Abstract
Eugenol is one of the most important phenylpropanoid volatiles in strawberry fruit. The DOF (DNA binding with One Finger) proteins are plant-specific transcription factors, which are involved in diverse biological processes. However, the molecular mechanism of how the DOF transcription factors regulate eugenol biosynthesis is poorly understood. In this study, the novel DOF transcription factor, Fragaria × ananassa DOF1 (FaDOF1), was identified and characterized. Analysis of subcellular localization using GFP showed that FaDOF1 was localized in the nucleus. FaDOF1 was highly expressed in flowers and peaked at small green fruit stage during maturity. Eugenol concentrations at different developmental stages and tissues had significant correlations with the transcription levels of FaDOF1. Transient overexpression and silencing of FaDOF1 promoted and repressed eugenol accumulation in strawberry fruit, respectively. Y1H, GUS, and dual-LUC assays indicated that FaDOF1 was bound at the promoters of the two key genes in eugenol biosynthesis, FaEGS1 and FaEGS2, and activated their transcripts. In summary, our results suggest that FaDOF1 acts as a positive regulator of eugenol metabolism, which provide new insights into the regulatory mechanisms that can improve the quality of strawberry fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenylpropanoids are important secondary metabolites including benenoids, phenylpropenes, stilbenes, lignins, and flavonoids/anthocyanins that have diverse metabolic functions in plant development, growth, and environmental adaptations. Lignins and hydroxycinnamic acids are complex biopolymers, embedded in the cell walls of xylem tissue, periderm, and pollen grains (Skirycz et al. 2007). Flavonoids are implicated in numerous key physiological processes, including stress tolerance, plant pigmentation, signal transduction, UV photoprotection, and various health benefits to human (Dixon et al. 1995).
Eugenol is an important phenylpropene volatile compound present in fruits, vegetables, spices, and herbs (Aragüez et al. 2013). Eugenol contributes to characteristic aromas in plants (e.g., nutmeg, cinnamon, clove) and plays key roles as pollinator attractant or defense compound. Eugenol is widely used in dental, food, cosmetics, and herbal medicine given its anti-bacterial, anti-fungal, anti-microbial, analgesic, and anti-inflammatory properties (Anu-Prathap et al. 2015; Bezerra et al. 2017).
DOF (DNA binding with One Finger) proteins are plant-specific transcription factors characterized by a highly conserved domain, which includes a single C2C2-type (Cys2/Cys2) zinc-finger-like motif in the N-terminal region, and a DOF domain that is crucial for specific DNA binding. However, the C-terminal of DOF proteins is less-conserved and with variable transcriptional regulation structure, which likely contribute to various functions of different DOF proteins (Yanagisawa 2001, 2004). DOF family members can act either as repressors or activators controlling several biological processes, including plant defence (Wei et al. 2023), cell division (Skirycz et al. 2008), leaf senescence (Wang et al. 2021), hormone-modulated biological processes (Rueda-Romero et al. 2011), phytochrome and cryptochrome signalling regulation (Iwamoto et al. 2009), carbon and nitrogen metabolism (Kurai et al. 2011), fatty acids biosynthesis (Wang et al. 2007), and flowering (Corrales et al. 2014; Li et al. 2009). Several DOF genes have been identified in plant species: 37 in Arabidopsis (Arabidopsis thaliana), 30 in rice (Oryza sativa) (Lijavetzky et al. 2003), 51 in maize (Zea mays) (Jiang et al. 2012), 34 in tomato (Solanum lycopersicum) (Cai et al. 2013), 28 in soybean (Glycine max) (Wang et al. 2007), one in green algae (Chlamydomonas reinhardtii) (Moreno-Risueno et al. 2007), 26 in barley (Hordeum vulgare) (Moreno-Risueno et al. 2007), 28 in sorghum (Sorghum bicolor) (Xiao et al. 2022), and 41 in poplar (Populus trichocarpa) (Yang et al. 2006).
The C2C2-type zinc-finger-like motif of DOF proteins can bind to specific cis-elements (AAAG) in the promoters of downstream genes to activate or repress their transcription. For example, OsDOF15 plays roles in primary root elongation by regulating the promoter activity of the ethylene biosynthesis gene OsACS1, thereby inhibiting ethylene production and modulating root meristems (Qin et al. 2019). In sweet cherry, PavDof2/6/15 modulates the transcription levels of cell wall metabolic genes (PavPL18, PavQRT3, PavXTH31, PavXTH26, and PavPME44) by binding to their promoters to directly control fruit softening (Zhai et al. 2022). In tomato, SlDof1 directly upregulates ACS2, NR, NOR, and PG2A expression and downregulates several transcription factors during fruit maturation (Wang et al. 2021). The Chinese flowering cabbage, BrDof2.4 was found to delay leaf senescence during postharvest though directly repressing BrAPM1, BrASPG2, and BrSAG12 transcription levels (Wang et al. 2021). In tea plant, CsDOF3 inhibits CsCLH1 expression and promotes chlorophyll accumulation by interacting directly with the AAAG cis-element in the CsCLH1 promoter in response to light (Liu et al. 2023). In strawberry, FaDOF2 is implicated in eugenol accumulation by stimulating the biosynthetic gene FaEGS2 (eugenol synthase) transcription (Molina-Hidalgo et al. 2017).
One hundred and eighteen DOFs were found in the strawberry genome. Many FaDOF genes (107) were expressed in crown tissues, suggesting their involvement in defense mechanism against crown rot (Luo et al. 2022). However, the regulatory function of most DOFs in strawberry fruit are still poorly addressed. The ripening-related transcription factors, FaEOBII and FaDOF2 are involved in eugenol production in strawberry fruit (Medina-Puche et al. 2015; Molina-Hidalgo et al. 2017). Eugenol concentration in strawberry is highest at the small green fruit stage, therefore, we hypothesized that there are other TFs regulating eugenol concentration at the early fruit developmental stages. In our previous study, four putative candidates were screened using the FaEGS1 promoters the bait in yeast one-hybrid (Y1H) library. These include FaMYB63 (Wang et al. 2022) and FaDOF1. Here, we identified FaDOF1 as a new regulator of eugenol metabolism in strawberry. FaDOF1 gene expression peaked in fruit at the small green stage. FaDOF1 was able to bind to the promoters of two key structural eugenol biosynthetic genes, FaEGS1 and FaEGS2. Furthermore, transient overexpression and silencing of FaDOF1 promoted and decreased the eugenol concentrations, respectively. These findings contribute towards understanding the complexity of regulatory networks controlling phenylpropanoid biosynthesis and metabolism affecting strawberry fruit quality.
Materials and methods
Plant materials and growth conditions
Octoploid strawberries (Fragaria × ananassa Duch. ‘Benihoppe’) were grown in pots with strawberry-specific substrate (Jinan, China) in a glasshouse at the Strawberry Germplasm Resource Garden of Anhui Agricultural University, Hefei, China, at 25oC (16 h light/8 h dark) and relative humidity of 60%. Fruit samples were harvested at seven different developmental stages: small green (G1), large green (G2), green-white (GW), white (W), red-turning (RT), red-ripening (R), and over-ripening (OR) at about 10, 15, 20, 25, 30, 35, and 40 days post anthesis (DPA), respectively. Samples were collected from 10 to 18 fruits at the same developmental stages and different tissues (roots, stolons, leaves, and flowers), immediately snap-frozen in liquid nitrogen and stored at -80oC for further analysis.
Subcellular localization
The full-length coding sequence of FaDOF1 was amplified using PCR primers (Table S1) and inserted into the BglII/SpeI-digested pCAMBIA1302 to generate the fusion protein pCAMBIA1302-FaDOF1-GFP. Agrobacterium tumefaciens strain GV3101 was transformed with plasmids, pCAMBIA1302 and pCAMBIA1302-FaDOF1-GFP following established protocols (Wang et al. 2022). The intact leaves of 1-month-old tobacco (Nicotiana benthamiana) were infiltrated with A. tumefaciens cells harbouring pCAMBIA1302 and pCAMBIA1302-FaDOF1-GFP, respectively. DAPI solution was used to stain cell nuclei of tobacco leaf tissues. Three days after infiltration, GFP and DAPI signals were monitored using a FV1000 fluorescence laser-scanning microscope (Olympus Corporation, Japan) at 488/500–515 nm and at 405/449–461 nm, respectively.
RNA extraction, sequence analysis, and gene expression pattern analysis
Total RNA was isolated from strawberry samples using a commercially available RNA Extraction Kit (Beijing Tsingke Biotech Co., Ltd., Beijing, China) user manual. First-strand cDNA was synthesized using the ReverTra Ace®’s First-Strand Synthesis System for RT-qPCR (Beijing Tsingke Biotech Co., Ltd., Beijing, China). Gene expression was determined by RT-qPCR using a CFX96™ Real-Time System machine (CFX96™ Optics Module, Bio-Rad, Singapore) and 2×TSINGKE® Master qPCR Mix (SYBR Green I) (Tsingke, Nanjing, China). Primers used for RT-qPCR analysis are listed in Table S2, including the reference genes interspacer 26 S–18 S. Multiple sequence alignment of FaDOF1 and its homologues from strawberry and tomato was performed using CLUSTALW and GeneDoc software. Phylogenetic analysis was performed using MEGA 7.0 software, employing the neighbor-joining method with 1000 bootstrap replicates. The prediction of conserved cis-acting elements in the promoter was analyzed via the PlantCARE database (Rojas-Gracia et al. 2019).
Plasmid constructions and fruit transient expression assays
The FaDOF1 coding sequence (1029 bp) was subsequently cloned into the pCAMBIA1302 vector to obtain overexpression constructs driven by the CaMV 35S promoter. A 300 bp FaDOF1 gene fragment was inserted into the pTRV2 vector to produce a knockdown (RNAi) construct (Jia and Shen 2013). The recombinant plasmid pCAMBIA1302-FaDOF1, pTRV2-FaDOF1, and empty vectors were then transformed into the A. tumefaciens strain, GV3101 by freeze-thaw method. Agrobacterium cells were harvested at an optical density (OD 600) of 0.8. Plant transformation was performed as previously described (Wang et al. 2022). At least seven strawberry fruits attached to the plant were injected with 1 mL of Agrobacterium suspensions containing the overexpression, RNAi and empty vector constructs at large green and green-white fruit stages, respectively. Fruits were harvested after seven days of transfection in seven biological replicates for each construct. Three FaDOF1-OVX (or FaDOF1-RNAi) fruits exhibiting the highest (or lowest) transcript levels of FaDOF1 were selected for further analysis.
Measurement of eugenol concentration by Gas Chromatography-Mass Spectrometry
Strawberry fruit and different tissues were ground to a fine powder in liquid nitrogen. Eugenol was extracted from aliquots of ground plant tissues (0.5 g) in 5 mL of saturated NaCl of a 25 mL vial. Solid-phase microextraction of eugenol was done in a 7890B GC system (Agilent Instruments, Santa Clara, CA, USA) coupled to a 7000 GC-MS Triple Qual quadrupole mass detector (Agilent). The condition and procedure used for gas chromatography-mass spectrometry (GC-MS) analysis were as described in previous report (Wang et al. 2022). The eugenol concentration was quantified using a eugenol reference standard curve (Sigma-Aldrich, St Louis, MO, USA).
Yeast one-hybrid
Y1H assay was conducted using a Matchmaker One-Hybrid Library Construction and Screening Kit (Clontech, USA) in accordance with the manufacturer’s guidelines. The promoters of FaEGS1 (1282 bp) and FaEGS2 (1424 bp) were amplified from ‘Benihoppe’ and cloned into the pAbAi vector to create bait vectors. The DNA binding domain of FaDOF1 was inserted into the pGADT7 vector. After the minimal inhibitory concentration of Aureobasidin A (AbA) was determined for the bait strains, each bait-prey interactions were conducted individually on an SD/-Leu/AbA plate, and the empty pGADT7 plasmid was used as the negative control. The primer used for the Y1H assay is listed in Table S1.
GUS and dual-luciferase reporter (dual-LUC) assays
To evaluate the effect of FaDOF1 on the transcripts of FaEGS1 and FaEGS2, a β-glucuronidase (GUS) and a dual-LUC transient expression systems were used according to the protocols described by Wang et al. (2022). For GUS staining, the promoter regions of FaEGS1 and FaEGS2 were cloned into the pCAMBIA1391Z vector with inducible GUS cassette. The CDS of FaDOF1 was inserted into the pGreenII 62-SK vector as an effector construct. The plasmids were co-infiltrated into N. benthamiana leaves via A. tumefaciens strain GV3101-mediated transformation with a pSoup helper vector. The negative control was the empty vector pGreenII62-SK. Histochemical staining of GUS was performed using 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid (X-Gluc). GUS activity was calculated using a standard curve of 4-methylumbelliferone (Sigma-Aldrich, St Louis, MO, USA), and each assay was repeated four times.
For the dual-LUC assay, the promoter sequences of FaEGS1 and FaEGS2 were infused with pGreenII 0800-LUC as reporter constructs. The 35S::REN insert served as an internal transfection efficiency control for all samples in the same vector. Each construct effector (pGreenII 62-SK-FaDOF1) and reporter plasmid pair was separately transfected into A. tumefaciens strain GV3101 and co-transformed into N. benthamiana leaves. Promoter activities of the samples are detected by Dual-Luciferase® Reporter Assay System (YEASEN, Shanghai, China) and expressed as the ratio of LUC to REN three days after agro-infiltration. Each pair combination was carried out with at least nine assays. Details of the primers used for GUS staining and dual-LUC assays can be found in Table S1.
Statistical analysis
All data are shown as the means ± standard deviations (SD) of at least three biological or technical replicates. Asterisks indicate that the values are significantly-different from the control samples using the student’s t-test. One-way ANOVA followed by Tukey’s test was used to analyze significant differences between the means, with a significance level set at P < 0.05.
Results
Identification of FaDOF1
In our previous study, we screened a strawberry fruit cDNA library by Y1H and identified four putative candidates using the promoter of FaEGS1 as the bait. One candidate has been characterized as the MYB transcription factor FaMYB63, and its role in the regulation of eugenol metabolism has been demonstrated (Wang at al. 2022). In this study, we aim to clarify the role of a second candidate, the DOF transcription factor FaDOF1, in eugenol biosynthesis in strawberry fruit. Sequence analysis showed that FaDOF1 has cDNA of 1029 bp long encoding a protein of 342 amino acids with a predicted molecular weight of 37 kDa. FaDOF1 contains a highly conserved DOF domain in the N-terminal region, with a C2C2 zinc finger domain that consists of 52 amino acid residues. The existence of a bipartite nuclear localization signal (NLS) suggests that FaDOF1 is a nuclear protein (Fig. 1A). Phylogenetic analysis revealed that FaDOF1 belongs to the C2.1 sub-clade, while FaDOF2 was clustered in C2.2 subgroup (Fig. 1B).
Multiple sequence alignment, phylogenetic analysis, and subcellular localization of FaDOF1. (A) Sequence alignment of FaDOF1 and DOFs from strawberry and tomato. Identical amino acids are highlighted. The putative bipartite NLS (nuclear localization sequence) is indicated by B1 and B2 (B) Phylogenetic relationship of FaDOF1 with homologous DOF proteins. Sl, Solanum lycopersicum; At, Arabidopsis thaliana; Os, Oryza sativa; Fa, Fragaria × ananassa. (C) Subcellular localization of FaDOF1 in Nicotiana benthamiana leaves. Bar = 20 μm
To validate FaDOF1 subcellular localization, the full-length sequence of FaDOF1 protein was fused in frame with GFP. The fluorescence signals of 35S::GFP protein were distributed along the entire cell. In contrast, 35S::FaDOF1-GFP signals were only localized in the nucleus (Fig. 1C).
Expression profiles of FaDOF1 in different developmental stages and tissues
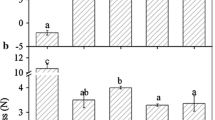
RT-qPCR analysis was conducted to analyse the transcript levels of FaDOF1 at seven fruit developmental stages (whole fruit, flesh, and achenes) and in five different tissues (root, stolon, leaf, flower, and red-ripening fruit). The transcript level of FaDOF1 was highest during the small-green stage (G1) and decreased thereafter in fruit (Fig. 2A). The expression pattern of FaDOF1 in strawberry flesh was similar to the profile observed in the whole fruit during fruit development (Fig. S1A). The expression levels of FaDOF1 in strawberry achenes at seven developmental stages were higher at small-green (G1) and white (W) stages when compared with other stages and then declined after white stage (Fig. S1B). FaDOF1 was expressed throughout all tissues, highest in flowers but much lower in red-ripening fruit (Fig. 2B). This finding is agreement with previous study showing that DOF genes have specific expression patterns, which are higher in vascular tissues of pericarp than in total pericarp (Wang et al. 2021; Gupta et al. 2015). Eugenol concentrations of fruit at different developmental stages and in various tissues were measured by GC-MS (Fig. 2). The eugenol amounts had a significantly positive correlation with the expression levels of FaDOF1 at different developmental stages of fruit (r = 0.982**, P < 0.01) and in various tissues (r = 0.940*, P < 0.05). These results imply that FaDOF1 may be involved in eugenol metabolism in strawberry.
The expression profiles of FaDOF1 and eugenol content at different developmental stages and organ tissues. (A) Transcription level of FaDOF1 and eugenol content during the strawberry fruit maturation. G1, small green fruit stage; G2, large green fruit stage; GW, green-white fruit stage; W, white fruit stage; RT, red-turning fruit stage; R, red-ripening fruit stage; OR, over-ripening fruit stage. (B) Expression of FaDOF1 and eugenol content in different organ tissues. Data were expressed as mean ± standard deviation (SD) for three replicates. Different letters above the bars indicate significant differences (P < 0.05, a one-way ANOVA, Tukey’s test)
FaDOF1 promotes eugenol accumulation in strawberry fruit
To test the function of FaDOF1 in eugenol metabolism pathway, transient overexpression and RNAi experiments were conducted in strawberry fruits. Agrobacterium carrying different FaDOF1 constructs or empty vector were injected into the fruit attached to strawberry plant. Three transgenic FaDOF1-overexpression fruits (FaDOF1-OVX1, FaDOF1-OVX2, and FaDOF1-OVX3) and three FaDOF1-RNAi fruits (FaDOF1-RNAiA, FaDOF1-RNAiB, and FaDOF1-RNAiC) were analysed. FaDOF1 was highly overexpressed in FaDOF1-OVX1, FaDOF1-OVX2, and FaDOF1-OVX3 than in empty vector fruit (Fig. 3A). Moreover, overexpression of FaDOF1 increased the amount of eugenol in FaDOF1-OVX1, FaDOF1-OVX2, and FaDOF1-OVX3. The eugenol content of fruits transiently overexpressing FaDOF1 were 2.5 to 4-fold higher than in the empty vector control fruit (Fig. 3B). In knock-down constructs, FaDOF1 expression was significantly lower in FaDOF1-RNAiA, FaDOF1-RNAiB, and FaDOF1-RNAiC fruit than in empty vector control fruit. This correlates with decreased eugenol concentrations in FaDOF1-RNAiA, FaDOF1-RNAiB, and FaDOF1-RNAiC, in which eugenol was reduced significantly by 27–33% compared with the empty vector control fruit (Fig. 3C and D). These results suggest that FaDOF1 has an important role in controlling eugenol accumulation in fruit.
Transient overexpression and RNAi of FaDOF1 in strawberry fruit affected eugenol accumulation and eugenol biosynthesis-related genes expression. (A and B) FaDOF1 expression and eugenol content in FaDOF1-overexpression fruits. (C and D) FaDOF1 expression and eugenol content in FaDOF1-RNAi fruits. (E) The expression of eugenol biosynthesis-related genes in FaDOF1-overexpression fruits. (F) The expression of eugenol biosynthesis-related genes in FaDOF1-RNAi fruits. Data are means ± SD of three technical replicates. Significant differences from empty vector control were determined by Student’s t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001)
The expression of genes related to eugenol metabolism in transient-transgenic strawberry fruit
To further understand the mechanism of FaDOF1 regulation of eugenol biosynthetic pathway in strawberry fruit, the expression of major eugenol metabolism genes in transient-transgenic strawberry fruit was measured by RT-qPCR. The eugenol biosynthesis pathway has been well characterized in petunia (Petunia × hybrida) and strawberry (Spitzer-Rimon et al. 2010, 2012; Van Moerkercke et al. 2011; Verdonk et al. 2005; Medina-Puche et al. 2015; Molina-Hidalgo et al. 2017; Wang et al. 2022). Eugenol synthesis starts from phenylalanine, which is de-ammonized to cinnamic acid by phenylalanine ammonia-lyase (PAL). Eugenol is subsequently generated via cinnamic acid-4-hydroxylase (C4H), 4-coumarate-CoA ligase (4CL), cinnamoyl-CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), and eugenol synthases (EGS), among others. EGSs, which include FaEGS1 and FaEGS2, are the main structural genes of eugenol synthesis. FaEGS1 is primarily expressed in green achene, while FaEGS2 transcript is significantly higher in the receptacles of red fruit than in other developmental stages (Aragüez et al. 2013). FaCAD1 catalyses the production of eugenol precursor, coniferyl alcohol, in eugenol metabolic pathway (Medina-Puche et al. 2015).
The expression of the three eugenol biosynthesis genes was significantly upregulated in the fruit transiently overexpressing FaDOF1, particularly FaEGS1 and FaEGS2 (Fig. 3E). Expression of the two genes was markedly down-regulated in the FaDOF1-RNAi fruits (Fig. 3F). Additionally, the transcript levels of the other four eugenol biosynthesis-related genes (FaPAL, FaC4H, Fa4CL, and FaCCR) were also affected in the transient-transgenic FaDOF1 fruits (Fig. S2). These results suggest that FaDOF1 promotes eugenol accumulation by regulating the expression of the key eugenol metabolic genes in transient-transgenic strawberry fruit.
FaDOF1 activates the transcript levels of FaEGS1 and FaEGS2 by binding to their promoters in a direct manner
Several DNA-binding motifs were identified in the promoters of FaEGS1 and FaEGS2. Both genes contain multiple potential DOF binding sites within the promoter regions (Fig. S3) indicating that they are directly regulated by FaDOF1. To further explore this, Y1H was carried out. After co-transformation, only the yeast strains containing the DNA binding domain of FaDOF1 and the promoters of FaEGS1 and FaEGS2 grew well on SD/-Leu and SD/-Leu/AbA media supplemented with AbA, indicating that FaDOF1 can specifically bind to the promoters of FaEGS1 and FaEGS2 in yeast (Fig. 4).
To further elucidate whether FaDOF1 regulates the transcripts of FaEGS1 and FaEGS2 and to test for promoter functionality, GUS and dual-LUC assays were performed in tobacco leaves. The two genes’ promoter sequences were fused to the the reporter vector pGreenII 0800. FaDOF1 was fused into the pGreenII 62 SK vector as an effector. The dual luciferase reporter assay showed that the LUC/REN ratios (reflecting transcriptional activity) of FaEGS1 and FaEGS2 promoters, were significantly up-regulated when the effector vector pGreenII 62 SK-FaDOF1 was present compared with the empty vector control, FaDOF1 increased the LUC/REN ratio of FaEGS1 promoter by 3.83-fold (P < 0.001; Fig. 5A) and of the FaEGS2 promoter by 2.15-fold (P < 0.001; Fig. 5B) than the empty vector controls.
Dual-luciferase and GUS assays showed that FaDOF1 activates the promoters of FaEGS1 (A and C) and FaEGS2 (B and D). The empty vector pGreenII62-SK was used as a negative control. Data are means ± standard deviation. Significant differences from the control were determined by Student’s t test, ***, P < 0.001
Furthermore, FaDOF1 was fused to the vector pGreenII 62 SK as an effector, while the FaEGS1 and FaEGS2 promoters were fused to the pCAMBIA1391Z vector to create reporters that drive GUS expression when activated (Fig. 5C and D). The FaDOF1 effector was co-transformed with a reporter into N. benthamiana leaves. Transformation of an empty effector (vector only) with either reporter was used as a negative control. The GUS activities were 3.72- and 6.67-fold (P < 0.001) higher in the tobacco plants transformed with FaDOF1 and co-cultivated with a reporter than the negative control, respectively. These results reveals that FaDOF1 can directly enhance the promoter activities of FaEGS1 and FaEGS2. Taken together, our findings highly suggest that FaDOF1 directly regulates FaEGS1 and FaEGS2 by binding to their promoters and positively regulating their expression, which then affects eugenol biosynthesis.
Discussion
The structural genes critical for diversity of branches leading to phenylpropanoid metabolism are usually highly expressed and finely regulated by various transcription factors such as MYB TFs, MBW ternary complex (R2R3-MYB, basic helix-loop-helix (bHLH), and WD40 proteins), NAC, and bZIP TFs (Gachon et al. 2005). The interaction of MYB and bHLH transcription factors was initially described for the activation of anthocyanin biosynthesis in Zea mays (Goff et al. 1992). Fruit growth and development are controlled by complex and transcriptionally-regulated metabolic networks (Sánchez-Gómez et al. 2022). Recently, Jiang et al. (2023) described how the strawberry FaEGL3 and FaLWD1/FaLWD1-like interact with the R2R3-FaMYB5 to form the MYB-bHLH-WD40 complex (MBW), regulating the accumulation of anthocyanins and proanthocyanidins by trans-activating of F3’H and LAR. MYB TFs have been more widely and profoundly investigated for their role in lignin deposition and flavonoid biosynthesis than other TFs. MYB58 and MYB63 are key regulators participated in lignin biosynthesis during the formation of secondary cell wall in Arabidopsis (Zhou et al. 2009). MdMYB1 (An et al. 2019) and VvMYB114 (Tirumalai et al. 2019) function as transcriptional activators or repressors to regulate the anthocyanins accumulation. In strawberry, MYB10 is a key transcription factor contributing to fruit color changes, while FaMYB1 is a repressor of anthocyanin biosynthesis (Aharoni et al. 2001; Castillejo et al. 2020). The R2R3-MYB transcription factor, FaEOBII is orthologous to the Petunia × hybrida PhEOBII and contributes to eugenol biosynthesis in petals by regulating FaCAD1 and FaEGS2 expression (Medina-Puche et al. 2015). Jiang et al. (2022) reported that DOF1.2 may be involved in repressing the FaC4H expression in the strawberry flesh. Moreover, DOF TFs also play roles in regulation of development and differentiation, metabolism, responses to biotic and abiotic stress (Yanagisawa 2004). FaDOF2 contributes to eugenol production in ripe fruit receptacles by controlling FaEGS2 expression and interacts with FaEOBII (Molina-Hidalgo et al. 2017). However, FaDOF2 and FaEOBII are fruit ripening-related genes whilst eugenol concentration peaks at the small green stage during early fruit development, when these transcription factors are expressed very low. In our previous work, a DOF transcription factor, FaDOF1, was found using FaEGS1 as the bait in Y1H screening library, but its function is still unknown.
We have identified FaDOF1, a transcription factor associated to eugenol biosynthesis in strawberry during early fruit development stages. Functional characterization by transient assays confirmed the role of FaDOF1 in eugenol biosynthesis. Overexpression of FaDOF1 increased eugenol production while silencing the gene by RNAi inhibited eugenol accumulation (Fig. 3). GUS staining, dual-LUC, and Y1H assays showed complementary results and indicated that FaDOF1 binds to the promoters of the structural eugenol biosynthetic genes, FaEGS1 and FaEGS2, and regulates their transcription (Figs. 4 and 5).
DOF family proteins contain a highly conserved amino acid regions that potentially serve as nuclear localization signals (NLSs) directing proteins to the nucleus as demonstrated in tomato (Rojas-Gracia et al. 2019), sweet cherry (Zhai et al. 2022), and banana (Feng et al. 2016). Similarly, we have confirmed the nuclear localization of FaDOF1 through the transient expression of the gene in tobacco leaves (Fig. 1C). FaDOF1 shows a transcriptional pattern similar to FaMYB63 (Wang et al. 2022), which also promotes eugenol production by directly or indirectly regulating the expression of structural genes (EGS1, EGS2, and CAD1), and other transcription factors (FaMYB10 and FaEOBII). Interestingly, FaEOBII expression was controlled by FaMYB10, affecting both anthocyanin and eugenol concentrations (Wang et al. 2022; Medina-Puche et al. 2015). Several studies showed that DOF TFs interact with other TFs, such as bZIP, MYB, ERF, and ZFP, and play divergent roles in light responsiveness, defense functions, seed development or germination, and phytohormone signaling (Feng et al. 2016; Yang et al. 2022). CsDOF3 and CsMYB308 regulate chlorophyllase production by forming a repressosome complex in young tea leaves (Liu et al. 2023). MaDof23 and MaERF9 participate in the regulation of banana fruit ripening by competitively binding to the promoters of ten ripening-related genes (Feng et al. 2016). However, the single zinc-finger Cycling Dof Factors (CDFs) and Phytochrome-Interacting Factors (PIFs) act as enhanceosome complexes promoting cell elongation (Gao et al. 2022). A similar interaction could occur between FaDOF1 and FaMYB63 transcription factors to control eugenol accumulation in early strawberry fruit development. Both transcription factors are not only co-expressed and up-regulated early in fruit development, but we have also shown how these two transcription factors can independently activate the promoter of FaEGS1, regulating the transcription of the eugenol biosynthetic gene FaEGS1 (present study; Wang et al. 2022). Further investigation would be needed to test the interaction between FaDOF1 and FaMYB63 and its molecular mechanism in modulating eugenol biosynthesis. In Petunia × hybrida petals, the three R2R3-MYB TFs (PhEOBII, ODO1, and EOBI) are well characterized and have unique interaction pattern. PhEOBII is involved in volatile phenylpropanoids production, such as eugenol and isoeugenol, and forms a regulatory triad with ODO1 and EOBI (Verdonk et al. 2005; Spitzer-Rimon et al. 2010, 2012; Van Moerkercke et al. 2011). Similar regulatory modules are established in Arabidopsis anthers where an orthologous protein triad (MYB99, MYB21, and MYB24) regulates various aspects of the complex phenylpropanoid pathway (Battat et al. 2019). However, the expression of FaDOF1 in fruit after the white stage was low, suggesting that it may have significant differences in gene expression and translation or maintains higher protein integrity and/or activity level in fruit than in other tissues (Wang et al. 2021; Colquhoun et al. 2011).
The phenylpropanoid pathway starts from phenylalanine, which is converted into p-coumaroyl-CoA by reactions catalyzed by PAL, C4H, and 4CL, mediating carbon flux from primary metabolisms to core and specialized phenylpropanoid branch pathways (Fraser et al. 2011). In our study, transient overexpression and RNAi assays in strawberry fruit confirmed that FaDOF1 was an activator of eugenol biosynthesis. Overexpressing FaDOF1 promoted eugenol accumulation, while silencing FaDOF1 repressed eugenol production. FaEGS1 and FaEGS2 in fruit were also significantly elevated when FaDOF1 was overexpressed, whilst downregulated when FaDOF1 was silenced (Fig. 3).
The DOF TFs typically bind to the AAAG sequence within the promoter regions of their downstream target genes to carry out their transcriptional regulatory function as demonstrated by BrDof2.4 in Brassica rapa (Wang et al. 2021), MaDof23 in Musa acuminata (Feng et al. 2016), FaDOF2 in strawberry (Aragüez et al. 2013), and PavDof2/6/15 in Prunus avium (Zhai et al. 2022). AtDOF4;2 acts as a positive and negative regulator of multiple target promoters in response to phenylpropanoid metabolism in Arabidopsis, such as FLS, DFR, LDOX, TT19, PAL1-2, C4H, and 4CL5, although EGS was not included in the screening (Skirycz et al. 2008). Our Y1H assay results suggest that FaDOF1 could directly bind to the DOF elements in the FaEGS1 and FaEGS2 promoters in yeast (Fig. 4), whilst the dual-LUC and GUS assays showed that FaDOF1 significantly activated the promoter of FaEGS1 and FaEGS2 (Fig. 5).
Molecular and phenotypic data of transient FaDOF1-overexpression and FaDOF1-RNAi fruits showed that FaDOF1 accelerated eugenol accumulation in strawberry fruit by activating the activities of EGS1 and EGS2 pomoters. Our study reveals a novel regulatory mechanism governing phenylpropanoid biosynthesis in strawberry, which could be exploited to better understand supplementary roles of phenylpropanoid in other horticultural fruits.
References
Aharoni A, De Vos CH, Wein M, Sun ZK, Greco R, Kroon A, Mol JN, O’Connell AP (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28(3):319–332. https://doi.org/10.1046/j.1365-313x.2001.01154.x
An JP, Zhang XW, You CX, Bi SQ, Wang XF, Hao YJ (2019) MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol 224(1):380–395. https://doi.org/10.1111/nph.16008
Anu Prathap MU, Wei C, Sun SN, Xu ZCJ (2015) A new insight into electrochemical detection of eugenol by hierarchical sheaf-like mesoporous NiCo2O4. Nano Res 8:2636–2645. https://doi.org/10.1007/s12274-015-0769-z
Aragüez I, Osorio S, Hoffmann T, Rambla JL, Medina-Escobar N, Granell A, Botella MÁ, Schwab W, Valpuesta V (2013) Eugenol production in achenes and receptacles of strawberry fruits is catalyzed by synthases exhibiting distinct kinetics. Plant Physiol 163(2):946–958. https://doi.org/10.1104/pp.113.224352
Battat M, Eitan A, Rogachev I, Hanhineva K, Fernie A, Tohge T, Beekwilder J, Aharoni A (2019) A MYB triad controls primary and phenylpropanoid metabolites for pollen coat patterning. Plant Physiol 180(1):87–108. https://doi.org/10.1104/pp.19.00009
Bezerra DP, Militão GCG, de Morais MC, de Sousa DP (2017) The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 9(12):1367. https://doi.org/10.3390/nu9121367
Cai XF, Zhang YY, Zhang CJ, Zhang TY, Hu TX, Ye J, Zhang JH, Wang TT, Li HX, Ye ZB (2013) Genome-wide analysis of plant-specific Dof transcription factor family in tomato. J Integr Plant Biol 55(6):552–566. https://doi.org/10.1111/jipb.12043
Castillejo C, Waurich V, Wagner H, Ramos R, Oiza N, Muñoz P, Triviño JC, Caruana J, Liu ZC, Cobo N, Hardigan MA, Knapp SJ, Vallarino JG, Osorio S, Martín-Pizarro C, Posé D, Toivainen T, Hytönen T, Oh Y, Barbey CR, Whitaker VM, Lee S, Olbricht K, Sánchez-Sevilla JF, Amaya I (2020) Allelic variation of MYB10 is the major force controlling natural variation in skin and flesh color in strawberry (Fragaria spp.) fruit. Plant Cell 32(12):3723–3749. https://doi.org/10.1105/tpc.20.00474
Colquhoun TA, Kim JY, Wedde AE, Levin LA, Schmitt KC, Schuurink RC, Clark DG (2011) PhMYB4 fine-tunes the floral volatile signature of Petunia × hybrida through PhC4H. J Exp Bot 62(3):1133–1143. https://doi.org/10.1093/jxb/erq342
Corrales AR, Nebauer SG, Carrillo L, Fernández-Nohales P, Marqués J, Renau-Morata B, Granell A, Pollmann S, Vicente-Carbajosa J, Molina RV, Medina J (2014) Characterization of tomato cycling Dof factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J Exp Bot 65(4):995–1012. https://doi.org/10.1093/jxb/ert451
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7(7):1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Feng BH, Han YC, Xiao YY, Kuang JF, Fan ZQ, Chen JY, Lu WJ (2016) The banana fruit Dof transcription factor MaDof23 acts as a repressor and interacts with MaERF9 in regulating ripening-related genes. J Exp Bot 67(8):2263–2275. https://doi.org/10.1093/jxb/erw032
Fraser CM, Chapple C (2011) The phenylpropanoid pathway in Arabidopsis. The Arabidopsis Book 9:e0152. https://doi.org/10.1199/tab.0152
Gachon CMM, Langlois-Meurinne M, Saindrenan P (2005) Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci 10(11):542–549. https://doi.org/10.1016/j.tplants.2005.09.007
Gao H, Song W, Severing E, Vayssières A, Huettel B, Franzen R, Richter R, Chai JJ, Coupland G (2022) PIF4 enhances DNA binding of CDF2 to co-regulate target gene expression and promote Arabidopsis hypocotyl cell elongation. Nat Plants 8(9):1082–1093. https://doi.org/10.1038/s41477-022-01213-y
Goff SA, Cone KC, Chandler VL (1992) Functional analysis of the transcriptional activator encoded by the maize B gene: evidence for a direct functional interaction between two classes of regulatory proteins. Gene Dev 6(5):864–875. https://doi.org/10.1101/gad.6.5.864
Gupta S, Malviya N, Kushwaha H, Nasim J, Bisht NC, Singh VK, Yadav D (2015) Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 241(3):549–562. https://doi.org/10.1007/s00425-014-2239-3
Iwamoto M, Higo K, Takano M (2009) Circadian clock- and phytochrome-regulated Dof-like gene, Rdd1, is associated with grain size in rice. Plant Cell Environ 32(5):592–603. https://doi.org/10.1111/j.1365-3040.2009.01954.x
Jia HF, Shen YY (2013) Virus-induced gene silencing in strawberry fruit. Methods Mol Biol 975:211–218. https://doi.org/10.1007/978-1-62703-278-016
Jiang Y, Zeng B, Zhao HN, Zhang M, Xie SJ, Lai JS (2012) Genome-wide transcription factor gene prediction and their expressional tissue-specificities in maize. J Integr Plant Biol 54(9):616–630. https://doi.org/10.1111/j.1744-7909.2012.01149.x
Jiang L, Yue M, Liu Y, Ye Y, Zhang Y, Lin Y, Wang X, Chen Q, Tang H (2022) Alterations of phenylpropanoid biosynthesis lead to the natural formation of pinkish-skinned and white-fleshed strawberry (Fragaria × ananassa). Int J Mol Sci 23(13):7375. https://doi.org/10.3390/ijms23137375
Jiang LY, Yue ML, Liu YQ, Zhang NT, Lin YX, Zhang YT, Wang Y, Li MY, Luo Y, Zhang Y, Wang XR, Chen Q, Tang HR (2023) A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol J 21(6):1140–1158. https://doi.org/10.1111/pbi.14024
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J 9(8):826–837. https://doi.org/10.1111/j.1467-7652.2011.00592.x
Li DJ, Yang CH, Li XB, Gan Q, Zhao XF, Zhu LH (2009) Functional characterization of rice OsDof12. Planta 229(6):1159–1169. https://doi.org/10.1007/s00425-009-0893-7
Lijavetzky D, Carbonero P, Vicente-Carbajosa J (2003) Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol Biol 3:17. https://doi.org/10.1186/1471-2148-3-17
Liu WM, Liu SY, Zhang KY, Xie MW, Sun HW, Huang XQ, Zhang LX, Li M (2023) Chlorophyllase is transcriptionally regulated by CsMYB308/CsDOF3 in young leaves of tea plant. Hortic Plant J 9(6):1162–1176. https://doi.org/10.1016/j.hpj.2022.12.001
Luo C, Hu YY, Shu B (2022) Identifying strawberry DOF family transcription factors and their expressions in response to crown rot. Not Bot Horti Agrobot Cluj-Napoca 50(1):12640. https://doi.org/10.15835/nbha50112640
Medina-Puche L, Molina-Hidalgo FJ, Boersma M, Schuurink RC, López-Vidriero I, Solano R, Franco-Zorrilla JM, Caballero JL, Blanco-Portales R, Muñoz-Blanco J (2015) An R2R3-MYB transcription factor regulates eugenol production in ripe strawberry fruit receptacles. Plant Physiol 168(2):598–614. https://doi.org/10.1104/pp.114.252908
Molina-Hidalgo FJ, Medina-Puche L, Cañete-Gómez C, Franco-Zorrilla JM, López-Vidriero I, Solano R, Caballero JL, Rodríguez-Franco A, Blanco-Portales R, Muñoz-Blanco J, Moyano E (2017) The fruit-specific transcription factor FaDOF2 regulates the production of eugenol in ripe fruit receptacles. J Exp Bot 68(16):4529–4543. https://doi.org/10.1093/jxb/erx257
Moreno-Risueno MÁ, Martínez M, Vicente-Carbajosa J, Carbonero P (2007) The family of DOF transcription factors: from green unicellular algae to vascular plants. Mol Genet Genomics 277(4):379–390. https://doi.org/10.1007/s00438-006-0186-9
Qin H, Wang J, Chen XB, Wang FF, Peng P, Zhou Y, Miao YC, Zhang YQ, Gao YD, Qi YD, Zhou JH, Huang RF (2019) Rice OsDOF15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol 223(2):798–813. https://doi.org/10.1111/nph.15824
Rojas-Gracia P, Roque E, Medina M, López-Martín MJ, Cañas LA, Beltrán JP, Gómez-Mena C (2019) The DOF transcription factor SlDOF10 regulates vascular tissue formation during ovary development in tomato. Front Plant Sci 10:216. https://doi.org/10.3389/fpls.2019.00216
Rueda-Romero P, Barrero-Sicilia C, Gómez-Cadenas A, Carbonero P, Oñate-Sánchez L (2012) Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J Exp Bot 63(5):1937–1949. https://doi.org/10.1093/jxb/err388
Sánchez-Gómez C, Posé D, Martín-Pizarro C (2022) Insights into transcription factors controlling strawberry fruit development and ripening. Front Plant Sci 13:1022369. https://doi.org/10.3389/fpls.2022.1022369
Skirycz A, Jozefczuk S, Stobiecki M, Muth D, Zanor MI, Witt I, Mueller-Roeber B (2007) Transcription factor AtDOF4;2 affects phenylpropanoid metabolism in Arabidopsis thaliana. New Phytol 175(3):425–438. https://doi.org/10.1111/j.1469-8137.2007.02129.x
Skirycz A, Radziejwoski A, Busch W, Hannah MA, Czeszejko J, Kwaśniewski M, Zanor MI, Lohmann JU, De Veylder L, Witt I, Mueller-Roeber B (2008) The DOF transcription factor OBP1 is involved in cell cycle regulation in Arabidopsis thaliana. Plant J 56(5):779–792. https://doi.org/10.1111/j.1365-313X.2008.03641.x
Spitzer-Rimon B, Farhi M, Albo B, Cna’ani A, Ben Zvi MM, Masci T, Edelbaum O, Yu YX, Shklarman E, Ovadis M, Vainstein A (2012) The R2R3-MYB-like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural scent-related genes in petunia. Plant Cell 24(12):5089–5105. https://doi.org/10.1105/tpc.112.105247
Spitzer-Rimon B, Marhevka E, Barkai O, Marton I, Edelbaum O, Masci T, Prathapani NK, Shklarman E, Ovadis M, Vainstein A (2010) EOBII, a gene encoding a flower-specific regulator of phenylpropanoid volatiles’ biosynthesis in petunia. Plant Cell 22(6):1961–1976. https://doi.org/10.1105/tpc.109.067280
Tirumalai V, Swetha C, Nair A, Pandit A, Shivaprasad PV (2019) miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J Exp Bot 70(18):4775–4792. https://doi.org/10.1093/jxb/erz264
Van Moerkercke A, Haring MA, Schuurink RC (2011) The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J 67(5):917–928. https://doi.org/10.1111/j.1365-313X.2011.04644.x
Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC (2005) ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17(5):1612–1624. https://doi.org/10.1105/tpc.104.028837
Wang CM, Zeng ZX, Su XG, Lakshmanan P, Shan W, Kuang JF, Lu WJ, Chen JY, Zhao YT (2021) A transcriptional repressor BrDof2.4 regulates protease genes involved in postharvest leaf senescence in Chinese flowering cabbage. Postharvest Biol Technol 181:111680. https://doi.org/10.1016/j.postharvbio.2021.111680
Wang HW, Zhang B, Hao YJ, Huang J, Tian AG, Liao Y, Zhang JS, Chen SY (2007) The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J 52(4):716–729. https://doi.org/10.1111/j.1365-313X.2007.03268.x
Wang SS, Shi MY, Zhang Y, Pan ZF, Xie XB, Zhang LZ, Sun PP, Feng H, Xue H, Fang CB, Zhao J (2022) The R2R3-MYB transcription factor FaMYB63 participates in regulation of eugenol production in strawberry. Plant Physiol 188(4):2146–2165. https://doi.org/10.1093/plphys/kiac014
Wang YY, Wang PW, Wang WH, Kong LX, Tian SP, Qin GZ (2021) Genome-wide binding analysis of the tomato transcription factor SlDof1 reveals its regulatory impacts on fruit ripening. Mol Hortic 1(1):9. https://doi.org/10.1186/s43897-021-00011-y
Wei JT, Zhao SP, Zhang HY, Jin LG, Yu TF, Zheng L, Ma J, Chen J, Zhou YB, Chen M, Fu JD, Ma YZ, Xu ZS (2023) GmDof41 regulated by the DREB1-type protein improves drought and salt tolerance by regulating the DREB2-type protein in soybean. Int J Biol Macromol 230:123255. https://doi.org/10.1016/j.ijbiomac.2023.123255
Xiao QL, Liu TT, Ling M, Ma QN, Cao W, Xing FY, Huang TH, Zhang YY, Duan H, Liu ZZ (2022) Genome-wide identification of DOF gene family and the mechanism dissection of SbDof21 regulating starch biosynthesis in Sorghum. Int J Mol Sci 23(20):12152. https://doi.org/10.3390/ijms232012152
Yanagisawa S (2001) The transcriptional activation domain of the plant-specific Dof1 factor functions in plant, animal, and yeast cells. Plant Cell Physiol 42:813–822. https://doi.org/10.1093/pcp/pce105
Yanagisawa S (2004) Dof domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol 45:386–391. https://doi.org/10.1093/pcp/pch055
Yang XH, Tuskan GA, Cheng MZ (2006) Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant Physiol 142(3):820–830. https://doi.org/10.1104/pp.106.083642
Yang YY, He ZL, Bing QH, Duan XY, Chen SY, Zeng M, Liu XG (2022) Two Dof transcription factors promote flavonoid synthesis in kumquat fruit by activating C-glucosyltransferase. Plant Sci 318:111234. https://doi.org/10.1016/j.plantsci.2022.111234
Zhai ZF, Xiao YQ, Wang YY, Sun YT, Peng X, Feng C, Zhang X, Du BY, Zhou X, Wang C, Liu Y, Li TH (2022) Abscisic acid-responsive transcription factors PavDof2/6/15 mediate fruit softening in sweet cherry. Plant Physiol 190(4):2501–2518. https://doi.org/10.1093/plphys/kiac440
Zhou JL, Lee CH, Zhong RQ, Ye ZH (2009) MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 21(1):248–266. https://doi.org/10.1105/tpc.108.063321
Funding
This work was supported by the Anhui Province Natural Science Foundation (2108085MC105), Key R&D Program of China (2022YFD1600700), and Natural Science Foundation of University in Anhui Province, China (2022AH050931 and 2023AH051043).
Author information
Authors and Affiliations
Contributions
Zhifei Pan, Rongyi Jiang, Congbing Fang, and Jing Zhao designed the experiments. Zhifei Pan and Rongyi Jiang performed the experiments. Zhifei Pan, Rongyi Jiang, and Tao Tao collected and analyzed data. Xingbin Xie, Guanghui Zheng, and Peipei Sun assisted in the design of this study. Congbing Fang and Jing Zhao wrote and edited the paper. Simona Nardozza and Mauren Jaudal revised the manuscript and provided discussion. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, Z., Jiang, R., Xie, X. et al. The DOF transcription factor, FaDOF1 affects eugenol accumulation in strawberry. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01213-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01213-2