Abstract
Hyperglycemia-induced oxidative stress in podocytes exerts a major role in the pathological process of diabetic nephropathy. Tripartite motif-containing protein 32 (TRIM32) has been reported to be a key protein in the modulation of cellular apoptosis and oxidative stress under various pathological processes. However, whether TRIM32 participates in the regulation of high glucose (HG)-induced injury in podocytes has not been investigated. This work aimed to assess the possible role of TRIM32 in mediating HG-induced apoptosis, oxidative stress, and inflammatory response in podocytes in vitro. Our results showed a marked increase in TRIM32 expression in HG-exposed podocytes and the glomeruli of diabetic mice. Loss-of-function experiments showed that TRIM32 knockdown improves the viability of HG-stimulated podocytes and suppresses HG-induced apoptosis, oxidative stress, and inflammatory responses in podocytes. Further investigation revealed that TRIM32 inhibition enhances the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, which is associated with the modulation of the Akt/glycogen synthase kinase-3β (GSK-3β) axis in podocytes following HG exposure. However, Akt suppression abrogated the TRIM32 knockdown-mediated activation of Nrf2 in HG-exposed podocytes. Nrf2 knockdown also markedly abolished the protective effects induced by TRIM32 inhibition o in HG-exposed podocytes. In summary, this work demonstrated that TRIM32 inhibition protects podocytes from HG-induced injury by potentiating Nrf2 signaling through modulation of Akt/GSK-3β signaling. The findings reveal the potential role of TRIM32 in mediating podocyte injury during the progression of diabetic nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Diabetic nephropathy is a severe complication of diabetes and a major contributor to end-stage renal disease [1]. Diabetic nephropathy is characterized by glomerular injury and accompanied by proteinuria [2]. Unfortunately, up to 40% of patients with diabetes will eventually develop diabetic nephropathy [3]. However, the treatment of diabetic nephropathy remains a challenging endeavor. Glomerular podocytes, the major components of the glomerular filtration barrier, play a vital role in the progression of diabetic nephropathy [4]. Persistent exposure to hyperglycemia evokes podocyte injury via induction of apoptosis, oxidative stress, and the inflammatory response, which is related to the pathogenesis of diabetic nephropathy [5,6,7]. The molecular mechanisms underlying hyperglycemia-induced podocyte injury remain incompletely understood. Therefore, a deeper understanding of the molecular mechanisms behind hyperglycemia-induced podocyte injury may offer new opportunities for the development of innovative therapeutic options for diabetic nephropathy.
Tripartite motif-containing protein 32 (TRIM32), a member of the TRIM family, is related to a number of physiological and pathological processes [8]. TRIM32 harbors the typical RING domain, confers E3 ligase activity, and is capable of ubiquitinating various substrates [9,10,11]. TRIM32 is widely expressed in adult tissues and plays a multifaceted role in diverse processes [12]. TRIM32 dysregulation is implicated in numerous pathological conditions, including myopathy, cardiomyopathy, neurological disorders, and various cancers [13,14,15,16,17,18,19,20]. Moreover, TRIM32 participates in the regulation of diverse cellular activities, including differentiation, proliferation, apoptosis, oxidative stress, and the inflammatory response [21,22,23,24].
Nuclear factor erythroid 2-related factor 2 (Nrf2) plays an essential role in organizing the cellular protection network under adverse stimuli [25]. Nrf2 translocates to the nucleus, where it binds to an antioxidant response element (ARE) in gene promoters to induce the expression of cytoprotective target genes [26]; the protein is involved in numerous pathological conditions by affecting apoptosis, oxidative stress, and the inflammatory response [27,28,29,30]. Increasing evidence shows that Nrf2 exerts a key effect on the pathogenesis of diabetic nephropathy [31,32,33]. Nrf2 activation ameliorates high glucose (HG)-induced apoptosis, oxidative stress, and inflammation of podocytes, which is conducive to curing diabetic nephropathy [34,35,36]. While Nrf2 activation is known to be modulated by various factors, such as Keap1 and Akt/glycogen synthase kinase-3β (GSK-3β) [37], the regulation of Nrf2 activation in diabetic nephropathy remains poorly understood.
To date, whether TRIM32 plays a role in diabetic nephropathy is unknown. In this study, we aimed to elucidate the potential role of TRIM32 in regulating the podocyte injury induced by HG. Our results showed marked increases in TRIM32 expression in podocytes following HG exposure. Functional studies showed that TRIM32 inhibition improves the viability of HG-stimulated podocytes and suppresses HG-induced apoptosis, oxidative stress, and the inflammatory response in podocytes. Further investigation revealed the regulatory effect of TRIM32 on Nrf2 signaling in podocytes following HG exposure. Akt suppression abrogated the TRIM32 knockdown-mediated activation of Nrf2 in HG-exposed podocytes. In addition, Nrf2 knockdown markedly abolished the protective effects induced by TRIM32 inhibition on HG-exposed podocytes. These results collectively show that TRIM32 inhibition protects podocytes from HG-induced injury by potentiating Nrf2 signaling via modulation of the Akt/GSK-3β axis.
MATERIALS AND METHODS
ANIMALS
All animal experiments conducted in this work were approved by the Ethical Committee of Xi’an Jiaotong University Health Science Center. Male diabetic mice (db/db) and age-matched male genetic control mice were purchased from Nanjing Biomedical Research Institute (Nanjing, China) and housed according to the standard protocols of the breeder. At 12 weeks of age, the mice were euthanatized and their kidneys were excised. Glomeruli were isolated by laser capture microdissection.
CELL CULTURE AND HG TREATMENT
The conditionally immortalized mouse podocyte cell line MPC5 was provided by BeNa Biotechnology (Beijing, China). The mouse podocytes were maintained in RPMI-1640 medium containing 10% fetal bovine serum and 10 U/ml interferon-γ at 33 °C for cell proliferation. The medium was replaced with fresh medium without interferon-γ, and cells were cultivated at 37 °C for 14 days to induce differentiation. The MPC5 cells were then placed in medium containing 30 mM glucose and cultivated for 48 h to induce HG injury in podocytes. Cells maintained in medium containing normal glucose (NG, 5.5 mM) were utilized as a control.
Real-Time Quantitative PCR
Total RNA was extracted from cultured podocytes and purified using the RNApure Tissue and Cell Kit (Cowin Biosciences, Beijing, China). The total RNA was reverse-transcribed into cDNA using the HiFi-Script cDNA Synthesis Kit (Cowin Biosciences). FastSYBR Mixture (Cowin Biosciences) was adopted to amplify cDNA by RT-qPCR with the appropriate primers. The data generated by real-time quantitative PCR (RT-qPCR) were assessed via the 2−ΔΔCt method, and relative gene expression was obtained using GAPDH for normalization.
WESTERN BLOT
Cultured podocytes were collected and lysed in lysis buffer (Solarbio, Beijing, China) supplemented with phosphatase inhibitors, proteinase inhibitors, and phenylmethanesulfonyl fluoride. Thereafter, the lysates were centrifuged and the supernatants were collected. Protein concentrations in the supernatants were quantified via the BCA Protein Assay Kit (Tiangen Biotech, Beijing, China). The same amounts of proteins were placed into each lane of sodium dodecyl sulfate polyacrylamide gels and separated via electrophoresis. Proteins were transferred to polyvinylidene fluoride membranes via an electron-transfer method using a Bio-Rad Trans-Blot apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were subsequently immersed in 5% skim milk for blocking, incubated with primary antibodies, hybridized with matched secondary antibodies, and then incubated with enhanced chemiluminescent reagents (Solarbio) to visualize the protein bands. The band intensity of the Western blot images was measured using Image-Pro Plus 6.0 software (MediaCybernetics, Rockville, MD, USA). Primary antibodies against TRIM32 (Abcam, Cambridge, UK), Akt (Proteintech Group, Wuhan, China), phospho-Akt (Ser473; Proteintech Group), GSK-3β (Proteintech Group), phopho-GSK-3β (Ser9; Proteintech Group), Nrf2 (Proteintech Group), GAPDH (Proteintech Group), and Lamin B1 (Proteintech Group) were used.
CELL TRANSFECTION
SiRNAs targeting TRIM32 or Nrf2 were synthesized by Genepharma (Shanghai, China) and then transfected into podocytes by using TransIntro EL Transfection Reagent (Transgen, Beijing, China) according to the manufacturer’s protocol. The downregulation of target genes was confirmed by RT-qPCR or Western blot after 48 h of transfection.
CELL VIABILITY ASSAY
MPC5 podocytes were cultured in a 96-well plate and transfected with the indicated siRNAs upon reaching ~ 70% confluence. After the cells were transfected for 48 h, the medium was replaced with fresh medium harboring HG. The podocytes were cultivated for another 48 h. Cell Counting Kit-8 (CCK-8) reagents (Solarbio, Beijing, China) were added to each well to determine podocyte viability. The absorbance of each well at 450 nm was measured via a microplate reader (BioTeke, Beijing, China).
TERMINAL DEOXYNUCLEOTIDYL TRANSFERASE DUTP NICK-END LABELING ASSAY
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed using the TransDetect In Situ Fluorescein TUNEL Cell Apoptosis Detection Kit (Transgen, Beijing, China) following the manufacturer’s protocol. In brief, podocytes were fixed with a formaldehyde fixing solution at the time of detection. Subsequently, 0.1% Triton X-100 solution was adopted to permeabilize the podocytes. The podocytes were incubated with TdT reagent and labeling solution at 37 °C for 1 h and protected from light. After staining with DAPI, the cells were visualized using a fluorescence microscope.
ANNEXIN V‐FITC/PI APOPTOSIS ASSAY
Annexin V‐FITC/PI apoptosis assay was performed via flow cytometry by using the Annexin V‐FITC/PI Apoptosis Kit (Solarbio, Beijing, China). In brief, cultured podocytes were dissociated by trypsin digestion and washed with ice-cold PBS. Podocytes were collected, resuspended in binding buffer, and then added with Annexin V‐FITC/PI solution. After cultivation for 15 min at room temperature away from light, the cells were assessed via a FACScan flow cytometry system.
DETECTION OF ROS GENERATION
Intracellular levels of ROS were evaluated via 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA), which could be oxidized to fluorescent DCFH. In general, at the time of detection, the old medium was discarded and fresh medium supplemented with 10 μM DCFH-DA (Beyotime, Shanghai, China) was added to cells. After cultivation for 30 min at 37 °C, the cells were washed with PBS and then analyzed via a FACScan flow cytometry system to quantify their fluorescence intensity.
MEASUREMENT OF MALONDIALDEHYDE AND SUPEROXIDE DISMUTASE ACTIVITY
The contents of malondialdehyde (MDA) and superoxide dismutase (SOD) in podocytes were respectively measured by the Lipid Peroxidation MDA Assay Kit (Beyotime, Shanghai, China) and the Total Superoxide Dismutase Assay Kit (Beyotime) according to the manufacturer’s protocols.
ENZYME-LINKED IMMUNOSORBENT ASSAY
The levels of pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IL-1β, in the supernatants of cultured podocytes were quantified using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s protocols.
Luciferase Activity Assays
The luciferase reporter vector pARE (Beyotime, Shanghai, China), which contains an ARE binding site, was adopted to measure the transcriptional activity of Nrf2. The luciferase reporter vector pNF-κB (Beyotime) was utilized to detect the transcriptional activity of NF-κB. TRIM32 siRNAs and the corresponding luciferase reporter vectors were cotransfected into MPC5 podocytes and cultivated for 48 h prior to HG stimulation. Cells were collected, lysed, and then subjected to luciferase activity measurements by using the Luciferase Reporter Gene Assay Kit (Beyotime).
STATISTICAL ANALYSIS
The experimental results were expressed as mean ± standard deviation. Statistical analysis and graphing were implemented using GraphPad Prism 8. Student’s t test was adopted for two-group comparisons. One-way ANOVA was used when three or more groups were compared. Differences were considered statistically significant when p < 0.05.
RESULTS
TRIM32 Expression Was Elevated in Podocytes Exposed to HG
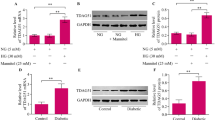
To determine the possible relevance of TRIM32 in diabetic nephropathy, we first determined the expression levels of TRIM32 in the glomeruli of diabetic mice. The results showed markedly upregulated mRNA levels of TRIM32 in the glomeruli of diabetic mice compared with that in control mice (Fig. 1A). Next, we investigated whether HG exposure could affect TRIM32 expression in cultured podocytes in vitro. Our results showed that TRIM32 mRNA levels are obviously elevated in podocytes after HG treatment (Fig. 1B). Western blot demonstrated significant increases in TRIM32 protein in HG-treated podocytes (Fig. 1C, D). These data indicate that TRIM32 is induced by HG in podocytes.
Effect of HG treatment on TRIM32 expression. (A) The mRNA level of TRIM32 in the glomeruli of diabetic mice was measured by RT-qPCR (n = 5, **p < 0.01). MPC5 cells were cultured for 48 h in the presence of NG or HG. (B) RT-qPCR was utilized to determine the effect of HG on TRIM32 mRNA levels. (C, D) Western blot was adopted to measure the effect of HG on TRIM32 protein levels. n = 5, **p < 0.01.
Inhibition of TRIM32 Repressed HG-Induced Apoptosis in Podocytes
To understand the biological role of TRIM32 in mediating HG-induced podocyte injury, we performed loss-of-function experiments on TRIM32. Transfection of TRIM32 siRNA into MPC5 cells markedly depleted TRIM32 expression with or without HG treatment (Fig. 2A–C). Viability assays showed that HG stimulation significantly reduces the viability of podocytes, which is markedly reversed by TRIM32 knockdown (Fig. 2D). TUNEL assay showed that HG-induced apoptosis in MPC5 cells is significantly attenuated by TRIM32 knockdown (Fig. 2E, F). The inhibitory effect of TRIM32 knockdown on HG-induced podocyte apoptosis was confirmed by the Annexin V-FITC/PI apoptosis assay (Fig. 2G, H). To confirm that HG induces apoptosis in podocytes, we detected the effect of the caspase inhibitor Z-VAD-FMK on HG-induced podocyte apoptosis. We found that treatment with Z-VAD-FMK markedly decreases HG-induced apoptosis in podocytes (Supplementary Fig. 1A and B). HG also induced the cleavage of PARP, which was abolished by Z-VAD-FMK treatment (Supplementary Fig. 1C). These data collectively suggest that TRIM32 inhibition alleviates HG-induced apoptosis in podocytes.
Effect of TRIM32 inhibition on HG-induced podocyte apoptosis. MPC5 cells were transfected with TRIM32 siRNA or control siRNA for 48 h and then exposed to HG. Downregulation of TRIM32 levels by siRNA transfection was confirmed by (A) RT-qPCR and (B, C) Western blot (n = 5). (D) The effect of TRIM32 inhibition on podocyte viability was assessed by CCK-8 assay (n = 5). The effect of TRIM32 inhibition on podocyte apoptosis was evaluated by (E, F) TUNEL assay and (G, H) Annexin V-FITC/PI assay (n = 5). **p < 0.01.
Inhibition of TRIM32 Relieved HG-Induced Oxidative Stress in Podocytes
To explore the role of TRIM32 in mediating HG-induced podocyte injury further, we investigated the effect of TRIM32 inhibition on HG-induced oxidative stress in MPC5 cells. HG exposure led to the extensive generation of ROS in podocytes, which was markedly decreased by TRIM32 knockdown (Fig. 3A, B). Moreover, HG-induced elevations in MDA contents were reduced by TRIM32 inhibition in podocytes (Fig. 3C). SOD activity, which was inhibited by HG, was significantly increased by TRIM32 inhibition in podocytes (Fig. 3D). Mitochondrial complex-I dysregulation has been linked to the excessive production of ROS. A previous study reported the critical role of TRIM32 in mediating the activity of complex-I [23]. The activity of complex-I was decreased by HG in podocytes (Supplementary Fig. 2). Interestingly, TRIM32 knockdown markedly restored the impaired activity of complex-I due to HG (Supplementary Fig. 2). To verify that oxidative stress contributes to the HG-induced cell death of podocytes, we detected whether the antioxidant tBHQ could rescue the HG-induced cell death of podocytes. Our data demonstrated that treatment with tBHQ significantly ameliorates the HG-induced apoptosis of podocytes (Supplementary Fig. 3). Altogether, these data indicate that TRIM32 inhibition relieves HG-induced oxidative stress in podocytes.
Effect of TRIM32 knockdown on HG-induced oxidative stress in podocytes. (A, B) The effect of TRIM32 inhibition on ROS generation was monitored by DCFH-DA staining (n = 5). (C) The effect of TRIM32 inhibition on MDA content was assessed by MDA assay (n = 5). (D) The effect of TRIM32 inhibition on SOD activity was evaluated by SOD assay (n = 5). *p < 0.05 and **p < 0.01.
Inhibition of TRIM32 Repressed the HG-Induced Inflammatory Response in Podocytes
We investigated the role of TRIM32 inhibition in the HG-induced inflammatory response in podocytes. We found that TRIM32 knockdown prominently decreases the release of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β (Fig. 4A–C). Moreover, TRIM32 knockdown markedly suppressed the activation of NF-κB in HG-exposed podocytes (Fig. 4D–F). To confirm that NF-κB activation contributes to the HG-induced cell death of podocytes, we detected the effect of NF-κB inhibition on HG-induced podocytes apoptosis. Interestingly, we found that NF-κB inhibition significantly attenuates the HG-induced apoptosis of podocytes (Supplementary Fig. 4). Overall, these data suggest that TRIM32 inhibition decreases the HG-induced inflammatory response in podocytes.
Effect of TRIM32 knockdown on the HG-induced inflammatory response in podocytes. The effect of TRIM32 inhibition on the concentration levels of (A) IL-6, (B) TNF-α, and (C) IL-1β in the culture supernatants was measured by ELISA (n = 5). (D, E) The effect of TRIM32 inhibition on the levels of nuclear NF-κB p65 protein was determined by Western blot (n = 5). Lamin B1 served as the loading control for nuclear proteins. (F) The effect of TRIM32 inhibition on the transcriptional activity of NF-κB was measured by luciferase reporter assay (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001.
Inhibition of TRIM32 Potentiated Nrf2 Signaling in HG-Exposed Podocytes
To determine the molecular mechanism underlying the effects of TRIM32 inhibition on HG-exposed podocytes, we investigated the role of TRIM32 inhibition in Nrf2 signaling contributing to the mediation of HG-induced apoptosis, oxidative stress, and the inflammatory response in podocytes. The results showed that HG treatment decreases nuclear Nrf2 levels, which could be significantly upregulated by TRIM32 knockdown (Fig. 5A, B). Furthermore, TRIM32 knockdown markedly increased the transcriptional activity of Nrf2 (Fig. 5C). TRIM32 knockdown also enhanced the expression of Nrf2 target genes, including HO-1 and NQO-1 (Fig. 5D). These data imply that TRIM32 inhibition potentiates Nrf2 signaling in HG-exposed podocytes.
Effect of TRIM32 inhibition on Nrf2 signaling in HG-exposed podocytes. (A, B) The effect of TRIM32 inhibition on the level of nuclear Nrf2 was measured by Western blot (n = 5). Lamin B1 served as the loading control for nuclear proteins. (C) The effect of TRIM32 inhibition on the transcriptional activity of Nrf2 was determined by luciferase reporter assay (n = 5). (D) The effect of TRIM32 inhibition on the levels of HO-1 and NQO-1 was assessed by Western blot. GAPDH served as the loading control. *p < 0.05 and **p < 0.01.
Inhibition of TRIM32 Enhanced Nrf2 Signaling via Modulation of the Akt/GSK-3β Axis in HG-Exposed Podocytes
TRIM32 plays a vital role in mediating the activation of the Akt/GSK-3β axis. Considering that the Akt/GSK-3β axis also regulates the activation of Nrf2 signaling, we sought to determine whether TRIM32 modulates Nrf2 signaling via the Akt/GSK-3β axis in HG-exposed podocytes. Interestingly, we found that TRIM32 inhibition increases the phosphorylation of Akt and GSK-3β in HG-exposed podocytes (Fig. 6A–C). Treatment with Akt inhibitors markedly decreased the phosphorylation of Akt and GSK-3β induced by TRIM32 inhibition in HG-exposed podocytes (Fig. 6A–C). TRIM32 inhibition-induced Nrf2 activation in HG-exposed podocytes was markedly abolished by Akt inhibition (Fig. 6D–F). In summary, these findings confirm that TRIM32 inhibition enhances Nrf2 signaling via modulation of the Akt/GSK-3β axis.
Effect of Akt inhibition on TRIM32-mediated Nrf2 signaling. MPC5 cells were transfected with TRIM32 siRNA for 48 h in the presence or absence of the Akt inhibitor, MK-2206 2HCl, and then treated with HG. Levels of (A–C) phospho-Akt and phospho-GSK-3β and (D, E) nuclear Nrf2 were determined by Western blot (n = 5). (F) Nrf2 transcriptional activity was monitored by luciferase activity (n = 5). *p < 0.05 and **p < 0.01.
Knockdown of Nrf2 Reversed TRIM32 Inhibition-Mediated Protective Effects in HG-Exposed Podocytes
To confirm whether TRIM32 inhibition protects podocytes from HG injury via Nrf2 signaling, we detected the effect of Nrf2 knockdown on the protective effects of TRIM32 inhibition on HG-exposed podocytes. Transfection of Nrf2 signaling markedly decreased the activation of Nrf2 induced by TRIM32 inhibition in HG-exposed podocytes (Fig. 7A–C). As expected, Nrf2 knockdown significantly abolished TRIM32 inhibition-induced suppressive effects on HG-induced apoptosis (Fig. 7D, E) and ROS generation (Fig. 7F, G). In addition, the inhibitory effects of TRIM32 inhibition on the HG-induced release of IL-6, TNF-α, and IL-1β were partially reversed by Nrf2 knockdown (Fig. 7H–J). In summary, these data confirm that TRIM32 inhibition protects podocytes from HG injury via Nrf2 signaling.
Effect of Nrf2 inhibition on the protective effects induced by TRIM32 inhibition in HG-exposed podocytes. TRIM32 siRNA and Nrf2 siRNA were cotransfected into MPC5 cells, which were then incubated for 48 h before HG exposure. (A–C) Levels of nuclear Nrf2 were examined by Western blot (n = 5). (D, E) Podocyte apoptosis was measured by Annexin V-FITC/PI assay (n = 5). (F, G) ROS levels in podocytes were assessed by DCFH-DA staining (n = 5). Concentration levels of (H) IL-6, (I) TNF-α, and (J) IL-1β in the culture supernatants of podocytes were monitored by ELISA (n = 5). *p < 0.05 and **p < 0.01.
DISCUSSION
In this work, we determined the pivotal role of TRIM32 in regulating the HG-evoked injury of podocytes. TRIM32 expression was significantly elevated in podocytes stimulated by HG. TRIM32 inhibition by siRNA-mediated gene silencing markedly suppressed HG-induced podocyte apoptosis, oxidative stress, and the inflammatory response. Moreover, we determined that TRIM32 inhibition confers anti-HG injury in podocytes by potentiating Nrf2 signaling via regulation of the Akt/GSK-3β axis (Fig. 8). Overall, our work demonstrates the vital role of the TRIM32/Akt/GSK-3β/Nrf2 axis in mediating HG-induced podocyte injury and highlights the possible relevance of TRIM32 in diabetic nephropathy.
TRIM32 exerts a key role in regulating the survival and apoptosis of various cell types. TRIM32 can inhibit the apoptosis of cancer cells and enhance cell survival following exposure to chemotherapeutics [38,39,40]. TRIM32 overexpression protects keratinocytes from apoptosis induced by ultraviolet B rays and TNF-α [23]. Besides the anti-apoptotic function of TRIM32, the pro-apoptotic role of TRIM32 in certain contexts has also been reported. Traumatic brain injury-induced cell apoptosis in the cortex is markedly reduced in TRIM32-knockout mice when compared with that in wild-type mice [41]. Moreover, TRIM32 knockdown alleviates oxygen glucose deprivation-induced apoptosis of hippocampal neurons [42]. TRIM32 silencing decreases the apoptosis of nucleus pulposus cells induced by IL-1β or TNF-α [43]. TRIM32 expression is notably induced by hydrogen peroxide or rotenone in human kidney cells, and TRIM32 upregulation enhances the sensitivity of human embryonic kidney cells to hydrogen peroxide- or rotenone-induced cell death [23]. These studies indicate that TRIM32 inhibition is conducive to kidney cell survival under noxious stimuli. However, whether TRIM32 is involved in regulating HG-induced apoptosis in podocytes remains unknown.
In this work, we confirmed that HG induces the apoptosis of podocytes. Our results showed that HG treatment induces the cleavage of the apoptosis indicator PARP. Moreover, treatment with caspase inhibitors significantly diminished the HG-induced apoptosis of podocytes. Our findings indicate that HG can trigger the apoptosis of cultured podocytes in vitro. Our work explores the role of TRIM32 in mediating HG-induced podocyte apoptosis. Our data demonstrated that TRIM32 expression is elevated in HG-exposed podocytes and that TRIM32 inhibition by siRNA-mediated gene silencing effectively attenuates HG-induced podocyte apoptosis. Thus, our work confirms that TRIM32 inhibition exerts an anti-apoptotic role in mediating HG-induced podocyte injury. The disparate functions of TRIM32 in mediating cell apoptosis may be associated with the cell type considered. Specifically, TRIM32 exerts a pro-apoptotic function in terminally differentiated cells, such as neurons and podocytes, but has an anti-apoptotic function in cells with high proliferation rates, such as cancer cells.
TRIM32 is involved in modulating oxidative stress. TRIM32 overexpression enhances the production of ROS induced by hydrogen peroxide or rotenone [23]. TRIM32 inhibition decreases the generation of ROS induced by oxygen glucose deprivation in hippocampal neurons and upregulates SOD contents [42]. In line with these findings, we found that TRIM32 knockdown inhibits the production of ROS and MDA but increases SOD contents in podocytes exposed to HG. Therefore, our work confirms the crucial role of TRIM32 in modulating oxidative stress. To verify that HG-induced oxidative stress contributes to the HG-induced cell death of podocytes, we investigated whether antioxidant treatment could rescue the HG-induced cell death of podocytes. Interestingly, we found that treatment with the antioxidant tBHQ effectively alleviates the HG-induced apoptosis of podocytes. Therefore, our data confirm that HG-induced oxidative stress contributes to the death of podocytes.
A recent study reported that TRIM32 regulates ROS production via the modulation of complex I activity in mitochondria [23]. Our data showed that HG treatment significantly decreases the activity of complex I, which may contribute to the excessive production of ROS. TRIM32 inhibition markedly restored the activity of complex I in HG-exposed podocytes. In line with previous findings, our work suggests that TRIM32 can affect HG-induced ROS production by regulating the activity of complex I.
TRIM32 plays a vital role in regulating the inflammatory response. TRIM32 knockout mice demonstrate reductions in pro-inflammatory cytokine and chemokine production post-Streptococcus suis infection [24]. TRIM32 overexpression promotes the release of pro-inflammatory cytokines in TNF-α-stimulated fibroblast-like synoviocytes [44]. Consistent with these studies, our work showed that TRIM32 inhibition markedly represses the production of pro-inflammatory cytokines induced by HG in podocytes. Moreover, TRIM32 regulates the inflammatory response associated with the modulation of NF-κB [44]. We demonstrated that TRIM32 inhibition impedes the nuclear translocation of NF-κB p65 and reduces the transcriptional activity of NF-κB. Therefore, TRIM32 inhibition may attenuate the HG-induced inflammation in podocytes associated with the downregulation of NF-κB activation. To confirm that the activation of NF-κB contributes to the HG-induced cell death of podocytes, we measured the effect of NF-κB inhibition on HG-induced podocyte apoptosis. Our results showed that NF-κB inhibition could significantly attenuate the HG-induced apoptosis of podocytes. Therefore, our work confirms that HG-induced NF-κB activation contributes to the cell death of podocytes.
Oxidative stress can upregulate the production of pro-inflammatory mediators, which contributes to the initiation of cell death [45]. In this work, we showed that TRIM32 inhibition restrains both oxidative stress and the inflammatory response induced by HG in cultured podocytes. Moreover, Nrf2 inhibition reversed TRIM32 inhibition-mediated inhibitory effects on HG-induced cell death and the inflammatory response, thereby indicating that TRIM32 inhibition restrains HG-induced cell death and inflammatory responses via Nrf2-regulated antioxidant effects.
Nrf2 signaling plays a vital role in modulating HG-induced apoptosis, oxidative stress, and inflammation in podocytes [34,35,36]. Interestingly, we found that TRIM32 inhibition potentiates Nrf2 signaling in HG-exposed podocytes, which is consistent with a recent study demonstrating that TRIM32 plays a role in regulating Nrf2 activation in oxygen glucose deprivation-induced neurons [42]. We found that TRIM32 inhibition shows no obvious effect on Nrf2 expression under normal conditions whereas Nrf2 expression decreases under HG conditions. Interestingly, Nrf2 expression in HG-exposed podocytes obviously increases to a greater extent compared with that under NG conditions following TRIM32 inhibition. Therefore, TRIM32 may regulate Nrf2 activation in a context-dependent manner. Under HG stress, Nrf2 activation is depressed by the high expression of TRIM32. WhenTRIM32 is inhibited, Nrf2 is drastically activated to antagonize HG-induced oxidative stress. We also found that TRIM32 inhibition potentiates Nrf2 signaling via modulation of the Akt/GSK-3β axis. The Akt/GSK-3β axis contributes to the modulation of Nrf2 activation [46,47,48], and TRIM32 may act as a vital regulator of the Akt/GSK-3β axis. TRIM32 inhibition enhances plakoglobin binding to PI3K, which leads to Akt activation [49]. TRIM32 knockout significantly increases the phosphorylation of Akt and GSK-3β in cardiomyocytes under hypertrophic stresses [50]. In this work, we showed that TRIM32 inhibition could increase the phosphorylation of Akt and GSK-3β in HG-exposed podocytes. Moreover, Akt inhibition markedly reversed TRIM32 inhibition-mediated Nrf2 activation in HG-exposed podocytes. Therefore, our data confirm that TRIM32 inhibition potentiates Nrf2 signaling via modulation of the Akt/GSK-3β axis.
Targeting TRIM32 to manipulate Nrf2 activation may have potential applications in the treatment of diabetic kidney disease. However, this strategy may present some limitations. Interventions upstream of the pathway (TRIM32-Akt/GSK-3β-Nrf2 axis), for example, are an indirect approach and could bring about greater concerns related to side effects or unexpected interactions compared with interventions farther downstream (Nrf2). Therefore, the precise therapeutic effects of TRIM32 inhibition on the treatment of diabetic kidney disease should be evaluated further by using animal models in vivo before this target can be used for therapy development.
CONCLUSION
The findings of this study demonstrate that TRIM32 inhibition ameliorates HG-induced apoptosis, oxidative stress, and inflammatory responses in podocytes by potentiating Nrf2 signaling via modulation of the Akt/GSK-3β axis. The data suggest that the TRIM32/Akt/GSK-3β/Nrf2 axis is a novel mechanism for regulating HG-induced injury in podocytes. Our findings indicate that TRIM32-mediated podocyte injury may play a vital role in the pathogenesis of diabetic nephropathy and suggest that TRIM32 may be a potential target for podocyte protection. However, the precise role of TRIM32 in mediating diabetic nephropathy requires further investigation by using animal models in vivo.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
References
Gnudi, L., R.J.M. Coward, and D.A. Long. 2016. Diabetic nephropathy: Perspective on novel molecular mechanisms. Trends in Endocrinology and Metabolism 27: 820–830.
Wada, J., and H. Makino. 2013. Inflammation and the pathogenesis of diabetic nephropathy. Clinical Science (London, England) 124: 139–152.
Gheith, O., N. Farouk, N. Nampoory, M.A. Halim, and T. Al-Otaibi. 2016. Diabetic kidney disease: World wide difference of prevalence and risk factors. Journal of Nephropharmacol 5: 49–56.
Armelloni, S., A. Corbelli, L. Giardino, M. Li, M. Ikehata, D. Mattinzoli, P. Messa, C. Pignatari, S. Watanabe, and M.P. Rastaldi. 2014. Podocytes: Recent biomolecular developments. Biomolecular Concepts 5: 319–330.
Mitjavila, M.T., and J.J. Moreno. 2012. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochemical Pharmacology 84: 1113–1122.
Elmarakby, A.A., and J.C. Sullivan. 2012. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics 30: 49–59.
Ram, C., A.K. Jha, A. Ghosh, S. Gairola, A.M. Syed, U.S. Murty, V.G.M. Naidu, and B.D. Sahu. 2020. Targeting NLRP3 inflammasome as a promising approach for treatment of diabetic nephropathy: Preclinical evidences with therapeutic approaches. European Journal of Pharmacology 885: 173503.
Watanabe, M., and S. Hatakeyama. 2017. TRIM proteins and diseases. Journal of Biochemistry 161: 135–144.
Cohen, S., B. Zhai, S.P. Gygi, and A.L. Goldberg. 2012. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. Journal of Cell Biology 198: 575–589.
Izumi, H., and Y. Kaneko. 2014. Trim32 facilitates degradation of MYCN on spindle poles and induces asymmetric cell division in human neuroblastoma cells. Cancer Research 74: 5620–5630.
Kano, S., N. Miyajima, S. Fukuda, and S. Hatakeyama. 2008. Tripartite motif protein 32 facilitates cell growth and migration via degradation of Abl-interactor 2. Cancer Research 68: 5572–5580.
Shieh, P.B., E. Kudryashova, and M.J. Spencer. 2011. Limb-girdle muscular dystrophy 2H and the role of TRIM32. Handbook of Clinical Neurology 101: 125–133.
Servian-Morilla, E., M. Cabrera-Serrano, E. Rivas-Infante, A. Carvajal, P.J. Lamont, A.L. Pelayo-Negro, G. Ravenscroft, R. Junckerstorff, J.M. Dyke, S. Fletcher, et al. 2019. Altered myogenesis and premature senescence underlie human TRIM32-related myopathy. Acta Neuropathologica Communications 7: 30.
Panicucci, C., M. Traverso, S. Baratto, C. Romeo, M. Iacomino, C. Gemelli, A. Tagliafico, P. Broda, F. Zara, C. Bruno, et al. 2019. Novel TRIM32 mutation in sarcotubular myopathy. Acta Myol 38: 8–12.
Borlepawar, A., A.Y. Rangrez, A. Bernt, L. Christen, S. Sossalla, D. Frank, and N. Frey. 2017. TRIM24 protein promotes and TRIM32 protein inhibits cardiomyocyte hypertrophy via regulation of dysbindin protein levels. Journal of Biological Chemistry 292: 10180–10196.
Ntim, M., Q.F. Li, Y. Zhang, X.D. Liu, N. Li, H.L. Sun, X. Zhang, B. Khan, B. Wang, Q. Wu, et al. 2020. TRIM32 deficiency impairs synaptic plasticity by excitatory-inhibitory imbalance via Notch pathway. Cerebral Cortex 30: 4617–4632.
Hillje, A.L., E. Beckmann, M.A. Pavlou, C. Jaeger, M.P. Pacheco, T. Sauter, J.C. Schwamborn, and L. Lewejohann. 2015. The neural stem cell fate determinant TRIM32 regulates complex behavioral traits. Frontiers in Cellular Neuroscience 9: 75.
Ruan, C.S., S.F. Wang, Y.J. Shen, Y. Guo, C.R. Yang, F.H. Zhou, L.T. Tan, L. Zhou, J.J. Liu, W.Y. Wang, et al. 2014. Deletion of TRIM32 protects mice from anxiety- and depression-like behaviors under mild stress. European Journal of Neuroscience 40: 2680–2690.
Yin, H., Z. Li, J. Chen, and X. Hu. 2019. Expression and the potential functions of TRIM32 in lung cancer tumorigenesis. Journal of Cellular Biochemistry 120: 5232–5243.
Wang, M., W. Luo, Y. Zhang, R. Yang, X. Li, Y. Guo, C. Zhang, R. Yang, and W.Q. Gao. 2020. Trim32 suppresses cerebellar development and tumorigenesis by degrading Gli1/sonic hedgehog signaling. Cell Death and Differentiation 27: 1286–1299.
Zhu, J.W., M.M. Zou, Y.F. Li, W.J. Chen, J.C. Liu, H. Chen, L.P. Fang, Y. Zhang, Z.T. Wang, J.B. Chen, et al. 2020. Absence of TRIM32 leads to reduced GABAergic interneuron generation and autism-like behaviors in mice via suppressing mTOR signaling. Cerebral Cortex 30: 3240–3258.
Wang, C., J. Xu, H. Fu, Y. Zhang, X. Zhang, D. Yang, Z. Zhu, Z. Wei, Z. Hu, R. Yan, et al. 2018. TRIM32 promotes cell proliferation and invasion by activating beta-catenin signalling in gastric cancer. Journal of Cellular and Molecular Medicine 22: 5020–5028.
Prajapati, P., D. Gohel, A. Shinde, M. Roy, K. Singh, and R. Singh. 2020. TRIM32 regulates mitochondrial mediated ROS levels and sensitizes the oxidative stress induced cell death. Cell Signal 76: 109777.
OuYang, X., J. Guo, Q. Lv, H. Jiang, Y. Zheng, P. Liu, T. Zhao, D. Kong, H. Hao, and Y. Jiang. 2020. TRIM32 drives pathogenesis in streptococcal toxic shock-like syndrome and streptococcus suis meningitis by regulating innate immune responses. Infection and Immunity 88: e00957-e1919.
Vomund, S., A. Schafer, M.J. Parnham, B. Brune, and A. von Knethen. 2017. Nrf2, the master regulator of anti-oxidative responses. International Journal of Molecular Sciences 18: 2772.
Zhang, D.D., and M. Hannink. 2003. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Molecular and Cellular Biology 23: 8137–8151.
Gum, S.I., and M.K. Cho. 2013. Recent updates on acetaminophen hepatotoxicity: The role of nrf2 in hepatoprotection. Toxicology Research 29: 165–172.
Ishii, Y., K. Itoh, Y. Morishima, T. Kimura, T. Kiwamoto, T. Iizuka, A.E. Hegab, T. Hosoya, A. Nomura, T. Sakamoto, et al. 2005. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. The Journal of Immunology 175: 6968–6975.
Kobayashi, E.H., T. Suzuki, R. Funayama, T. Nagashima, M. Hayashi, H. Sekine, N. Tanaka, T. Moriguchi, H. Motohashi, K. Nakayama, et al. 2016. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature Communications 7: 11624.
Anedda, A., E. Lopez-Bernardo, B. Acosta-Iborra, M. Saadeh Suleiman, M.O. Landazuri, and S. Cadenas. 2013. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radical Biology & Medicine 61: 395–407.
Adelusi, T.I., L. Du, M. Hao, X. Zhou, Q. Xuan, C. Apu, Y. Sun, Q. Lu, and X. Yin. 2020. Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed Pharmacother 123: 109732.
Warren, A.M., S.T. Knudsen, and M.E. Cooper. 2019. Diabetic nephropathy: An insight into molecular mechanisms and emerging therapies. Expert Opinion on Therapeutic Targets 23: 579–591.
Keri, K.C., N.S. Samji, and S. Blumenthal. 2018. Diabetic nephropathy: Newer therapeutic perspectives. Journal of Community Hospital Internal Medicine Perspectives 8: 200–207.
Chang, T.T., Y.A. Chen, S.Y. Li, and J.W. Chen. 2020. Nrf-2 mediated heme oxygenase-1 activation contributes to the anti-inflammatory and renal protective effects of Ginkgo biloba extract in diabetic nephropathy. Journal of Ethnopharmacology 266: 113474.
Wang, D., M. Jin, X. Zhao, T. Zhao, W. Lin, Z. He, M. Fan, W. Jin, J. Zhou, L. Jin, et al. 2019. FGF1(DeltaHBS) ameliorates chronic kidney disease via PI3K/AKT mediated suppression of oxidative stress and inflammation. Cell Death & Disease 10: 464.
Yang, S.M., S.M. Ka, H.L. Wu, Y.C. Yeh, C.H. Kuo, K.F. Hua, G.Y. Shi, Y.J. Hung, F.C. Hsiao, S.S. Yang, et al. 2014. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 57: 424–434.
Shaw, P., and A. Chattopadhyay. 2020. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. Journal of Cellular Physiology 235: 3119–3130.
Horn, E.J., A. Albor, Y. Liu, S. El-Hizawi, G.E. Vanderbeek, M. Babcock, G.T. Bowden, H. Hennings, G. Lozano, W.C. Weinberg, et al. 2004. RING protein Trim32 associated with skin carcinogenesis has anti-apoptotic and E3-ubiquitin ligase properties. Carcinogenesis 25: 157–167.
Su, X., B. Wang, Y. Wang, and B. Wang. 2020. Inhibition of TRIM32 induced by miR-519d increases the sensitivity of colorectal cancer cells to cisplatin. Oncotargets and Therapy 13: 277–289.
Du, Y., W. Zhang, B. Du, S. Zang, X. Wang, X. Mao, and Z. Hu. 2018. TRIM32 overexpression improves chemoresistance through regulation of mitochondrial function in non-small-cell lung cancers. Oncotargets and Therapy 11: 7841–7852.
Zhang, Z.B., L.L. Xiong, B.T. Lu, H.X. Zhang, P. Zhang, and T.H. Wang. 2017. Suppression of Trim32 enhances motor function repair after traumatic brain injury associated with antiapoptosis. Cell Transplantation 26: 1276–1285.
Wei, L., J.S. Zhang, S.F. Ji, H. Xu, Z.H. Zhao, L. Zhang, L. Pang, J.F. Zhang, P.B. Yang, and H. Ma. 2019. Knockdown of TRIM32 protects hippocampal neurons from oxygen-glucose deprivation-induced injury. Neurochemical Research 44: 2182–2189.
Chen, F., Q. Guo, Q. Chen, Z. Han, X. Zhou, L. Wu, X. Guo, B. Ni, and J. Yang. 2020. TRIM32 triggers beta-catenin signaling through ubiquitylation of AXIN1 to promote inflammatory factor-induced apoptosis of rat nucleus pulposus cells. American Journal of Physiology. Cell Physiology 318: C695–C703.
Liang, T., M. Song, K. Xu, C. Guo, H. Xu, H. Zhang, and L. Xu. 2020. TRIM32 promotes inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. Scandinavian Journal of Immunology 91: e12876.
Kalogeris, T., Y. Bao, and R.J. Korthuis. 2014. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology 2: 702–714.
Yu, H., J. Zhen, Y. Yang, J. Du, J. Leng, and Q. Tong. 2020. Rg1 protects H9C2 cells from high glucose-/palmitate-induced injury via activation of AKT/GSK-3beta/Nrf2 pathway. Journal of Cellular and Molecular Medicine 24: 8194–8205.
Chen, R., Y.Y. Zhang, J.N. Lan, H.M. Liu, W. Li, Y. Wu, Y. Leng, L.H. Tang, J.B. Hou, Q. Sun, et al. 2020. Ischemic postconditioning alleviates intestinal ischemia-reperfusion injury by enhancing autophagy and suppressing oxidative stress through the Akt/GSK-3beta/Nrf2 pathway in mice. Oxidative Medicine and Cellular Longevity 2020: 6954764.
Li, X., Y. Zou, J. Xing, Y.Y. Fu, K.Y. Wang, P.Z. Wan, and X.Y. Zhai. 2020. Pretreatment with roxadustat (FG-4592) attenuates folic acid-induced kidney injury through antiferroptosis via Akt/GSK-3beta/Nrf2 pathway. Oxidative Medicine and Cellular Longevity 2020: 6286984.
Cohen, S., D. Lee, B. Zhai, S.P. Gygi, and A.L. Goldberg. 2014. Trim32 reduces PI3K-Akt-FoxO signaling in muscle atrophy by promoting plakoglobin-PI3K dissociation. Journal of Cell Biology 204: 747–758.
Chen, L., J. Huang, Y. Ji, X. Zhang, P. Wang, K. Deng, X. Jiang, G. Ma, and H. Li. 2016. Tripartite motif 32 prevents pathological cardiac hypertrophy. Clinical Science (London, England) 130: 813–828.
Funding
This study was supported by the Science and Technology Research and Development Program of Shaanxi Province (2016SF-176).
Author information
Authors and Affiliations
Contributions
Zhao Chen designed the study, performed the experiments, and drafted the manuscript. Lifang Tian, Li Wang, and Xiaotao Ma performed the experiments. Fuqian Lei and Xianghui Chen collected and analyzed the data. Rongguo Fu designed the study and reviewed the manuscript. All authors have read this manuscript and approve of its final version.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors agree to the publication of this paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Tian, L., Wang, L. et al. TRIM32 Inhibition Attenuates Apoptosis, Oxidative Stress, and Inflammatory Injury in Podocytes Induced by High Glucose by Modulating the Akt/GSK-3β/Nrf2 Pathway. Inflammation 45, 992–1006 (2022). https://doi.org/10.1007/s10753-021-01597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01597-7