Abstract
Diabetic nephropathy (DN), a serious complication of hyperglycemia, is one of the most common causes of end-stage renal disease (ESRD). Glomerular podocyte injury is a major mechanism that leads to DN. However, the mechanisms underlying podocyte injury are ambiguous. In this study, we sought to investigate the contribution of SET domain-containing protein 6 (SETD6) to the pathogenesis of podocyte injury induced by glucose (GLU) and palmitic acid (PA), as well as the underlying mechanisms. Our results showed that GLU and PA treatment significantly decreased SETD6 expression in mouse podocytes. Besides, Cell Counting Kit-8 (CCK-8) and flow cytometry assay demonstrated that silencing of SETD6 silence obviously enhanced cell viability, and suppressed apoptosis in GLU and PA-induced podocytes. We also discovered that downregulation of SETD6 suppressed GLU and PA-induced ROS generation and podocyte mitochondrial dysfunction. Nrf2-Keap1 signaling pathway was involved in the effect of SETD6 on mitochondrial dysfunction. Taken together, silencing of SETD6 protected mouse podocyte against apoptosis and mitochondrial dysfunction through activating Nrf2-Keap1 signaling pathway. Therefore these data provide new insights into new potential therapeutic targets for DN treatment.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is one of the major chronic complications of diabetes (Cao and Cooper 2011). It is widely acknowledged that DN is the leading cause of end-stage renal disease (ESRD) (Bommer 2001). Hyperglycemia and hyperlipidemia can cause glomerular basement membrane thickening, mesangial expansion, and extracellular matrix hyperplasia in DN patients, further leading to glomerular hyperfiltration, proteinuria, and finally progressing to ESRD (Ahmad 2015). Podocytes are terminally differentiated glomerular visceral epithelial cells that maintains the integrity of the glomerular filtration barrier (GFB) and prevents urinary protein loss (Belinda et al. 2012). Glucose (GLU) and palmitic acid (PA)-induced podocyte injury, such as podocyte apoptosis and mitochondrial dysfunction, has been demonstrated to intimately associated with GFB destruction and proteinuria in DN (Michele et al. 2003; Katalin et al. 2006; Siu et al. 2006). Thus, Searching the target to preventing podocyte injury will provide a novel target for DN therapy.
SET domain-containing protein 6 (SETD6), an important member of the lysine methyltransferase family (Chang et al. 2011), was recently identified as an important regulator of multiple signaling pathways through methylating protein substrates (Vershinin et al. 2016; Mukherjee et al. 2017). On the other hand, it can also interact with nuclear receptor signaling factors and transcriptional regulators to stimulate transcriptional activity, affecting cell proliferation, inflammation, and oxidative stress response (Chen et al. 2016; Binda et al. 2013). Notably, several studies demonstrated that SETD protein was abnormally expressed in mice with high-carbonhydrate diet (Villeneuve et al. 2010), which was closely related to occurrence and development of diabetes (Adamska-Patruno et al. 2019; Korsmo-Haugen et al. 2019). Nevertheless, there is no related studies focused on the role of SETD6 in DN.
Nuclear factor-erythroid-2-related factor 2 (Nrf2) is universally involved in diabetic renal injury (Cheng et al. 2019), mainly expressed in mesangial cell (Lin et al. 2006), renal tubular epithelial cell (Du et al. 2018), and podocyte injury (Wang et al. 2011, 2014). These cell damage can further cause glomerular sclerosis and renal interstitial fibrosis, which were the pathological features of DN (Bose et al. 2017). Aberrant expression of Nrf2 was found in mice with diabetic retinopathy (Xu et al. 2014). Induction of Nrf2 enhanced the antioxidant response during mitochondrial stress (Shih et al. 2005). Recently, Nrf2 was revealed to be regulated by SETD6, and enhance the expression of antioxidant genes under oxidative stress (Chen et al. 2016). In our research, we investigated the function of SETD6 in GLU and PA-induced podocyte apoptosis and mitochondrial dysfunction. And we further explored whether Nrf2-Keap1 pathway was involved in the mediation of GLU and PA-induced podocyte injury. Our findings are expected to provide some theoretical basis to search for a new solution for the DN treatment.
Materials and methods
Cell culture and treatments
High glucose and high fatty acids was widely used to mimic a pathological state of early podocyte injury in human DN (Sanjay et al. 2011; Hou-Yong et al. 2011; Wang et al. 2019), and the injury of mouse podocytes was induced by GLU and PA in this study. Mouse podocytes,which were previously reported to express podocytes-specific markers(Liu et al. 2018), were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). Briefly, podocytes were incubated at humidified incubator (33 °C, 5% CO2) in RPMI-1640 (Sigma, St. Louis, MO, USA) medium which consisted of 10% Fetal bovine serum (FBS, Gibco, Rockville, MD, USA), 100 U/mL penicillin, 100 µg/mL streptomycin and 1× insulin transferrin-selenium (ITS) (Sigma, St. Louis, MO, USA). When podocytes reached 70–80% confluence, the cells were induced to differentiate using ITS-free medium for 10–14 days. Then the podocytes were treated with normal-gulcose(5.5 mmol/L) or high-glucose (30 mmol/L, GLU) (Ha et al. 2013) and different concentrations of palmitic acid (50, 100, 200, 300 and 400 µmol/L, PA) for various periods of time (12, 24, and 48 h).
Plasmids
Plasmids used for suppression in podocytes were SETD6 siRNA (si-SETD6). The siRNAs with sequences that do not target any gene productwas a negative control (Vector, 1 µg/mL). The si-SETD6 (1 µg/mL) was transfected into immortalized mouse podocytes (6-well plate, 1.0 × 106/cm2). After transfection for 24 h, the podocytes were treated with 30 mmol/L GLU and 200 µmol/L PA for 24 h.
Western blot analysis
At the end of experiments, podocytes were lysed on ice for 15 min in RIPA lysis buffer (Sigma, St. Louis, MO, USA). The supernatants were collected after centrifugation at 12,000×g at 4 °C for 20 min. Total protein concentration from podocyte lysate was detected by BCA protein concentration detection kit (Solarbio, Beijing, China). Then total protein was separated by 8% SDS polyacrylamide gel electrophoresis and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% zero fat milk powder and incubated with corresponding target protein antibodies and rabbit anti-β-actin monoclonal antibody. Then, the membranes were incubated with a goat anti-rabbit HRP antibody (Sigma, St. Louis, MO, USA). The protein complexes were detected by the enhanced chemiluminescence reagents (Abcam, Cambridge, UK). Primary antibodies used were as Table 1.
CCK-8 proliferation vitality assay
Mouse podocyte proliferation was measured using the Cell Counting Kit 8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). The cell viability was measured at specified time points using microplate reader (Thermo Fisher Scientific, Massachusetts, USA) by spectrophotometry at 450 nm.
Apoptosis assay
Mouse podocyte apoptosis was detected using the Cell Apoptosis Detection Kit (Qiagen, Valencia, CA, USA). After 24 h cultured with 30 mmol/L GLU and 200 µmol/L PA, si-SETD6-infected podocytes were incubated with 10 µL FITC Annexin-V and propidiumiodide (Becton, Heidelberg, Germany) at room temperature for 15 min. Then, flow cytometry was performed according to the manufacturer’s instructions.
Mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) measurement
The MMP of podocytes was monitored using Tetrechloro-tetraethylbenzimidazol carbocyanine iodide (JC-1, Beyotime Biotechnology, Shanghai, China). And the generation of ROS in podocytes was evaluated by 2′,7′-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe. Briefly, podocytes were washed twice with PBS, and incubated in the dark with JC-1 (2 µmol/L, 30 min at 37 °C) or DCFH-DA (10 µM, 20 min at 37 °C). Then, the cells were fixed using 4% polyformaldehyde (Beyotime Biotechnology, Shanghai, China) for 10 min. JC-1 (590/520 nm) and DCFH-DA (520 nm) fluorescence levels were detected by confocal microscopy and flow cytometry.
Statistical analysis
The data were represented as means ± SEM (standard error of mean) and each experiment was performed in triplicate in this study. One-way ANOVA (analysis of variance) and Student’s unpaired t test were used to analyze statistical significance. p value < 0.05 were considered statistically significant.
Results
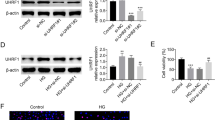
SETD6 was downregulated in GLU and PA-induced mouse podocytes
Podocytes were treated with GLU (30 mmol/L) and PA (50, 100, 200, 300 and 400 µmol/L) to establish a podocyte injury model. CCK-8 assay evidenced that GLU and PA decreased the viability of podocytes in a dose-dependent and time-dependent manner (Fig. 1a–c). What’s more, the result showed that SETD6 protein level was reduced by GLU and PA treatment (Fig. 1d).
SETD6 was downregulated in GLU and PA-induced mouse podocytes. Mouse podocytes were treated with 30 mmol/L glucose (GLU) and different concentrations of palmitic acid (50, 100, 200, 300 and 400 µmol/L, PA) for the indicated time points (12 h, 24 or 48 h). a–c The vibility of podocytes was measured by CCK-8 assay. *p < 0.05 (vs. Control). d Western blot analysis was used to detect the SETD6 expression in podocytes treated with 30 mmol/L GLU and 200 µmol/L PA for 24 h. β-actin was used as an internal control. *p < 0.05 (vs. 0 h). *#p < 0.05 (vs. 12 h). The columns were presented as the mean ± SEM (n ≥ 03)
Downregulation of SETD6 inhibited GLU and PA-induced podocyte apoptosis
To investigate the effect of SETD6 on the cell growth in GLU and PA-induced podocytes, podocytes were transfected with SETD6 siRNA (si-SETD6) or negative control (Vector) for 24 h and then the cells were treated with or without 30 mmol/L GLU and 200 µmol/L PA for 24 h. Our Western blotting results displayed the efficiency of si-SETD6 (Fig. 2a, b). As expected, CCK-8 and flow cytometry analysis respectively demonstrated that transfection of si-SETD6 obviously enhanced cell viability and suppressed apoptosis of podocytes (Fig. 2c, d). Meanwhile, the results evidenced that SETD6 silence significantly reduced caspase-3 and Bax (Bcl-2 Assaciated X protein) expression and enhanced BCL-2 (B-cell lymphoma-2) expression in GLU and PA-induced podocytes (Fig. 2e–h).
Down-regulation of SETD6 inhibited GLU and PA-induced podocyte apoptosis. Mouse podocytes (1.0 × 106/cm2) were transfected with SETD6 siRNA (si-SETD6, 1 µg/mL) or negative control (Vector, 1 µg/mL) for 24 h and then the cells were treated with or without 30 mmol/L GLU and 200 µmol/L PA for 24 h. a and b Western blot analysis of SETD6 expression in podocytes. c The viability of podocytes was evaluated by CCK-8 assay. d The apoptosis of podocytes was detected by flow cytometry analysis. e–h The expression of caspase-3, Bax, and BCL2 were assayed by Western blot analysis. β-actin was used as an internal control. *p < 0.05 (vs. vector). #p < 0.05 (vs. vector + GLU + PA). The columns were presented as the mean ± SEM (n ≥ 03)
Downregulation of SETD6 suppressed GLU and PA-induced ROS generation and podocyte mitochondrial dysfunction
Several studies have indicated podocyte apoptosis usually manifests mitochondrial dysfunction and abundant generation of ROS (reactive oxygen species) (Herrera et al. 2001; Fan et al. 2019). Thus we then investigate the effect of SETD6 on mitochondrial dysfunction in GLU and PA-induced podocyte. As shown in Fig. 3a, the level of MMP was reduced in the presence of GLU and PA, which was strongly reversed by SETD6 downregulation. Strikingly, our findings also revealed that silencing of SETD6 markedly decreased ROS generation (Fig. 3b). Consistent with this result, SETD6 deletion significantly facilitated the expression of SOD1/2, GPX1, and GCLC in GLU and PA-induced podocytes (Fig. 3c–g).
Down-regulation of SETD6 suppressed GLU and PA-induced podocyte mitochondrial dysfunction and ROS generation. Mouse podocytes (1.0 × 106/cm2) were transfected with SETD6 siRNA (si-SETD6, 1 µg/mL) or negative control (Vector, 1 µg/mL) for 24 h and then the cells were treated with or not 30 mmol/L GLU and 200 µmol/L PA for 24 h. a Manifests mitochondrial membrane potential was measured by JC-1 using fluorescence microscope (original magnification, ×400). b Endogenous ROS content was assayed via DCFH-DA fluorescent probe. c–g Western blot analysis of the SOD1/2, GPX1 and GCLC expression in podocytes. β-actin was used as an internal control. *p < 0.05 (vs. vector). #p < 0.05 (vs. vector + GLU + PA). The columns were presented as the mean ± SEM (n ≥ 03).
Downregulation of SETD6 protected against podocyte apoptosis and mitochondrial dysfunction through activating Nrf2-Keap1 signaling pathway
Considering that Nrf2 signaling alleviates podocyte injury (Shen et al. 2019), and SETD6 has a negative effect on Nrf2 expression (Chen et al. 2016). We wonder that Nrf2 signaling pathway maybe participate in the mediation of SETD6-induced podocyte injury. The results showed that GLU and PA treatment decreased the expression of Nrf2, and increased the expression of Keap-1, which were inversed by silencing of SETD6 (Fig. 4a–c). Then we study the effect of Nrf2 signaling pathway blockage on podocyte apoptosis and mitochondrial dysfunction. The deactivating of Nrf2-Keap1 pathway was established by brusatol (Selleck, USA) treatment. Strikingly, it was observed that Nrf2 blockage strongly attenuated the protection effect of SETD6 on GLU and PA-induced podocyte apoptosis (Fig. 4d, e). At the same time, ROS reduction and MMP elevation induced by SETD6 downregulation were damaged through Nrf2 inhibitor co-treatment (Fig. 4f, g).
SETD6 silence protected against podocyte apoptosis and mitochondrial dysfunction through activating Nrf2-Keap1 pathway. Mouse podocytes (1.0 × 106/cm2) were transfected with SETD6 siRNA (si-SETD6, 1 µg/mL) or negative control (Vector, 1 µg/mL) or treated with Nrf2 inhibitor brusatol (10 µM) for 24 h. And then the cells were treated with or not 30 mmol/L GLU and 200 µmol/L PA for 24 h. a–c Western blot analysis of the Nrf2 and Keap1 expression in podocytes. β-actin was used as an internal control. d The vibility of podocytes was measured by CCK-8 assay. e The apoptosis of podocytes was detected by flow cytometry analysis. f Manifests mitochondrial membrane potential was measured by JC-1 fluorescent probe. g Endogenous ROS content was assayed via DCFH-DA fluorescent probe. *p < 0.05 (vs. vector). #p < 0.05 (vs. vector + GLU + PA). &p < 0.05 (vs. si-SETD6 + GLU + PA). The columns were presented as the mean ± SEM (n ≥ 03)
Discussion
Podocytes serve as a pivotal role in the DN progression and the development of proteinuria (Mundel and Reiser 2010). Both basic and clinical findings indicated that DN patients presented with long-term hyperglycemia (Tagawa et al. 2016). High glucose resulted in glomerular podocyte injury, and the apoptosis and detachment of podocytes eventually led to the appearance of proteinuria (Khaled et al. 2012; Eid et al. 2009; Choel et al. 2009). Reducing or blocking the apoptosis of podocytes that involved in the DN development (Zhang et al. 2019) is an effective treatment strategy for DN. In this study, we reported that SETD6 was significantly downregulated in podocytes treated with GLU and PA treatment, and down-regulation of SETD6 rescued the podocyte apoptosis.
SETD6, a lysine methyltransferase, express a high level in liver and kidney (Chen et al. 2015). Previous studies report that SETD6 was a negative regulator of antioxidant stress response (Chen et al. 2016), and dysfunction of redox homeostasis may lead to mitochondria damage. Our further study demonstrated that SETD6 prevents podocytes against mitochondrial dysfunction by inhibiting the accumulation of ROS. As a critical factor in apoptosis, Mitochondrial oxidative stress and ROS are increased in HG-treated podocytes(Qiao et al. 2018). Maintaining the balance of MMP and suppressing intracellular ROS production is beneficial to prevent podocyte against apoptosis, and thus have protective effects on DN (Fan et al. 2019; Cai et al. 2016). Thus SETD6 may protect podocytes against apoptosis via mediating mitochondrial dysfunction.
SETD proteins usually function as a methyltransferase to monomethylated H2AZ at lysine 7 (H2AZK7me1) and finally maintained embryonic stem cell self-renewal (Binda et al. 2013). Similarly, inhibition of SET domain–containing lysine methyltransferase 7/9, two other members of the lysine methyltransferase family, suppressed methylation of lysine 4 of histone H3 (H3K4) and finally ameliorated renal fibrosis (Sasaki et al. 2016). SETD6 also methylated the lysine at position 310 of RelA (p65) protein, which restrained p65 phosphorylation. Recently, Nrf2 was revealed to be regulated by SETD6, and SETD6 down-regulation increased the Nrf2 protein expression (Chen et al. 2016). Consistent with previous reports, we uncovered novel findings that the Nrf2-Keap1 pathway were activated by GLU and PA in podocytes. The Nrf2-Keap1 pathway plays a vital role in regulation of nephron development, glomerular podocyte motility, adhesion, as well as apoptosis. In normal conditions, actin binding repressor protein-Keap1 make sure that Nrf2 located in the cytoplasm. Once oxidative stress occurs, Nrf2 easily unwinds bound with Keap1, translocates into the nucleus, combine with antioxidant response genes (Palsamy and Subramanian 2011; Nakai et al. 2013). Our further study confirmed that silencing of SETD6 induced the activation of Nrf2 signaling pathway, preventing the over-accumulation of ROS and mitochondrial dysfunction, eventually inhibiting apoptosis.
In conclusion, we showed that SETD6 expression was decreased in mouse podocytes treated with GLU and PA. SETD6 silencing obviously suppressed apoptosis of GLU and PA-induced mouse podocytes. Meanwhile, down-regulation of SETD6 suppressed GLU and PA-induced mouse podocyte mitochondrial dysfunction and ROS generation. Additionally, Down-regulation of SETD6 protected against GLU and PA-induced mouse podocyte apoptosis and mitochondrial dysfunction through activating the Nrf2-Keap1 pathway.
Data availability
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
Adamska-Patruno E, Samczuk P, Ciborowski M, Godzien J, Pietrowska K, Bauer W, Gorska M, Barbas C, Kretowski A (2019) Metabolomics reveal altered postprandial lipid metabolism after a high-carbohydrate meal in men at high genetic risk of diabetes. J Nutr 149(6):915–922
Ahmad J (2015) Management of diabetic nephropathy: recent progress and future perspective. Diabetes Metab Syndr Clin Res Rev 9(4):343–358
Belinda J, Mythili G, Andi Q, Ying F, Chuang PY, Cohen HW, Maria A, Thomas DB, He JC (2012) Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS ONE 7(5):e36041
Binda O, Sevilla A, LeRoy G, Lemischka IR, Garcia BA, Richard S (2013) SETD6 monomethylates H2AZ on lysine 7 and is required for the maintenance of embryonic stem cell self-renewal. Epigenetics 8(2):177–183
Bommer J (2001) Attaining long-term survival when treating diabetic patients with ESRD by hemodialysis. Adv Ren Replace Ther 8(1):13–21
Bose M, Almas S, Prabhakar S (2017) Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med Off Publ Am Feder Clin Res 65(8):1093
Cai X, Bao L, Ren J, Li Y, Zhang Z (2016) Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1alpha axis in vitro. Food Funct 7(2):805–815
Cao Z, Cooper ME (2011) Pathogenesis of diabetic nephropathy. J Diabetes Investig 2(4):243–247
Chang Y, Levy D, Horton JR, Peng J, Zhang X, Gozani O, Cheng X (2011) Structural basis of SETD6-mediated regulation of the NF-kB network via methyl-lysine signaling. Nucleic Acids Res 39(15):6380–6389
Chen JJ, Xia XH, Wang LF, Jia YF, Nan P, Li L, Chang ZJ (2015) Identification and comparison of gonadal transcripts of testis and ovary of adult common carp Cyprinus carpio using suppression subtractive hybridization. Theriogenology 83(9):1416–1427
Chen A, Feldman M, Vershinin Z, Levy D (2016) SETD6 is a negative regulator of oxidative stress response. Biochimica et Biophysica Acta (BBA)s 1859(2):420–427
Cheng D, Gao L, Su S, Sargsyan D, Wu R, Raskin I, Kong AN (2019) Moringa isothiocyanate activates Nrf2: potential role in diabetic nephropathy. AAPS J 21(2):31
Choel LS, Hyeok S, Ji H, Ha LJ, Dong-Sub LS, Seung-Jae J, Hye KSeung, Ki K, Tae-Hyun KD, Hyun KJ (2009) Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int 76(8):838–848
Du X, Yu J, Sun X, Qu S, Zhang H, Hu M, Yang S, Zhou P (2018) Impact of epigallocatechin3gallate on expression of nuclear factor erythroid 2related factor 2 and gammaglutamyl cysteine synthetase genes in oxidative stressinduced mouse renal tubular epithelial cells. Mol Med Rep 17(6):7952–7958
Eid AA, Yves G, Fagg BM, Rita M, Barnes JL, Karen B, Abboud HE (2009) Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 58(5):1201
Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, Liang W, Ding G (2019) Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int J Biol Sci 15(3):701
Ha TS, Choi JY, Park HY, Han GD (2013) Changes of podocyte p130Cas in diabetic conditions. J Nephrol 26(5):870–876
Herrera B, Álvarez AM, Sánchez A, Fernández M, Roncero C, Benito M, Fabregat I (2001) Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor β in fetal hepatocytes. Faseb J 15(3):741
Hou-Yong D, Min Z, Lin-Li L, Ri-Ning T, Kun-Ling M, Dan L, Min W, Bi-Cheng L (2011) The roles of connective tissue growth factor and integrin-linked kinase in high glucose-induced phenotypic alterations of podocytes. J Cell Biochem 113(1):293–301
Katalin S, Raff AC, Mario S, Bottinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55(1):225
Khaled A, Abdul H, Ajay S, Sanjay M, Dan R (2012) Podocyte foot process effacement in postreperfusion allograft biopsies correlates with early recurrence of proteinuria in focal segmental glomerulosclerosis. Transplantation 96(3):1238–1244
Korsmo-Haugen HK, Brurberg KG, Mann J, Aas AM (2019) Carbohydrate quantity in the dietary management of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 21(1):15–27
Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS (2006) Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 17(10):2812–2820
Liu Y, Xu Z, Ma F, Jia Y, Wang G (2018) Knockdown of TLR4 attenuates high glucose-induced podocyte injury via the NALP3/ASC/Caspase-1 signaling pathway. Biomed Pharmacother 107:1393–1401
Michele DV, Alessandra M, Maria A, Alois R, Gaetano S, Paola F (2003) Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52(4):1031–1035
Mukherjee N, Cardenas E, Bedolla R, Ghosh R (2017) SETD6 regulates NF-kappaB signaling in urothelial cell survival: implications for bladder cancer. Oncotarget 8(9):15114–15125
Mundel P, Reiser J (2010) Proteinuria: an enzymatic disease of the podocyte? Kidney Int 77(7):571–580
Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, Hirata M, Shinohara M, Fukagawa M, Nishi S (2013) Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens 27(4):586–595
Palsamy P, Subramanian S (2011) Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling. Biochimica et Biophysica Acta (BBA) 1812(7):719–731
Qiao C, Ye W, Li S, Wang H, Ding X (2018) Icariin modulates mitochondrial function and apoptosis in high glucose-induced glomerular podocytes through G protein-coupled estrogen receptors. Mol Cell Endocrinol 473:146–155
Sanjay J, Laura DP, Masato H, Shreeram A, Rajshekhar C, Helen L (2011) Expression profiles of podocytes exposed to high glucose reveal new insights into early diabetic glomerulopathy. Lab Invest 91(4):488–498
Sasaki K, Doi S, Nakashima A, Irifuku T, Yamada K, Kokoroishi K, Ueno T, Doi T, Hida E, Arihiro K (2016) Inhibition of SET domain-containing lysine methyltransferase 7/9 ameliorates renal fibrosis. J Am Soc Nephrol 27(1):203
Shen Y, Chen S, Zhao Y (2019) Sulfiredoxin-1 alleviates high glucose-induced podocyte injury though promoting Nrf2/ARE signaling via inactivation of GSK-3β. Biochem Biophys Res Commun 516(4):1137–1144
Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH (2005) Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280(24):22925–22936
Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC (2006) Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. Bmc Nephrol 7(1):6–6
Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S-I, Koya D, Asanuma K (2016) Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65(3):755–767
Vershinin Z, Feldman M, Chen A, Levy D (2016) PAK4 methylation by SETD6 promotes the activation of the Wnt/β-catenin pathway. J Biol Chem 291(13):6786–6795
Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R (2010) Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes 59(11):2904–2915
Wang D, Dai C, Li Y, Liu Y (2011) Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int 80(11):1159
Wang C, Li C, Peng H, Ye Z, Zhang J, Liu X, Lou T (2014) Activation of the Nrf2-ARE pathway attenuates hyperglycemia-mediated injuries in mouse podocytes. Cell Physiol Biochem 34(3):891–902
Wang Y, Xue J, Li Y, Zhou X, Qiao S, Han D (2019) Telmisartan protects against high glucose/high lipid-induced apoptosis and insulin secretion by reducing the oxidative and ER stress. Cell Biochem Funct 37(3):161–168
Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung E-R, Huang H, Wu L, Eberhart C, Handa JT (2014) NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 57(1):204–213
Zhang S, Qiu X, Dong S, Zhou L, Zhu Y, Wang M, Jin L (2019) MicroRNA-770-5p is involved in the development of diabetic nephropathy through regulating podocyte apoptosis by targeting TP53 regulated inhibitor of apoptosis 1. Eur Rev Med Pharmacol Sci 23(3):1248–1256
Funding
The study was approved by Shaanxi Provincial Health and Family Planning Commission Scientific Research Fund (2016D018), the National Natural Sciences Foundation of China (81473010) and Shaanxi Province Key Research and Development Project fund (2018SF-173).
Author information
Authors and Affiliations
Contributions
XW, CXH and RL conceived and designed the study, and revised the draft; QLL and SW contributed to the acquisition, analyses, and interpretation of data; XW, QLL, DQK and ZL performed the cell culture and transfection experiments; XW, QLL, and YFG designed the experiments, performed data analyses, and drafted the manuscript. All authors contributed to writing the manuscript, reviewed and approving the final version. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, Q., Kong, D. et al. Down-regulation of SETD6 protects podocyte against high glucose and palmitic acid-induced apoptosis, and mitochondrial dysfunction via activating Nrf2-Keap1 signaling pathway in diabetic nephropathy. J Mol Hist 51, 549–558 (2020). https://doi.org/10.1007/s10735-020-09904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-020-09904-6