Abstract
High salinity leads to a reduction in growth, germination, metabolic stability, and production of pepper (Capsicum annuum L.) plants worldwide. GABA priming showed a positive effect on plant growth and development and improved plant stress tolerance. The current study aimed to investigate the effect of exogenous GABA treatments on endogenous GABA shunt pathway in germinating seeds of green bell pepper (Capsicum annuum L.) under salt stress (0, 25, 50, 75, 100 and 200 mM NaCl) through the characterization of seed germination pattern, seedling growth, seed moisture content, GABA shunt metabolite levels (GABA, Alanine, and Glutamate), the level of oxidative damage in terms of the accumulation of reactive oxygen substances and the expression of pepper dehydrin gene (CaDHN3) in response to all salt stress treatments that were examined in this study. Pre-treatment of pepper seeds with GABA improved seed germination by enhancing germination percentage, germination rate, seedling length, seedling fresh and dry weights, seed moisture content, and decreasing mean time to germinate under salt stress. Data showed an increase with positive correlation between internal GABA metabolite, alanine, and glutamate levels and NaCl concentrations in response to all GABA priming treatments. The MDA content increased as NaCl concentration increased under all GABA treatments. However, there was a significant reduction in MDA content in all GABA treatments and hydro-primed pepper seeds when compared to untreated seeds under all NaCl concentrations. The expression of pepper dehydrin gene (CaDHN3) was significantly increased with the increase of NaCl concentrations under all GABA treatments. Priming pepper seeds with exogenous GABA significantly activates GABA shunt and accumulate GABA internally to maintain C: N balance, stabilize internal metabolism, sustain amino acid metabolism, enhance scavenging of reactive oxygen species (ROS) by activating defense mechanisms, and significantly increase the expression of CaDHN3 to prevent lipid peroxidation, maintain metabolic stability and enzymes function and prevent dehydration during seeds germination in response to salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most consumed vegetables worldwide is pepper (Capsicum annuum L.), which mainly contains high vitamins A, C, and minerals. Peppers contain a wide range of phytochemicals, such as capsaicinoids, phenolics, ascorbic acid, and carotenoids (Kumar and Tata 2009). Capsaicinoids are the ingredients in pepper that are responsible for its pungent taste (Sarpras et al. 2016). The level of capsaicin ranged from 0 to 3636 µg/g in the mature green stage and from 0 to 4820 µg/g in the red/yellow stage, while the concentration of dihydrocapsaicin ranged from 0 to 2148 µg/g in the mature green stage and from 0 to 2162 µg/g in the red/yellow stage (Hamed et al. 2019). Pepper (Capsicum annuum L.) belongs to the family Solanaceae which includes tomato (Solanum lycopersicum), potato (Solanum. tuberosum), eggplant (Solanum. melongena), tobacco (Nicotiana tabacum), and petunia (Petunia spp.) (Kelley et al. 2009). The origin of pepper is Mexico and Central America countries, but generally, it is produced in every country all over the world. In addition to its nutritional importance, also pepper is used in various pharmaceutical industries due to its antioxidant, anticancer, and antimicrobial properties (Safari et al. 2017; Iranbakhsh et al. 2018). Peppers are picked green and immature, but when they ripen on plant, they become full-sized and sweeter due to high vitamin content. Additionally, as fruits become mature, they gradually accept their genetic color; such as red, yellow, green, and orange (Zhang et al. 2002). The nutrients quantity and vitamins content of red pepper is higher than green pepper due to the extra time they stay attached to the plant before fruit harvesting (Jovicich et al. 2004). More than 200 common names for pepper, including bell pepper, Jalapenos, Cayenne, and Christmas pepper (ornamental) (Latham 2009).
Salinity is determined by increasing sodium (Na+) and chloride (Cl−) ions concentration in the soil which can be induced by either ionic stress or osmotic stress (Ismail et al. 2014). Salinity is considered as a limiting factor for the production of crops worldwide (Zhu 2002, 2016), because it has a negative effect on all aspects of plant growth and development (Cuartero et al. 2006; Abbasi et al. 2012). Reduction in shoot system fresh and dry weight under salt stress was observed in beans (Vicia faba L.) (Qados 2011), lettuce (Lactuca sativa L) (Andriolo et al. 2005) and cowpea (Vigna unquiculata L) (Dantas et al. 2005). Likewise, salt stress disturbed plant internal water relations such as decreasing the osmotic potential in beans (Vicia faba L.) (Qados 2011), sea buckthorn (Hippophae rhamoides L) (Qin et al. 2009) and barley leaves (Hordeum vulgare L) (Yağmur et al. 2006). Additionally, salinity changes ion equilibrium, mineral nutrition, water status, and efficiency of photosynthesis (Nabati et al. 2011). In pepper (Capsicum annuum L.), salt stress significantly leads to severe reduction in leaf numbers, leaf area, root length, and chlorophyll contents (Kaouther et al. 2012) which was consistent with reduction of leaf area in canola (Bybordi 2010) and leaf number in groundnut (Mensah et al. 2006) and sprout plant (Al-Thabet et al. 2004).
Gamma-aminobutyric acid (GABA) is a non-protein amino acid. In animal cells, it has vital roles roles in neurology and signal communications (Watanabe et al. 2002). When the concentration of GABA is altered in the brain it may contribute to various neurological disorders including parkinson, epilepsy, and seizures (Ting Wong et al. 2003). In plant cells, the first discovery of GABA was in potato tubers and it is synthesized through the GABA shunt pathway (Michaeli and Fromm 2015). The metabolism of GABA is performed by multistep starting from glutamate by glutamate decarboxylase that later is converted to succinate semialdehyde (SSA) and succinate enter the tricarboxylic acid (TCA) cycle (Krishnan et al. 2013). GABA shunt involved three main reactions directed by glutamate decarboxylase (GAD), GABA transaminase (GABA-T), and succinate semialdehyde dehydrogenase (SSADH) enzymes (Michaeli and Fromm 2015). Decarboxylation of glutamate to GABA is catalyzed by GAD and controlled by Calmodulin\Ca+ 2 complex (Snedden and Fromm 2001). GABA transaminase catalyzed the conversion of GABA to SSA by using pyruvate or α-ketoglutarate as amino-group acceptor that leads to the production of alanine or glutamate (Geigenberger and Stitt 1993; Fernie et al. 2001). A significant correlation between SSADH and GAD genes expression was observed under various abiotic stresses (Steinhauser et al. 2004; Usadel et al. 2005). The accumulation of GABA is mainly in the cytosol; which is later transported into mitochondria (Michaeli et al. 2011). GABA plays a critical role in plant metabolism under biotic/abiotic stresses to maintain carbon: nitrogen (C: N) balance, scavenging reactive oxygen species (ROS) (AL-Quraan et al. 2013), and regulating cytosolic pH (Steinhauser et al. 2004).
The process in which plants attain a unique primed or physiological state by pretreatment with any chemical priming agent is called priming, and this process (preconditioning) influences the plants’ ability to respond to stressful conditions (Cohen et al. 2007). Several studies proved the importance of priming in improving seed germination, seedling emergence, growth nodulation, and productivity in various crops such as wheat (Tabassum et al. 2018), rice (Jisha and Puthur 2016) and sunflower (Moghanibashi et al. 2013). Furthermore, GABA is used as a priming agent for alleviating drought stress (Vijayakumari and Puthur 2016) and heat stress (Nayyar et al. 2014). A study performed by Tian et al. (2005) proved that GABA priming reduced the effect of NaCl treatments by increasing enzyme activity such as catalases (CAT) and superoxide dismutase (SOD) in maize seed. Moreover, exogenous GABA treatment affected stress-related genes expression in the roots of Caragana intermedia under NaCl stress (Shi et al. 2010). GABA treatment significantly alleviated the chilling injury in tomato seedlings by increasing the activity of antioxidant enzymes and scavenging the accumulation of ROS in response to cold stress (Malekzadeh et al. 2014). Additionally, exogenous GABA treatment can regulate weight loss, chilling injury index and cell death and maintain lower rate of electrolyte leakage during postharvest storage of various crops fruits, including zucchini, banana, and peaches (Madebo et al. 2021). Ramzan et al. (2023) study showed that exogenous treatment of pepper (Capsicum annuum L.) seedlings with GABA and glutathione increased the salt tolerance by activating the antioxidant defense mechanisms, enhancing the activity of respiratory enzymes and up-regulating the expression of CaXTHs stress-related genes. Also, GABA application enhanced the drought stress tolerance in various crops, including grapevines (Malabarba et al. 2019), tomato (Gramazio et al. 2020), and white clover (Zhou et al. 2021) by increasing the water use efficiency and improving the nitrogen assimilation.
The accumulation of GABA in plants is related to environmental stress, and this accumulation might interrupt the plant growth and development (Bouche and Fromm 2004). Inducing the accumulation of GABA in tobacco caused alteration of vegetative development due to reduction in cell elongation of stems (Baum et al. 1996). However, a previous study conducted on a hulless barley suggested that GABA was essential for mediating NaCl stress-induced antioxidant system enhancement and phenolic compound accumulation in germinated seeds (Ma et al. 2019). In white clover, applying exogenous GABA showed no effect on seed germination under normal conditions but its application at a low concentration under salt stress significantly improved seed germination by decreasing the osmotic potential, soluble sugar, and free proline content (Cheng et al. 2018). Under long-term salt stress, exogenous GABA significantly influenced the germination rate, improved photosynthesis, and decreased root dry mass in wheat (Li et al. 2010, 2016a). Various studies suggested that GABA has an essential role in germination by providing building blocks for metabolic reorganization via the TCA cycle (Fait et al. 2006; Gutierrez et al. 2007). In addition, during seed germination; GABA activated gene expression of α-amylase in the aleurone of barley seeds and promoted the degradation of seed starch in a dose-dependent pattern (Sheng et al. 2018). In contrast, excess amounts of exogenous GABA could inhibit primary root growth and seed germination by inducing a change in the balance of nitrogen and carbon metabolism to maintain storage and seeds dormancy (Du et al. 2020).
Plant treated with GABA showed a crucial role in sustaining internal metabolism under stress. Applying exogenous GABA to crop plants can alleviate different metabolic pathways in response to salinity (Kumar et al. 2017). Several studies revealed that plants exposed to NaCl accompanied with GABA treatment showed higher photosynthesis rate and stomatal conductance, enhanced resistance against adverse environmental conditions, improved seed germination rate, reduced chloroplast and mitochondrial damage, and decreased membrane leakage (Wang et al. 2017; Cheng et al. 2018). A study on rice (Oryza sativa L.) under salinity conditions showed that GABA priming significantly controls reactive oxygen species (ROS) levels by inducing secondary metabolism and antioxidant enzymes during seed germination (Sheteiwy et al. 2019). In tomato (Solanum lycopersicum L.), treatment with GABA significantly inhibited the effect of salt stress on seedling height, reduced the net Na+ efflux in leaves and roots, and prevented the accumulation of Na+ in tissues (Wu et al. 2020).
Moreover, applying GABA at low concentration increased adventitious root growth in poplar (Liriodendron tulipifera L.) (Xie et al. 2020) and increased the content of endogenous GABA in the leaves and stem of tomato (Lycoperiscan esculentum) seedlings under salt stress (Çekiç 2018). In lettuce (Lactuca sativa L.), the application of GABA inhibited the negative effect of NaCl in early growth stage at both cellular (oxidative stress) and biophysical (chlorophyll content) levels (Kalhor et al. 2018). Furthermore, GABA can enhance nitric oxide (NO) accumulation in muskmelon (Cucumis melo), where NO acts as a signaling molecule for GABA pathway induction to enhance salt stress tolerance by improving the antioxidant system, ion homeostasis, proline metabolism, and promoted growth and photosynthetic efficiency (Xu et al. 2021). However, NO induction to produce GABA was also reported in unstressed wheat (Triticum aestivum L.), which in turn was associated with various physiological improvements (Khanna et al. 2021). Additionally, exogenous GABA can protect plants from oxidative damage and reduce ROS accumulation in tomato in response to long-term cold treatment (Shang et al. 2011). In mungbean (Vigna radiate L.) plants, GABA had a significant effect on reducing malondialdehyde (MDA) and H2O2 levels, improving the antioxidant activities in anthers and leaves, upregulating osmolytes synthesis, and improving C-fixation and assimilation to maintain leaf water status under heat stress (Priya et al. 2019).
As a result of its significant role in plant metabolism, growth and development; exogenous GABA showed a significant result in inducting plant responses under various abiotic stresses. Therefore, this study was aimed to investigate the effect of exogenous GABA treatments on endogenous GABA shunt pathway in germinating seeds of green bell pepper (Capsicum annuum L.) under salt stress through the characterization of seed germination pattern, seedling growth, GABA shunt metabolite levels (GABA, Alanine, and Glutamate), the level of oxidative damage in terms of the accumulation of MDA and the expression of pepper dehydrin gene (CaDHN3) in response to all NaCl treatments that were used in this study. The present study determined the effects of exogenous GABA on pepper (Capsicum annuum L.) seeds’ tolerance to NaCl treatments and investigated the functional role of GABA priming in the induction of the GABA shunt pathway endogenously and metabolic stability during seed germination under salt stress.
Materials and methods
Plant materials and seed surface sterilization
Freshly harvested green bell pepper seeds (Capsicum annuum L., Sweet pepper, variety: California Wonder) were provided by Oula Seeds International company (Irbid, Zarqa Highway, Al-Mafraq, Jordan). This pepper variety was used in this study since it is not genetically modified and it is the most cultivated pepper variety in Jordan. Surface sterilization of the seeds was performed by suspending seeds in 100% bleach (v/v, 6% sodium hypochlorite) for 5 min followed by five times washing with sterile distilled water (Lindsey et al. 2017).
Seeds priming with GABA
The GABA solutions (0.5, 1.0, 1.5, 2.0, or 2.5 mM) were prepared by dissolving GABA (Sigma-Aldrich, USA) in distilled water (d. H2O). The solution pH was adjusted to 5.6 using 1 M NaOH solution. Surface sterilized seeds (25,000 seeds) were treated by submerging in 0.5, 1.0, 1.5, 2.0, or 2.5 mM GABA solutions, and distilled water, separately for 24 h at 25˚C. Treatment with distilled water is referred to as hydro-priming. After soaking, the seeds were allowed to air dry to return to their original moisture at room temperature for 3 days. Untreated dry seeds (seeds that were not treated with distilled water or GABA solutions) were used as a control group.
NaCl treatments and growth conditions
The sterilized hydro-primed and GABA-treated seeds (0.5, 1.0, 1.5, 2.0, or 2.5 mM) in addition to untreated seeds, were grown on filter paper in Petri dishes supplemented with different concentrations of sodium chloride (NaCl): 0, 25, 50, 75, 100, and 200 mM (3 mL in each Petri dish), separately. All experiments were conducted in the laboratory by incubating the treated seeds at 25ºC for 8 days (AL-Quraan et al. 2023).
Seed moisture content
Seed moisture content was measured for three replicates of 30 seeds each immediately after imposing seed treatments with GABA solutions and after drying the seeds to their original moisture content. Seed moisture content was measured according to the International Seed Testing Association (ISTA) by calculating the difference in seed fresh weight before and after drying them in an oven at 80 oC for 72 h (oven-dry weight). This difference was then divided by the seed fresh weight and was expressed as a percentage (%) of the wet weight using the following equation:
Equation (1)
Seeds and growth sensitivity to NaCl treatments
Thirty seeds from each GABA treatments (0.5, 1.0, 1.5, 2.0, or 2.5 mM) in addition to distilled water (hydro-priming), and non-treated seeds as a control group, were planted on two filter papers supplemented with 0, 25, 50, 75, 100, and 200 mM NaCl, separately. The seeds were incubated at 25 ˚C for 8 days. Seeds were considered as germinated seeds when the radicle had protruded and grown out of the covering seed layers. Seeds with radicle protrusion was scored after 1, 2, 3, 4, 5, 6, 7, and 8 days after planting. The effect of NaCl on seed germination was calculated using the following equation (Bhardwaj et al. 2012):
Equation (2)
Germination percentage (G%) after 8 days was compared to the untreated seeds (control group). An average of three replicate plates were used for each treatment. Eight days after planting, only germinated seeds were used for further experiments (GABA metabolites extraction, MDA analysis, and CaDHN3 expression). The mean time to germination (MTG) was calculated using the following equation (Samarah et al. 2016):
Equation (3)
Where ni is the number of newly germinated seeds at time ti; ti is the number of days from the beginning of planting (imbibition); and Σ ni is the total number of seeds germinated. An average of three replicate plates were used for each treatment. The germination rate (GR) was calculated using the following equation (Bhardwaj et al. 2012):
Equation (4)
Where ni is the number of seeds germinated at time ti; ti is the number of days from the beginning of planting (imbibition). An average of three replicate plates were used for each treatment.
Seedling length (cm), seedling fresh weight (g), and dry weight (g) were determined for each treatment on the 8th day after planting. Seedling length (cm) was measured from the seed radicle to the shoot tip using a ruler. Seedling fresh weight (g) was determined by collecting the seedling samples separately and weighing them directly. Seedling dry weight (g) was determined after oven drying at 70 °C of each seedling sample for 72 h. An average of three replicate plates were used for each treatment.
GABA-metabolites extraction
GABA metabolites were extracted according to Zhang and Bown (1997) with the following modification: 500 mg of germinating seeds (seeds and the emerged seedlings) at 1st day, 4th day, and 8th day after planting for each NaCl treatment (0, 25, 50, 75, 100, and 200 mM) separately were grounded with mini pestle and mortar and placed in 1.5 mL microcentrifuge tubes. To each tube, 400 µL methanol was added and the samples were mixed for 10 min. Liquid from the samples was removed by regular evaporation overnight (tubes were kept open to allow methanol evaporation). Then 500 µL of 70 mM lanthanum chloride was added to each tube. The tubes were mixed for 15 min and subsequently centrifuged at 15,000 rpm for 5 min. The supernatant was removed to new tubes and was mixed with 160 µL of 1 M potassium hydroxide (KOH). The tubes were mixed for 10 min and then centrifuged at 15,000 rpm for 5 min. The supernatant containing metabolites was transformed into a new tube and was used for GABA shunt metabolites (GABA, Alanine, and Glutamate) level determination. An average of three replicates was used for each treatment.
GABA-metabolite level determination
GABA level was measured according to Zhang and Bown (1997) with the following modifications: the reaction mixture contained 50 µL of sample extract, 14 µL of 4 mM NADP+, 19 µL of 0.5 M potassium pyrophosphate at pH 8.6, 10 µL of (2 u µL− 1) GABASE enzyme (Gabase enzyme powder was suspended in 0.1 M potassium pyrophosphate at pH 7.2 containing 12.5% glycerol and 5 mM β-mercaptoethanol) and 10 µL of α-ketoglutarate. Change in spectrophotometric absorbance at 340 nm was recorded after 90 min incubation at 25˚C using the microplate reader (Synergy HTX, BioTek Instruments, USA). The level of GABA (nmol mg− 1 (FM) was determined using the NADPH standard curve (range from 0 to 10 nmol). An average of three replicates was used for each treatment.
Alanine level determination
Alanine level was measured according to Bergmeyer and Grassl (1988) with the following modifications: the reaction mixture contained 180 µL of 0.05 M Na-carbonate buffer pH 10, 7 µL of 30 mM β-NAD+, 50 µL of sample extract, and 5 µL of 0.3 u µL− 1 alanine dehydrogenase (Sigma-Aldrich, USA) enzyme suspension. Change in spectrophotometric absorbance at 340 nm after the addition of alanine dehydrogenase was recorded after 60 min incubation at 25oC using the microplate reader (Synergy HTX, BioTek Instruments, USA). The level of alanine as nmol mg− 1 (FM) was determined using the NADH standard curve (range from 0 to 5 nmol). An average of three replicates was used for each treatment.
Glutamate level determination
Glutamate level was measured according to Bergmeyer and Grassl (1983) with the following modifications: the deamination reaction mixture contained 180 µL of 0.1 M Tris-HCl pH 8.3, 8 µL of 7.5 mM β-NAD+, 50 µL of sample extract, and 5 µL of 0.8 u mL− 1 glutamate dehydrogenase enzyme suspension (Sigma-Aldrich, USA). Change in spectrophotometric absorbance at 340 nm after the addition of glutamate dehydrogenase was recorded after 60 min incubation at 25oC using the microplate reader (Synergy HTX, BioTek Instruments, USA). The level of glutamate was determined as nmol mg− 1 (FM) using the NADH standard curve (range from 0 to 5 nmol). An average of three replicates was used for each treatment.
Oxidative damage and MDA assay
The level of malondialdehyde (MDA) as a reference for reactive oxygen species in germinating seeds was determined as the following: 100 mg tissue was grounded using a mini pestle and mortar then placed in 1.5 mL microcentrifuge tubes at 1st day, 4th day, and 8th−day after planting for each NaCl treatment (0, 25, 50, 75, 100, and 200 mM), separately. The lipid peroxidation (MDA) assay kit (colorimetric) (ab118970, Abcam, Waltham, USA) was used according to the manufacturer’s instructions. In this kit: lipid peroxidation was determined by the reaction of free MDA (present in the sample) with thiobarbituric acid (TBA) to generate a MDA-TBA adduct that formed a colorimetric (532 nm) product, proportional to the MDA present in the sample. The absorbance was measured spectrophotometrically at 532 nm using the microplate reader (Synergy HTX, BioTek Instruments, USA). The level of malondialdehyde (MDA) was determined as nmol mg− 1 (FM) from a standard curve of MDA (range from 0 to 5 nmol). An average of three replicate plates was used for each treatment.
Total RNA extraction
Total RNA from fresh samples was extracted by using the IQeasy™ plus Plant RNA Extraction Kit from Intron Biotechnology (South Korea) according to the manufacturer’s instructions. Total RNA was extracted from germinating seeds on 8th−day after planting for each sodium chloride (NaCl) concentration (0, 25, 50, 75, 100, and 200 mM), separately and suspended in RNase-free water. RNA concentrations were determined by their absorbance A260 using a nanodrop spectrophotometer (ND-100, NanoDrop Technologies, USA). The integrity of RNA was determined after separation of RNA on a 1.5% (w/V) agarose gel after electrophoresis and stained with RedSafe nucleic acid staining solution and was visualized using a UV trans-illuminator and detection system.
CaDHN3 mRNA expression level by reverse transcriptase-PCR
Gene-specific primers for the pepper dehydrin gene (CaDHN3) (forward primer 5` ATGGCACATAACGGTACTAGCC 3` (reverse primer 5` CCCTTCATCTTTCTTCATAGCAT 3`) (Jing et al. 2016) were used for RT-PCR analysis of steady-state mRNA levels in pepper seeds that were used in this study under all treatments separately. A one-step reverse transcriptase-PCR (RT-PCR) reaction was performed using primer pairs specific for pepper dehydrin gene (CaDHN3) (Jing et al. 2016), SuperScript TM III one-step RT-PCR system with platinum® Taq DNA polymerase according to the manufacturer’s instructions (Intron Biotechnology, Korea) as the following: one cycle of reverse transcription reaction (45℃ 30 min− 1) and denaturation of RNA: cDNA hybrid (94℃ 5 min− 1) followed by three step cycling (denaturation (94 ͦ C 45 min− 1), annealing (56℃ 45 s− 1), extension (72℃ 1 min− 1) for 35 cycles then final extension (72℃ 10 min− 1) for one cycle. RT-PCR amplification products were separated on 2% agarose gels and were stained with RedSafe nucleic acid staining solution. Transcript abundance of CaDHN3 (850 bp) was calculated according to AL-Quraan et al. (2010) as the following: the expression level of CaDHN3 in all treatments (GABA-treated seeds, hydro-primed seeds and untreated seeds under all NaCl treatments) were determined by measuring the fluorescence of RT-PCR amplicon band (850 bp) using the Gel Documentation system and Image Analysis System (Alpha Innotech.CA, USA). The amount of fluorescence in a cDNA amplicon representing specific RNA in each sample was used as a measure of the level of expression. The level of CaDHN3 RNA in each tube was normalized with respect to the fluorescence of the 18 S RNA (1800 bp) (forward primer 5`CCACCCATAGAATCAAGAAAGAG3` and reverse primer 5`GCAAATTACCCAATCCTGAC3`) (Hassan et al. 2015) as internal control. The background fluorescence on agarose gels was subtracted from the fluorescence value of each band. Each determination represents an average of three different biological replicates with standard deviation. Data was represented as Log2 fold expression in the level of CaDHN3 gene expression on day 8 post-germination in GABA-treated seeds, hydro-primed seeds and untreated seeds after each NaCl treatment compared to normal growth (0 mM NaCl).
Data statistical analysis
The experimental design for all studies was a completely randomized design (CRD). Treatments were replicated three times. All assays and measuring parameters were conducted in triplicate. Mean and standard deviation (SD) values were determined for all assay parameters. Normality tests of one-way analysis of variance (ANOVA) using the least significant difference (LSD) multiple comparison tests on the means were used for data analysis at a 95% confidence level (P-value < 0.05) for each GABA concentration, hydro-primed and untreated seeds under all NaCl treatments for each measuring parameters and assays (Germination percentage (G%), seedling length, seedling fresh weight, seedling dry weight, germination rate (GR), mean time to germinate (MTG), seed moisture content, GABA shunt metabolites, MDA level, and Log2 fold expression of CaDHN3 gene). Pearson correlation coefficient (r) was used to show the trend between the NaCl concentrations and the means of measured parameters for each GABA concentration, hydro-primed and untreated seeds. All statistical analyses were done using the SPSS version 25.0 software.
Results and discussion
Effects of GABA priming on pepper seeds germination and seedlings growth under NaCl stress
Seed germination percentage (G%), seedling length, and seedling fresh and dry weight of pepper (Capsicum annuum L.) under salt stress were recorded. Significant differences (P ≤ 0.05) in germination percentage, seedling length, fresh and dry weights were observed after all GABA treatments irrespective of different NaCl concentrations. Seeds G% was negatively (r = -0.85 to -0.953) affected by salt stress under all GABA treatments (Table 1). Treatment with 0.5 mM GABA showed high G% under all NaCl concentrations even at 200 mM NaCl (80%) compared to untreated seeds (45%). For 1.0 mM GABA treatment, high G% was observed under low NaCl treatments (90–93%) and then G% gradually reduced with the increase in NaCl concentration (73% at 200 mM NaCl). Treatments with 1.5, 2, and 2.5 mM GABA displayed similar results by enhancing the G% (80–98%) under all NaCl concentrations used in this study. Like GABA priming, hydro-primed seeds (H2O) showed high germination percentage under all NaCl concentrations (75–98%) compared to untreated seeds (45–86%).
Seedling lengths were significantly (P ≤ 0.05) reduced and negatively correlated (r = -0.937 to -0.991) by the increase of NaCl concentrations among all seeds treated with GABA, in addition to hydro-primed (H2O) and untreated seeds (Table 1). Under 0 mM NaCl, pepper seedlings showed an increase in seedling length (5.6 to 7.04 cm) gradually by increasing the concentrations of GABA treatment (from 0.5 to 2.5 mM GABA) compared to other NaCl concentrations. Seedling length in hydro-primed and untreated seeds was decreased (5.6 to 0.87 cm) by increasing NaCl concentrations. All GABA treatments significantly (P ≤ 0.05) enhanced the growth of pepper seedlings under all NaCl treatments compared to hydro-primed and untreated seeds. Moreover, at 200 mM NaCl treatment, all GABA treatments except 2 mM GABA showed a significant increase in seedling length compared to hydro-primed and untreated seeds. Seedling fresh and dry weights were significantly (P ≤ 0.05) increased in response to seed priming with GABA and distilled water compared to untreated seeds. All pepper seeds that were primed with GABA and distilled water had significantly (P ≤ 0.05) higher fresh weight compared to untreated seeds under all NaCl concentrations. Furthermore, seeds treated with 0.5 mM GABA showed a reduction in seedling dry weight compared to untreated seeds, while seeds treated with 1.0, 1.5, 2.0, 2.5 mM GABA and hydro-priming showed slight enhancement in seedling dry weight among all NaCl concentrations compared to untreated seeds (Table 1).
Seed germination and early seedling growth are important stages for plant development which are affected by many environmental factors such as high temperature and salinity. Generally, high NaCl treatment inhibited both seed germination and seedling growth in various cultivated crops (Wang et al. 2018). Ullah et al. (2023b) reported that salt stress negatively affects the growth performance of chufa (Cyperus esculentus L. var. Sativus Boeck) resulting in a significant decline in length of root and shoot and fresh and dry weights. A study on sees of rapeseed (Brassica napus L.) under NaCl stress showed a significant reduction in germination percentage, seedling length, and fresh and dry weights when NaCl treatments increased consistently (Zhang et al. 2023). However, exogenous priming with GABA inhibited the negative impact of NaCl and accelerated germination index, seedling root length, and fresh and dry weights of rapeseed (Brassica napus L.) (Zhang et al. 2023). In the mung bean (Vigna radiate L.), exogenous GABA treatment increased salt tolerance by improving seedling growth and fresh weight (Ullah et al. 2023a).
Moreover, exogenous GABA significantly improved the root length, shoot length, and fresh and dry weight of maize (Zea mays L.) seedlings under various abiotic stresses (Wang et al. 2017). Priming rice (Oryza sativa L.) seeds with 0.5 mM GABA showed significant improvement in the mobilization of sugar and amino acids which in turn enhanced rice seedlings growth and weight under salt stress (Sheteiwy et al. 2019). In agreement with our data, lettuce (Lactuca sativa L.) seed germination and plant growth were significantly improved under saline conditions due to exogenous GABA treatments (Kalhor et al. 2018). The application of exogenous GABA with different concentrations on sweet pepper (Capsicum annuum L.) seeds under drought stress significantly increased root and shoot length and fresh and dry weights (Iqbal et al. 2023). Our data indicated that GABA priming efficiently alleviated the effect of salt stress during pepper seed germination and significantly enhanced pepper seedlings growth under all NaCl treatments. Furthermore, exogenous GABA treatment increased pepper seeds water absorption by adjusting the seed osmolarity, maintaining membrane integrity, increasing the turgor pressure, preserving the water balance and metabolic stability in Capsicum annuum L. tissues, which in turn boosted the seedlings growth under salt stress.
Pepper seeds germination rate (GR) and mean time to germinate (MTG) in response to GABA priming
Germination rate (GR) and mean time to germinate (MTG) for pepper seeds were recorded 8 days after planting (Table 2). The effect of salt stress was recorded on pepper seeds by decreasing the GR with a negative correlation and increasing the MTG with a positive correlation under all NaCl treatments. Seeds treated with 0.5 mM GABA showed the highest GR and lower MTG compared to other treatments under all NaCl concentrations. Consequently, seeds treated with GABA or hydro-primed seeds showed enhancement in GR and MTG under all NaCl treatments compared to untreated seeds as shown in Table 2. GABA treatments has increased the germination capacity of pepper seeds under salt stress through increasing the activity of α- and β-amylases to promote starch metabolism for energy production during seed germination (Cheng et al. 2018). Also, exogenous GABA treatment induced the production of endogenous GABA that performed osmo-regulatory function and maintained ion homoeostasis inside pepper seeds to boost water uptake under NaCl treatments (Hayat et al. 2023). High concentrations of salt inhibited the activity of enzymes that contributed to seed germination and germination rate in cotton (Maryum et al. 2022). However, cotton (Gossypium hirsutum L.) seeds treated with GABA significantly improved seeds GR and MTG under the same concentrations of NaCl used in this study (Dong et al. 2024).
Similar reports suggested that exogenous GABA treatment had a positive effect on the germination rate of lettuce (Kalhor et al. 2018), citrus (Ziogas et al. 2017), and wheat (Suhel et al. 2023). Seed germination and growth of wheat (Triticum aestivum L.) seedlings was dramatically delayed by high NaCl concentration, whereas wheat seeds treated with GABA were less affected by salinity and showed improvement in seeds germination rate and seedlings growth (Li et al. 2016b). Also, exogenous treatment of onion (Allium cepa L.) seeds with amino acids showed the highest germination rate compared with non-treated seeds (Abdelkader et al. 2023).
Pepper (Capsicum annuum L.) seeds growth and development were significantly affected by heavy metals. In contrast, melatonin treatment significantly reduced these effects by promoting pepper seeds growth characteristics (Rizwan et al. 2024). In addition, exogenous treatment of pepper (Capsicum annuum L.) with sodium hydrogen sulfide reduced salt toxicity and enhanced growth rate in response to salt stress (Kaya et al. 2024). All these studies come in agreement with our findings which proved the effectiveness of exogenous GABA treatment in inducing salt tolerance of pepper seeds by increasing the germination rate and reducing the mean time to germinate under all NaCl treatments. Taken together, GABA treatments increased the germination capacity of pepper seeds to countercurrent the salt toxicity by reducing the accumulation of salt ions, and maintaining metabolic homeostasis to restore seedlings growth under salt stress.
Pepper seeds moisture content in response to GABA priming
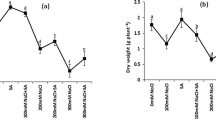
Seed moisture content was measured directly after soaking seeds for 24 h with different concentrations of GABA (0.5, 1.0, 1.5, 2.0, 2.5 mM) and hydro-priming treatment. The pepper seed moisture content ranged from 45.55 to 60.19% for all GABA treatments except in untreated seeds (9.48) (Table 3). Results showed a significant (P ≤ 0.05) difference in seed moisture content between all GABA-treated seeds, and hydro-primed seeds in comparison with untreated seeds (Fig. 1). GABA treatments significantly (P ≤ 0.05) increased the seed moisture content of pepper which in turn improved the capability of seeds to germinate and grow under NaCl treatments.
Seed moisture content of pepper (Capsicum annum L.) seeds immediately after imposing treatments with 0.5, 1.0, 1.5, 2.0, 2.5 mM GABA, d.H2O, and untreated seeds separately for 24 h at 25ºC and after drying the treated seeds in oven at 80˚C for 72 h. Columns with different letters are statistically different (P ≤ 0.05) by LSD
Various studies showed that NaCl caused an increase in both stem and root Na+ content which decreased water content and changed cell wall properties. Priming wheat (Triticum aestivum L.) seeds with GABA greatly alleviated Na + increase and significantly upsurge water absorption (Wang et al. 2019; Khanna et al. 2021). Exogenous GABA treatment in maize (Zea mays L.) seedlings reduced the accumulation of substances that caused growth damage through significant enhancement of seed moisture content and water avilability, which consequently improved plant fresh and dry mass under NaCl stress (Wang et al. 2017).
Moreover, GABA treatment significantly improved the relative water content of pepper (Capsicum annuum L.) seedlings and significantly enhanced drought stress tolerance (Iqbal et al. 2023). Soaking wheat (Triticum durum L.) seeds with different concentrations of chitosan exhibited significant elevation in seed moisture content, and fresh and dry weights compared to untreated seeds (AL-Quraan et al. 2023). Similarity, Alkahtani et al. (2020) study reported that pepper (Capsicum annuum L.) seeds primed with chitosan increased relative water uptake and seed moisture content under salt stress. Likewise, exogenous GABA effectively improved salt stress tolerance of Malus hupehensis by promoting seedlings’ growth and development (seedling length, biomass (fresh and dry weights), and water content of seeds) (Li et al. 2020). Taken together, our data indicated that GABA significantly aided pepper seeds to absorb more water even when return into their original moisture (data not shown) and maintained seed moisture content compared to untreated seeds (Table 3; Fig. 1).
The effect of GABA priming on GABA shunt metabolism under NaCl stress in pepper seedlings
The GABA shunt pathway is one of the critical metabolic pathways in plants that activated by abiotic stress such as salt stress (Hayat et al. 2023). In this study, the endogenous level of GABA shunt metabolites (GABA, Alanine, and Glutamate) in treated and untreated pepper (Capsicum annuum L.) seeds were measured on the 1st, 4th, and 8th day after planting at different NaCl concentrations. Current data showed a significant (P ≤ 0.05) increase with positive correlation between internal GABA, alanine, and glutamate levels and NaCl concentration in all GABA priming treatments (Table 4). The highest internal GABA level was recorded on the 4th day of germination for seeds treated with 0.5 mM GABA. Seeds treated with 0.5, 1.0, and 1.5 mM GABA concentrations had a higher accumulation of GABA content in all days recorded, while 2.0 and 2.5 mM GABA and hydro-primed seed had lower accumulation of GABA but it was relatively high compared to untreated seeds. For alanine; 0.5, 1.0, and 1.5 mM GABA-primed seeds had the highest level, and the level of alanine was increased with days among all NaCl concentrations. The alanine level in 2.0 and 2.5 mM GABA and hydro-primed seed was lower but still high compared to untreated seeds under all NaCl treatments. The same trend was observed in glutamate, where glutamate content was increased on the 1st, 4th, and 8th days of germination in all GABA-primed seeds under all NaCl treatments, respectively (Table 4).
The GABA shunt pathway had a critical role in connecting amino acid metabolism with other organic acid intermediates. Also, it serves as a carbon and nitrogen source to supply the carbon: nitrogen (C: N) deficit and maintain metabolic stability in plants under abiotic conditions (Batushansky et al. 2014; Che-Othman et al. 2020). Under salt stress, exogenous GABA priming successfully enhanced pepper seeds to accumulate GABA internally to supply the metabolic intermediate and nourish the citric acid cycle during seed germination (AL-Quraan et al. 2023; Dabravolski and Isayenkov 2023). Also, the elevation of GABA level internally inhibited the accumulation of ROS, provided oxidative protection, maintained the C: N metabolic stability and alleviated the negative impact of salt stress during pepper seed germination.
Many previous studies showed that exogenous GABA induced the accumulation of endogenous GABA and glutamate contents and improved plant tolerance to stress (Ramesh et al. 2018; Li et al. 2020). Under salt stress, supplemented maize seedlings with exogenous GABA increased antioxidant enzymes activity, improved photosynthetic performance, enhanced sugar accumulation, activated nitrogen metabolism and improved the C: N balance (Wang et al. 2023). Under heat and salinity stresses, an increased level of internal GABA was observed in wheat (Triticum aestivum L.) seeds primed with exogenous GABA (Yu et al. 2023). Likewise, exogenous treatment of apple (Malus domestica, c.v. Cripps Pink) fruit with GABA caused an increase in endogenous GABA and glutamate contents under drought stress (Cheng et al. 2023). Heat stress decreased GABA content in kiwifruits (Actinidia spp.), while exogenous GABA application elevated GABA content up to 3.36 times higher than non-treated fruits. However, glutamate level did not change under normal conditions, but it was significantly increased after GABA application in response to heat stress (Huo et al. 2023). Under cold stress, growth and yield were inhibited in various plants (Liu et al. 2020). In tea (Camellia sinensis (L.) O. Kuntze) seedlings, exogenous application of GABA significantly improved metabolic stability by increasing endogenous GABA content under cold stress (Zhu et al. 2019). Similarly, exogenous 5-aminolevulinic acid (ALA) treatment increased GABA, alanine, and glutamate levels and significantly improved anti-oxidation and cell expansion of tomato (Solanum lycopersicum) seedlings under cold stress (Liu et al. 2020). In agreement with previous studies, our data demonstrated that exogenous GABA treatments enhanced elevation in endogenous GABA, alanine, and glutamate levels to increase the capability of pepper seeds to stabilize C: N metabolism during seed germination under salt stress. Furthermore, GABA-regulated intermediate metabolites participated in tricarboxylic acid cycle, GABA shunt, antioxidant defense system, carbohydrates and lipid metabolism, and nitrogen assimilation (Dabravolski and Isayenkov 2023; Dong et al. 2024) which played positive roles in reactive oxygen species scavenging, energy conversion, soluble sugar accumulation, osmotic adjustment, and ion homeostasis in GABA-primed pepper seeds in response to NaCl treatments.
Oxidative damage in pepper seedlings from GABA primed seeds under NaCl stress
Malondialdehyde (MDA) is an organic compound that is measured to indicate the level of lipid peroxidation in response to abiotic stresses. The content of MDA in GABA-treated, hydroprimed and untreated pepper (Capsicum annuum L.) seeds was recorded at the 1st, 4th, and 8th days after germination at different NaCl concentrations (Table 5). Current results showed a significant (P ≤ 0.5) increase in MDA content with a positive correlation to increasing NaCl concentrations. Pepper seeds treated with 2.0 mM GABA had the lowest amount of MDA with a trend of small elevation on 4th and 8th days of germination. All other GABA-treated and hydro-primed seeds showed a significant reduction in MDA content compared to untreated seeds under all NaCl concentrations. The untreated seeds had the highest MDA content, especially on day 4th of germination at 100 and 200 mM NaCl treatments (Table 5). These results indicated that priming pepper seeds with 0.5, 1.0, and 1.5 mM GABA under salt stress significantly (P ≤ 0.5) reduced the MDA content by activating ROS scavenging defense mechanisms to mitigate the oxidative damage caused by NaCl treatments during seed germination.
Salt stress increased ROS and oxidative damage of cell membranes which leads to increased MDA content under stress in plants (AL-Quraan et al. 2023). Exogenous application of GABA reduced MDA level under salt stress in chufa (Cyperus esculentus L. var. Sativus Boeck) seedlings (Ullah et al. 2023b). The accumulation of endogenous GABA as a result of exogenous GABA treatment under abiotic stress to countercurrent the oxidative damage on internal metabolism had been reported in various plants (Qi et al. 2019). Under heat stress, application of GABA decreased the relative conductivity and reduced accumulation of MDA amount in wheat (Triticum aestivum L.) seedlings (Wang et al. 2022). Foliar spray with GABA on carrot (Daucus carota L.) grown under drought stress gave similar results to our study, where GABA enhanced tolerance to drought stress by increasing the antioxidant enzymes activities and decreased ROS and MDA contents (Bashir et al. 2021).
Moreover, GABA treatment significantly reduced ROS and MDA contents that resulted from chilling in cucumber (Cucumis sativus L.) (Malekzadeh et al. 2017), cold stress in Banana (Musa acuminata) fruits (Wang et al. 2016), and drought stress in pepper (Capsicum annuum L.) seedlings (Iqbal et al. 2023) to boost plant tolerance and inhibit the stress negative effect on growth and yield production. Golnari et al. (2021) study showed higher membrane stability and lower H2O2 and MDA contents when used GABA as a priming agent in strawberry (Fragaria × ananassa Duch.) compared with untreated plants. Furthermore, AL-Quraan et al. (2023) showed that treated durum wheat (Triticum durum L.) seeds with chitosan reduced MDA content on 1st, 4th and 8th days of germination under different concentrations of NaCl compared to untreated seeds, which indicated that seeds priming with bio-activators significantly enhanced ROS scavenging against salt stress. Our data come in agreement with all previous studies that supported the fact that cellular damage induced by NaCl treatments is controlled by exogenous GABA application as shown by lower MDA content in GABA primed seeds compared to untreated seeds during pepper seeds germination. GABA treatments significantly reduced the oxidative damage and the ROS production by increasing the activity of antioxidant enzymes to maintain membrane integrity and reduce metabolic disruption (Shala et al. 2024) which in turn increased the pepper seeds tolerance to salt stress.
The effect of GABA on CaDHN3 mRNA transcript level in response to NaCl stress in pepper seedlings
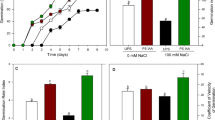
The expression of dehydrins is usually considered as a significant marker for abiotic stress tolerance in various plants (Clarke et al. 2015). Expression of pepper (Capsicum annuum L.) dehydrin gene (CaDHN3) mRNA transcript of GABA-primed pepper seeds in response to different concentrations of NaCl after 8 days of germination was determined (Table 6; Fig. 2). Data showed a significant increase (P ≤ 0.05) in the level of CaDHN3 mRNA transcript with the increase of NaCl concentrations under all GABA treatments. Pepper seeds treated with 0.5 mM GABA showed an upsurge in CaDHN3 transcription with the increase in NaCl concentration compared to other GABA treatments and untreated seeds. Also, the expression of CaDHN3 in seeds treated with 1.0 and 2.5 mM GABA was significantly elevated with the increase in NaCl concentration compared to untreated seeds. On the other hand, the expression of CaDHN3 in seeds treated with 1.5 and 2 mM GABA was lower than that in untreated seeds. Hydro-primed seeds showed lower CaDHN3 transcription level compared to untreated seeds under all NaCl concentrations (Table 6; Fig. 2). The elevation in CaDHN3 transcription in GABA-treated pepper seeds might be connected with the diverse protective effects of dehydrin during the later stages of seed embryogenesis and development to prevent electrolyte leakage and lipid peroxidation, stabilize cell membranes and cellular molecules, maintain enzyme function and prevent dehydration (Zhou et al. 2021; Cheng et al. 2018) that eventually promoted seed germination and seedlings growth of pepper in response to salt stress.
Log2 fold expression of CaDHN3 in in pepper (Capsicum annum L.) seeds exposed to seven treatments (0.5, 1.0, 1.5, 2.0, 2.5 mM GABA, d.H2O, and untreated seeds) supplemented with (0, 25, 50, 75, 100, and 200) mM of NaCl. For each seeds treatment under different NaCl treatments, Means followed by different letters are statistically different (P ≤ 0.05) by LSD
Dehydrins (DHNs) are the most abundant proteins in seeds that used in the later stage of embryonic development and also accumulated in plants under various abiotic stresses including high or low temperature, dehydration, and salinity (Graether and Boddington 2014). During the late stage of seed germination, the accumulation of DHNs is associated with several vital functions such as ROS scavenging, sustaining flow-ability of cell sap, and stabilizing structure and function of proteins to countercurrent the impact of environmental stresses (Allagulova et al. 2003; Hundertmark and Hincha 2008). Studies showed that overexpression of dehydrins (DHNs) in crops and ornamental plants significantly enhanced tolerance to cold, drought, and salt stress (Sun et al. 2021; AL-Quraan et al. 2022). Overexpression of DHNs genes enhanced tolerance to salt and drought stresses and improved the growth rate of Arabidopsis thaliana seedlings exposed to 100 mM NaCl (Lv et al. 2018). Furthermore, soaking white clover (Trifolium repens) seeds with different concentrations of GABA significantly exhibited higher DHNs (SK1, Y2K, Y2SK, and dehydrin b) genes expression levels (using a real-time quantitative polymerase chain reaction (qRT-PCR) than both seeds treated with water and untreated seeds under the same NaCl treatments (Cheng et al. 2018).
In pepper, the overexpression of CaDHN2 enhanced drought tolerance by increasing the antioxidant enzymes activities, and lowering ROS content (Li et al. 2023). In the current study, GABA-treated pepper (Capsicum annuum L.) seeds showed higher dehydrin gene (CaDHN3) expression levels under all NaCl treatments. Our study comes in agreement with Zhou et al. (2021) who found that priming white clover (Trifolium repens) seeds with GABA improved seed germination and seedlings stress tolerance by enhancing antioxidant metabolism, elevation of DREB gene expression and increasing the accumulation of dehydrin proteins under water stress Similarly, elevation of dhn and wcor dehydrins genes expression in wheat (Triticum durum L.) seedlings enhanced drought tolerance during post-germination stage by protection of cells membrane, cryoprotection of enzymes and proteins and prevention ROS accumulation (AL-Quraan et al. 2022). Our data suggested that the maintenance of higher CaDHN3 gene expression to synthesize dehydrin protein could be one of the most significant survival mechanisms regulated by GABA treatments in pepper during seeds germination in response to salt stress.
Conclusion
Salt stress is a global problem for farmers, and its solution requires the usage of multiple approaches to enhance crop growth and production. Seeds priming with GABA could be an effective practice to alleviate salt-caused inhibition of seeds germination and maintain successful germination and seedlings growth under salt stress. In this study, data showed that salt stress had a negative effect on seed germination and seedling growth of pepper (Capsicum annuum L.). Salt stress significantly decreased seedling length, fresh and dry weights, as well as the seeds germination rate of pepper. Pre-treatment of pepper seeds with GABA improved seed germination by increasing the seed moisture content and enhancing germination percentage and seedlings growth under salt stress. Exogenous GABA treatment significantly enhanced pepper seeds water absorption by adjusting seeds osmolarity and maintaining membrane integrity which ultimately preserved the water balance and metabolic stability in the Capsicum annuum L. tissues that resulted in improving and boosting the seedlings growth under salt stress. Also, GABA treatments increased germination rate and decreased pepper seeds MTG under salt stress by improving the germination capacity to countercurrent the salt toxicity, decreasing cell membrane damage and maintaining metabolic homeostasis. GABA priming activated the endogenous GABA shunt pathway and increased the capability of pepper seeds to maintain internal metabolic stability by significantly increasing the endogenous GABA levels in pepper seeds to produce more GABA for C: N metabolic stability, oxidative protection, energy conversion, osmotic adjustment, and ion homeostasis in response to NaCl treatments. Significant reduction in MDA contents was observed in all GABA treatments and hydro-primed pepper seeds when compared to untreated seeds under all NaCl concentrations. Priming pepper seeds with GABA protected cell membranes and enhanced ROS scavenging abilities under salt stress. The level of pepper dehydrin gene (CaDHN3) was significantly increased with the increase of NaCl concentrations under all GABA treatments. The significant elevation in CaDHN3 transcription in GABA-treated pepper seeds might be connected with the important role of dehydrin during the later stages of seed embryogenesis and development to prevent electrolyte leakage and lipid peroxidation, stabilize cellular molecules, maintain metabolic stability and enzymes function and prevent dehydration during seeds germination in response to salt stress. Our data suggested that the maintenance of higher CaDHN3 gene expression is significantly important to improve salt stress tolerance in pepper during seeds germination to boost growth and maintain metabolic stability regulated by GABA. Collectively, the current study provided significant evidence that priming Capsicum annuum L. seeds with GABA could successfully reduce salinity-induced adverse effect on seed germination, seedlings growth and internal metabolism. Further research should be directed toward investigating the effect of GABA treatment on nutritional composition of pepper and the production of valuable phytochemicals.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbasi GH, Akhtar J, Anwar-ul-Haq M, Ahmad N (2012) Screening of maize hybrids for salt tolerance at seedling stage under hydroponic condition. Soil Environ 31:83. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20123198658
Abdelkader M, Voronina L, Puchkov M, Shcherbakova N, Pakina E, Zargar M, Lyashko M (2023) Seed priming with exogenous amino acids improves Germination Rates and enhances photosynthetic pigments of Onion seedlings (Allium cepa L). Horticulturae 9:80. https://doi.org/10.3390/horticulturae9010080
AL-Quraan NA, Locy RD, Singh NK (2010) Expression of calmodulin genes in wild type and calmodulin mutants of Arabidopsis thaliana under heat stress. Plant Physiol Biochem 48:697–702. https://doi.org/10.1016/j.plaphy.2010.04.011
AL-Quraan NA, Sartawe FA, Qaryouti MM (2013) Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J Plant Physiol 170:1003–1009. https://doi.org/10.1016/j.jplph.2013.02.010
AL-Quraan NA, Samarah NH, Tanash AA (2022) Effect of drought stress on wheat (Triticum durum) growth and metabolism: insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Funct Plant Biol 51:FP22177. https://doi.org/10.1071/fp22177
AL-Quraan NA, Samarah NH, Rasheed EI (2023) The role of chitosan priming in induction of GABA shunt pathway during wheat seed germination under salt stress. Biol Plant 67:234–248. https://doi.org/10.32615/bp.2023.029
Al-Thabet SS, Leilah AA, Al-Hawass I (2004) Effect of NaCl and incubation temperature on seed germination of three canola (Brassica napus L.) cultivars. Sci King Faisal Univ (Basic Appl Sciences) 5:81–92. https://services.kfu.edu.sa/scientificjournal/Handlers/FileHandler.ashx?file=b514.pdf&Folder=UploadFiles
ALKahtani MDF, Attia KA, Hafez YM, Khan N, Eid AM, Ali MAM, Abdelaal KAA (2020) Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 10, 1180. https://doi.org/10.3390/agronomy10081180
Allagulova CR, Gimalov FR, Shakirova FM, Vakhitov VA (2003) The plant dehydrins: structure and putative functions. Biochem (Moscow) 68:945–951. https://doi.org/10.1023/a:1026077825584
Andriolo JL, Luz GL, da, Witter MH, Godoi RdosS, Barros GT, Bortolotto OC (2005) Growth and yield of lettuce plants under salinity. Horticultura Brasileira 23:931–934. https://doi.org/10.1590/s0102-05362005000400014
Bashir R, Riaz HN, Anwar S, Parveen N, Khalilzadeh R, Hussain I, Mahmood S (2021) Morpho-physiological changes in carrots by foliar γ-aminobutyric acid under drought stress. Brazilian J Bot 44:57–68. https://doi.org/10.1007/s40415-020-00676-7
Batushansky A, Kirma M, Grillich N, Toubiana D, Pham PA, Balbo I, Fromm H, Galili G, Fernie AR, Fait A (2014) Combined transcriptomics and metabolomics of Arabidopsis thaliana seedlings exposed to exogenous GABA suggest its role in plants is predominantly metabolic. Mol Plant 7:1065–1068. https://doi.org/10.1093/mp/ssu017
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15:2988–2996. https://doi.org/10.1002/j.1460-2075.1996.tb00662.x
Bergmeyer HU, Grassl M (1988) Methods of enzymatic analysis. Verlag Chemie, vol 2, 3rd edn. VCH, London, Weinheim
Bhardwaj J, Anand A, Nagarajan S (2012) Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol Biochem 57:67–73. https://doi.org/10.1016/j.plaphy.2012.05.008
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends in plant science. 9:110–115. https://doi.org/10.1016/j.tplants.2004.01.006
Bybordi A (2010) The influence of salt stress on seed germination, growth and yield of canola cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38:128–133. https://doi.org/10.15835/nbha4017493
Çekiç FÖ (2018) Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress. Celal Bayar Univ J Sci 14:61–64. https://doi.org/10.18466/cbayarfbe.348935
Che-Othman MH, Jacoby RP, Millar AH, Taylor NL (2020) Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol 225:1166–1180. https://doi.org/10.1111/nph.15713
Cheng B, Li Z, Liang L, Cao Y, Zeng W, Zhang X, Ma X, Huang L, Nie G, Liu W (2018) The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K + transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int J Mol Sci 19:2520. https://doi.org/10.3390/ijms19092520
Cheng P, Yue Q, Zhang Y, Zhao S, Khan A, Yang X, He J, Wang S, Shen W, Qian Q (2023) Application of γ-aminobutyric acid (GABA) improves fruit quality and rootstock drought tolerance in apple. J Plant Physiol 280:153890. https://doi.org/10.1016/j.jplph.2022.153890
Cohen Y, Baider A, Gotlieb D, Rubin E (2007) Control of Bremia lactucae in field-grown lettuce by DL-3-amino-n-butanoic acid (BABA). https://orgprints.org/id/eprint/10318/1/cohen-etal-2007-bremia_lactucae.pdf
Cuartero J, Bolarin MC, Asins MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57:1045–1058. https://doi.org/10.1093/jxb/erj102
Dabravolski SA, Isayenkov SV (2023) The role of the γ-aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae 9:230. https://doi.org/10.3390/horticulturae9020230
Dantas BF, Ribeiro L, de Aragão S, C.A (2005) Physiological response of cowpea seeds to salinity stress. Revista Brasileira De Sementes 27:144–148. https://doi.org/10.1590/s0101-31222005000100018
Dong Z, Huang J, Qi T, Meng A, Fu Q, Fu Y, Xu F (2024) Exogenous γ-Aminobutyric acid can improve seed germination and seedling growth of two cotton cultivars under salt stress. Plants 13:82. https://doi.org/10.3390/plants13010082
Du C, Chen W, Wu Y, Wang G, Zhao J, Sun J, Ji J, Yan D, Jiang Z, Shi S (2020) Effects of GABA and vigabatrin on the germination of Chinese chestnut recalcitrant seeds and its implications for seed dormancy and storage. Plants 9:449. https://doi.org/10.3390/plants9040449
Fait A, Angelovici R, Less H, Ohad I, Urbanczyk-Wochniak E, Fernie AR, Galili G (2006) Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol 142:839–854. https://doi.org/10.1104/pp.106.086694
Fernie AR, Roessner U, Trethewey RN, Willmitzer L (2001) The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213. https://doi.org/10.1007/s004250100521
Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189:329–339. https://doi.org/10.1007/bf00194429
Golnari S, Vafaee Y, Nazari F, Ghaderi N (2021) Gamma-aminobutyric acid (GABA) and salinity impacts antioxidative response and expression of stress-related genes in strawberry cv. Aromas. Brazilian J Bot 44:639–651. https://doi.org/10.1007/s40415-021-00750-8
Graether SP, Boddington KF (2014) Disorder and function: a review of the dehydrin protein family. Front Plant Sci 5:576. https://doi.org/10.3389/fpls.2014.00576
Gramazio P, Takayama M, Ezura H (2020) Challenges and prospects of new plant breeding techniques for GABA improvement in crops: tomato as an example. Front Plant Sci 11:577980. https://doi.org/10.3389/fpls.2020.577980
Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C (2007) Combined networks regulating seed maturation. Trends Plant Sci 12:294–300. https://doi.org/10.1016/j.tplants.2007.06.003
Hamed M, Kalita D, Bartolo ME, Jayanty SS (2019) Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: comparative study of the effect of ripening stage and cooking methods. Antioxidants 8:364. https://doi.org/10.3390/antiox8090364
Hassan NM, El-Bastawisy ZM, El-Sayed AK, Ebeed HT, Alla MMN (2015) Roles of dehydrin genes in wheat tolerance to drought stress. J Adv Res 6:179–188. https://doi.org/10.1016/j.jare.2013.11.004
Hayat F, Khan U, Li J, Ahmed N, Khanum F, Iqbal S, Altaf MA, Ahmad J, Javed HU, Peng Y, Ma X (2023) γ aminobutyric acid (GABA): a key player in alleviating abiotic stress resistance in horticultural crops: current insights and future directions. Horticulturae 9:647. https://doi.org/10.3390/horticulturae9060647
Hundertmark M, Hincha DK (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:1–22. https://doi.org/10.1186/1471-2164-9-118
Huo L, Chen Y, Zhang Y, Zhang H, Wang H, Xu K, Sun X (2023) Exogenous γ-aminobutyric acid enhances heat tolerance of kiwifruit plants by protecting photosynthetic system and promoting heat shock proteins expression. Annals Agricultural Sci 68:137–147. https://doi.org/10.1016/j.aoas.2023.12.003
Iqbal B, Hussain F, Khan MS, Iqbal T, Shah W, Ali B, Al Syaad KM, Ercisli S (2023) Physiology of gamma-aminobutyric acid treated Capsicum annuum L.(Sweet pepper) under induced drought stress. PLoS ONE 18:e0289900. https://doi.org/10.1371/journal.pone.0289900
Iranbakhsh A, Ardebili ZO, Ardebili NO, Ghoranneviss M, Safari N (2018) Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology. Acta Physiol Plant 40:1–11. https://doi.org/10.1007/s11738-018-2730-8
Ismail A, Takeda S, Nick P (2014) Life and death under salt stress: same players, different timing? J Exp Bot 65:2963–2979. https://doi.org/10.1093/jxb/eru159
Jing H, Li C, Ma F, Ma J-H, Khan A, Wang X, Zhao L-Y, Gong Z-H, Chen R-G (2016) Genome-wide identification, expression diversication of dehydrin gene family and characterization of CaDHN3 in pepper (Capsicum annuum L). PLoS ONE 11:e0161073. https://doi.org/10.1371/journal.pone.0161073
Jisha KC, Puthur JT (2016) Seed priming with BABA (β-amino butyric acid): a cost-effective method of abiotic stress tolerance in Vigna radiata (L). Wilczek Protoplasma 253:277–289. https://doi.org/10.1007/s00709-015-0804-7
Jovicich E, Cantliffe DJ, Sargent SA, Osborne LS (2004) Production of greenhouse-grown peppers in Florida. Document HS979. The Department of Horticultural sciences. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. http://edis.ifas.ufl.edu/hs228
Kalhor MS, Aliniaeifard S, Seif M, Asayesh EJ, Bernard F, Hassani B, Li T (2018) Enhanced salt tolerance and photosynthetic performance: implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol Biochem 130:157–172. https://doi.org/10.1016/j.plaphy.2018.07.003
Kaouther Z, Mariem BF, Fardaous M, Cherif H (2012) Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescence of Tunisian cultivars of Chili pepper (Capsicum frutescens L). J Stress Physiol Biochem 8:236–252. https://doi.org/10.5053/ejobios.2012.6.0.6
Kaya C, Uğurlar F, Ashraf M, Alyemeni MN, Dewil R, Ahmad P (2024) Mitigating salt toxicity and overcoming phosphate deficiency alone and in combination in pepper (Capsicum annuum L.) plants through supplementation of hydrogen sulfide. J Environ Manage 351:119759. https://doi.org/10.1016/j.jenvman.2023.119759
Kelley WT, Boyhan GE, Harrison KA, Granberry DM, Langston DB, Sparks AN, Culpepper S, Hurst WC, Fonsah EG (2009) Commercial pepper production handbook, vol 1309. Bulletin. University of Georgia.USAhttps://hdl.handle.net/10724/12339
Khanna RR, Jahan B, Iqbal N, Khan NA, AlAjmi MF, Rehman MT, Khan MIR (2021) GABA reverses salt-inhibited photosynthetic and growth responses through its influence on NO-mediated nitrogen-sulfur assimilation and antioxidant system in wheat. J Biotechnol 325:73–82. https://doi.org/10.1016/j.jbiotec.2020.11.015
Krishnan S, Laskowski K, Shukla V, Merewitz EB (2013) Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ–aminobutyric acid on perennial ryegrass. J Am Soc Hortic Sci 138:358–366. https://doi.org/10.21273/jashs.138.5.358
Kumar OA, Tata SS (2009) Ascorbic acid contents in Chili peppers (Capsicum, L). Notulae Scientia Biologicae 1:50–52. https://doi.org/10.15835/nsb113445
Kumar S, Jangde S, Priya T, Yashu BR (2017) Effect of GABA on morphology, yield and yield attributes in Black Gram (Vigna mungo (L.) Hepper) under salt stress condition. Int J Pure Appl Biosci 5:1223–1228. https://doi.org/10.18782/2320-7051.5927
Latham E (2009) The colourful world of chillies. New Zealand: Stuff. co. nz. https://www.stuff.co.nz/life-style/food-wine/1756288/The-colourful-world-of-chillies
Li Y, Bai Q, Jin X, Wen H, Gu Z (2010) Effects of cultivar and culture conditions on γ-aminobutyric acid accumulation in germinated fava beans (Vicia faba L). J Sci Food Agric 90:52–57. https://doi.org/10.1002/jsfa.3778
Li MF, Guo SJ, Yang XH, Meng QW, Wei XJ (2016a) Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol Plant 60:123–131. https://doi.org/10.1007/s10535-015-0559-1
Li MF, Guo SJ, Yang XH, Meng QW, Wei XJ (2016b) Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol Plant 60:123–131. https://doi.org/10.1007/s10535-015-0559-1
Li Y, Liu B, Peng Y, Liu C, Zhang X, Zhang Z, Liang W, Ma F, Li C (2020) Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci Hort 261:108982. https://doi.org/10.1016/j.scienta.2019.108982
Li X, Feng H, Liu S, Cui J, Liu J, Shi M, Zhao J, Wang L (2023) Dehydrin CaDHN2 enhances Drought Tolerance by affecting ascorbic acid synthesis under Drought in Peppers. Plants 12:3895. https://doi.org/10.3390/plants12223895
Lindsey III, Rivero BE, Calhoun L, Grotewold CS, Brkljacic E, J (2017) Standardized method for high-throughput sterilization of Arabidopsis seeds. JoVE (Journal Visualized Experiments) e56587. https://doi.org/10.3791/56587-v
Liu T, Jiao X, Yang S, Zhang Z, Ye X, Li J, Qi H, Hu X (2020) Crosstalk between GABA and ALA to improve antioxidation and cell expansion of tomato seedling under cold stress. Environ Exp Bot 180:104228. https://doi.org/10.1016/j.envexpbot.2020.104228
Lv A, Su L, Liu X, Xing Q, Huang B, An Y, Zhou P (2018) Characterization of Dehydrin protein, CdDHN4-L and CdDHN4-S, and their differential protective roles against abiotic stress in vitro. BMC Plant Biol 18:1–13. https://doi.org/10.1186/s12870-018-1511-2
Ma Y, Wang P, Wang M, Sun M, Gu Z, Yang R (2019) GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem 270:593–601. https://doi.org/10.1016/j.foodchem.2018.07.092
Madebo MP, Hu S, Zheng Y, Jin P (2021) Mechanisms of chilling tolerance in melatonin treated postharvest fruits and vegetables: a review. J Future Foods 1(2):156–167. https://doi.org/10.1016/j.jfutfo.2022.01.005
Malabarba J, Reichelt M, Pasquali G, Mithöfer A (2019) Tendril coiling in grapevine: jasmonates and a new role for GABA? J Plant Growth Regul 38:39–45. https://doi.org/10.1007/s00344-018-9807-x
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biology Plants 20:133–137. https://doi.org/10.1007/s12298-013-0203-5
Malekzadeh P, Khosravi-Nejad F, Hatamnia AA, Sheikhakbari Mehr R (2017) Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol Mol Biology Plants 23:827–836. https://doi.org/10.1007/s12298-017-0475-2
Maryum Z, Luqman T, Nadeem S, Khan SMUD, Wang B, Ditta A, Khan MKR (2022) An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front Plant Sci 13:907937. https://doi.org/10.3389/fpls.2022.907937
Mensah JK, Akomeah PA, Ikhajiagbe B, Ekpekurede EO (2006) Effects of salinity on germination, growth and yield of five groundnut genotypes. Afr J Biotechnol 5:1973–1979. http://www.academicjournals.org/AJB
Michaeli S, Fromm H (2015) Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? 6:419. Frontiers in plant sciencehttps://doi.org/10.3389/fpls.2015.00419
Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 67:485–498. https://doi.org/10.1111/j.1365-313x.2011.04612.x
Moghanibashi M, Karimmojeni H, Nikneshan P (2013) Seed treatment to overcome drought and salt stress during germination of sunflower (Helianthus annuus L). J Agrobiology 30:89–96. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20153165956
Nabati J, Kafi M, Nezami A, Moghaddam PR, Masoumi A, Mehrjerdi MZ (2011) Effect of salinity on biomass production and activities of some key enzymatic antioxidants in kochia (Kochia scoparia). Pak J Bot 43:539–548. https://www.pakbs.org/pjbot/PDFs/43(1)/PJB43(1)539.pdf
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33:408–419. https://doi.org/10.1007/s00344-013-9389-6
Priya M, Sharma L, Kaur R, Bindumadhava H, Nair RM, Siddique KHM, Nayyar H (2019) GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-44163-w
Qados AMA (2011) Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L). J Saudi Soc Agricultural Sci 10:7–15. https://doi.org/10.1016/j.jssas.2010.06.002
Qi C, Lin X, Li S, Liu L, Wang Z, Li Y, Bai R, Xie Q, Zhang N, Ren S (2019) SoHSC70 positively regulates thermotolerance by alleviating cell membrane damage, reducing ROS accumulation, and improving activities of antioxidant enzymes. Plant Sci 283:385–395. https://doi.org/10.1016/j.plantsci.2019.03.003
Qin J, Dong W, He K, Chen J, Wang Z (2009) Short-term responses to salinity of seabuckthorn (Hippophae rhamnoides L.) seedlings in the extremely cold and saline Qinghai region of China. 11:231. Forestry Studies in Chinahttps://doi.org/10.1007/s11632-009-0039-9
Ramesh SA, Kamran M, Sullivan W, Chirkova L, Okamoto M, Degryse F, McLaughlin M, Gilliham M, Tyerman SD (2018) Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 30:1147–1164. https://doi.org/10.1105/tpc.17.00864
Ramzan M, Shah AA, Ahmed MZ, Bukhari MA, Ali L, Casini R, Elansary HO (2023) Exogenous application of glutathione and gamma amino-butyric acid alleviates salt stress through improvement in antioxidative defense system and modulation of CaXTHs stress-related genes. South Afr J Bot 157:266–273. https://doi.org/10.1016/j.sajb.2023.04.008
Rizwan M, Nawaz A, Irshad S, Manoharadas S (2024) Exogenously applied melatonin enhanced chromium tolerance in pepper by up-regulating the photosynthetic apparatus and antioxidant machinery. Sci Hort 323:112468. https://doi.org/10.1016/j.scienta.2023.112468
Safari N, Iranbakhsh A, Ardebili ZO (2017) Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci Technol 19:055501. https://doi.org/10.1088/2058-6272/aa57ef
Samarah NH, Wang H, Welbaum GE (2016) Pepper (Capsicum annuum) seed germination and vigour following nanochitin, chitosan or hydropriming treatments. Seed Sci Technol 44:609–623. https://doi.org/10.15258/sst.2016.44.3.18
Sarpras M, Gaur R, Sharma V, Chhapekar SS, Das J, Kumar A, Yadava SK, Nitin M, Brahma V, Abraham SK, Ramchiary N (2016) Comparative analysis of fruit metabolites and pungency candidate genes expression between Bhut Jolokia and other Capsicum species. PLoS ONE 11:e0167791. https://doi.org/10.1371/journal.pone.0167791
Shala AY, Aboukamar AN, Gururani MA (2024) Exogenous application of Gamma Aminobutyric Acid improves the Morpho-physiological and biochemical attributes in Lavandula Dentata L. under salinity stress. Horticulturae 10:410. https://doi.org/10.3390/horticulturae10040410
Shang H, Cao S, Yang Z, Cai Y, Zheng Y (2011) Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem 59:1264–1268. https://doi.org/10.1021/jf104424z
Sheng Y, Xiao H, Guo C, Wu H, Wang X (2018) Effects of exogenous gamma-aminobutyric acid on α-amylase activity in the aleurone of barley seeds. Plant Physiol Biochem 127:39–46. https://doi.org/10.1016/j.plaphy.2018.02.030
Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B (2019) GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci 20:5709. https://doi.org/10.3390/ijms20225709
Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Zhang SG (2010) Effects of exogenous GABA on gene expression of Caragana Intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ 33:149–162. https://doi.org/10.1111/j.1365-3040.2009.02065.x
Snedden WA, Fromm H (2001) Calmodulin as a versatile calcium signal transducer in plants. New Phytol 151:35–66. https://doi.org/10.1046/j.1469-8137.2001.00154.x
Steinhauser D, Usadel B, Luedemann A, Thimm O, Kopka J (2004) CSB. DB: a comprehensive systems-biology database. Bioinformatics 20:3647–3651. https://doi.org/10.1093/bioinformatics/bth398
Suhel M, Husain T, Pandey A, Singh S, Dubey NK, Prasad SM, Singh VP (2023) An appraisal of ancient molecule GABA in abiotic stress tolerance in plants, and its crosstalk with other signaling molecules. J Plant Growth Regul 42:614–629. https://doi.org/10.1007/s00344-022-10610-8
Sun Y, Liu L, Sun S, Han W, Irfan M, Zhang X, Zhang L, Chen L (2021) An DHN, a dehydrin protein from Ammopiptanthus Nanus, mitigates the negative effects of drought stress in plants. Front Plant Sci 12:788938. https://doi.org/10.3389/fpls.2021.788938
Tabassum T, Farooq M, Ahmad R, Zohaib A, Wahid A, Shahid M (2018) Terminal drought and seed priming improves drought tolerance in wheat. Physiol Mol Biology Plants 24:845–856. https://doi.org/10.1007/s12298-018-0547-y
Tian XL, Wu XL, Li Y, Zhang SQ (2005) The effect of gamma-aminobutyric acid in superoxide dismutase, peroxidase and catalase activity response to salt stress in maize seedling. Shi Yan Sheng Wu Xue bao. (in Chinese) 38:75–79
Ting Wong CG, Bottiglieri T, Snead III, O.C (2003) Gaba, γ-hydroxybutyric acid, and neurological disease. Annals Neurology: Official J Am Neurol Association Child Neurol Soc 54:S3–S12. https://doi.org/10.1002/ana.10696
Ullah A, Ali I, Noor J, Zeng F, Bawazeer S, Eldin SM, Asghar MA, Javed HH, Saleem K, Ullah S (2023a) Exogenous γ-aminobutyric acid (GABA) mitigated salinity-induced impairments in mungbean plants by regulating their nitrogen metabolism and antioxidant potential. Front Plant Sci 13:1081188. https://doi.org/10.3389/fpls.2022.1081188
Ullah A, Tariq A, Zeng F, Noor J, Sardans J, Asghar MA, Zhang Z, Peñuelas J (2023b) Application of GABA (γ-aminobutyric acid) to improve saline stress tolerance of chufa (Cyperus esculentus L. var. Sativus Boeck) plants by regulating their antioxidant potential and nitrogen assimilation. South Afr J Bot 157:540–552. https://doi.org/10.1016/j.sajb.2023.04.027
Usadel B, Kuschinsky AM, Steinhauser D, Pauly M (2005) Transcriptional co-response analysis as a tool to identify new components of the wall biosynthetic machinery. Plant Biosystems-An Int J Dealing all Aspects Plant Biology 139:69–73. https://doi.org/10.1080/11263500500059827
Vijayakumari K, Puthur JT (2016) γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. Plants subjected to PEG-induced stress. Plant Growth Regul 78:57–67. https://doi.org/10.1007/s10725-015-0074-6
Wang Y, Luo Z, Mao L, Ying T (2016) Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem 197:333–339. https://doi.org/10.1016/j.foodchem.2015.10.118
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S (2017) γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7:1–13. https://doi.org/10.1038/srep43609
Wang J, Zhong XM, Lv XL, Shi ZS, Li FH (2018) Photosynthesis and physiology responses of paired near-isogenic lines in waxy maize (Zea mays L.) to nicosulfuron. Photosynthetica 56:1059–1068. https://doi.org/10.1007/s11099-018-0816-6
Wang X, Dong H, Hou P, Zhou H, He L, Wang C (2019) Effects of exogenous gamma-aminobutyric acid on absorption and regulation of ion in wheat under salinity stress, in: Computer and Computing Technologies in Agriculture XI: 11th IFIP WG 5.14 International Conference, CCTA 2017, Jilin, China, August 12–15, 2017, Proceedings, Part II 11. Springer, pp. 347–357. https://doi.org/10.1007/978-3-030-06179-1_35
Wang X, Wang, Xiaodong, Peng C, Shi H, Yang J, He M, Zhang M, Zhou Y, Duan L (2022) Exogenous gamma-aminobutyric acid coordinates active oxygen and amino acid homeostasis to enhance heat tolerance in wheat seedlings. J Plant Growth Regul 41:2787–2797. https://doi.org/10.1007/s00344-021-10474-4
Wang Y, Cao H, Wang S, Guo J, Dou H, Qiao J, Yang Q, Shao R, Wang H (2023) Exogenous γ-aminobutyric acid (GABA) improves salt-inhibited nitrogen metabolism and the anaplerotic reaction of the tricarboxylic acid cycle by regulating GABA-shunt metabolism in maize seedlings. Ecotoxicol Environ Saf 254114756. https://doi.org/10.1016/j.ecoenv.2023.114756
Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H (2002) GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 213:1–47. https://doi.org/10.1016/s0074-7696(02)13011-7
Wu X, Jia Q, Ji S, Gong B, Li J, Lü G, Gao H (2020) Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating na + uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol 20:1–21. https://doi.org/10.1186/s12870-020-02669-w
Xie T, Ji J, Chen W, Yue J, Du C, Sun J, Chen L, Jiang Z, Shi S (2020) GABA negatively regulates adventitious root development in poplar. J Exp Bot 71:1459–1474. https://doi.org/10.1093/jxb/erz520
Xu J, Liu T, Qu F, Jin X, Huang N, Wang J, Hu X (2021) Nitric oxide mediates γ-aminobutyric acid-enhanced muskmelon tolerance to salinity–alkalinity stress conditions. Sci Hort 286:110229. https://doi.org/10.1016/j.scienta.2021.110229
Yağmur M, Kaydan D, Neşe O (2006) Potasyum uygulamasının tuz stresindeki arpanın fotosentetik pigment içeriği, ozmotik potansiyel, K+/Na + oranı ile bitki büyümesindeki etkileri. J Agricultural Sci 12:188–194. https://doi.org/10.1501/tarimbil_0000000472
Yu S, Lian Z, Yu L, Guo W, Zhang C, Zhang Y (2023) Gamma-aminobutyric acid improves wheat seed germination under salinity and heat stress. Reprints Res Square com. https://doi.org/10.21203/rs.3.rs-3362927/v1
Zhang G, Bown AW (1997) The rapid determination of γ-aminobutyric acid. Phytochemistry 44:1007–1009. https://doi.org/10.1016/s0031-9422(96)00626-7
Zhang Z, Lu A, Dárcy WG (2002) Capsicum annuum Linnaeus, Special Plant. Flora China 17:313–313
Zhang S, Khan A, Zhao L, Feng N, Zheng D, Shen X (2023) Effect of GABA on seed germination and seedling growth of rapeseed under salt stress. Reprints Res Square com. https://doi.org/10.21203/rs.3.rs-3132215/v1
Zhou M, Hassan MJ, Peng Y, Liu L, Liu W, Zhang Y, Li Z (2021) γ-aminobutyric acid (GABA) priming improves seed germination and seedling stress tolerance associated with enhanced antioxidant metabolism, DREB expression, and dehydrin accumulation in white clover under water stress. Front Plant Sci 12:776939. https://doi.org/10.3389/fpls.2021.776939
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Zhu J-K (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W (2019) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19:1–20. https://doi.org/10.1186/s12870-019-1646-9
Ziogas V, Tanou G, Belghazi M, Diamantidis G, Molassiotis A (2017) Characterization of β-amino-and γ-amino butyric acid-induced citrus seeds germination under salinity using nanoLC–MS/MS analysis. Plant Cell Rep 36:787–789. https://doi.org/10.1007/s00299-016-2063-2
Funding
This work was supported by grant number [512/2022] from the Deanship of Research, Jordan University of Science and Technology, Jordan.
Author information
Authors and Affiliations
Contributions
Nisreen A. AL-Quraan: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data collection, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Nezar H. Samarah: Methodology, Validation, Resources, Data collection, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Aroub M. AL-Fawaz: Methodology, Validation, Data curation, Writing - original draft, Visualization, Investigation.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AL-Quraan, N.A., Samarah, N.H. & AL-Fawaz, A.M. The physiological effect of GABA priming on pepper (Capsicum annuum L.) during seed germination under salt stress. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01207-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01207-0