Abstract
Main conclusion
Auxin and abscisic acid regulate strawberry fruit ripening and senescence through cross-talk of their signal transduction pathways that further modulate the structural genes related to physico-chemical properties of fruit.
The physiological and transcriptomic changes in harvested strawberry fruits in responses to IAA, ABA and their combination were analyzed. Exogenous IAA delayed the ripening process of strawberries after harvest while ABA promoted the postharvest ripening. However, treatment with a combination of IAA and ABA did not slow down nor accelerate the postharvest ripening in the strawberry fruits. At the molecular level, exogenous IAA up regulated the expressions of genes related to IAA signaling, including AUX/IAA, ARF, TOPLESS and genes encoding E3 ubiquitin protein ligase and annexin, and down regulated genes related to pectin depolymerization, cell wall degradation, sucrose and anthocyanin biosyntheses. In contrast, exogenous ABA induced genes related to fruit softening, and genes involved in signaling pathways including SKP1, HSPs, CK2, and SRG1. Comparison of transcriptomes in responses to individual treatments with IAA or ABA or the combination revealed that there were cooperative and antagonistic actions between IAA and ABA in fruit. However, 17 % of the differentially expressed unigenes in response to the combination of IAA and ABA were unique and were not found in those unigenes responding to either IAA or ABA alone. The analyses also found that receptor-like kinases and ubiquitin ligases responded to both IAA and ABA, which seemed to play a pivotal role in both hormones’ signaling pathways and thus might be the cross-talk points of both hormones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The octoploid strawberry (Fragaria × ananassa) is one of the important economic fruit species, cultivated and consumed around the world for its pleasant flavor and nutritional values (Li et al. 2013). However, strawberry as a perishable fruit is prone to water loss, decay, physiological deterioration and fungal pathogen infection, due to its rapid ripening and senescence after harvest (Shin et al. 2008). The ripening and senescence in strawberry fruits are genetically programmed complex processes that involve many changes in gene expression and metabolism, including anthocyanin accumulation, chlorophyll degradation, cell wall breakdown, biosynthesis of sugars, flavors and aroma volatiles (Knee et al. 1977; Miszczak et al. 1995; Ornelas-Paz et al. 2013; Chen et al. 2014).
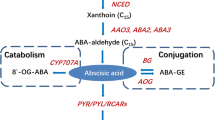
The initiation and progression of fruit ripening and senescence can be regulated by such plant hormones as abscisic acid (ABA) and ethylene (Mcatee et al. 2013). Ethylene is a vital hormone for climacteric fruit ripening and ABA is believed to indirectly regulate ripening via ethylene. In contrast, for non-climacteric fruit, ABA plays a major role in regulating the ripening and senescence (Mcatee et al. 2013). Previous studies showed that exogenous ABA application could increase anthocyanin contents, enhance sucrose accumulation and accelerate the ripening processes in strawberry (OfosuAnim et al. 1996; Jiang and Joyce 2003; Jia et al. 2011).
The earlier work involving a single plant hormone species greatly advanced our understanding of the effects of hormones on fruit ripening and senescence. However, it is now evident that the ripening and senescence processes are regulated in a complex way involving cross-talks among different classes of plant hormones (Munne-Bosch and Muller 2013). For example, IAA, an auxin compound, has been reported to cross-talk with ethylene during ripening in tomato and peach because ethylene production increases with elevation of IAA contents and the transcript levels of IAA-signaling components, and the increased ethylene production can in turn up-regulate IAA biosynthesis and transcription of IAA-signaling components (Jones et al. 2002; Trainotti et al. 2007). In strawberry fruit, IAA suppresses the expression of the 9-cis-epoxycarotenoid dioxygenase genes FaNCED1, FaNCED2, and FaCYP707A1 that are involved in ABA biosynthesis (Ji et al. 2012). Naphthalene acetic acid (NAA), a synthetic auxin compound, has also been observed to delay anthocyanin accumulation, chlorophyll loss, softening and the subsequent ripening of strawberry (Given et al. 1988a; Symons et al. 2012). It has also been reported that the levels of IAA in strawberry fruit are high during its early development but reduce to low levels prior to coloration, which is in contrast to ABA levels that are low at anthesis and gradually increase with fruit development and ripening (Symons et al. 2012). Similar changes in ABA and IAA levels also occurred during storage of strawberry fruits that were harvested at white stage (Chen et al. 2014). Given et al. (1988b) proposed that the decline in IAA concentration in the achenes of strawberry maturing fruits modulated the speed of fruit ripening. All these researches suggest that the ripening and senescence processes of non-climacteric fruits involve complex cross-talks among multiple plant hormones. The underlying mechanisms of the cross-talks between ABA and IAA or ethylene in fruit ripening are yet to be deciphered.
Our previous study found that there was an accelerated reduction in IAA contents and an increase in ABA levels in detached strawberry fruits compared to those in in planta fruits, which might be the reason for accelerated senescence in harvested fruits (Chen et al. 2014). To further understand the molecular basis of the cross-talk between ABA and IAA in regulating the postharvest ripening and senescence of strawberry fruit, we used de novo assembly and digital gene expression (DGE) methods to establish three transcriptomes of harvested strawberry fruits in response to individual treatments with IAA, ABA or their combination, and we then performed comparative analyses of these transcriptomes.
Materials and methods
Plant materials and treatments
Strawberry plants (Fragaria × ananassa) were grown in a plastic greenhouse (Chen et al. 2014), and fruits at 23 days after anthesis were harvested. The harvested fruits were of similar size, and free of diseases and insect pests. The fruits were quickly transported to the laboratory within 1 h of harvest. The fruits were surface sterilized by immersion in 0.1 % sodium hypochlorite solution for 2 min, followed by three rinsings in ddH2O (distilled and deionized water) and brief surface drying in a laminar flow hood. Then, these fruits were randomly divided into four groups, with each containing eighty fruits. For hormone treatments, hormone solution or water (as a control) was injected with 100-µL microsyringe into the fruit core from the pedicel. Group IAA fruits were injected with 100 µL of 1 mM IAA. Similarly, Group ABA fruits were injected with 100 µL of 1 mM ABA, and Group IAA + ABA with 100 µL of mixture solution of IAA (1 mM) and ABA (1 mM). The control fruits were injected with 100 µL water. All these fruits were kept at 20 ± 2 °C, with 90 ± 5 % relative humidity under darkness for 2 days, then quickly frozen in liquid nitrogen and stored in a −80 °C freezer for further analyses.
Determination of tristimulus color and firmness
Tristimulus color was measured on two opposite sides (equatorial area) of each fruit using Chroma Meter CR-400 (Konica Minolta Sensing Inc., Osaka, Japan) and a* value representing red/green ratio was recorded.
Firmness was also measured, using the TA-XT2i Texture Analyzer (Stable Micro Systems Ltd., Surrey, UK), on the area where tristimulus color was quantified. The depth of puncture was 7 mm at a rate of 0.5 mm/s. The maximum force (in Newton) needed to puncture the fruits was recorded.
Determination of endogenous ABA and IAA levels in strawberry fruits
ABA and IAA contents were simultaneously determined using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) as described by Symons et al. (2012). Briefly, the frozen fruit sample (10 g) was ground in liquid nitrogen using a mortar and pestle. One gram powder was mixed with 10 mL 80 % methanol containing 1 mg of 2, 6-di-tert-butyl-4- methylphenol (BHT > 99.0 %, Aladdin Industrial Inc., Shanghai, China). The mixture was kept at 4 °C overnight, and then filtered through a Whatman No. 1 filter paper. The liquid was concentrated to less than 1 mL using speed vacuum at 35 °C. The concentrate was then taken up in 3 × 3 mL of 10 % (v/v) methanol-0.4 % (v/v) acetic acid and injected into a Sep-Pak C18 cartridge (6 cc/500 mg, Waters Corporation, Milford, MA, USA). Plant hormones were eluted from the Sep-Pak with 0.4 % (v/v) acetic acid–methanol solution after a rinse with 10 % (v/v) methanol-0.4 % (v/v) acetic acid. The eluates were vacuum dried, the residue was re-suspended in 20 % (v/v) methanol-0.4 % (v/v) acetic acid and centrifuged at 12,000g for 3 min. The ABA and IAA content in the sample were then analyzed using an Agilent 6460 Triple quadruple LC/MS system (Agilent Technologies Inc., Santa Clara, CA, USA).
Isolation of total RNA
Strawberry fruits were grounded in liquid nitrogen and one gram powder was collected for total RNA extraction using cetyltrimethylammonium bromide (CTAB) protocol as described by Chang et al. (1993).
Construction of cDNA libraries and RNA sequencing
To obtain a transcriptome of Fragaria × ananassa fruit as a reference for DGE, a cDNA library was constructed using the total RNAs pooled from aforementioned four groups (i.e., IAA, ABA, IAA + ABA, and the control) described above. Individual cDNA libraries representing each group of fruit samples were constructed with total RNA from five fruits in that group. Two biological replicates per group were used. Three µg total RNA per group was used as input material for the RNA sample preparations. mRNA was purified from total RNA using poly-T oligo-attached magnetic beads.
Sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instruction. PCR products were purified using the AMPure XP system and library quality was assessed using an Agilent Bioanalyzer 2100 system.
The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq SR Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instruction. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 2000/2500 platform and 125 bp paired-end reads (50 bp single-end reads for DGE) were generated.
De novo assembly and gene functional annotation
For quality control, raw data (raw reads) of fastq format were processed through the in-house perl scripts to remove low-quality reads and reads containing adapters and/or poly (N), resulting in clean data (clean reads). Q20, Q30, GC content and sequence duplication levels of the clean data were also calculated. The high-quality clean data were used for all further analyses.
For transcriptome assembly, ref-1 files and ref-2 files from all libraries were pooled to form one big left.fq file and one big right.fq file, respectively. The left.fq and right.fq files were then subject to the Trinity assembly process (Grabherr et al. 2011) with min-kmer-cov set to 2 and all other parameters to default settings.
Gene function was annotated based on the following databases: NCBI non-redundant protein sequences (Nr), NCBI non-redundant nucleotide sequences (Nt), Protein family (Pfam), Clusters of Orthologous Groups of proteins (KOG/COG), the manually annotated and reviewed protein sequence database (Swiss-Prot), KEGG Ortholog database (KO), and Gene Ontology (GO).
Quantification of gene expression levels and differential expression analysis
Gene expression levels were estimated by RSEM (Li and Dewey 2011) for each sample. Prior to differential gene expression analysis, for each sequenced library, the read counts were adjusted by edgeR program package through one scaling normalized factor. Differential expression analysis of two samples was performed using the DEGseq R package (Wang et al. 2010). P value was adjusted using Q value (Storey and Tibshirani 2003). Q value <0.005 and |log2 (fold change)| > 1 was set as the threshold for significantly differential expression.
RT-qPCR verification of RNA sequencing transcripts
Real-time quantitative PCR (RT-qPCR) was used to validate the digital gene expression data obtained by RNA sequencing. The cDNA of group IAA, ABA, IAA + ABA and control obtained was 10 × diluted and 5 µL was used as the template in each qPCR reaction using the SYBR® Premix Ex Taq™ (TaKaRa Biotechnology, Dalian, China) and the reactions were run using the Applied Biosystems 7500 Real-Time PCR System (Life Technologies Corporation, Beverly, MA, USA). Genes and primers for the qPCR were listed in Supplemental Table S1. Relative expression was determined with the 2−ΔΔT algorithm by normalizing to the transcript levels of related genes in the control group.
Results
Changes in maturity and hormone levels in strawberry fruits in response to exogenous IAA and ABA
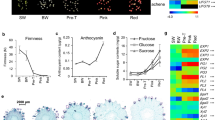
Strawberry fruits at white stage (approximately 23 days after anthesis) were harvested and subject to IAA and ABA treatments. Two days after the respective treatments, a* value, an indicator of color, increased in all four groups, including IAA, ABA, IAA + ABA, and control (Fig. 1a). The IAA-treated fruits had the lowest a* value. Although the ABA-treated fruits appeared to have the highest a* value, there was no significant difference among ABA, IAA + ABA and control (Fig. 1a). In contrast to the color changes, the firmness of all fruits decreased 2 days after harvest, and there was no significant difference in firmness among the four groups of fruits (Fig. 1b).
Color a and firmness b of strawberry fruits in response to exogenous IAA and ABA. 0 day represents the freshly harvested fruits (23 days after anthesis). Control, IAA, ABA, and IAA + ABA represent fruits treated with water, IAA, ABA, and a mixture of IAA and ABA for 2 days, respectively. Lower-case letters (a–c) on the bar chart indicate the significant differences between different treatments (Duncan’s test, P < 0.05)
There were lower levels of IAA in the control and ABA groups and higher levels of IAA in IAA and IAA + ABA groups 2 days after harvest than those of day 0 (freshly harvested) fruits (Fig. 2a). Regarding ABA contents, higher levels of ABA were detected in all groups of fruits on day 2, compared with those of day 0 fruits. In addition, fruits treated with ABA and IAA + ABA had high levels of ABA that were nearly three times of those in control and IAA groups (Fig. 2b).
IAA a and ABA b contents in strawberry fruits 0 day, Control, IAA, ABA, IAA + ABA are noted as in the legend to Fig. 1. Lower-case letters (a–d) on the bar chart indicate the significant differences between different treatments (Duncan’s test, P < 0.05)
RNA sequencing, de novo assembly and gene annotation of Fragaria × ananassa fruit transcriptome
To obtain an overview of Fragaria × ananassa fruit transcriptome, cDNA library constructed from the total RNA of fruits was subject to 125 bp pair-end reads using Illumina Hiseq platform. After eliminating adapters, ambiguous nucleotides and low-quality sequences, 86,234,158 clean reads of 10.78 Gb were obtained, and the average GC content was 45.78 % (Table S2). The clean reads were then assembled into 44,457 unigenes, with an N50 length of 1595 bp and an average size of 852 bp (Table S1). Assembled unigenes ranged from 201 to 15,670 bp and about half of them (24,791, 55.76 %) were 200–500 bp in length (Fig. S1).
To functionally annotate these unigenes, sequences were compared against public databases for Blast analysis, with a threshold of 10−5. 58.51 % of unigenes (26,013) were annotated (Table S3), 46.78 % of them or 20,801 unigenes had significant similarity to known sequences in the NCBI non-redundant protein sequences (Nr) database (Table S3). 23,377 (52.58 %) unigenes were annotated based on sequence search against the NCBI non-redundant nucleotide sequences (Nt) database and 15,675 (35.25 %) the SwissProt database (Table S3). In addition, querying against the Protein family (Pfam) suggested that there were 15,645 (35.19 %) unigenes containing known protein domains. To functionally classify the transcriptomes, the assembled unigenes were also searched against such databases as Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO) and euKaryotic Ortholog Groups (KOG), which allowed us to place 6360 unigenes into 262 KEGG pathways and 8497 unigenes in all 26 KOG functional categories (Table S3; Figs. S2, S3). However, 17,310 unigenes were assigned to 47 level-two GO terms (Table S3; Fig. S4).
Global transcriptional profiling of harvested strawberry fruit in responses to IAA and ABA
To reveal the molecular events in immature strawberry fruits (23 days after anthesis) in response to IAA and ABA, four DGE libraries representing transcripts from the control, IAA, ABA and their combination (IAA + ABA) fruits, respectively, were constructed. Using an Illumina 2000 RNA sequencing platform, we obtained a total of 11.41 G reads with an average length of 50 bp, approximately 93.31–93.97 % clean reads were mapped into the transcriptome reference database described above (Table S4).
RNA sequence data analyses revealed that, when compared to the control, there were only 153 genes that were significantly differentially transcribed in the IAA-treated fruits (Q value < 0.005 and |log2 (fold change)| > 1). Among them, 111 unigenes were up-regulated and 42 unigenes were down-regulated (Table S5). In the ABA-treated fruits, 132 unigenes were significantly differential expressed, with 48 up-regulated and 84 down-regulated genes (Table S6). In the IAA + ABA fruits, there were 147 significantly differentially expressed unigenes, including 54 up-regulated and 94 down-regulated genes (Table S7).
H cluster display revealed that strawberry fruits treated with a combination of exogenous IAA and ABA had a different transcript pattern from that treated with IAA or ABA alone (Fig. 3a). As shown in the Venn diagrams in Fig. 3b, there was an overlap of 27 unigenes between IAA and ABA groups, 58 unigenes between IAA and IAA + ABA, and 84 unigenes between ABA and IAA + ABA (Fig. 3b). There were only 20 significantly differential unigenes that were commonly shared in IAA, ABA and IAA + ABA groups (Fig. 3b). Interestingly, there were 25 unigenes that were specific to the IAA + ABA group (Fig. 3b). These data suggested that the expression pattern in response to the combination of ABA and IAA was not just an additive or subtractive algorithm of those responding to IAA or ABA alone.
Expression analysis of postharvest strawberry fruit in response to exogenous IAA
Among the transcripts significantly elevated in IAA group, there were some unigenes involved in IAA conjugation and signaling pathways (Tables 1, S5, S8). For example, comp50466_c0, a significantly up-regulated unigene, encodes an indole-3-acetic acid-amido synthetase (GH3) and is possibly involved in IAA conjugation (Tables 1, S5). Another unigene named comp45854_c0 having a 2.2-fold increase in its transcript levels encodes an IAA-responsive protein and belongs to the AUX/IAA family (Tables 1, S5). Comp45854_c0 and comp46511_c0, up-regulated by 2.2- and 24.5-fold, respectively, are two auxin response factor (ARF) unigenes (Tables 1, S5). Two putatively encoding annexin unigenes (comp44021_c0 and comp44021_c1) were substantially increased by 15.6- and 13.2-fold, respectively (Tables 1, S5). Intriguingly, the unigene comp53054_c0, encoding a putative E3 ubiquitin protein ligase, was down-regulated while another E3 ubiquitin protein ligase-like gene (comp46194_c0) was up-regulated (Tables 1, S5). Similarly, transcript levels of three putative receptor-like kinases genes (comp51887_c0, comp52501 and comp47297_c0) were decreased while the transcript levels of two other putative receptor-like kinase genes (comp51143_c0, comp47443_c0) were increased (Tables 1, S5).
Another class of significantly differentially expressed unigenes upon IAA treatment was heat-shock protein-related genes. For example, the unigene comp43575 with 3.9-fold induction by IAA encodes an arg2-like heat-shock protein (Tables 1, S5). Arg2 was shown to be an IAA-induced heat-shock protein gene in mung bean (Vigna radiata) hypocotyls (Yamamoto et al. 1992). Transcript levels of some unigenes related to degradation of pectin and other cell wall components were also shown to be significantly elevated (Tables 1, S5). These genes are generally associated with fruit softening. There was an increase in the expression of comp48185_c0 and comp54649_c0; these two unigenes encode putative xylosyltransferase 1 (XT1) and xyloglucan endotransglucosylase/hydrolase (XTH), respectively (Tables 1, S5), which have roles of catalyzing the endo-cleavage of xyloglucan polymers and transferring of the newly generated reducing ends to other xyloglucans.
Two unigenes, comp54082_c0 and comp49931_c0 encoding proteins having acetyl-CoA-carboxylase activity that catalyzes the ATP-dependent formation of malonyl-CoA from acetyl-CoA and bicarbonate (Ohlrogge and Jaworski 1997), were significantly down-regulated by exogenous IAA, with 2.4- and 2.00-fold, respectively (Tables 1, S5). However, the unigene encoding butyrate-CoA synthase was up-regulated. The transcript of a putative β-ketoacyl-CoA synthase (KAS) family gene (comp55300_c0) notably increased by 8.4-fold, which encodes an enzyme that catalyzes the synthesis of long saturated acyl chains of fatty acid (Fofana et al. 2004). The cytochrome P450 86B1-like gene (comp39724_c0) encoding protein owning fatty acid ω-hydroxylase activity associated with suberin synthesis, increased by twofold (Tables 1, S5).
Exogenous IAA also decreased the transcript of the unigene comp42246_c0 encoding sucrose synthase (Tables 1, S5), the activity of which increased with the increase in sucrose concentration during the ripening of strawberries (Hubbard et al. 1991). Additionally, the unigene comp48366_c0 encoding phenylalanine ammonia-lyase (PAL) was down-regulated with the low activity of UDP-rhamnose: rhamnosyltransferase gene (FaRT1) owning glycosyltransferase 4 (FaGT4) activity (Tables 1, S5), suggesting a suppression of IAA to anthocyanin biosynthesis as reported by Given et al. (1988a). Interestingly, two putative UDP-glucose flavonoid 3-O-glucosyltransferase 3 (FaGT3) unigenes comp46753_c0 and comp48158_c0 were up-regulated (Tables 1, S5) although FaGT3 did not form glucose (Glc) esters and was not involved in the biosynthesis of phenylpropanoyl- and benzoyl-D-Glc derivatives (Lunkenbein et al. 2006).
Glutathione S-transferase (GST) was involved in the metabolism during fruit senescence (Shi et al. 2014). There were three putative GST genes (comp43212_c0, comp38169_c0, comp44552) increased by exogenous IAA (Tables 1, S4), which was similar to PpGST1 and PpGST2 remarkably induced by IAA treatment in pear fruits (Shi et al. 2014).
Expression analysis of harvested strawberry fruit in response to exogenous ABA
ABA generally induces genes involved in ABA metabolism and signaling. A putative E3 ubiquitin ligase encoding unigene comp41565_c0 was significantly up-regulated. Another unigene named comp54510_c0 encoding a SKP1-like protein with homology to a subunit of E3 ubiquitin ligase was also significantly up-regulated (Tables 2, S6). The SKP1-like protein has been shown to be involved in ABA signaling in Arabidopsis thaliana (Li et al. 2012). In contrast to the above up-regulated unigenes, the transcript levels of comp4449_c0 decreased by 7.0-fold upon the ABA treatment. Interestingly, comp44449_c0 has 98 % identity with SAR5 (Genbank: L44142.1, an auxin-repressed protein gene) (Tables 2, S6); SAR5 has been shown to be absent or at very low levels in breaker and ripe fruits (Reddy and Poovaiah 1990). ABA also increased the transcripts of two putative annexin (comp44021_c0 and comp44021_c1) and a putative receptor-like kinase (comp47443_c0) (Tables 2, S6). However, the exogenous ABA treatment did not strongly elevate the transcript levels of genes involved in ABA biosynthesis and signaling (Tables S6, S9). The transcripts of unigenes comp50323_c0 (a homologue of NCED1), comp41907_c0 (FaPYR1) and comp46342_c0 and comp46342_c1 (both are ABI1 protein phosphatase 2C family gene) were slightly up-regulated (only 1.3-, 1.5-, 1.5- and 1.2-fold, respectively) (Table S9).
Exogenous ABA treatment also effected the expression of heat-shock proteins (HSPs) genes. The unigene comp47381_c0 encoding a low-molecular-weight heat-shock protein (LMW HSP17.4) was down-regulated (Tables 2, S6). In contrast, the unigene comp50216_c0, a putative HSP 70 protein gene, was up-regulated (Tables 2, S6). The homologue of this putative HSP 70 protein in maize was shown to regulate the ABA-induced antioxidant in response to combined drought and heat stress (Hu et al. 2010). In addition, a Bcl-2-associated athanogene 6-like (BAG6-like) gene comp53095_c0 was down-regulated in the strawberry fruits treated with ABA (Tables 2, S6). The BAG6-like gene encodes a BAG protein that mediates direct interaction with the ATPase domain of HSP70 molecular chaperones (Kabbage and Dickman 2008).
Several fruit softening-related genes were also affected by exogenous ABA. The unigenes comp42142_c0 (a glucan endo-1,3-β-glucosidase-like gene), comp34077_c0 and comp34077_c1 (pectate lyase gene), comp54649_c0 (xyloglucan endotransglucosylase gene) and comp54649_c0 (a putative XTH gene) were up-regulated (Tables 2, S6). Comp18699_c0 and comp43648_c0 (both are cinnamyl alcohol dehydrogenase (CAD)-like genes) were also up-regulated (Tables 1, 2). In contrast, two expansin genes, comp39227_c0 and comp43102_c0, were down-regulated (Tables 2, S6).
In addition, some fruit ripening and senescence-related genes were up-regulated by the ABA treatment. The transcript levels of comp39127_c0 had 2.2-fold increase (Tables 2, S6). This unigene is a homologue of AtCK2α1 encoding a subunit of casein kinase CK2 (formerly known as casein kinase II)-like protein; this protein was shown to be involved in responses to various hormones (Mulekar et al. 2012). Similarly, comp40864_c0, a putative senescence-related gene (SRG1) was up-regulated; SRG1 protein is a member of the Fe(II)/ascorbate oxidase superfamily (Callard et al. 1996). The expression of comp41637-c0 (a metallothionein 1-like gene MET2) also had 1.4-fold increase (Tables 2, S6). In contrast to these up-regulated unigenes, comp41879_c0 encoding a putative metallothionein-like protein 1 was down-regulated (Tables 2, S6). The metallothionein has been reported to be both developmental and ripening genes (Esaka et al. 1992; Whitelaw et al. 1997; Garci´a-Hernández et al. 1998).
Interestingly, ABA enhanced Fe recycling-related unigenes. comp50647_c0, a unigene encoding a putative ferric reductase defective 3 (FRD3), was up-regulated (Tables 2, S6). The unigene comp54516_c0 encoding an isoflavone reductase (IFR)-like protein was also up-regulated (Tables 2, S6). IFR may promote production of phenolics that facilitate the reuse of Fe (Lei et al. 2014). In Arabidopsis, ABA was shown to be involved in the reutilization and transport of Fe from root to shoot (Lei et al. 2014).
Expression analysis of harvested strawberry fruit in response to the combination of ABA and IAA
The treatment with combination of IAA and ABA shared an overlap of 58 unigenes with the IAA treatment (Fig. 3b). Except for 15 unigenes whose functions are unknown, most of these unigenes were related to fruit softening. Some unigenes (e.g., unigenes encoding pectate lyase, 1,3-β-glucosidase and pectinesterase) in response to the treatment with both IAA and ABA displayed changes similar to those responding to the treatment with IAA alone but some other unigenes (e.g., unigene encoding β-galactosidase) showed an opposite pattern in the transcript levels (Tables 1, 3).
In contrast, the treatment with combination of IAA and ABA shared an overlap of 84 unigenes with the ABA treatment, including 21 uncharacterized unigenes (Fig. 3b). The majority of these unigenes encode HSP, BAG, fatty acid desaturase, lipoxygenase, metallothionein, defensin, oleosin, and vicilin-like antimicrobial peptides (Tables 2, 3).
However, some unigenes were uniquely dysregulated by the combination treatment, such as, comp48717_c0 (a 17.2 kDa class II heat-shock protein), comp50306_c0 (a MAPKKK-like gene), and comp50021_c0 (a putative cytochrome c oxidase gene).
Notably, the expression of some unigenes involved in IAA or ABA signaling was only moderately altered by the treatment with a mixture of IAA and ABA. For example, the transcripts of comp41907_c0 (FaPYR1), comp46342_c0 and comp46342_c1 (two ABI1 protein phosphatase 2C family genes) were decreased by 1.4-, 1.6-, and 1.3-fold, respectively (Tables S7, S10). The transcripts of comp44817_c0 and comp45854_c0 (two putative AUX/IAA family genes) were increased by 2.1-fold and twofold, respectively (Tables S7, S10).
RT-qPCR validation of digital expression patterns revealed by RNA sequencing
Twelve unigenes that were significantly differentially expressed as revealed by above-mentioned digital analyses of RNA sequencing data were randomly selected for validation using RT-qPCR. Overall, the RT-qPCR results were consistent with the RNA-seq data. Linear regression analysis indicated that there was a high correlation between the RT-qPCR data and the RNA-seq data (R 2 = 0.95) (Fig. S5).
Discussion and conclusion
Our previous study showed that postharvest strawberries experienced accelerated ripening and senescence processes that were correlated with enhanced decrease of endogenous auxin (IAA) levels and increase of ABA contents, compared to those in planta fruits(Chen et al. 2014). The physico-chemical parameters examined in this study revealed that exogenous IAA delayed the ripening processes of harvested strawberry fruits, whereas ABA promoted the processes; the treatment with a mixture of IAA and ABA, however, showed little effect on the ripening processes. More importantly, the current study investigated into the underlying molecular events by establishing and comparative analyses of four strawberry fruit transcriptomes in response to respective treatments with IAA, ABA, IAA + ABA, or water (control).
Auxin is produced in the achenes and stimulates receptacle expansion during fruit development, but inhibits fruit ripening (Given et al. 1988b). For harvested strawberries, exogenous IAA delayed pectin depolymerization and degradation of cell wall components by suppressing genes encoding pectate lyase, β-D-xylosidase, endoglucanase, β-galactosidase, endo-1,3-β-glucosidase, endo-1,3(4)-β-glucanase. Interestingly, IAA promoted the transcript levels of pectinesterase/pectinesterase inhibitor-like gene (Table 1), which at least in part accounts for IAA’s role in hindering pectin degradation during ripening of harvested strawberries. IAA also promoted the reorganization of cellulose–xyloglucan framework by activating the expressions of XT1 and XTH (Table 1), which is consistent with a previous report that tomato LeEXT1 (XTH) was up-regulated by IAA (Catalá et al. 2000). In addition, exogenous IAA suppressed the expression of genes encoding sucrose synthase, phenylalanine ammonia-lyase and glycosyltransferase (Table 1). IAA also induced the expression of glutathione S-transferases (GSTs) as reported in pear fruit (Shi et al. 2014), which would protect tissues against oxidative stress in fruit development and senescence. These findings were consistent with the quality attributes shown in Fig. S6, suggesting that exogenous IAA delayed the ripening processes in strawberry fruits after harvest.
As expected, the auxin signaling pathway and components for keeping auxin homeostasis in harvested strawberry fruits were activated by exogenously applied IAA. AUX/IAA proteins, the repressors heterodimerizing with auxin responsive factor (ARF), may provide specificity in IAA responses (Pierre-Jerome et al. 2013). Exogenous IAA induced the expression of putative AUX/IAA as well as ARF in this study (Table 1). Similar result was also reported by Liu et al. (2011) who found that naphthalene acetic acid (NAA, a synthetic species of auxin) activated the accumulations of AUX/IAA when applied either at large green or white stage of in planta strawberry fruits. Notably, the putative GH3.1 gene encoding GH3 that conjugates IAA to amino acids was also induced by IAA (Table 1), confirming its involvement in the establishment and maintenance of low IAA levels during fruit ripening (Böttcher et al. 2010). In grape berry (Vitis Vinifera L.), the transcript levels of VvGH3-1 increased at the onset of ripening (veraison), accompanying the IAA sequestration in the ripening berries (Böttcher et al. 2010). Therefore, the intervention of exogenous IAA influenced the IAA homeostasis between anabolism and catabolism, and made the dynamic balance towards the conjugation of IAA in fruits.
Very-long-chain fatty acids (VLCFAs) have been found to be probably required for polar auxin transport or tissue patterning in plants, which has been demonstrated in pas1 (acetyl-CoA carboxylase PASTICCINO3) mutants of Arabidopsis (Roudier et al. 2010). In this study, exogenous IAA also affected fatty acid metabolism in harvested strawberry fruits by altering the transcript levels of acetyl-CoA-carboxylase, cytochrome P450 86B1-like protein, especially β-ketoacyl-CoA synthase (KAS) (Table 1). KAS is the first-step elongase in the VLCFA biosynthesis; VLCFAs function in cell expansion as essential lipids at plasma membrane (Roudier et al. 2010).
The role of ubiquitin in hormone signaling cascades has been previously reported. For example, E3 ubiquitin ligase CUL1 (cullin) and RBX1 (RING-H2 finger protein) have been shown to be subunits of the SCFTIR1 complex, which is required for the response to auxin (Gray et al. 2002). Reduced auxin response is associated with increased RELATED TO UBIQUITIN (RUB) modification of CUL1 when overexpression of RBX1 in Arabidopsis (Gray et al. 2002). In the harvested strawberries, the RING-H2 finger protein-like gene as well as ubiquitin-conjugating enzyme E2-like gene induced by IAA (Table 1) suggests that ubiquitin may affect the IAA signaling through its E3 ubiquitin ligase activity controlling the sensitivity of fruits to IAA, and subsequently regulate fruit development. In addition, Arg2 and TOPLESS-like gene also respond to IAA in harvested fruits in mung bean and Arabidopsis (Yamamoto et al. 1992; Szemenyei et al. 2008). Arg2 has been speculated to be a heat-shock cognate gene and TOPLESS (TPL) is a transcriptional co-repressor that can direct interaction with ARF5/MONOPTEROS regulated by IAA12/BDL (Yamamoto et al. 1992; Szemenyei et al. 2008). The specific regulatory mechanisms of auxin in fruit development and ripening need to be further studied.
ABA has a more dominant role in ripening of non-climacteric fruits including strawberries than climacteric fruits (Mcatee et al. 2013). In contrast to IAA, exogenous ABA had antagonist effects on the expression of cell wall degradation-related genes such as glucan endo-1, 3-β-glucosidase and pectate lyase gene (Table 2), resulting in promoting fruit softening. The transcript levels of an XTH gene (comp54649_c0), a homologous gene of FcXTH1 (Genbank: GQ367550), were also induced by ABA (Table 2). In addition, two expansin genes (comp39227_c0 and comp43102_c0) were also negatively regulated by ABA (Table 2). Thus, the repression of cell expansions may be one of the aspects that ABA suppressed the development and promoted the ripening of strawberries.
ABA moderately up-regulated genes in ABA biosynthesis and signaling, including FaNCED1, FaPYR1 and FaABI1 (Table S6) and strongly activated a putative RING-H2 finger E3 ubiquitin ligase gene and a subunit of the SCF complex E3 ligases, SKP1 (Table 2). Previous studies showed that ABA-insensitive 3 (ABI3) derogation was mediated, at least in part, by the ABI-interacting protein 2 (AIP2), a RING-type E3 ubiquitin ligase (Zhang et al. 2005). These findings suggest that ubiquitin-mediated proteolysis also acts upstream of the ABA signaling cascades.
Heat-shock proteins (HSPs)/chaperones play crucial roles in protecting plants against stresses by re-establishing normal protein conformation and thus cellular homeostasis (Wang et al. 2004). In strawberry, low-molecular weight HSP (LMW HSP) was expressed in fruits at elongation stages and gradually decreased in the turning stage and the red stage, which was not controlled by auxin (Medina-Escobar et al. 1998). In this study, LMW HSP was found not to be regulated by IAA too (Tables 1, S5), but was significantly decreased by the ABA treatment (Table 2). The transcript levels of HSP70 were elevated by ABA, whereas the BAG6-like gene was repressed (Table 2). The knock-out mutant of BAG6 exhibited early flowering and a hastened senescence in Arabidopsis (Doukhanina et al. 2006). Therefore, the interaction of HSPs and BAG may participate in the regulation of ABA-mediated ripening and senescence in strawberry fruits.
This study also revealed several critical genes that have not been fully characterized yet were regulated by ABA. Casein kinase CK2-like protein, a ubiquitous Ser/Thr kinase, consists two catalytic α subunits and two regulatory β subunits (Mulekar et al. 2012). In Arabidopsis, CK2α mutants displayed hyposensitive responses to ABA associated with later flowering under both long- and short-day conditions (Mulekar et al. 2012). In harvested strawberries, a putative CK2α1 gene was found to be induced by exogenous ABA (Table 2), suggesting that CK2α1 may also involve in the ABA signaling. In addition, ABA may participate in the Fe recycling during the postharvest ripening in strawberries. The Fe(II)/ascorbate oxidase superfamily gene SRG1 and metallothionein MET2 were also induced by ABA. Exogenous ABA up-regulated the genes related to Fe reutilization, FRD3 and isoflavone reductase gene IFR (Table 2).
In strawberry fruits treated with a combination of IAA and ABA, there was overlap of genes with fruits treated with IAA or ABA alone. The overlap mainly involved in pectin esterlysis, cell wall degradation, fatty acid metabolism, and transmembrane signal transduction. To some extent, the responses to the combination treatment displayed the cooperation or antagonism between IAA and ABA treatments. For example, both genes encoding pectinesterase/pectinesterase inhibitors induced by IAA and genes encoding HSPs induced by ABA were also regulated by their mixture. However, expansins, which were induced by IAA and/or repressed by ABA, were not significantly altered by the IAA and ABA combination.
The strawberry fruits responded to the treatment with the mixture of IAA and ABA in a different way than individual treatments with IAA or ABA alone. Approximately 17 % of differentially expressed unigenes responding to the combination were not found in the differentially expressed unigenes responding to IAA or ABA. Among these unigenes, 17.2 kDa class II heat-shock protein (LMW HSP) was up-regulated specifically by the combination but neither by IAA nor ABA (Table 3). MAPKKK-like gene was also uniquely elevated by the combination (Table 3). In Arabidopsis, MAPKKKs are known to participate in hormone-mediated signaling and defense responses (Rodriguez et al. 2010). In contrast, cytochrome c oxidase gene was specially down-regulated by the combination (Table 3); this gene encodes the key enzyme in cell respiration. These unique genes may represent novel gene networks in response to the combination of IAA and ABA.
Annexins belong to an evolutionarily conserved multigene family of Ca2+-dependent and phospholipid binding proteins and play a significant role in protecting plants from both abiotic and biotic stresses (Jami et al. 2012). A previous study also certified that annexins mediated osmotic stress and ABA signal transduction in Arabidopsis (Lee et al. 2004). In this study, two annexins were involved in the response of harvested fruits to both IAA and ABA (Tables 1, 2, 3), suggesting annexins in harvested fruits may not only participate in ABA signal transduction but also in IAA signal transduction.
It is known that receptor-like kinases (RLKs) play critical roles in signaling pathways that regulate growth, development, hormone perception, and pathogenic defense responses (Gou et al. 2010; ten Hove et al. 2011). Both IAA and ABA repressed the expression of leucine-rich repeat receptor-like protein kinase (LRR-RLK) (Tables 1, 2). LRR-RLKs represent one of the largest transmembrane receptor kinase families. In Arabidopsis thaliana roots, no LRR-RLKs were observed to respond to ABA but T-DNA insertion lines corresponding to 16 RLK genes showed a consistently enhanced root length, indicating an increased resistance to IAA (ten Hove et al. 2011). So far, the functions of the majority of LRR-RLKs have not been elucidated. In addition, a putative serine/threonine kinase (STK), one of the RLK subclass, was induced by IAA and ABA (Tables 1, 2), suggesting that RLKs may play important roles in plant hormone signaling pathways during postharvest fruit ripening.
In conclusion, the physiological processes of fruit ripening and senescence are regulated in a complex way by cross-talks among multiple hormones. This study advanced our understanding of global molecular events in harvested strawberry fruits in responding to exogenous hormones. As proposed in Fig. 4, either exogenous IAA or ABA transmits its signaling to intracellular substances through receptor-like kinases (RLKs) and further affects the inherent signaling pathways of hormones. The signaling of exogenous IAA disrupts intracellular IAA homeostasis and its original signaling by regulating such genes as GH3, Ubiquitin ligase (Ub), TOPLESS (TPL). On the other hand, the exogenous ABA signaling disturbs the normal expression of such genes as CK2 and HSPs that may interact with intracellular ABA signaling. SKP1 functions as a signal in the downstream of the ABA signaling that may regulate some structural genes. The negative regulation of ARP by ABA implies complex roles of ARP in the ripening and senescence processes of the strawberry fruits. The response of fruits to the mixture of IAA and ABA can be either cooperative or antagonistic between the two hormones. Our findings suggest that a marginal fluctuation in plant hormone signaling may cause substantial changes in metabolism probably due to their cascading effects. In addition, genes encoding receptor-like kinases and ubiquitin ligases may play pivotal roles in IAA and ABA signaling pathways and may be the cross-talk points of the hormones.
A putative regulatory network of auxin and ABA on ripening and senescence of postharvest strawberry fruits. Dashed arrows hypothetical pathways. AA amino acid, ABA abscisic acid, ABAR ABA responsive proteins, ABI1 ABA-insensitive 1, ARF IAA-responsive factor, ARP IAA-repressor protein, AUX/IAA IAA/indole-3-acetic acid protein, CK2 casein kinase 2, HSPs heat-shock proteins, LRR leucine-rich repeat receptor-like protein kinase, MAP3K mitogen-activated protein kinase kinase kinase 3, PYR1 pyrabactin resistance 1, RCAR regulatory components of ABA receptors, SKP1 S-phase kinase-associated proteins 1, SnRK2 sucrose non-fermenting protein-related kinase, STK serine/threonine receptor-like protein kinase, TPL TOPLESS, Ub Ubiquitin ligase, VLCFA very-long-chain fatty acid
Author contribution statement
MLC and CJX conceived and designed the experiments. CJX and LWJ performed the experiments. CJX analyzed the data and wrote the manuscript. YTJ and LZS also have contributed to the data interpretation and writing.
Abbreviations
- ABA:
-
Abscisic acid
- ABI3:
-
Abscisic acid insensitive 3
- AIP2:
-
ABI-interacting protein 2
- ARF:
-
Auxin response factor
- ARP:
-
Auxin-repressed protein
- BAG:
-
Bcl-2 associated athanogene family
- CAD:
-
Cinnamyl alcohol dehydrogenase
- CK:
-
Casein kinase
- DGE:
-
Digital gene expression
- GH3:
-
Indole-3-acetic acid-amido synthetase
- HSPs:
-
Heat-shock proteins
- IAA:
-
Auxin
- KO:
-
KEGG Ortholog database
- KOG/COG:
-
Clusters of orthologous groups of proteins
- NCED:
-
9-cis-epoxycarotenoid dioxygenase
- RLKs:
-
Receptor-like kinases
- SKP1:
-
S-phase kinase-associated proteins 1
- VLCFA:
-
Very-long-chain fatty acid synthesis
- XT1:
-
Xylosyltransferase 1
- XTH:
-
Xyloglucan endotransglucosylase/hydrolase
References
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of IAA by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of IAA conjugation during ripening. J Exp Bot 61:3615–3625
Callard D, Axelos M, Mazzolini L (1996) Novel molecular markers for late phases of the growth cycle of Arabidopsis thaliana cell-suspension cultures are expressed during organ senescence. Plant Physiol 112:705–715
Catalá C, Rose JKC, Bennett AB (2000) IAA-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol 122:527–534
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chen JX, Mao LC, Mi HB, Zhao YY, Ying TJ, Luo ZS (2014) Detachment-accelerated ripening and senescence of strawberry (Fragaria × ananassa Duch. cv. Akihime) fruit and the regulation role of multiple phytohormones. Acta Physiol Plant 36:2441–2451
Doukhanina EV, Chen S, van der Zalm E, Godzik A, Reed J, Dickman MB (2006) Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 281:18793–18801
Esaka M, Fujisawa K, Goto M, Kisu Y (1992) Regulation of ascorbate oxidase expression in pumpkin by auxin and copper. Plant Physiol 100:231–237
Fofana B, Duguid S, Cloutier S (2004) Cloning of fatty acid biosynthetic genes β-ketoacyl CoA synthase, fatty acid elongase, stearoyl-ACP desaturase, and fatty acid desaturase and analysis of expression in the early developmental stages of flax (Linum usitatissimum L.) seeds. Plant Sci 166:1487–1496
García-Hernández M, Murphy A, Taiz L (1998) Metallothioneins 1 and 2 have distinct but overlapping expression patterns in Arabidopsis. Plant Physiol 118:387–397
Given NK, Venis MA, Grierson D (1988a) Phenylalanine ammonia-lyase activity and anthocyanin synthesis in ripening strawberry fruit. J Plant Physiol 133:25–30
Given NK, Venis MA, Gierson D (1988b) Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174:402–406
Gou XP, He K, Yang H, Yuan T, Lin HH, Clouse SD, Li J (2010) Genome-wide cloning and sequence analysis of leucine-rich repeat receptor-like protein kinase genes in Arabidopsis thaliana. BMC Genom 11:19
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng QD (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Gray WM, Hellmann H, Dharmasiri S, Estelle M (2002) Role of the Arabidopsis RING-H2 protein RBX1 in RUB modification and SCF function. Plant Cell Online 14:2137–2144
Hu XL, Liu RX, Li YH, Wang W, Tai FJ, Xue RL, Li CH (2010) Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. Plant Growth Regul 60:225–235
Hubbard NL, Pharr DM, Huber SC (1991) Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species. Physiol Plant 82:191–196
Jami SK, Clark GB, Ayele BT, Ashe P, Kirti PB (2012) Genome-wide comparative analysis of annexin superfamily in plants. Plos One 7:11
Ji K, Chen P, Sun L, Wang YP, Dai SJ, Li Q, Li P, Sun YF, Wu Y, Duan CR, Leng P (2012) Non-climacteric ripening in strawberry fruit is linked to ABA, FaNCED2 and FaCYP707A1. Funct Plant Biol 39:351–357
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Jiang YM, Joyce DC (2003) ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul 39:171–174
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an IAA-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 65:1390–1402
Knee M, Sargent JA, Osborne DJ (1977) Cell-wall metabolism in metabolism in developing strawberry fruits. J Exp Bot 28:377–396
Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, Park OK (2004) Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 16:1378–1391
Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ (2014) Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ 37:852–863
Li B, Dewey C (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323
Li CJ, Liu ZJ, Zhang QR, Wang RZ, Xiao LT, Ma H, Chong K, Xu YY (2012) SKP1 is involved in abscisic acid signalling to regulate seed germination, stomatal opening and root growth in Arabidopsis thaliana. Plant Cell Environ 35:952–965
Li H, Mao WJ, Liu W, Dai HY, Liu YX, Ma Y, Zhang ZH (2013) Deep sequencing discovery of novel and conserved microRNAs in wild type and a white-flesh mutant strawberry. Planta 238:695–713
Liu D, Chen J, Lu W (2011) Expression and regulation of the early IAA-responsive Aux/IAA genes during strawberry fruit development. Mol Biol Rep 38:1187–1193
Lunkenbein S, Bellido M, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, Muñoz-Blanco J, Schwab W (2006) Cinnamate metabolism in ripening fruit. characterization of a UDP-glucose: cinnamate glucosyltransferase from strawberry. Plant Physiol 140:1047–1058
Mcatee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4:79
Medina-Escobar N, Cardenas J, Munoz-Blanco J, Caballero JL (1998) Cloning and molecular characterization of a strawberry fruit ripening-related cDNA corresponding a mRNA for a low-molecular-weight heat-shock protein. Plant Mol Biol 36:33–42
Miszczak A, Forney CF, Prange RK (1995) Development of aroma volatiles and color during postharvest ripening of `Kent’ strawberries. J Am Soc Hortic Sci 120:650–655
Mulekar JJ, Bu Q, Chen F, Huq E (2012) Casein kinase II α subunits affect multiple developmental and stress-responsive pathways in Arabidopsis. Plant J 69:343–354
Munne-Bosch S, Muller M (2013) Hormonal cross-talk in plant development and stress responses. Front Plant Sci 4:529
OfosuAnim J, Kanayama Y, Yamaki S (1996) Sugar uptake into strawberry fruit is stimulated by abscisic acid and indoleacetic acid. Physiol Plant 97:169–174
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol 48:109–136
Ornelas-Paz J, Yahia EM, Ramírez-Bustamante N, Pérez-Martínez JD, Escalante-Minakata MP, Ibarra-Junquera V, Acosta-Muñiz C, Guerrero-Prieto V, Ochoa-Reyes E (2013) Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem 138:372–381
Pierre-Jerome E, Moss BL, Nemhauser JL (2013) Tuning the IAA transcriptional response. J Exp Bot 64:2557–2563
Reddy ASN, Poovaiah BW (1990) Molecular cloning and sequencing of a cDNA for an IAA-repressed mRNA: correlation between fruit growth and repression of the IAA-regulated gene. Plant Mol Biol 14:127–136
Rodriguez MCS, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Roudier F, Gissot L, Beaudoin F, Haslam R, Michaelson L, Marion J, Molino D, Lima A, Bach L, Morin H, Tellier F, Palauqui JC, Bellec Y, Renne C, Miquel M, DaCosta M, Vignard J, Rochat C, Markham JE, Moreau P, Napier J, Faure JD (2010) Very-long-chain fatty acids are involved in polar IAA transport and developmental patterning in Arabidopsis. Plant Cell 22:364–375
Shi HY, Li ZH, Zhang YX, Chen L, Xiang DY, Zhang YF (2014) Two pear glutathione S-transferases genes are regulated during fruit development and involved in response to salicylic acid, IAA, and glucose signaling. PloS One 9(2):e89926. doi:10.1371/journal.phone.0089926
Shin Y, Ryu JA, Liu RH, Nock JF, Watkins CB (2008) Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol Technol 49:201–209
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 63:4741–4750
Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates IAA-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319:1384–1386
ten Hove C, Bochdanovits Z, Jansweijer VA, Koning F, Berke L, Sanchez-Perez G, Scheres B, Heidstra R (2011) Probing the roles of LRR RLK genes in Arabidopsis thaliana roots using a custom T-DNA insertion set. Plant Mol Biol 76:69–83
Trainotti L, Tadiello A, Casadoro G (2007) The involvement of IAA in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58:3299–3308
Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Whitelaw CA, Le Huquet JA, Thurman DA, Tomsett AB (1997) The isolation and characterisation of type II metallothionein-like genes from tomato (Lycopersicon esculentum L.). Plant Mol Biol 33:503–511
Yamamoto KT, Mori H, Imaseki H (1992) Novel mRNA sequences induced by indole-3-acetic acid in sections of elongating hypocotyls of mung bean (Vigna radiata). Plant Cell Physiol 33:13–20
Zhang X, Garreton V, Chua NH (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev 19:1532–1543
Acknowledgments
This research is supported by the National Basic Research Program (973 program) of China (2013CB127101) and the National Natural Science Foundation of China (31372113).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
Fig. S1 Length distribution of unigenes (TIFF 1055 kb)
Supplementary material 2
Fig. S2 KEGG classification of unigenes (TIFF 1772 kb)
Supplementary material 3
Fig. S3 KOG classification of unigenes (TIFF 1966 kb)
Supplementary material 4
Fig. S4 GO classification of unigenes (TIFF 3190 kb)
Supplementary material 5
Fig. S5 Correlation analysis of gene expression levels revealed by RT-qPCR and RNA-seq digital data (12 randomly selected unigenes are comp48373_c0, comp49239_c0, comp29094_c0, comp45854_c0, comp44817_c0, comp9725_c0, comp38169_c0, comp44449_c0, comp50216_c0, comp34077_c1, comp54510_c0, comp46511_c0) (TIFF 1078 kb)
Supplementary material 6
Fig. S6 Soluble solid (a), soluble sugar (b) and total anthocyanin (c) contents of strawberry fruits in response to exogenous IAA and ABA. 0, Control, IAA, ABA, IAA+ABA are noted as in the legend to Fig. 1. Lower-case letters (a, b, c, d) on the bar chart indicate the significant differences between different treatments (Duncan’s test, P < 0.05) (TIFF 1840 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Mao, L., Lu, W. et al. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 243, 183–197 (2016). https://doi.org/10.1007/s00425-015-2402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2402-5