Abstract
Main conclusion
The structural, functional and in-silico studies of Dof transcription factor attempted so far reveals immense opportunity to analyze the plant genomes in terms of number of Dof genes and discuss in light of the evolution. The multiple functions of Dof genes needs to explored for crop improvement.
Transcription factors play a very vital role in gene regulation at transcriptional level and are being extensively studied across phylas. In recent years, sequencing of plant genomes has led to genome-wide identification and characterizations of diverse types of plant-specific transcription factor gene family providing key insights into their structural and functional diversity. The DNA binding with one finger (Dof), a class belonging to C2H2-type zinc finger family proteins, is a plant-specific transcription factor having multiple roles such as seed maturation and germination, phytohormone and light-mediated regulation and plant responses to biotic and abiotic stresses. Dof proteins are present across plant lineage, from green algae to higher angiosperm, and represent a unique class of transcription factor having bifunctional binding activities, with both DNA and proteins, to regulate the complex transcriptional machinery in plant cells. The structural and functional diversity of the Dof transcription factor family along with the bioinformatics analysis highlighting the phylogeny of Dof families is reviewed in light of its importance in plant biotechnology for crop improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription factors (TFs) recognize specific DNA sequence elements within promoter and are responsible for activating or repressing the activity of RNA polymerase vis-a-vis controlling the temporal and spatial expression of a target gene. TFs manifest their function through direct or indirect (through co-activators) interactions with the basal apparatus that lead to changes in the properties of RNA polymerase. A typical plant transcription factor contains a DNA-binding domain, an oligomerization site, a transcription regulation domain and a nuclear localization signal. Most of the transcription factors have only one type of DNA-binding region and oligomerization region although some lack transcription regulation domain or a specific DNA-binding region. The DNA-binding domain of a TF interacts with DNA at the cis -sequence elements owing to the presence of basic amino acid and thus determines the specificity of the transcription factor. The spatial arrangement of these amino acid residues in DNA-binding domain seems to affect their affinity and selectivity. The oligomerization region is responsible for determining protein–protein interactions, as most transcription factors can form hetero and/or homodimers, which ultimately affects sequence element specificity, trans-activation efficiency or DNA-binding affinity and nuclear localization (Kono et al. 2012). It is estimated that approximately 5 % of eukaryotic genes encode TFs (Riechmann et al. 2000; Riechmann and Ratcliffe 2000). To date, more than 100 different DNA-binding domains have been recognized (Kummerfeld and Teichmann 2006). Based on their DNA-binding domains, TFs are classified into different families, some of which are found in all eukaryotes (e.g. MYB or bHLH) whilst others are kingdom specific (DOF, DREB, WRKY, NAC). A list of plant-specific transcription factors, their structural features, DNA-binding Domain, biological roles and a representative of 3D structure with their PDB entry is summarized in supplementary table 1.
DNA binding with one finger (Dof) transcription factors
The Dof family is one of the well-characterized plant-specific transcription factor having diverse roles in plant growth and development. In recent years, the sequencing of plant genomes has led to the identification of multiple Dof genes from different plant species. Dof transcription factors play key role in a variety of biological processes like photosynthetic carbon assimilation, light-regulated gene expression, accumulation of seed storage proteins, germination, dormancy, response to phytohormones, flowering time, guard cell-specific gene expression and many more that are considered to be unique to plants. Dof genes are widely distributed in plant kingdom ranging from green unicellular algae to vascular plants and are characterized by the presence of two major domains namely an N-terminal conserved DNA-binding domain and a C-terminal transcriptional regulation domain. Dof proteins generally comprise 200–400 amino acids having a highly conserved domain (Dof domain) of 50–52 amino acids including a C2C2-type zinc finger motif at N-terminal end. Transcriptional regulation domain having diverse amino acid sequences reflects the expected varied functions of the Dof proteins. The typical structure of Dof domain as elucidated from sequence clone of Elusine coracona (EU760631) is shown in Fig. 1 (Kushwaha et al. 2008). The serine stretches that are frequently located immediately downstream of the Dof domains might be the molecular hinges linking the two domains. The strong similarity among Dof DNA-binding domains suggested that all Dof proteins display similar DNA-binding specificity. Indeed, an AAAG sequence or its reversibly oriented sequence, CTTT, is always found in the binding sequence of individual Dof proteins.
Structure of Dof domain of E. coracana and its amino acid sequence (Kushwaha et al. 2008)
DNA-binding domain consists of CX2CX21CX2C motif that has been predicted to have the ability to form a single zinc finger and hence named Dof (DNA binding with one finger) domain proteins (Yanagisawa 2002). The typical feature of Dof families of proteins is the presence of four cysteine residues in the conserved Dof domain region, as shown in Fig. 2. The sequence-specific DNA binding of the Dof domain proteins has been established by many in vitro and in vivo experiments. All Dof proteins analysed so far, except for protein of pumpkin, recognized an AAAG motif as the essential sequence element in DNA-binding assays in vitro (Yanagisawa 2002). Therefore, the conserved Dof domain alone was able to confer its sequence-specific DNA binding (Kisu et al. 1998; Yanagisawa 1995) and the regions outside the Dof domain do not appear to play a major role in interaction with DNA (Yanagisawa and Schmidt 1999). A study revealed that maize Dof1 can bind an AAAG motif in the surface of the nucleosome reconstructed in vitro as well as the motif on naked DNA, although the binding was dependent on the position of the AAAG motif in nucleosome (Cavalar et al. 2003).
Transcription factors representing same family might have diverse activity owing to the presence of transcriptional regulation domain, which may act as repressor or activator, depending on whether they inhibit or stimulate the transcription of target genes. Repression of gene expression may occur via exclusion of activators from target promoters by competitive binding between transcription factors for the same cis-acting element. It may also be due to masking of regulation domains by dimerization of transcription factors, as well as interaction of repression domains with transcription factors. A barley Dof protein (BPBF) is reported to activate the transcription from a putative target promoter, but BPBF with a mutation on the cysteine residue results in its inactivation (Mena et al. 1998).
Dof domain shows bifunctional binding activities
The Dof domain earlier identified as a DNA-binding domain is now regarded as a bifunctional domain showing both DNA-binding and protein–protein interaction activities. The first protein–protein interaction was observed with an Arabidopsis Dof domain protein (OBP1), which was identified as a protein interacting with bZIP proteins associated with stress responses. OBPl specifically increased the binding of the OBF proteins to ‘ocs’ element sequences (Zhang et al. 1995). The Dof2 transcription factor of maize interacts with five different maize High Mobility Group (HMGB) proteins and is stimulated by them for its DNA target site with different efficiencies (Krohn et al. 2002). OsDof3 of rice regulates the gibberellin response with interaction of a GAMYB as studied in a yeast two-hybrid assay (Washio 2003). In another report from Diaz et al. 2002, HvGAMYB and BPBF in barley are part of a regulatory complex wherein (1) both factors display physical interactions in vivo in the yeast two-hybrid system; and (2) for the interaction to occur in the developing endosperm, both TFs were expressed at the same time in this tissue, as documented both by Northern blotting and by in situ hybridization analyses. Another Dof protein from barley, SAD that activates transcription of endosperm-specific genes interacts with R2R3MYB protein (GAMYB) that was studied via bimolecular fluorescent complex (BiFC) approach (Diaz et al. 2005).
PBFs (P-box binding factor) can bind to Dof box in the promoter of storage protein gene and also interact with other transcription factors which bind to the same promoter in the adjacent region such as PBF with O2 in maize (Vicente-Carbajosa et al. 1997) and RPBF with RISBZ in rice (Kawakatsu et al. 2009). WPBF, a wheat PBF Dof, interacts with a protein TaQM, identified from a wheat root cDNA library and activates transcription of an alpha-gliadin gene during wheat seed development (Dong et al. 2007). The BPBF has also been reported to form a ternary complex with two other seed transcription factors, BLZ2 and HvMYBS3 (a R1MYB) from barley in the yeast three-hybrid system. HvMYBS3 is also an activator of gene expression during endosperm development (Rubio-Somoza et al. 2006).
Regulation of a MYB32b in barley under GA signaling is mediated through modulation by two protein complexes, one for activation includes SAD and HvGAMYB while the other WRKY38 and BPBF for repression (Zou et al. 2008). AtDOF4.7 which is involved in regulation of floral organ abscission interacts with another abscission-related transcription factor, Arabidopsis ZINC FINGER PROTEIN2 (Wei et al. 2010). AtDof3.2 which acts as a negative regulator of seed germination was reported to interact in a yeast two-hybrid system and in planta with TCP14, a positive regulator of seed germination and opposes TCP14 function in the regulation of a specific set of ABA-related genes (Rueda-Romero et al. 2012). A list of key Dof interacting proteins reported till date from different plant species along with their functions is summarized in Table 1.
Evolution and expansion of Dof gene family across plant lineage
The complete or nearly complete genome sequence information of rice, Arabidopsis and many other vascular and non-vascular plants provides an opportunity to identify Dof gene family through genome annotation and genome-wide comparative analysis. Using tools of bioinformatics and availability of genome sequence information, attempts have been made to predict the number of Dof genes in different crops (Table 2). There exists great diversity in terms of number of Dof genes in different crops, which possibly reveals their multiple and diverse gene functions across crops.
A genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families revealed the presence of at least 30 and 36 Dof genes, respectively. Further analysis led to their classification into four major clusters of orthologous genes and showed gene loss and duplication events in Arabidopsis and rice that occurred before and after the last common ancestor of the two species (Lijavetzky et al. 2003). Similar types of phylogenetic analyses have been reported for other plant TF families such as the WRKY, R2R3–MYB, bZIP, MADS, GATA, and others (Eulgem et al. 2000; Jakoby et al. 2002; Martin and Paz-Ares 1997; Parenicova et al. 2003; Reyes et al. 2004). A comparison of Dof gene family among poplar, Arabidopsis and rice has been made with emphasis on evolution of Dof genes (Yang et al. 2006). A total of 41 Dof genes were predicted in poplar and their analysis with Arabidopsis and rice based on ancestral paralogous sequences concluded that after a gene duplication event, the evolution of the duplicated genes is driven by purifying selection, Darwinian positive selection, local duplication, translocations and domain co-option (or domain shuffling) (Yang et al. 2006).
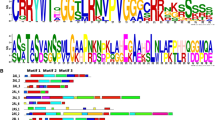
The origin and evolution of the Dof transcription factor family based on phylogenetic analysis of Dof sequences across the representative organisms belonging to green unicellular algae to vascular plants have also been reported. A total of 116 Dof genes representing green unicellular alga (Chlamydomonas reinhardtii), moss (Physcomitrella patens), fern (Selaginella moellendorffii), gymnosperm (Pinus taeda), dicotyledoneous angiosperm (Arabidopsis thaliana) and monocot (Oryza sativa and Hordeum vulgare) have been in silico characterized. The phylogenetic tree constructed (Fig. 3) revealed the existence of six major clusters of orthologous and paralogous genes that probably originated by gene duplication events from a paraphyletic basal grade (Moreno-Risueno et al. 2007b).
Phylogenetic tree constructed using full-length Dof protein sequences from Chlamydomonas reinhardtii (1), Selaginella moellendorfii (10), Physcomitrella patens (5), Pinus taeda (7), Arabidopsis thaliana (36), Oryza sativa Japonica (30), Sorghum bicolor (28), Glycine max (78), Hordeum vulgare (21), Nicotiana tobacum (17), Populus trichocarpa (43), Triticum asetivum (17), Zea mays (44), Brychypodium distactylon (25), Solanum lycopersicum (30), Solanum tuberosum (34), Jatropha curcas (23) and Cajanus cajan (38) using MEGA5.2.2 by NJ method. The full expanded figure has been provided as supplementary Fig. 1
Attempts have also been made to use various bioinformatics tools for characterizing Dof gene family in cereals and millets. Sequence analysis of PCR-amplified 13 Dof domains of cereals (rice, wheat, sorghum, barley, oat, maize, barnyard millet, proso millet, little millet, kodo and foxtail millet) and five Dof genes (rice, wheat, barley, maize and finger millet) revealed its identity to Dof-like proteins. Further, in silico investigation of the cloned Dof genes of finger millet, barley, wheat and maize revealed its identity to PBF Dof, based on the presence of motifs related to regulation of endosperm-specific seed storage protein genes (Kushwaha et al. 2008).
In another study, 31 Dof gene family members of wheat have been identified through extensive analysis of nucleotide databases (Dong et al. 2007). Expression analysis of the TaDof family across all major organs using quantitative RT-PCR revealed that the majority of TaDof members were predominately expressed in vegetative organs. A large number of TaDof members were downregulated by drought and/or were responsive to the light and dark cycle. Light-responsive Dof genes showed their involvement in photosynthesis or sucrose transport. TaDof family might have an important role in light-mediated gene regulation, including involvement in the photosynthetic process (Shaw et al. 2009).
Similarly, 28 Dof genes were identified and in silico characterized in Sorghum bicolor (L.) Moench (Kushwaha et al. 2011). Analysis of intron/exon organization revealed majority of the predicted Dof genes to be intronless as observed in case of rice and Arabidopsis. The cis-regulatory element analysis of the predicted Dof genes revealed the major putative functions as regulation of genes associated with seed storage proteins, abiotic and biotic stresses, photoperiod, growth hormone and meristem. The in silico prediction of two-dimensional and three-dimensional structures of Dof proteins of Sorghum has been generated by multiple threading and iterative structural assembly simulations (Kushwaha et al. 2013). Further, based on gene ontology (GO) terms in I-TASSER server, putative functions of modelled SbDof proteins have been predicted. Very recently, the genome-wide in silico characterization of 25 Dof genes of sugarcane along with comparative phylogenetic analysis with rice, Arabidopsis and sorghum has also been reported (Gupta et al. 2014).
Brachypodium distachyon, the first Pooideae grass sequenced, has been analysed for Dof family members (Hernando-Amado et al. 2012). A total of 27 Dof genes were identified in this grass and a phylogenetic comparison with Oryza sativa and Hordeum vulgare Dofs classified the DOF proteins into four Major Cluster of Orthologous Groups (MCOGs). Using qRT-PCR analysis, the expression profiles of the annotated BdDof genes across four organs (leaves, roots, spikes and seeds was investigated, which further classified them into two distinct groups based on their expression patterns across the tested tissue types. In tomato, 34 Dof genes distributed on 11 chromosomes along with their gene structures, chromosomal locations, phylogeny, protein motifs and evolution pattern have been reported recently (Cai et al. 2013). The expression profiling of these SlDof genes displayed differential expression either in their transcript abundance or in their expression patterns under normal growth conditions (Cai et al. 2013).Recently, 78 putative Dof genes have been reported in soybean, which constitute the largest Dof gene family known from any plant species (Guo and Qiu 2013). These predicted GmDof genes are non-randomly distributed within and across 19 out of 20 chromosomes. The expression pattern of some duplicate genes is partially redundant while others are showing functional diversity, suggesting the occurrence of sub-functionalization during subsequent evolution. These Dof proteins were phylogenetically clustered into nine distinct subgroups. The analysis of whole genome sequence of pigeonpea has identified 38 putative Dof genes (Malviya et al. 2014). Thus there exists a huge diversity of Dof gene members, and their phylogenetic clustering across plant species, which possibly suggest a high degree of functional diversity of Dof proteins in plants.
Expression and functional diversity of Dof proteins
Dof transcription factors are involved in the regulation of biological processes unique to plants such as photosynthetic carbon assimilation, phytochrome signaling, seed maturation and germination, the salicylic acid response, guard cell-specific gene expression, photoperiodic flowering, dormancy, response to phytohormones, accumulation of seed storage proteins, nitrogen assimilation, biosynthesis of glucosinolates and phenyl propanoid metabolism, regulation of oil content in seeds, regulation of glutamine synthetase (GS) gene expression, light-mediated gene regulation, regulation of interfascicular cambium formation and vascular tissue development and leaf axial patterning by promoting revoluta transcription. Recently, it has been reported that some Dof transcription factors also have motif for intercellular protein trafficking spanning highly conserved zinc finger motif (Chen et al. 2013). Dof transcription factor has also been reported to be involved in regulation of drought and salt stress in tomato (Corrales et al. 2014). The variable functions attributed to Dof transcription factor in different crops are summarized in Table 3.
Tissue-specific expression of Dof genes in plants
Regulation of gene expression is crucial for a variety of essential processes in plants such as growth, development, differentiation, metabolic regulation and adaptation to the environment. In maize, Dof1 is a transcriptional activator which shows different activity in response to greening and etiolation of protoplast, whilst Dof2 appears to be a repressor that can block trans-activation of Dof1 (Yanagisawa and Sheen 1998). The Dof protein, PBF, binds to the prolamin box that is a strong candidate for a cis-element responsive for endosperm-specific gene expression (Vicente-Carbajosa et al. 1997). The DAG1 Dof gene in Arabidopsis is specifically expressed in the phloem of all organs of the plant but not in the seed or in the embryo at any stage of development (Papi et al. 2002). The binding of Dof proteins to diverse plant promoters suggests that Dof proteins may be involved in a variety of signal-response and/or tissue-specific gene expressions in plants. Interestingly, all biological processes that are speculated to be mediated by Dof proteins are plant specific e.g. expression of a photosynthetic gene, seed-specific genes and a plant oncogene, and pathogen-responsive gene expression (Kuriakosea et al. 2009).
Dof associated with biotic stress
The role of Dof transcription factors in biotic stress tolerance has been investigated in barley seeds based on their interaction with cystatin gene (Martinez et al. 2005). Cystatins are a group of cysteine proteinase inhibitors that have been identified in vertebrates, invertebrates and plants (Barrett 1987). The pathogen produces cysteine proteinases and peptidase which help them to colonize and proliferate into the host cells. The activities of all these degrading enzymes are influenced by the host cystatin proteins, which resist the pathogen proliferation by inhibiting these enzymes (Barrett 1981). Two Dof proteins, SAD and BPBF, interact specifically in vitro with oligonucleotides containing Dof binding sites derived from the cystatin gene promoter. The OBP1 protein from Arabidopsis that interacts with an OCS element-binding factor OBF4 is also shown to be involved in biotic stress (Singh et al. 2002; Zhang et al. 1995).
Dof as regulator of plant hormone response
Phytohormone signaling pathways are quite complex and their regulation is achieved by binding and coordinated interactions of many transcription factors at cis-acting elements present in the promoter of phytohormones-responsive genes (Yanagisawa 1997). Dof proteins are regulators for plant hormone-responsive genes and have been shown to mediate the response of gibberellins (Washio 2003) and auxins (Baumann et al. 1999). Gibberellins are diterpenoid hormones that play crucial roles in plant growth and development including seed germination, leaf expansion, stem elongation, flower and fruit development (Hooley 1994). The analysis of the alpha-amylase promoters from barley, wheat and rice have identified a conserved cis-element required for GA induction, termed as GA-responsive element (GARE). Although this GARE may not always be tripartite, most often it includes three sequence motifs, the TAACAAA box or GA-responsive element (GARE), the pyrimidine box CCTTTT and the TATCCAC box (Gubler and Jacobsen 1992; Rogers and Rogers 1992; Skriver et al. 1991). The presence of CCTTTT pyrimidine box in GARE, the recognition core motif of Dof proteins, clearly indicates the role of Dof in gibberellins response. The pattern of expression of NtBBF1, a Dof protein known to play a pivotal role in regulating the rolB expression, might provide the possible mechanism of auxin induction (Baumann et al. 1999). Ascorbate oxidases have been reported to be specifically expressed in the quiescent centre of maize root and are involved in organization of root meristems through auxin-dependent expression (Kerk and Feldman 1995). Auxin-responsive cis-region of the pumpkin ascorbate oxidase gene has been identified using a transient assay method (Kisu et al. 1998) and it is likely to be involved in cell growth or division of higher plants.
Dof associated with seed storage protein accumulation
During cereal seed development, nitrogen and sulphur are stored in the starchy endosperm cells, mainly in the form of a complex group of proteins, the prolamines. The genes encoding prolamines are co-ordinately expressed in the developing endosperm where they are under spatial and temporal transcription control, involving cis-acting motifs in their promoters and trans-acting transcription factors. A PBF (Prolamin box)-DOF associated with regulation of endosperm-specific seed storage proteins has been studied in maize, barley, wheat, rice and finger millet (Noguero et al. 2013). The first PBF has been cloned from maize and identified as a transcriptional activator of zein gene, a seed storage protein of maize (Vicente-Carbajosa et al. 1997). A cDNA encoding a DNA-binding protein of the Dof class of transcription factor has been isolated from a barley endosperm library (Mena et al. 1998). Transient expression experiments in developing barley endosperms demonstrated that BPBF trans-activates transcription from the P-box element of a native Hor2 (hordein) promoter. Similarly, rice PBF (RPBF), isolated from rice cDNA expressed sequence tag clones, is known to play important role as an activator for seed storage protein genes and involved in quantitative regulation by combinatorial interactions with RISBZ1 in determining endosperm-specific expression (Yamamoto et al. 2006). A transient expression experiment also demonstrated that the wheat prolamin-box binding factor (WPBF), isolated from wheat endosperm, could trans-activate transcription of native alpha-gliadin promoter by binding to the intact PB (Dong et al. 2007).
Dof associated with carbon and nitrogen metabolism
Dof proteins are found to be an activator for multiple genes associated with the organic acid metabolism, including expression of phosphoenolpyruvate carboxylase (PEPC) gene (Yanagisawa 2000). The maize Dof1 has been reported to be an activator of PEPC gene expression and enhances transcription from the promoters of orthophosphate dikinase and pyruvate kinase (Yanagisawa 2000), as expression of Dof1-specific antisense RNA or the DNA-binding domain of Dof1 alone reduced the expression of these genes. Nitrogen assimilation is an essential process for the growth and development of plants, where inorganic nitrogen in the soil in the form of nitrate and ammonia is initially converted into glutamine and glutamate by two enzymes, glutamine synthase and glutamate synthase. Nitrogen assimilation requires not only inorganic nitrogen but also the carbon skeleton, 2-oxogluterate. Because Dof1 appears to be a key regulator in the coordinated gene expression involved in carbon skeleton production and there is an intimate link between carbon and nitrogen metabolism, there is a speculation that Dof1 modulates nitrogen assimilation as well. Elementary analysis of Dof1 over-expression/knock-down transgenic Arabidopsis plants revealed increased nitrogen content in transgenic plants (30 %), indicating promotion of net nitrogen assimilation (Yanagisawa 2004). Most significantly, the Dof1 transgenic plants exhibit improved growth under low nitrogen conditions. Another Dof-type zinc finger transcription factor gene SRF1 was isolated from sweet potato (Ipomoea batatas) preferentially expressing in storage roots and reported to modulate the carbohydrate metabolism in the storage roots through negative regulation of a vacuolar invertase gene (Tanaka et al. 2009).
Dof proteins involved in light responses
Plants can perceive subtle changes in light quality and quantity through a set of photoreceptors, including phytochromes and cryptochromes. Upon perception, these photoreceptors initiate signal transduction pathways leading to photomorphogenic changes in development. The Dof proteins are also involved in light responses to plants. OBP3, an Arabidopsis Dof protein, is a component in both phyB and cryptochrome 1 (cry1) signaling pathways, acting as a positive and negative regulator, respectively (Ward et al. 2005). Another Arabidopsis Dof protein, COG1 negatively regulates both phytochrome A and phytochrome B signaling pathway (Park et al. 2003). In Arabidopsis, several Dof transcription factors namely CDF1, CDF2, CDF3 and CDF5 are reported to be essential for photoperiodic control of flowering, and mutation in CDF is responsible for photoperiod-insensitive early flowering by increasing CONSTANTS expression levels (Fornara et al. 2009; Imaizumi et al. 2005). Two of the rice Dof genes, OsDof12 and Rdd1 are also phy-regulated genes. OsDof12 regulates flowering in long-day condition and is inhibited by dark treatment (Li et al. 2009), while Rdd1 regulates grain size in rice and its expression differs in continuous dark and light conditions (Iwamoto et al. 2009). Dof transcription factor genes also exhibit circadian rhythms and play a crucial role in the control of flowering time by photoperiodic perception in plants. Two full-length cDNA, JcDof1 and JcDof3 isolated from Jatropha curcas seedlings by yeast one-hybrid library reveal their expression under long day, short day and continuous light conditions and have been shown to interact with F-box proteins to regulate photoperiodic flowering (Yang et al. 2011). Recently, a full-length Dof1 of finger millet, associated with circadian cycle, has been reported (Kumar et al. 2014).
Dof influencing plant morphology
In Arabidopsis, Dof proteins related with plant morphological pattern have recently been reported. Guo et al. (2009) revealed the involvement of AtDof5.6 in regulation of interfascicular cambium formation and vascular tissue development (Guo et al. 2009). Expression of three Dof proteins of Arabidopsis namely Dof2.1, Dof4.6 and Dof5.3 has been reported at preprocambial stage in leaves suggesting their role in preprocambial development (Gardiner et al. 2010). Promoter activation of AtDof2.4 and AtDof5.8 in procambial cells of leaf primordial, roots, embryo and prospective veins in leaf primordia of seedlings, cotyledons of developing embryo has also been reported (Konishi and Yanagisawa 2007). AtDof5.1 has been reported to regulate leaf axial patterning (Kim et al. 2010). In a recent study, it was found that AtDof4.2 regulates shoot branching and seed coat formation in Arabidopsis. Further, AtDof4.2-overexpressing plants exhibit an increased branching phenotype, and mutation of the T-M-D motif in AtDof4.2 significantly reduces branching in transgenic plants. The seeds of an AtDof4.2-overexpressing plant showed a collapse-like morphology in the epidermal cells of the seed coat. The Dof4.2 mutant did not exhibit changes in branching or its seed coat though there was substantial increase in the silique length and seed yield (Zou et al. 2013).
Dof involved in guard cell development
SCAP1, a Dof-type transcription factor isolated from Arabidopsis has been reported to express in maturing guard cell but not in guard mother cell. SCAP1 was reported to regulate expression of genes encoding key elements of stomatal functioning and morphogenesis, such as K+ channel protein, MYB60 transcription factor and pectin methyl esterases (Negi et al. 2013). This clearly indicates that SCAP1 regulates essential processes of stomatal guard cell maturation and functions as a key transcription factor regulating the final stages of guard cell differentiation and ultimately influences the morphological development of plant. Dof transcription factor from potato, StDof1, has also been reported to control guard cell-specific gene expression (Plesch et al. 2001). Presence of Dof binding site in the promoter of guard cell-specific genes has been reported in Arabidopsis (Galbiati et al. 2008). Requirement for a specific number of the Dof binding site in the promoter region for guard cell-specific expression has been demonstrated in GAL-GFP trap lines in Arabidopsis (Gardner et al. 2009). Dof binding sites also contribute to guard cell-specific expression of AtMYB60 of Arabidopsis (Cominelli et al. 2011).
Dof associated with seed germination
Various reports in Arabidopsis have shown that Dof proteins are also involved in regulation of seed germination (Noguero et al. 2013). The Arabidopsis Dof gene, DAG1 (Dof Affecting Germination) is involved in the maternal control of seed germination. The DAG1 gene is expressed specifically in the vascular system of the mother plant but not in the embryo or anywhere else in the seed at any stage of development (Papi et al. 2000, 2002). Another member of the Dof gene family in Arabidopsis, DAG2, also showed its involvement in the control of seed germination. The DAG2 mutant seeds have germination properties opposite to those of seeds from the DAG1 knockout type, and the effect of the mutation of DAG2 is maternal. Moreover, seeds from plants that overexpressed DAG1 showed germination characteristics analogous to those of dag2 mutant seeds, whereas seeds from double mutant dag2dag1 plants behave like dag1 seeds. Based on these studies, it was reported that DAG2 and DAG1 have opposite regulatory roles (Gualberti et al. 2002). Similarly, another Dof gene of Arabidopsis, Dof6 influencing seed germination has also been reported. The transcript levels of this gene accumulate in dry seeds and decay gradually after ripening and also upon seed imbibition. While constitutive over-expression of DOF6 produced aberrant growth and sterility in the plant, its over-expression induced upon seed imbibition triggered delayed germination, abscisic acid (ABA)-hypersensitive phenotypes and increased expression of the ABA biosynthetic gene ABA1 and ABA-related stress genes. Wild-type germination and gene expression were gradually restored during seed after ripening, despite DOF6-induced over-expression. These results indicate that DOF6 negatively affects seed germination (Rueda-Romero et al. 2012).
Conclusion
The gene regulation is a complex process involving repertoire of regulatory elements, the transcription factor being one of the most important elements influencing gene regulation at the level of transcription by modulating the cis-regulatory sequences of diverse genes. The significance of transcription factors is being realized based on their quantitative and ubiquitous representation across the plant genomes and subsequently several plant-specific transcription factors have been deciphered. DNA binding with one finger (Dof) constitutes a large family of transcription factors that are associated with various biological processes unique to plants. The functional attributes of Dof have been elucidated in several crops, and the expansion of Dof gene family is being witnessed based on genome-wide analysis of crops whose genome sequences are available. The possible interactions with other transcription factors regulating diverse biological processes, functional characterization of in silico-identified Dof gene families of different crops, elucidating downstream genes being regulated by Dof transcription factors are some of the key issues to be addressed. Further, there is a need to understand developmental and evolutionary dynamics of regulatory networks formed by Dof domain across the phyla and identify Dof genes associated with newer functions like abiotic stresses. The structural diversification of Dof mediated by gene duplication might be closely associated with the development of complex regulatory network during plant evolution but still the epigenetic role associated with the evolution of duplicated Dof genes needs to be investigated. This review highlights the structural, functional and bioinformatics aspect of Dof transcription factor reported till date and tries to identify the gaps for further study.
Author contribution
DY conceived, framed and wrote the final review. SG, NM, HK composed the structural and functional aspects of Dof transcription factor. VKS, NM and JN framed the bioinformatics aspects of Dof. NM composed the supplementary table comprising of description about different plant specific transcription factors and also finalized the phylogenetic tree. NCB revised the initial draft and reframed by finalizing the sub-headings with the required content. All authors coordinated to draft, read and approve the final manuscript.
References
Barajas-Lopez JD, Tezycka J, Travaglia CN, Serrato AJ, Chueca A, Thormahlen I, Geigenberger P, Sahrawy M (2012) Expression of the chloroplast thioredoxins f and m is linked to short-term changes in the sugar and thiol status in leaves of Pisum sativum. J Exp Bot 63(13):4887–4900
Barrett A (1981) Characterization of a genomic sequence coding for potato multicystatin, an eight-domain cystein proteinase inhibitor. Methods Enzymol 80:771–778
Barrett AJ (1987) The cystatin: a new class of peptidase inhibitors. Trends Biochem Sci 12:193–196
Baumann K, De Paolis A, Costantino P, Gualberti G (1999) The DNA binding site of the Dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 11:323–334
Cai X, Zhang Y, Zhang C, Zhang T, Hu T, Ye J, Zhang J, Wang T, Li H, Ye Z (2013) Genome-wide analysis of plant-specific Dof transcription factor family in tomato. J Integr Plant Biol 55:552–566
Cavalar M, Moller C, Offermann S, Krohn NM, Grasser KD, Peterhansel C (2003) The interaction of DOF transcription factors with nucleosomes depends on the positioning of the binding site and is facilitated by maize HMGB5. Biochemistry 42:2149–2157
Chen H, Ahmad M, Rim Y, Lucas WJ, Kim JY (2013) Evolutionary and molecular analysis of Dof transcription factors identified a conserved motif for intercellular protein trafficking. New Phytol 198:1250–1260
Cominelli E, Galbiati M, Albertini A, Fornara F, Conti L, Coupland G, Tonelli C (2011) DOF-binding sites additively contribute to guard cell-specificity of AtMYB60 promoter. BMC Plant Biol 11:162
Corrales AR, Nebauer SG, Carrillo L, Fernandez-Nohales P, Marques J, Renau-Morata B, Granell A, Pollmann S, Vicente-Carbajosa J, Molina RV, Medina J (2014) Characterization of tomato Cycling Dof Factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J Exp Bot 65:995–1012
De Paolis A, Sabatini S, De Pascalis L, Costantino P, Capone I (1996) A rolB regulatory factor belongs to a new class of single zinc finger plant proteins. Plant J 10:215–223
Diaz I, Vicente-Carbajosa J, Abraham Z, Martinez M, Isabel-La Moneda I, Carbonero P (2002) The GAMYB protein from barley interacts with the DOF transcription factor BPBF and activates endosperm-specific genes during seed development. Plant J 29:453–464
Diaz I, Martinez M, Isabel-LaMoneda I, Rubio-Somoza I, Carbonero P (2005) The DOF protein, SAD, interacts with GAMYB in plant nuclei and activates transcription of endosperm-specific genes during barley seed development. Plant J 42:652–662
Dong G, Ni Z, Yao Y, Nie X, Sun Q (2007) Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Mol Biol 63:73–84
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Ruhl M, Jarillo JA, Coupland G (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86
Galbiati M, Simoni L, Pavesi G, Cominelli E, Francia P, Vavasseur A, Nelson T, Bevan M, Tonelli C (2008) Gene trap lines identify Arabidopsis genes expressed in stomatal guard cells. Plant J 53:750–762
Gardiner J, Sherr I, Scarpella E (2010) Expression of DOF genes identifies early stages of vascular development in Arabidopsis leaves. Int J Dev Biol 54:1389–1396
Gardner MJ, Baker AJ, Assie JM, Poethig RS, Haseloff JP, Webb AA (2009) GAL4 GFP enhancer trap lines for analysis of stomatal guard cell development and gene expression. J Exp Bot 60:213–226
Gabriele S, Rizza A, Martone J, Circelli P, Costantino P, Vittorioso P (2010) The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J 61:312–323
Gualberti G, Papi M, Bellucci L, Ricci I, Bouchez D, Camilleri C, Costantino P, Vittorioso P (2002) Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 14:1253–1263
Gubler F, Jacobsen JV (1992) Gibberellin-responsive elements in the promoter of a barley high-pI alpha-amylase gene. Plant Cell 4:1435–1441
Guo Y, Qiu LJ (2013) Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS One 8:e76809
Guo Y, Qin G, Gu H, Qu LJ (2009) Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell 21:3518–3534
Gupta S, Kushwaha H, Singh VK, Bisht NC, Sarangi BK, Yadav D (2014) Genome wide in silico characterization of Dof transcription factor gene family of sugarcane and its comparative phylogenetic analysis with Arabidopsis, rice and sorghum. Sugar Tech 16(4):372–384
Hernando-Amado S, Gonzalez-Calle V, Carbonero P, Barrero-Sicilia C (2012) The family of DOF transcription factors in Brachypodium distachyon: phylogenetic comparison with rice and barley DOFs and expression profiling. BMC Plant Biol 12:202
Hooley R (1994) Gibberellins: perception, transduction and responses. Plant Mol Biol 26:1529–1555
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309:293–297
Isabel-LaMoneda I, Diaz I, Martinez M, Mena M, Carbonero P (2003) SAD: a new DOF protein from barley that activates transcription of a cathepsin B-like thiol protease gene in the aleurone of germinating seeds. Plant J 33:329–340
Iwamoto M, Higo K, Takano M (2009) Circadian clock- and phytochrome-regulated Dof-like gene, Rdd1, is associated with grain size in rice. Plant Cell Environ 32:592–603
Jakoby M, Weisshaar B, Droge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7:106–111
Jiang Y, Zeng B, Zhao H, Zhang M, Xie S, Lai J (2012) Genome-wide transcription factor gene prediction and their expressional tissue-specificities in maize. J Integr Plant Biol 54:616–630
Kang HG, Foley RC, Onate-Sanchez L, Lin C, Singh KB (2003) Target genes for OBP3, a Dof transcription factor, include novel basic helix-loop-helix domain proteins inducible by salicylic acid. Plant J 35:362–372
Kawakatsu T, Yamamoto MP, Touno SM, Yasuda H, Takaiwa F (2009) Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J 59:908–920
Kerk NM, Feldman LJ (1995) A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development 121:2825–2833
Kim HS, Kim SJ, Abbasi N, Bressan RA, Yun DJ, Yoo SD, Kwon SY, Choi SB (2010) The DOF transcription factor Dof5.1 influences leaf axial patterning by promoting Revoluta transcription in Arabidopsis. Plant J 64:524–535
Kisu Y, Ono T, Shimofurutani N, Suzuki M, Esaka M (1998) Characterization and expression of a new class of zinc finger protein that binds to silencer region of ascorbate oxidase gene. Plant Cell Physiol 39:1054–1064
Konishi M, Yanagisawa S (2007) Sequential activation of two Dof transcription factor gene promoters during vascular development in Arabidopsis thaliana. Plant Physiol Biochem 45:623–629
Kono H, Imanishi M, Negi S, Tatsutani K, Sakaeda Y, Hashimoto A, Nakayama C, Futaki S, Sugiura Y (2012) Rational design of DNA sequence-specific zinc fingers. FEBS Lett 586:918–923
Krohn NM, Yanagisawa S, Grasser KD (2002) Specificity of the stimulatory interaction between chromosomal HMGB proteins and the transcription factor Dof2 and its negative regulation by protein kinase CK2-mediated phosphorylation. J Biol Chem 277:32438–32444
Kumar A, Kanwal P, Gupta AK, Singh BR, Gaur VS (2014) A full-length Dof1 transcription factor of finger millet and its response to a circadian cycle. Plant Mol Biol Rep 32:419–427
Kummerfeld SK, Teichmann SA (2006) DBD: a transcription factor prediction database. Nucleic Acids Res 34:D74–81
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J 9:826–837
Kuriakosea B, Aruna V, Gnanamanickama SS, Thomas G (2009) Tissue-specific expression in transgenic rice and Arabidopsis thalianaplants ofGUS gene driven by the 50 regulatory sequences of an anther specific rice gene YY2. Plant Sci 177:390–397
Kushwaha H, Gupta N, Singh VK, Kumar A, Yadav D (2008) In silico analysis of PCR amplified DOF (DNA binding with one finger) transcription factor domain and cloned genes from cereals and millets. Online J Bioinform 9:130–143
Kushwaha H, Gupta S, Singh VK, Rastogi S, Yadav D (2011) Genome wide identification of Dof transcription factor gene family in sorghum and its comparative phylogenetic analysis with rice and Arabidopsis. Mol Biol Rep 38:5037–5053
Kushwaha H, Gupta S, Singh VK, Bisht NC, Sarangi BK, Yadav D (2013) Cloning, in silico characterization and prediction of three dimensional structure of SbDof1, SbDof19, SbDof23 and SbDof24 proteins from Sorghum [Sorghum bicolor (L.) Moench]. Mol Biotechnol 54:1–12
Li D, Yang C, Li X, Gan Q, Zhao X, Zhu L (2009) Functional characterization of rice OsDof12. Planta 229:1159–1169
Lijavetzky D, Carbonero P, Vicente-Carbajosa J (2003) Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol Biol 3:17
Malviya N, Gupta S, Singh VK, Yadav MK, Bisht NC, Sarangi BK, Yadav D (2014) Genome wide in silico characterization of Dof gene families of pigeonpea (Cajanus cajan (L) Millisp.). Mol Biol. doi:10.1007/s11033-014-3797-y
Martin C, Paz-Ares J (1997) MYB transcription factors in plants. Trends Genet 13:67–73
Martinez M, Rubio-Somoza I, Fuentes R, Lara P, Carbonero P, Diaz I (2005) The barley cystatin gene (Icy) is regulated by DOF transcription factors in aleurone cells upon germination. J Exp Bot 56:547–556
Mena M, Vicente-Carbajosa J, Schmidt RJ, Carbonero P (1998) An endosperm-specific DOF protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. Plant J 16:53–62
Moreno-Risueno MA, Diaz I, Carrillo L, Fuentes R, Carbonero P (2007a) The HvDOF19 transcription factor mediates the abscisic acid-dependent repression of hydrolase genes in germinating barley aleurone. Plant J 51:352–365
Moreno-Risueno MA, Martinez M, Vicente-Carbajosa J, Carbonero P (2007b) The family of DOF transcription factors: from green unicellular algae to vascular plants. Mol Genet Genomics 277:379–390
Negi J, Moriwaki K, Konishi M, Yokoyama R, Nakano T, Kusumi K, Hashimoto-Sugimoto M, Schroeder JI, Nishitani K, Yanagisawa S, Iba K (2013) A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr Biol 23:479–484
Noguero M, Atif RM, Ochatt S, Thompson RD (2013) The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci Int J Exp Plant Biol 209:32–45
Papi M, Sabatini S, Bouchez D, Camilleri C, Costantino P, Vittorioso P (2000) Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev 14:28–33
Papi M, Sabatini S, Altamura MM, Hennig L, Schafer E, Costantino P, Vittorioso P (2002) Inactivation of the phloem-specific Dof zinc finger gene DAG1 affects response to light and integrity of the testa of Arabidopsis seeds. Plant Physiol 128:411–417
Parenicova L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B, Angenent GC, Colombo L (2003) Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15:1538–1551
Park DH, Lim PO, Kim JS, Cho DS, Hong SH, Nam HG (2003) The Arabidopsis COG1 gene encodes a Dof domain transcription factor and negatively regulates phytochrome signaling. Plant J 34:161–171
Plesch G, Ehrhardt T, Mueller-Roeber B (2001) Involvement of TAAAG elements suggests a role for Dof transcription factors in guard cell-specific gene expression. Plant J 28:455–464
Reyes JC, Muro-Pastor MI, Florencio FJ (2004) The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol 134:1718–1732
Riechmann JL, Ratcliffe OJ (2000) A genomic perspective on plant transcription factors. Curr Opin Plant Biol 3:423–434
Riechmann JL, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Rogers JC, Rogers SW (1992) Definition and functional implications of gibberellin and abscisic acid cis-acting hormone response complexes. Plant Cell 4:1443–1451
Rubio-Somoza I, Martinez M, Abraham Z, Diaz I, Carbonero P (2006) Ternary complex formation between HvMYBS3 and other factors involved in transcriptional control in barley seeds. Plant J 47:269–281
Rueda-Lopez M, Crespillo R, Canovas FM, Avila C (2008) Differential regulation of two glutamine synthetase genes by a single Dof transcription factor. Plant J 56:73–85
Rueda-Romero P, Barrero-Sicilia C, Gomez-Cadenas A, Carbonero P, Onate-Sanchez L (2012) Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J Exp Bot 63:1937–1949
Shaw LM, McIntyre CL, Gresshoff PM, Xue GP (2009) Members of the Dof transcription factor family in Triticum aestivum are associated with light-mediated gene regulation. Funct Integr Genomics 9:485–498
Shimofurutani N, Kisu Y, Suzuki M, Esaka M (1998) Functional analyses of the Dof domain, a zinc finger DNA-binding domain, in a pumpkin DNA-binding protein AOBP. FEBS Lett 430:251–256
Singh K, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, Mueller-Roeber B, Witt I (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47:10–24
Skirycz A, Jozefczuk S, Stobiecki M, Muth D, Zanor MI, Witt I, Mueller-Roeber B (2007) Transcription factor AtDOF4;2 influences phenylpropanoid metabolism in Arabidopsis thaliana. New Phytol 175: 425–438
Skriver K, Olsen FL, Rogers JC, Mundy J (1991) cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc Natl Acad Sci USA 88:7266–7270
Sugiyama T, Ishida T, Tabei N, Shigyo M, Konishi M, Yoneyama T, Yanagisawa S (2012) Involvement of PpDof1 transcriptional repressor in the nutrient condition-dependent growth control of protonemal filaments in Physcomitrella patens. J Exp Bot 63(8):3185–3197
Tanaka M, Takahata Y, Nakayama H, Nakatani M, Tahara M (2009) Altered carbohydrate metabolism in the storage roots of sweet potato plants overexpressing the SRF1 gene, which encodes a Dof zinc finger transcription factor. Planta 230:737–746
Vicente-Carbajosa J, Moose SP, Parsons RL, Schmidt RJ (1997) A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc Natl Acad Sci USA 94:7685–7690
Wang HW, Zhang B, Hao YJ, Huang J, Tian AG, Liao Y, Zhang JS, Chen SY (2007) The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J 52:716–729
Ward JM, Cufr CA, Denzel MA, Neff MM (2005) The Dof transcription factor OBP3 modulates phytochrome and cryptochrome signaling in Arabidopsis. Plant Cell 17:475–485
Washio K (2001) Identification of Dof proteins with implication in the gibberellin-regulated expression of a peptidase gene following the germination of rice grains. Biochim Biophys Acta 1520:54–62
Washio K (2003) Functional dissections between GAMYB and Dof transcription factors suggest a role for protein-protein associations in the gibberellin-mediated expression of the RAmy1A gene in the rice aleurone. Plant Physiol 133:850–863
Wei PC, Tan F, Gao XQ, Zhang XQ, Wang GQ, Xu H, Li LJ, Chen J, Wang XC (2010) Overexpression of AtDOF4.7, an Arabidopsis DOF family transcription factor, induces floral organ abscission deficiency in Arabidopsis. Plant Physiol 153:1031–1045
Yamamoto MP, Onodera Y, Touno SM, Takaiwa F (2006) Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes. Plant Physiol 141:1694–1707
Yanagisawa S (1995) A novel DNA-binding domain that may form a single zinc finger motif. Nucleic Acids Res 23:3403–3410
Yanagisawa S (1997) Dof DNA-binding domains of plant transcription factors contribute to multiple protein-protein interactions. Eur J Biochem 250:403–410
Yanagisawa S (2000) Dof1 and Dof2 transcription factors are associated with expression of multiple genes involved in carbon metabolism in maize. Plant J 21:281–288
Yanagisawa S (2002) The Dof family of plant transcription factors. Trends Plant Sci 7:555–560
Yanagisawa S (2004) Dof domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol 45:386–391
Yanagisawa S, Schmidt RJ (1999) Diversity and similarity among recognition sequences of Dof transcription factors. Plant J 17:209–214
Yanagisawa S, Sheen J (1998) Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell 10:75–89
Yang X, Tuskan GA, Cheng MZ (2006) Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant Physiol 142:820–830
Yang J, Yang MF, Wang D, Chen F, Shen SH (2010) JcDof1, a Dof transcription factor gene, is associated with the light-mediated circadian clock in Jatropha curcas. Physiol Plant 139:324–334
Yang J, Yang MF, Zhang WP, Chen F, Shen SH (2011) A putative flowering-time-related Dof transcription factor gene, JcDof3, is controlled by the circadian clock in Jatropha curcas. Plant Sci Int J Exp Plant Biol 181:667–674
Zhang B, Chen W, Foley RC, Buttner M, Singh KB (1995) Interactions between distinct types of DNA binding proteins enhance binding to ocs element promoter sequences. Plant Cell 7:2241–2252
Zou X, Neuman D, Shen QJ (2008) Interactions of two transcriptional repressors and two transcriptional activators in modulating gibberellin signaling in aleurone cells. Plant Physiol 148:176–186
Zou HF, Zhang YQ, Wei W, Chen HW, Song QX, Liu YF, Zhao MY, Wang F, Zhang BC, Lin Q, Zhang WK, Ma B, Zhou YH, Zhang JS, Chen SY (2013) The transcription factor AtDOF4.2 regulates shoot branching and seed coat formation in Arabidopsis. Biochem J 449:373–388
Acknowledgements
The financial support by UP Council of Agricultural Research, Lucknow in the form of research grant (Letter No.738/DY/CROPS/RF/2014 dated 30/7/2014) to D Yadav and Department of Science and Technology, Government of India, New Delhi in the form of Women Scientist-A fellowship (SR/WOS-A/LS-110/2012(G) to N. Malviya is thankfully acknowledged. S. Gupta acknowledges the CSIR, New Delhi for Senior Research Fellowship. The author wishes to acknowledge the Head, Department of Biotechnology, D.D.U Gorakhpur University, Gorakhpur, INDIA for infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, S., Malviya, N., Kushwaha, H. et al. Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 241, 549–562 (2015). https://doi.org/10.1007/s00425-014-2239-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2239-3