences 20. https://doi.org/10.3390/ijms20205238.
Baraldi, A., G. Zambruno, L. Furci, et al. 1994. Beta-1 integrins in the normal human glomerular capillary wall: An immunoelectron microscopy study. Nephron 66: 295–301. https://doi.org/10.1159/000187826.
Li, M.R., C.T. Lei, H. Tang, et al. 2022. MAD2B promotes podocyte injury through regulating numb-dependent notch 1 pathway in diabetic nephropathy. International Journal of Biological Sciences 18: 1896–1911. https://doi.org/10.7150/ijbs.68977.
Dou, Y., Y. Shang, Y. Shen, et al. 2020. Baicalin alleviates adriamycin-induced focal segmental glomerulosclerosis and proteinuria by inhibiting the Notch1-snail axis mediated podocyte EMT. Life Sciences 257: 118010. https://doi.org/10.1016/j.lfs.2020.118010.
von Spee-Mayer, C., E. Siegert, D. Abdirama, et al. 2016. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Annals of the Rheumatic Diseases 75: 1407–1415. https://doi.org/10.1136/annrheumdis-2015-207776.
Shao, M., J. He, R. Zhang, et al. 2019. Interleukin-2 deficiency associated with renal impairment in systemic lupus erythematosus. Journal of Interferon & Cytokine Research 39: 117–124. https://doi.org/10.1089/jir.2018.0016.
Acknowledgements
We thank Dr. Jiaheng Xie, for his kind suggestion in the revision of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2020YFA0710800), the Key Program of National Natural Science Foundation of China (81930043), the Jiangsu Provincial Key Research and Development Program (BE2020621) and the National Science Foundation of Jiangsu Province (BK20210016).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. WD, YGH and SLY designed experiments. WD and JTT carried out experiments. Data collection and analysis were performed by WD, ZSY, HMX, ZY and CL. The first draft of the manuscript was written by WD. All authors commented on previous versions of the manuscript. ZYY, ZDD and YHH performed the language modifying. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki, and approved by the Ethics Committee of Nanjing Drum Tower Hospital. This study followed the NIH Guide for the Care and Use of Laboratory Animals. All animal experimental protocols were approved by the Ethics Committee of Nanjing Drum Tower Hospital.
Consent to Participate
Written informed consent was obtained from the parents.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 271 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, D., Jiang, T., Zhang, S. et al. Blockade of Notch1 Signaling Alleviated Podocyte Injury in Lupus Nephritis Via Inhibition of NLRP3 Inflammasome Activation. Inflammation 47, 649–663 (2024). https://doi.org/10.1007/s10753-023-01935-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01935-x