Abstract
Gamma-aminobutyric acid (GABA), a non-proteinaceous amino acid, is reported in prokaryotes and eukaryotes, since ancient times. However, it has gained attention in the present time because of its rapid accumulation during stressed conditions in plants as well as in the cyanobacteria. In plants, it regulates the number of physiological processes such as pollen tube growth, root growth, TCA cycle, N2-metabolism, and osmoregulation. Several biotic and abiotic stresses prevail in the environment, which lead to enhanced accumulation of reactive oxygen species (ROS) thus causing oxidative damage. However, a rapid increase in the accumulation of GABA during stress in various plant forms like bacteria, cyanobacteria, fungi, and plants indicates its putative role in stress regulation and acclimation. This review summarizes the biosynthesis of GABA, its role in abiotic stress tolerance, and its crosstalk with ROS, nitric oxide, Ca+2 ions, phytohormones, and polyamines in stress acclimation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA) is a natural, non-proteinogenic, ubiquitous, and four-carbon molecule that exists in heterotrophs and autotrophs (Shelp et al. 2012a, b; Gilliham and Tyerman 2016; Ramesh et al. 2016). The presence of GABA was firstly reported in potato tuber while analyzing the different biochemical components by Steward et al. (1949). In recent years, GABA has been extensively studied as an important neuro-inhibitor in animals (Babateen 2016). It is mainly produced through GABA shunt. Enzymes of GABA shunt are evolutionarily conserved, which indicates the ancient origin of GABA from bacteria together with reports in plants and vertebrates also support this hypothesis (Michaeli and Fromm 2015). Studies showed that GABA also responds to various biotic and abiotic stresses (Shi et al. 2010; Seifikalhor et al. 2019; Ramos-Ruiz et al. 2019) marked with enhanced reactive oxygen species (ROS) accumulation in cells (Finkel 2011; Cui et al. 2011). Various studies show that GABA rapidly accumulates in plants under adverse conditions, such as high temperature, salt, drought, low light, cold, hypoxia, and UV-B (Rizhsky et al. 2004; Jiao et al. 2019; Su et al. 2019; Xu et al. 2021). Daş et al. (2016), Suhel et al. (2022) and Kumar et al. (2017) have reported about concentration-dependent accumulation of GABA under heavy metal exposure such as cadmium (Cd), zinc (Zn), calcium (Ca2+), aluminum (Al), and arsenic (As) in different plant species. Because of its significant increase during different kinds of stresses, extensive work is needed to determine its role during stress. The signaling role of GABA has also been studied in the modulation of plant growth and development by its interaction with other signaling molecules like nitric oxide (NO), hydrogen peroxide(H2O2), calcium–calmodulin (Ca2+/CaM) complex, cyclic guanosine-3′,5′-monophosphate (cGMP), mitogen-activated protein kinase (MAPK), etc. (Yu and Eldred 2005; Jiao et al. 2019). Apart from this, GABA also regulates pollen tube and root growth in plants through aluminum-activated malate transporters (ALMTs) (Ramesh et al. 2015).
Furthermore, it has also been reported that a specific concentration of GABA regulates plant growth antioxidant system and transcription of genes of antioxidant enzymes; hence, it appears that GABA may alleviate the oxidative damage in plants (Li et al. 2017). Under salt stress, GABA may act as a non-toxic osmolyte and also scavenges ROS under stressful conditions (Carillo 2018). GABA has been shown to enhance salt stress tolerance in barley and Nicotiana sylvestris by modulating physiological responses (Akçay et al. 2012; Ma et al. 2018). Exogenously applied GABA was also reported to regulate osmotic balance in plants and thus enhanced stress tolerance (Vijayakumari and Puthur 2016). Moreover, the study showed that under salt stress, the production of H2O2 is effectively inhibited by exogenously applied GABA which also regulates the gene expression of H2O2-producing enzymes in Caragana intermedia (Shi et al. 2010). It has also been known that seed germination percentage significantly increases upon seed priming with GABA and reduces the salt stress in wheat and maize (Luo et al. 2011). Several findings have suggested that exogenously applied GABA also protects the plant from heat stress damage (Liu et al. 2019; Nayyar et al. 2014; Priya et al. 2019).
Apart from GABA, many other amino acids like polyamines (PA), proline (Pro), some hormones, and plants metabolites such as jasmonates (JA) and salicylic acid (SA) control the downstream stress responses of GABA and regulate various biochemical reactions by interacting with stress signals (Tuteja and Sopory 2008; Scholz et al. 2017; Shelp and Zarei 2017; Sheteiwy et al. 2019; Zhu et al. 2019).
The fungus Fusarium graminearum, which grows on the plant cell wall, also upregulates the genes of GABA shunt to produce the GABA (Carapito et al. 2008). GABA also triggers gene expression in Agrobacterium tumefaciens (Planamente et al. 2010). In Caragana intermedia, GABA has been studied to control the expression of genes that are involved in diverse roles and are conserved in plants and animals (Shi et al. 2010). Unlike animals, a receptor for GABA is not yet discovered in plants, so a modulator site for GABA is proposed to be present at the glutamate decarboxylase (GAD) (Michaeli and Fromm, 2015). In this review article, we have appraised the biosynthesis of GABA under abiotic stress and its crosstalk with ROS, NO, Ca2+ ions, phytohormones, and polyamines in stress regulation and acclimation.
GABA Metabolism in Plants
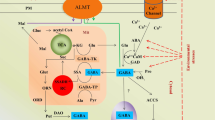
GABA metabolism inside the plant cell is linked through the tri-carboxylic acid cycle (TCA), nitrogen metabolism, polyamine degradation, and proline metabolism as shown in Fig. 1 (Bor and Turkan 2019). In plants and animals, it is produced by GABA shunt, which is not a common feature in many cyanobacteria because of the absence of the GAD gene in most of them, however, reported in the cyanobacteria such as Aphanothece halophytica, Synechocystis, etc. (Boonburapong et al. 2015; Jantaro and Kanwal 2017). Under oxidative stress conditions, it is also produced through different pathways such as polyamines degradation as reported in mammals, plants, and cyanobacteria (Fig. 2) (Signorelli et al. 2015; Jantaro and Kanwal 2017). Glutamate is the pivotal molecule in the GABA shunt for which experimental pieces of evidence have been provided by the study of Boonburapong et al. (2015) in which exogenous glutamate increased the GABA accumulation in Aphanothece halophytica under acid stress. Biologically, the enzyme glutamate dehydrogenase is responsible for the synthesis of glutamate from α-ketoglutarate (Jantaro and Kanwal 2017). There are three leading enzymes, i.e., GABA transaminase (GABA-T), glutamate decarboxylase (GAD), and succinic semialdehyde dehydrogenase (SSADH) involved in GABA shunt in plants and cyanobacteria, interlinking of these enzymes has been shown in Fig. 1. (I) The GADs convert l-glutamate, a proteinogenic amino acid into GABA (Fig. 1) (Jantaro and Kanwal 2017); this is an irreversible reaction occurring in the cytosol (Michaeli and Fromm 2015). Several kinds of GADs are found in different plant species, i.e., four in Prunus species and nine in Arabidopsis thaliana (Shelp and Zarei 2017). (II) GABA transaminase (GABA-T) converts GABA into succinic semialdehyde (SSA) and utilizes pyruvate as an amino acid acceptor in the plants (Fig. 1) (Michaeli and Fromm 2015). In animals, GABA-TK carries this step. Li et al. (2021) have noticed the major role of GABA-TP in the GABA catabolism in Arabidopsis mutants, showing 100 times high GABA due to lack of GABA-TP, and its effect on the plant. However, inhibition of GABA transaminase in plants caused high GABA accumulation (Bown and Shelp 2016). III) Enzyme SSADH converts succinic semialdehyde (SSA) into succinate in GABA shunt (Fig. 1). The value of Km is lower for SSA (5–15 µM) than for NAD (166–460 µM) which possesses a high Km value. Studies also provide evidence for GABA production through nitrogen metabolism and GABA accumulation on supply of nitrogenous compounds in Synechocystis sp. (Boonburapong et al. 2015; Kanwal and Incharoensakdi 2019). They reported enhanced GABA accumulation on the supply of nitrate (NO3−) followed by nitrite (NO2−), ammonium (NH4+), and urea (Boonburapong et al. 2015). Studies showed that the biosynthesis of GABA is also linked to carbon metabolism, as GABA production is enhanced upon supply of glucose (Fig. 1) (Jantaro and Kanwal 2017; Zhong et al. 2017).
(1) Glutamate is converted into GABA, (2) GABA is converted into succinic semialdehyde, (3) succinic semialdehyde is converted into succinate, (4, 5) exogenous nitrate and ammonium produce glutamine that produces glutamate and ultimately GABA, (6) under stressed conditions Ca2+ ions are increased in the cytosol, which activates calmodulin and it activates GAD, (7) high proton concentration under stress (anoxia) activates GAD, (8) under oxidative stress proline forms GABA, (9) GABA is synthesized through polyamine degradation
(1) NaCl stress, (2) Genes CaGR61 and, (3) CaGR62 are highly expressed and produce H2O2, (4) on exogenous GABA supplementation CaGR60 expression is enhanced through NADPH oxidase H2O2 is produced which serves as secondary messenger and, (5) leads to lowering in H2O2 levels, (6) exogenous GABA supplementation, under NaCl stress enhances expression of CaGR30 and CaGR31 thus synthesize ethylene, (7) similarly, CaGR28 expression is enhanced which produces abscisic acid, (8) exogeneous GABA supplementation under NaCl stress leads to polyamine biosynthesis, ultimately H2O2 biosynthesis in roots cells, (9) exogeneous GABA enhances activities of SOD, POD, and CAT
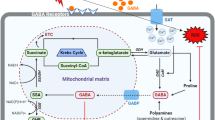
Plants are adopted in several different ways to enhance GABA accumulation during stress in the cell such as GAD activation through Ca–calmodulin, high proton concentrations (Fig. 6), polyamines, and proline degradation (Fan et al. 2012; Scholz et al. 2017; Woodrow et al. 2017). Polyamines are nitrogenous bases having an amino group with low molecular weight, found in prokaryotes as well as in eukaryotes. Putrescine (Put), spermine (Spm), and spermidine (Spd) are the major polyamines present in plants (Chen et al. 2019). Apart from regulating other essential processes, polyamines are involved in stress responses in plants (Chen et al. 2019). Increased biosynthesis of polyamines has been noticed under abiotic stress in plants (Fig. 3) (Jiao et al. 2019). It has been studied in Arabidopsis thaliana that GABA is produced through the degradation of polyamine (putrescine and spermine) in plant peroxisomes (Fig. 2) (Seifikalhor et al. 2019; Corpas et al. 2019). Xing et al. (2007) have reported about GABA formation through polyamine degradation, under salt stress. S-adenosyl-methionine (SAM) is a common precursor of polyamine and ethylene biosynthesis pathway, because of which their synthesis occurs through a common pathway as shown in Fig. 3. In order to affect the polyamine synthesis, GABA is reported to enhance the activities of arginine decarboxylase (ADC), S-adenosyl-methionine decarboxylase (SAMDC), and ornithine decarboxylase (ODC) (Fig. 3) in muskmelon seedlings under salt stress (Hu et al. 2015). The GABA alleviates polyamines biosynthesis by enhancing the activity of the major enzyme in polyamine biosynthesis, SAMDC (Fig. 3) (Yong et al. 2017). The GABA also regulates polyamine formation through the arginase pathway (Mohapatra et al. 2010; Majumdar et al. 2016).
Exogeneous GABA supplementation enhances activities of (1) ADC and (2) ODC, (3) exogenous GABA enhances activity of SAMDC, ultimately contributes to polyamine biosynthesis. Schematic representation to show the enzymes whose activities are enhanced by GABA application. GABA is represented by a red-outlined pentagon (Color figure online)
GABA Accumulation and Abiotic Stress
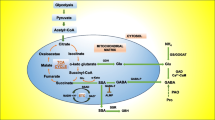
In a stress situation, GABA is produced by the polyamine degradation in the plants (Fig. 2) (Jiao et al. 2019). The diamine oxidase (DAO) which is a rate-limiting enzyme converts putrescine into gamma-aminobutyraldehyde. Further, AMADH converts it into GABA (Fig. 2). GABA content decreased by 30% when DAO activity is blocked through its inhibitor aminoguanidine (AG) in fava beans as reported by Yang et al. (2013). Increased activity of two enzymes is thought to be responsible for the increased production of GABA during stress, firstly, increased glutamate decarboxylase activity in maize plants (Wang et al. 2017), and secondly, enhanced diamine oxidase (DAO) activity in the Glycine max (Xing et al. 2007). The GABA accumulation also occurs during viral infection in the plants (Kinnersley and Turano 2000; Bouche and Fromm 2004). The GABA rapidly accumulates during stress in plants, fungi, bacteria, and cyanobacteria (Wang et al. 2017; Mekonnen et al. 2016). Certain plants show different intervals of time for the maximum GABA accumulation, which ranges from minutes to days.
Drought Stress
Drought is the most prevalent stress in the current scenario. Drought limits plant growth but GABA treatment results in the expression of genes involved in stress regulation in creeping bentgrass (Li et al. 2018). During drought, plants such as Arabidopsis, soybean, sesame, wheat, and turnip have been reported to accumulate GABA (Bown and Shelp 2016; Ramesh et al. 2017; Saeidia et al. 2017; Marček et al. 2019). Results of these studies show a high increase in glucose, an unidentified disaccharide, GABA, l-threonine 1, stearic acid, and palmitic acid in drought-stressed conditions. Mutant Arabidopsis plant which lacked the expression of GAD gene-gad 12 showed sensitivity toward drought stress by undergoing negative physiological changes (Mekonnen et al. 2016). Studies reveal the role of GABA in reducing oxidative stress by regulating the antioxidant system under drought stress (Krishnan et al. 2013; Sheteiwy et al. 2019).
Salt Stress
Previous studies have reported that millions of hectares of land are affected by salinity (about 7% of total land) and thus has become a major factor that alters various biochemical, physiological, and morphological characteristics of plant species (Ashraf 1993; Munns 2002; Cheng et al. 2018). Several studies suggested that GABA may act as a prominent osmolyte to maintain osmotic balance and ROS scavenger under salt-stressed conditions (Yu et al. 2014; Vijayakumari and Puthur 2016; Carillo 2018). Exogenous application of GABA alleviated salt-induced toxicity in Caragana intermedia by regulating the expression of genes responsible for H2O2 and peroxidase production (Shi et al. 2010) (Fig. 4). Luo et al. (2011) demonstrated that GABA also enhanced seed germination and reduced salt injury in wheat and maize (Fig. 5). The findings of Wang et al. (2017) suggested that exogenously applied GABA enhances the endogenous production of GABA which provides resistance against salt tolerance. High salt concentration negatively affects photosynthetic machinery (Che-othman et al. 2019), and GABA shunt is required for proper growth during salt stress. Under salt stress, a high amount of assimilated carbon gets diverted toward respiration (Che-othman et al. 2019). During this situation, a high supply of ATP is required to detoxify ROS, for ion exclusion, solute synthesis to maintain osmotic balance and the TCA cycle is unable to run; however, GABA shunt meets the ATP requirement for the survival of the cell.
Represents a combined crosstalk of GABA, NO, calcium, H2O2, polyamines, and phytohormones (1) under stress NO is produced; (2) NO induces cGMP production; (3) cGMP activates PKG, (4) PKG enhanced expression of MAPK; (5) cGMP opens Ca2+ channels; (6) Ca2+ binds to synthesized MAPK and activates it; (7) Active MAPK leads to expression of DAO and AMADH; (8) DAO and (9) AMADH enzyme produce GABA in the plastids; (10) Increased intracellular Ca2+ levels activate calmodulin, this activates GAD which produces GABA; (11) GABA enhances expression of ACS and ACO; (12) ACS enzyme converts SAM into ACC; (13) ACO enzymes converts ACC into ethylene; (14) H2O2 produced in peroxisomes and plastids opens Ca2+ channels, Ca2+ ions along with GABA close the stomata under stress, (15) co-transportation of GABA along with malate occurs, negatively charged malate neutralizes Al3+ toxicity in root cells
(1) GABA is accumulated under drought, chill, and drought stress, (2) on providing exogenous GABA proline is biosynthesized which, (3) protects tomato seedlings under chill stress, (4) exogenous GABA alleviates chill stress injury in peach and banana during storage, (5) exogenous GABA improves seed germination under salt stress in wheat and maize
Chilling Stress
The temperature on the Earth varied according to the locality, season and also day and night. Chilling temperature ranges from 0 to 15 °C in plants system (Guan et al. 2009; Malekzadeh et al. 2012). Low temperature is a key factor among various factors which affect the productivity of plants (Aghdam et al. 2012). Shang et al. (2011) reported that chilling stress declined by the exogenously applied GABA. It has also been reported that GABA could play a significant role in the mitigation of chilling stress in wheat and by increasing proline content in tomato seedlings (Malekzadeh et al. 2012; 2014). Another finding showed that GABA content does increase under cold stress (Mazzucotelli et al. 2006; Wang et al. 2014). Exogenously applied GABA also alleviates chilling injury of banana peel and peach fruit during cold storage (Shang et al. 2011; Yang et al. 2011; Wang et al. 2014) (Fig. 5). GABA enhances the postharvest period of various fruits and increases tolerance to chilling stress (Aghdam et al. 2015; Malekzadeh et al. 2017; Sheng et al. 2017). It has been studied that GABA shunt stimulated during chilling stress in cold tolerant as well as in cold-sensitive species of Zucchini (Carvajal et al. 2015; Palma et al. 2015). Mazzucotelli et al. (2006) have reported an increase in GABA accumulation in wheat and barley seedlings through GABA shunt in tolerant varieties whereas through GAD in sensitive varieties (Mazzucotelli et al. 2006). A cold shock to fungal cell culture also causes GABA accumulation. In plants, GABA shunt also plays a role in cell signaling, pH regulation, oxidative stress, osmoregulation, TCA cycle, and N2-metabolism and thus defends from the insects (Al-Quraan et al. 2014; Al-Quran and Al-Omari 2017). Accumulation of this non-proteinogenic amino acid is an adaptive strategy of the plants to adapt during oxidative stress (Al-Quran and AL-Omari 2017).
GABA and Its Interaction with Other Signaling Molecules and Hormones
The increased accumulation of GABA is linked with its significant role under stress conditions in plants, bacteria, and fungi. Recent studies have focused to understand the signaling routes which are involved during stress in plants. Several biotic and abiotic stresses prevail in the environment (such as herbivory, plant–pathogen interaction, salt stress, drought, metal stress, radiations, high light intensity, and so on) which leads to excess production of ROS in the cell resulting in oxidative damage (Krishnamurthy and Rathinasabapathi 2013; Dimlioğlu et al. 2015). Oxidative stress causes lipid peroxidation in cellular membranes, breakage of DNA molecules, denaturation of proteins, oxidation of carbohydrate molecules, pigments damage, and losses in enzymatic activities (Bhattacharjee 2014; Talbi et al. 2015; Al-Quran and Al-Omari 2017; Dutta et al. 2018). It has been studied that ROS are produced in abiotic stress to execute the downstream response against stress and play the role of a signaling molecule to help the plant in stress tolerance (Krishnamurthy and Rathinasabapathi 2013; Bose et al. 2014). Basal metabolism of ROS and reactive nitrogen species (RNS) occurs inside the peroxisomes in the plant cell, which leads to the formation of H2O2, NO, and GABA through polyamine degradation (Corpas et al. 2019). This nitro-oxidative metabolism gets exacerbated during stress conditions. Chloroplasts, mitochondria, and peroxisomes are the primary sites of ROS production (Bose et al. 2014). It is assumed that peroxisomes receive some signal during stress and thus enhance the formation of ROS and RNS (Corpas et al. 2019). Peroxisomes serve as the source of ROS and NO, which play the role of signal molecules in the plant cell (Sandalio et al. 2013; Astier et al. 2018; Turkan 2018; Corpas et al. 2019). The NO produced here, regulates the activity of peroxisomal enzymes, through nitration or S-nitrosylation (Begara-Morales et al. 2018; Corpas et al. 2019). Increased levels of GABA under various kinds of stress (Jantaro and Kanwal 2017; Carillo 2018) indicate that it is not utilized by the Krebs cycle; rather, it has a signaling role. GABA has a significant role in the pathways involved in the elimination of ROS, as a drastic increase in ROS is observed in mutants that do not express GABA shunt genes, indicating about shunt's role in ROS elimination (AL-Quraan 2015). GABA shunt is said to be involved as a signaling and metabolic pathway to adapt plants against abiotic stress (Michaeli and Fromm 2015; Li et al 2016; AL-Quran and AL-Omari 2017). Whether GABA functions inside the plant cell as a signal molecule or as a metabolite is still a matter of discussion (Shi et al. 2010). GABA binding proteins have also been reported in the bacteria such as Pseudomonas, as being ubiquitous, it is assumed to be a communicating molecule between the prokaryotes and eukaryotes (Dagron et al. 2013).
Interaction of GABA with Nitric Oxide (NO)
NO is an important participant in signal transduction during stress in plants (Li et al. 2019). Guo et al. (2014) reported that polyamines stimulated NO signaling for enhancing cold tolerance in Nicotiana tobaccum, and water stress through enhanced enzymatic activity in Trifolium repens plant. Exogenous SNP (a NO donor) leads to GABA biosynthesis during UV-B stress in soybean sprouts (Li et al. 2019; Jiao et al. 2019; Jalil et al. 2019). On other hand, exogenous GABA supplementation in water-stressed Trifolium repens has resulted in 90% more NO content in plants than in the controls (Li et al. 2019). It can be said that NO leads to GABA accumulation and GABA also causes NO accumulation. In soybean sprouts, NO produces cGMP/PKG as a second messenger that activates gene protein expression of mitogen-activated protein kinase (MAPK). NO activates MAPK through the transient influx of calcium, as reported in tobacco cells (Jiao et al. 2019). MAPK in turn activates gene and protein expression of two enzymes, DAO and NAD+-dependent amino aldehyde dehydrogenase (AMADH) (Jiao et al. 2019) as shown in Fig. 4. The DAO converts putrescine to γ-aminobutyraldehyde, which is converted into GABA through AMADH in germinating soybean (Yin et al. 2014). In soybean sprouts, an enhanced level in the protein expression of AMADH occurred on the second day of NO exposure.
Interaction of GABA with H2O2
Despite being produced along with NO and H2O2 (signal molecules), the role of GABA is unknown in the signaling pathway. On one side, H2O2 shows damaging effects on plants such as it causes apoptosis in lentil seedlings (Diaz-Vivancos et al. 2015; AL-Quran and AL-Omari 2017; De Simone et al. 2017). Studies reported that H2O2 and superoxide radicals behave as damaging molecules as well as signaling molecules during stress in the plants (Shi et al. 2010). In pancreatic beta cells, GABA pretreatment is reported to decrease the ROS production, caused by the application of H2O2 due to a hampered antioxidant system (Tang et al. 2018). Their studies focused to identify an endogenous antioxidant molecule that could decrease oxidative stress to protect it. GABA is reported to regulate the redox homeostasis in the pancreatic beta cells (Tang et al. 2018).
ROS behave as rate-limiting secondary messengers in Abscissic acid (ABA) signaling. Through SSH, and by comparing the homology between the sequences of the genes expressed, it is seen that GABA does increase the expression of enzymes involved in the H2O2 production namely NADPH oxidase, peroxidase, and amine oxidase (AO) during stress in C. intermedia (Shi et al. 2010) (Fig. 4). Peroxidases produce H2O2 by oxidizing auxin (Fig. 2). Exogenous GABA enhances NADPH oxidase gene expression. Expression of NADPH oxidase is more pronounced at 12 h of the GABA treatment (Shi et al. 2010). In C. intermedia, enhanced expression of peroxidase and amine oxidase (AO) has been studied during NaCl stress. H2O2 production increases by the enhanced activity of the enzymes namely found in peroxisomes due to their enhanced gene expression. Exogenous GABA application significantly reduced H2O2 accumulation in these conditions through reduced NADPH oxidase activity (Fig. 2) (Shi et al. 2010).
The same study has also reported that a higher concentration of GABA up to 20 mM is unable to inhibit H2O2 accumulation in the plant. GABA is reported to regulate the genes that are involved in the transport of the hormones, polyamine biosynthesis, ROS generation, and also in signal transduction (Shi et al. 2010). As shown in Fig. 2, exogenous treatment of GABA during NaCl stress caused expression of NADPH oxidase enzyme that produced H2O2 which served as a second messenger, which further regulated H2O2 production in cells of C. intermedia (Shi et al. 2010). However, it indeed needs further experimental proof in future studies.
Interaction of GABA with Ca2+
During flooding, a decrease in the cytosolic pH causes glutamate decarboxylase activation, which increases GABA production at low pH (Fig. 6) (Cui et al. 2020). Other stresses such as cold and heat utilize the Ca2+–calmodulin pathway to activate GAD and enhance the GABA concentration (Fig. 4) (Kinnersley and Turano 2000). The GAD is activated through two pathways either by high proton concentration or through increased calcium concentration (Kinnersley and Turano 2000) (Fig. 6). In yeast and E. coli, GAD activity is enhanced to regulate oxidative stress under low pH. Under various stresses, increased levels of ROS enhance the cytosolic Ca2+ concentration by opening the calcium channels secondly by generating NO which increases calcium levels (Fig. 4) (Jiao et al. 2019; Bor and Turkan 2019; Podlesakova et al. 2019). Increased calcium level ameliorates stress by regulating the activity of calcium-dependent proteins which play various roles in the adaptation under stress conditions (Bor and Turkan 2019). Most of the glutamate decarboxylases present in plants possess a calmodulin-binding domain (Bor and Turkan 2019; Shelp and Zarei 2017). Nuclear magnetic resonance also provides evidence of the interaction of GAD with calmodulin. Further, calmodulin interacts with two peptides at the calmodulin-binding domain of GAD (Bouche and Fromm 2004) (Fig. 1). It is still unknown whether all GADs found in plants possess a calmodulin-binding domain or not. Then, it is a matter of curiosity to elucidate the significance of calmodulin interaction in GAD's activity. In the Arabidopsis plant, five genes for GAD are reported, and different GADs are expressed in different organs which show their tissue-specific distribution (Bouche and Fromm 2004).
In abiotic stress such as light intensity and nutrient deficiency, GABA signaling is involved to increase Ca2+ in the cytosol, which combines with calmodulin to activate glutamate decarboxylase (GAD) (Fig. 1). Under the above-mentioned stress, regulation and gene expression of enzymes involved in GABA metabolism do increase and ultimately GABA levels get enhanced. The GABA enters mitochondria and participates in the tri-carboxylic acid (TCA) cycle and also GABA may be involved to regulate the expression of several genes related to signaling and metabolism (Michaeli and Fromm 2015). An increase in cytosolic calcium concentration is linked with GABA production and the use of calcium chelator blocked the gene and protein expression of DAO and AMADH in the soybean sprouts, which resulted in the inhibition of GABA production (Fig. 4) (Jiao et al. 2019).
Interaction of GABA with Phytohormones
Plant hormones also participate in stress responses, which are seen in variations in their gene expression during the stress in plants (Podlesakova et al. 2019). The GABA production increases on the application of abscisic acid (ABA) in wheat roots and on the application of auxin in rice root tips (Fig. 2) (Kinnersley and Turano 2000).
Abscisic Acid
In the guard cells of Vicia faba, ABA regulates copper amine oxidase (CAO) activity, which catalyzes putrescine oxidation in the peroxisomes and produces H2O2 as a byproduct. H2O2 leads to increased cytosolic Ca2+ levels and finally stomatal closure (Podlesakova et al. 2019) (Fig. 4). Studies report that Arabidopsis mutants which lacked GABA biosynthesis were unable to close the stomata. Lack of GABA accumulation due to mutation in the AMADH gene in Arabidopsis plants makes the plant more sensitive to salinity. The AMADH enzyme produces GABA, from γ-aminobutyraldehyde. Another study indicates the stomatal closure by ABA through NO (Podlesakova et al. 2019). Thus, studies indicate GABA production through ABA and its role in stomatal closure.
Auxin
The role of IAA/ABA is seen to regulate the stress of Al, as they lead to gene expression of aluminum-activated malate transporters (ALMTs) (Podlesakova et al. 2019) (Fig. 2). The ALMTs are not generally activated by Al3+ except as in Triticum aestivum (TaALMTs). The GABA regulates these ALMTs as reported by Ramesh et al. (2018). GABA negatively regulates malate efflux through ALMTs in the plants, under Al stress (Ramesh et al. 2018). GABA through ALMTs regulates tolerance in roots toward low pH, high pH, and Al3+ ions (Ramesh et al. 2018; Podlesakova et al. 2019) (Fig. 2). Hormones activate the polyamine metabolism that generates GABA, ROS, and NO which enhance calcium concentration, and these chemical changes execute responses against stress (Podlesakova et al. 2019).
Ethylene
Several pieces of evidence suggested that GABA is involved in ethylene signaling (Ji et al. 2018). GABA increases the expression of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) genes and then increases the expression of ethylene (ET) (Shi et al. 2010) (Fig. 2). A correlation is established between the sequential increase of Ca2+, GABA, and ethylene concentrations under stressed conditions (Gilliham and Tyerman 2016; Kinnersley and Turano 2000) (Fig. 2). The GABA regulates the mRNA level of ACC synthase and thereby regulates ethylene synthesis (Kinnersley and Turano 2000). As, during NaCl stress, GABA mediates its effect through ethylene, and stem elongation inhibited by GABA was reduced up to a significant level on the use of aminoethoxy vinyl glycine (AVG), an inhibitor of ethylene biosynthesis, and silver thiosulfate (ITS), an inhibitor of ethylene action (Shi et al. 2010). The findings of Ji et al. (2018) suggested that GABA-mediated alleviation of salt stress is linked with ET, ABA, and H2O2 (Table1).
In plants, amino acids are not catabolized to provide energy but are utilized to support other metabolic pathways; this indicates that GABA has a signaling role in plants.
Molecular Mechanisms of GABA Signaling in Plants
GABA alters gene expression in cold stress but what role GABA has at molecular and the genetic level is not known (Li et al. 2016). Increased GABA production has been studied in different stresses in plants. The role of GABA during stress is proved but the fact that plants become more sensitive when they are mutated for GAD1 and GAD2 (Ramesh et al. 2017). The glutamate decarboxylase gene has been found to be expressed at a high level under NaCl stress in C. intermedia (Shi et al. 2010). During NaCl stress, polyamine biosynthetic genes are expressed by GABA, in Cattleya intermedia roots (Shi et al. 2010), and H2O2 concentration inside the cell is also decreased by GABA application (Fig. 4) (Shi et al. 2010).
GABA is believed to play an important role not only in signaling and stress tolerance but also in several physiological processes (Shelp et al. 2012c; Gilliham and Tyerman 2016; Ramesh et al. 2016). Various signaling molecules including Ca2+, phytohormones, amino acids like proline (Pro), and polyamines (PAs) are linked with GABA and regulate different physiological responses (Podlesakova et al. 2019). During fruit development in tomatoes, Nonaka et al. (2017) reported the expression of three GAD genes (SlGAD1–3), one SSADH gene (SlSSADH), and three GABA-T genes, and Solanum lycopersicum GABA transaminases (SlGABA-T1–3). Expressions of these genes are critical for GABA metabolism (Koikeet al. 2013; Takayamaet al. 2015). Koike et al. (2013) demonstrated that SlGABA-T1 and SlGABA-T3 suppressed GABA accumulation that led to dwarf phenotype in tomato plants. On the other hand, deletion of an autoinhibitory domain of GAD2 and GAD3 shows the increase in GABA pool resulting in high plant growth and fruit yields (Nonaka et al. 2017). However, high accumulation of GABA in tomato fruits causes reduced ethylene synthesis resulting in less sensitive fruits to ethylene (Takayama et al. 2017). It has been studied that genes of GABA shunt express differentially in different stress conditions (Shelp et al. 2012c). Overexpression of MYB55 (high-temperature-induced transcription factor) increases the level of GABA by binding to glutamate decarboxylase 3 (GAD3) gene (El-kereamy et al. 2012). However, WRKY40 and STOP1 repress the expression of the GAD4 gene (Mirabella et al. 2015). Kobayashi et al. (2014) observed that in atstop1 mutant GAD1 and GABA-T expression is low resulting in low accumulation of GABA, while GAD4 expression increases. The expression of STOP1 is associated with the expression of ALMT1, a key gene involved in aluminum tolerance. Gilliham and Tyerman (2016) postulated that GABA acts by binding on GABA binding motif present on ALMT1.
GABA is also produced in plants via terminal oxidation of polyamines (putrescine and spermidine) by CuAO and PAO; these oxidation results in the production of 3-aminopropanal (APAL) and 4-aminobutanal (ABAL) (Fincato et al. 2012; Shelp et al. 2012a, b; Planas-Portell et al. 2013; Zarei et al. 2015a, b) (Fig. 2). Further oxidation of ABAL carried out by aminoaldehyde dehydrogenases (AMADH) leading to the generation of GABA (Shelpet al. 2012a, b; Tiburcio et al. 2014) (Fig. 2). Previously, it has been reported that two apples (Malus domestica) AMDAH genes (MdAMADH1and MdAMADH2) in its active state produce GABA and beta-alanine in the presence of ABAL or APAL and NAD+ (Zarei et al. 2015a, b).
Many plant species showed heat adaptation by regulating the expression of HSPs and APXs genes such as through HSFs (heat shock transcription factors) pathway (Mittler et al. 2012; Wang et al. 2016). The APXs are involved in the antioxidant defense system and linked with stress tolerance in plants (Li et al. 2018). The HSPs play important roles in the stabilization of proteins in heat tolerance (Xu et al. 2011). Lin et al. (2018) studied that overexpressing miR160 Arabidopsis shows higher heat tolerance by upregulating different HSPs, while miR398 acts as a key regulator in abiotic stress tolerance (Zhu et al. 2011). Studies showed that miR398 downregulates the expression of HSPs and HSFs in plants (Guan et al. 2013; Lu et al. 2013). In creeping bentgrass, miR398s were downregulated by pretreatment of GABA; on the other hand, HSFs, HSPs, and APXs expression were upregulated by GABA (Li et al. 2019). Li et al. (2018) demonstrated that foliar spray of GABA enhances the expression of HSP90 and APX which are linked with heat tolerance. These studies show that GABA is involved in heat tolerance through HSFs pathways.
Plant hormone, like ABA, is a key messenger in non-stressed as well as in stressed conditions. ABA regulates stomatal movement by regulating the Ca2+ concentration in the cytosol (Kim et al. 2010; Raghavendra et al. 2010). Cytosolic Ca2+ increases under stress conditions directly or indirectly (Singh et al. 2017). An increase in cytosolic Ca2+ level induces the activity of GADs by Ca2+-CaM signaling, resulting in the conversion of glutamate to GABA (Virdi et al. 2015; Shelp and Zarei 2017). Increased GABA accumulation regulates the expression of aluminum-activated malate transporters [ALMT], and these transporters regulate the stomatal movement, seed germination, and grain formation under stress conditions (Meyer et al. 2010; De Angeli et al. 2013; Xu et al. 2015; Palmer et al. 2016; Zarei et al. 2017). However, Zarei et al. (2017) observed that high accumulation of GABA is related to high expression of ALMT2. Expression of genes related with spermidine synthase 1 (SPDS1), arginine decarboxylase 2 (ADC2), spermine synthase (SPMS), and PAs is significantly increased during drought stress in Arabidopsis while in ABA-insensitive (abi1-1) and ABA-deficient (aba2-3) mutants, these responses are reduced (Alcazar et al. 2006). Spermidine (Spd) and spermine (Spm) induce H2O2 generation in Vicia faba increasing cytosolic Ca2+ and stomatal closure (Podlesakova et al. 2019). It has been studied that GABA is produced by PAs degradation and GABA synthesis-deficient mutant of Arabidopsis exhibits the distorted shape of stomata (Podlesakova et al. 2019). Additionally, amino aldehyde dehydrogenase (AMADH) homolog mutant plants involved in GABA synthesis were less tolerant to salinity than wild type because of reduction in GABA content (Zarei et al. 2016). These findings suggest that PAs, ABA, and GABA are associated with the regulation of stomatal closure.
Conclusions and Future Perspectives
Studies provide information about the production of GABA and its role in stress acclimation in plants as well as in some cyanobacteria. Three enzymes, GAD, SSADH, and GABA-T, play key roles in GABA shunt in plants and cyanobacteria. GABA metabolism is linked with carbon (TCA cycle), nitrogen, and proline metabolism. Polyamine degradation increases the GABA level; however, on the other hand, GABA also regulates the biosynthesis of polyamines. Exogenous as well as endogenous GABA plays a significant role in stress (drought, temperature, metal, chilling, etc.) tolerance. GABA significantly reduces oxidative stress by interacting with other signaling molecules like NO, H2O2, Ca2+, and phytohormones. On the other hand, GABA also increases the H2O2 level by increasing the NADPH oxidase activity; hence, it is an area of future research to investigate the pathway of GABA action in regulating H2O2 homeostasis. The level of NO increases by exogenously applied GABA and exogenously applied NO increases the GABA level and thus, investigations on GABA-NO signaling implication in regulating plant growth, development, and stress acclimation remain to be done. It also declines stressful conditions by altering the expression of some genes like those involved in polyamine synthesis. Expression of genes (GAD, GABA-T, and SSADH) involved in GABA metabolism are up/downregulated under various stress conditions. GABA also induces the expression of HSPs. Therefore, this review gives comprehensive information that how the equilibrium of GABA is maintained at biochemical, physiological, and molecular levels which subsequently renders abiotic stress tolerance in plants. But a clear mechanism that how GABA interacts with other signaling molecules and phytohormones is still an open research area in plant biology.
References
Aghdam MS, Asghari M, Farmani B, Mohayeji M, Moradbeygi H (2012) Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci Hortic 144:116–120
Aghdam MS, Naderi R, Sarcheshmeh MAA, Babalar M (2015) Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol Technol 110:70–76
Akçay N, Bor M, Karabudak T, Ozdemir F, Türkan I (2012) Contribution of Gamma aminobutyric acid (GABA) to salt stress responses of Nicotianasylvestris CMSII mutant and wild type plants. J Plant Physiol 169:452–458
Alcazar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876. https://doi.org/10.1007/s10529-006-9179-3
Al-Quraan NA, Al-Sharbati M, Dababneh Y, Al-Olabi M (2014) Effect of temperature, salt, and osmotic stresses on seed germination and chlorophyll contents in lentil (Lens culinaris Medik). Acta Hortic. https://doi.org/10.17660/ActaHortic.2014.1054.4
AL-Quraan NA, AL-Omari HA (2017) GABA accumulation and oxidative damage responses to salt, osmotic and H2O2 treatments in two lentil (Lens culinaris Medik) accessions. Plant Biosys 151:148–157
AL-Quraan NA, (2015) GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol Plant. https://doi.org/10.1007/s11738-015-1836-5
Ashraf M (1993) Effect of sodium chloride on water relations and some organic osmotica in arid zone plant species Melilotus indica (L.) All Der. Tropen Land Wirt 94:95–102
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3421
Babateen OM (2016) GABA signaling regulation by GLP-1 receptor agonists and GABA—a receptors modulator. Dissertation. Acta Universitatis Upsaliensis, Uppsala. ISBN 978-91-554-9548-0
Begara-Morales JC, Chaki M, Valderrama R, Sánchez-Calvo B, Mata-Pérez C, Padilla MN, Huorpas FJ, Barroso JB (2018) Nitric oxide buffering and conditional nitric oxide release in stress response. J Exp Bot 69:3425–3438
Bhattacharjee S (2014) Membrane lipid peroxidation and its conflict of interest: the two faces of oxidative stress. Curr Sci 107:11
Boonburapong B, Laloknam S, Incharoensakdi A (2015) Accumulation of gamma-aminobutyric acid in the halotolerant cyanobacterium Aphanothece halophytica under salt and acid stress. J Appl Phycol 28:1
Bor M, Turkan I (2019) Is there a room for GABA in ROS and RNS signalling? Environ Exp Bot 161:67–73
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bown AW, Shelp BJ (2016) Plant GABA: not just a metabolite. Trends in Plant Sci 21:811–813
Carapito R, Hatsch D, Vorwerk S, Petkovski E, Jeltsch JM, Phalip V (2008) Gene expression in Fusarium graminearum grown on plant cell wall. Fungal Genet Biol 45:738–748
Carillo P (2018) GABA shunt in durum wheat. Front Plant Sci 9:100
Carvajal F, Palma F, Jamilena M, Garrido D (2015) Preconditioning treatment induces chilling tolerance in zucchini fruit improving different physiological mechanisms against cold injury. Ann Appl Biol 166:340–354
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1945
Cheng B, Li Z, Liang L, Cao Y, Zeng W, Zhang X, Ma X, Huang L, Nie G, Liu W, Peng Y (2018) The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int J Mol Sci 19:9
Che-Othman MH, Jacoby RP, Millar AH, Taylor NL (2019) Wheat mitochondrial respiration shifts from the TCA cycle to the GABA shunt under salt stress. New Phytol 225(3):1166–1180
Corpas FJ, Del Rio LA, Palma JM (2019) Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J Integr Plant Biol 61(7):803–816
Cui H, Kong Y, Zhang H (2011) Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012:13
Cui Y, Miao K, Niyaphorn S, Qu X (2020) Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int J Mol Sci 21:995
Dagron A, Chapalain A, Mijouin L, Hillion M, Duclairoir-Poc C, Chevalier S, Taupin L, Orange N, Feuilloley MGJ (2013) Effect of gaba, a bacterial metabolite, on Pseudomonas fluorescens surface properties and cytotoxicity. Int J Mol Sci 14(6):12186–12204
Daş ZA, Dimlioğlu G, Bor M, Özdemir F (2016) Zinc induced activation of GABA-shunt in tobacco (Nicotiana tobaccum L.). Environ Exp Bot 122:78–84
De Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4:1804
De Simone A, Hubbard R, De la TNV, Velappan Y, Wilson M, Considine MJ, Soppe WJJ, Foyer CH (2017) Redox changes during the cell cycle in the embryonic meristem of Arabidopsis thaliana. Antioxid Redox Signal 27:1505–1519
Diaz-Vivancos P, De SA, Kiddle G, Foyer CH (2015) Glutathione-linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1104
Dimlioğlu G, Daş ZA, Bor M, Özdemir F, Türkan İ (2015) The impact of GABA in harpin-elicited biotic stress responses in Nicotiana tabaccum. J Plant Physiol 188:51–57
Dutta S, Mitra M, Agarwal P, Mahapatra K, De S, Sett U, Roy S (2018) Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal Behav 13:8
El-kereamy A, Ranathunge Bi Y-M, K, Beatty PH, Good AG, Rothstein SJ, (2012) The Rice R2R3-MYB Transcription Factor OsMYB55 Is Involved in the Tolerance to High Temperature and Modulates Amino Acid Metabolism. PLoS ONE. 7(12):e52030
Fan LQ, Yang LW, Gao HB, Wu XL, Xia Q, Gong BB (2012) Effects of exogenous gamma-aminobutyric acid on polyamine metabolism of melon seedlings under hypoxia stress. Ying Yong Sheng Tai XueBao 23(6):1599–1606
Fincato P, Moschou PN, Spedaletti V, Tavazza R, Angelini R, Federico R, Roubelakis AKA, Tavladoraki P (2012) Functional diversity inside the Arabidopsis polyamine oxidase gene family. J Exp Bot 62:1155–1168
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15
Gilliham M, Tyerman SD (2016) Linking metabolism to membrane signaling: the GABA-malate connection. Trends Plant Sci 21(4):295–301
Guan YJ, Hu J, Wang XJ, Shao CX (2009) Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B 10(6):427–433
Guan Q, Lu X, Zeng H, Zhang Y, Zhu J (2013) Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J 74:840–851
Guo Z, Tan J, Zhuo C, Wang C, Xiang B, Wang Z (2014) Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosyl methionine synthetase in Medicago sativa sub sp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol J 12:601–612
Hu XH, Xu ZR, Xu WN, Li JM, Zhao N, Zhou Y (2015) Application of γ- aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol Biochem 92:1–10
Jalil SU, Iqbal M, Khan R, Ansari MI (2019) Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana. Curr Plant Biol 19:100119
Jantaro S, Kanwal S (2017) Low-Molecular-Weight Nitrogenous Compounds (GABA and Polyamines) in Blue-Green Algae. Algal Green Chemistry. https://doi.org/10.1016/B978-0-444-63784-0.00008-4
Ji J, Yue J, Xie T, Chen W, Du C, Chang E, Shi S (2018) Roles of γ-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: Involving in abscisic acid and ethylene-signalling pathways. Planta 248(3):675–690
Jiao C, Duan Y, Lin Q (2019) MAPK mediates NO/cGMP-induced GABA accumulation in soybean sprouts. Food Sci Technol 100:253–262
Kanwal S, Incharoensakdi A (2019) The role of GAD pathway for regulation of GABA accumulation and C/N balance in Synechocystis sp. PCC6803. J Appl Phycol 31:3503–3514
Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61:561–591
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Kobayashi Y, Ohyama Y, Kobayashi Y, Ito H, Iuchi S, Fujita M, Zhao CR, Tanveer T, Ganesan M, Kobayashi M, Koyama H (2014) STOP2 activates transcription of several genes for Al- and low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol Plant 7(2):311–322
Koike S, Matsukura C, Takayama M, Asamizu M, Ezura H (2013) Suppression of γ -aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol 54:793–807
Krishnamurthy A, Rathinasabapathi B (2013) Auxin and its transport play a role in plant tolerance to arsenite induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ 36(10):1838–1849
Krishnan S, Laskowski Shukla V, Merewitz EB (2013) Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ-aminobutyric acid on perennial ryegrass. J Am Soc Hortic Sci 138(5):358–366
Kumar N, Dubey AK, Upadhyay AK, Gautam A, Ranjan R, Srikishna S, Sahu N, Behera SK, Mallick S (2017) GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci Rep 7:8786
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6:30338
Li Y, Fan Y, Ma Y, Zhang Z, Yue H, Wang L, Li J, Jiao Y (2017) Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul 36(2):436–449
Li Z, Peng Y, Huang B (2018) Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera). Int J Mol Sci 19:1623
Li Z, Cheng B, Zeng W, Liu Z, Peng Y (2019) The transcriptional and post-transcriptional regulation in perennial creeping bentgrass in response to γ-aminobutyric acid (GABA) and heat stress. Environ Exper Bot 162:515–524
Li L, Dou N, Zhang H, Wu C (2021) The versatile GABA in plants. Plant Signal Behav 16:1862565. https://doi.org/10.1080/15592324.2020.1862565
Lin JS, Kuo CC, Yang IC, Tsai WA, Shen YH, Lin CC, Liang YC, Li YC, Kuo YW, King YC, Lai HM, Jeng ST (2018) MicroRNA160 modulates plant development and heat shock protein gene expression to mediate heat tolerance in Arabidopsis. Front Plant Sci 9:68
Liu T, Liu Z, Li Z, Peng Y, Zhang X, Ma X, Huang L, Liu W, Nie G, He L (2019) Regulation of heat shock factor pathways by γ-aminobutyric acid (GABA) associated with thermotolerance of creeping Bentgrass. Int J Mol Sci 20(19):4713
Lu X, Guan Q, Zhu J (2013) Down regulation of CSD2 by a heat-inducible miR398 is required for thermotolerance in Arabidopsis. Plant Signal Behav. 8:e24952
Luo HY, Gao Yang LW, Xiao-Lei HB, WU, Liu HH, (2011) Physiological mechanism of GABA soaking to tomato seed germination and seedling development under NaCl stress. Acta Bot Boreal Occid Sin 31:2235–2242
Ma Y, Wang P, Chen Z, Gu Z, Yang R (2018) GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J Plant Physiol 231:192–201
Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78
Malekzadeh P, Khara J, Heidari R (2012) Effect of exogenous Gamma-aminobutyric acid on physiological tolerance of wheat seedlings exposed to chilling stress. Iran J Plant Physiol 3:1
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants 20(1):133–137
Malekzadeh P, Khosravi-Nejad F, Hatamnia AA, Mehr RS (2017) Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol Mol Biol Plants 23:827–836
Marček T, Hamow KÁ, Végh B, Janda T, Darko E (2019) Metabolic response to drought in six winter wheat genotypes. PLoS ONE 14:2
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57(14):3755–3766
Mekonnen DW, Flügge UI, Ludewig F (2016) Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci 245:25–34
Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63:1054–1062
Michaeli S, Fromm H (2015) Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front Plant Sci 6:419
Mirabella R, Rauwerda H, Allmann S, Scala A, Spyropoulou EA, De VM, Boersma MR, Breit TM, Haring MA, Schuurink RC (2015) WRKY40 and WRKY6 act downstream of the green leaf volatile E-2-hexenal in Arabidopsis. Plant J 83:6
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Mohapatra S, MinochaLong RS, Minocha SC (2010) Transgenic manipulation of a single polyamine in poplar cells affects the accumulation of all amino acids. Amino Acids 38:4
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33:408–419
Nonaka S, Arai C, Takayama M, Matsukura C, Ezura H (2017) Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci Rep 7(1):7057
Palma F, Carvajal F, Ramos JM, Jamilena M, Garrido D (2015) Effect of putrescine application on maintenance of zucchini fruit quality during cold storage: contribution of GABA shunt and other related nitrogen metabolites. Postharvest Biol Technol 99:131–140
Palmer AJ, Baker A, Muench SP (2016) The varied functions of aluminium-activated malate transporters–much more than aluminium resistance. BiochemSoc Trans 44:856–862
Planamente S, Vigouroux A, Mondy S, Nicaise M, Faure D, Morera S (2010) A conserved mechanism of GABA binding and antagonism is revealed by structure-function analysis of the periplasmic binding protein Atu2422 in Agrobacterium tumefaciens. J Biol Chem. 285(39):30294–30303
Planas-Portell J, Gallart M, Tiburcio AF, Altabella T (2013) Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol 13:109
Podlesakova K, Ugena L, Spichal L, Dolezal K, De DN (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48:53–65
Priya M, Sharma L, Kaur R, Bindumadhava H, Nair RM, Siddique KHM, Nair H (2019) GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci Rep 9:7788. https://doi.org/10.1038/s41598-019-44163-w
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Ramesh S, Tyerman S, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijó JA, Ryan PR, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6:7879
Ramesh SA, Tyerman SD, Gilliham M, Xu B (2016) γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci 74:1577–1603
Ramesh SA, Tyerman SD, Gilliham M, Xu B (2017) g-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci 74:1577–1603
Ramesh SA, Kamran M, Sullivan W, Chirkova L, Okamoto M, Degryse F, McLaughlin M, Gilliham M, Tyerman SD (2018) Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 30:1147–1164
Ramos-Ruiz R, Martinez F, Knauf-Beiter G (2019) The effects of GABA in plants. Cogent Food Agric 5(1):1670553
Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Saeidia M, Moradib F, Abdolic M (2017) Impact of drought stress on yield, photosynthesis rate, and sugar alcohols contents in wheat after anthesis in semiarid region of Iran. Arid Land Res Manag 31(2):204–218
Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, Del RLA (2013) Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Sub cell Biochem. https://doi.org/10.1007/978-94-007-6889-5_13
Scholz SS, Malabarba J, Reichelt M, Heyer M, Ludewig F, Mithofer A (2017) Evidence for GABA-induced systemic GABA accumulation in Arabidopsis upon wounding. Front Plant Sci 8:388
Seifikalhor M, Aliniaeifard S, Hassani B, Niknam V, Lastochkina O (2019) Diverse role of γ-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. https://doi.org/10.1007/s00299-019-02396-z
Shang H, Cao S, Yang Z, Cai Y, Zheng Y (2011) Effect of exogenous gamma-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem 59(4):1264–1268
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012a) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193(194):130–135
Shelp BJ, Bozzo GG, Simpson ZA, JP, Trobache CP, Allan WL, (2012b) Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. II. Integr Anal Bot 90(9):781–793
Shelp BJ, Bozzo GG, Zarei A, Simpson JP, Trobacher CP, Allan WL (2012c) Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. Integr anal Bot 90(9):781–793
Shelp BJ, Zarei A (2017) Subcellular compartmentation of 4-aminobutyrate (GABA) metabolism in Arabidopsis: an update. Plant Signal Behav 12:e1322244
Sheng L, Shen D, Luo Y, Sun X, Wang J, Luo T, Zeng Y, Xu J, Deng X, Cheng Y (2017) Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem 216:138–145
Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B (2019) GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci 20(22):5709
Shi SQ, Zheng S, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Zhang SG (2010) Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ 33:149–162
Signorelli S, Dans PD, Coitino EL, Borsani O, Monza J (2015) Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 10(3):e0115349
Singh R, Parihar P, Singh S, Mishra RK, Singh VP, Prasad SM (2017) Reactive oxygen species signaling and stomatal movement: current updates and future perspectives. Redox Biol 11:213–218
Steward F, Thompson J, Dent C (1949) γ-Aminobutyric acid, a constituent of the potato tuber. Sci 110:439–440
Su N, Wu Q, Chen J, Shabala L, Mithöfer A, Wang H, Qu M, Yu M, Shabala CJ, S, (2019) GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J Exp Bot 70:6349–6361
Suhel M, Husain T, Prasad SM, & Singh VP (2022) GABA Requires Nitric Oxide for Alleviating Arsenate Stress in Tomato and Brinjal Seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10576-7
Takayama M, Koike S, Kusano M, Matsukura C, Saito K, Ariizumi T, Ezura H (2015) Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol 56:1533–1545
Takayama M, Matsukura C, Ariizumi T, Ezuram H (2017) Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Re 36:103–116
Talbi SMC, Romero-Puertas A, Hernandez L, Terron A, Ferchichi LMS (2015) Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ Exp Bot 111:114–126
Tang X, Yu R, Zhou Q, Jiang S, Le G (2018) Protective effects of γ-aminobutyric acid against H2O2-induced oxidative stress in RIN-m5F pancreatic cells. Nutr Metab (lond) 15:60
Tiburcio AF, Altabella T, Bitrian M, Alcazar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240:1–18
Turkan I (2018) ROS and RNS: key signalling molecules in plants. J Exp Bot 69(14):3313–3315
Tuteja N, Sopory SK (2008) Chemical signaling under abiotic stress environment in plants. Plant Signal Behav 3:525–536
Vijayakumari K, Puthur J (2016) γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul 78(1):57–67
Virdi AS, Singh S, Singh P (2015) Abiotic stress responses in plants: roles of calmodulin regulated proteins. Front Plant Sci 6:809
Wang Y, Luo Z, Huang X, Yang K, Gao S, Du R (2014) Effect of exogenous c-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic-Amst 168:132–137
Wang X, Huang W, Yang Z, Liu J, Huang B (2016) Transcriptional regulation of heat shock proteins and ascorbate peroxidase by CtHsfA2b from African bermudagrass conferring heat tolerance in Arabidopsis. Sci Rep 6:28021
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S (2017) γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7:43609
Woodrow P, Ciarmiello LF, Annunziata MG, Pacifico S, Iannuzzi F, Mirto A, D’Amelia L, Dell’Aversana E, Piccolella S, Fuggi A, Carillo P (2017) Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol Plant 159:290–312
Xing SG, Jun YB, Hau ZW, Liang LY (2007) Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem. 45(8):560–6
Xu Y, Zhan C, Huang B (2011) Heat shock proteins in association with heat tolerance in grasses. Int J Proteomics. https://doi.org/10.1155/2011/529648
Xu M, Gruber BD, Delhaize E, White RG, James RA, You J, Yang Z, Ryan PR (2015) The barley anion channel, HvALMT1, has multiple roles in guard cell physiology and grain metabolism. Physiol Plant 153:183–193
Xu B, Long Y, Feng X, Zhu X, Sai N, Chirkova L, Betts A, Herrmann J, Edwards EJ, Okamoto M (2021) GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat Commun 12:1952
Yang A, Cao S, Yang Z, Cai Y, Zheng Y (2011) γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem 129:1619–1622
Yang R, Guo Q, Gu Z (2013) GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem 136:152–159
Yin Y, Yang R, Gu Z (2014) Calcium regulating growth and GABA metabolism pathways in germinating soybean (Glycine max L.) under NaCl stress. Eur Food Res Technol 239(1):149–156
Yong B, Xie H, Li Z, Li Y-P, Zhang Y, Nie G, Zhang XQ, Ma X, Huang LK, Yan YH, Peng Y (2017) Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol 8:1107
Yu D, Eldred WD (2005) Glycine and GABA interact to regulate the nitric oxide/cGMP signaling pathway in the turtle retina. Visual Neuro Sci 22(6):825
Yu C, Zeng L, Sheng K, Chen F, Zhou T, Zheng X, Yu T (2014) γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem 159:29–37
Zarei A, Trobacher CP, Shelp BJ (2015a) NAD+-aminoaldehyde dehydrogenase candidates for 4-aminobutyrate (GABA) and β-alanine production during terminal oxidation of polyamines in apple fruit. FEBS Lett 589:2695–2700
Zarei A, Trobacher CP, Cooke AR, Meyers AJ, Hall JC, Shelp BJ (2015b) Apple fruit copper amine oxidase isoforms: peroxisomal MdAO1 prefers diamines as substrates, whereas extracellular MdAO2 exclusively utilizes monoamines. Plant Cell Physiol 56:137–147
Zarei A, Trobacher CP, Shelp BJ (2016) Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci Rep 6:35115
Zarei A, Chiu GZ, Yu G, Trobacher CP, Shelp BJ (2017) Salinity-regulated expression of genes involved in GABA metabolism and signalling. Botany 95:6
Zhong H, Tong L, Gu N, Gao F, Lu Y, Xie RG, Zhang X (2017) Endocannabinoid signaling in hypothalamic circuits regulates arousal from general anesthesia in mice. J Clin Investig 127(6):2295–2309
Zhu C, Ding Y, Liu H (2011) MiR398 and plant stress responses. Physiol Plant 143:1–9
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W (2019) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19:43
Acknowledgements
Mohammad Suhel is grateful to the University Grants Commission, New Delhi, for granting fellowship. Tajammul Husain thankful to the CSIR for providing fellowship. Dr. Samiksha Singh is grateful to the University Grants Commission for providing Dr. D.S. Kothari Post-Doctoral Fellowship (No. F.4-2/2006(BSR)/OT/19-20/0006) to carry out this work. Aparna Pandey is thankful as SRF (CSIR UGC-NET Dec. 2018, Ref. No. 627).
Author information
Authors and Affiliations
Contributions
VPS and SMP conceptualized the idea. MS, TH, AP, and SS wrote the review. NKD, SMP, and VPS corrected review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Naeem Khan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suhel, M., Husain, T., Pandey, A. et al. An Appraisal of Ancient Molecule GABA in Abiotic Stress Tolerance in Plants, and Its Crosstalk with Other Signaling Molecules. J Plant Growth Regul 42, 614–629 (2023). https://doi.org/10.1007/s00344-022-10610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10610-8