Abstract
Abscisic acid (ABA) research has focused on improving plant resistance under stress; however, the effects of exogenous ABA application in plants under non-stress conditions have not been thoroughly analyzed. Here, we explored the regulatory effects of exogenous ABA application on the growth of a model plant, tobacco, in an environment with no stressors by spraying leaves at the seedling stage. At the same time, as an important cash crop in the world, ABA can improve the growth ability of tobacco seedling stage and have a positive impact on the later growth.We found a significant increase in tobacco biomass under a 0.5 mg/L ABA treatment. Chlorophyll fluorescence parameters, including PIabs, Fm, Phi (Eo), Phi (Po), Sm, and N in the ABA group increased in different degrees compared with the control (CK group). Ultrastructure observation of tobacco leaves showed that chloroplast, plasmid, and mitochondria ultrastructure were significantly different from those in the CK group. qRT-PCR confirmed the reliability of the transcriptome results. ABA may increase the biomass of tobacco by affecting the expression of receptor genes of plant hormones such as auxin and gibberellin, as well as the synthesis of alkaloids and metabolism of amino acids such as phenylalanine. We found that genes expressed in related resistance pathways, such as CNGCs and CDPK, were down-regulated, suggesting that they may affect the tobacco plants’ ability to defend itself against pathogens. These preliminary findings provide a reference for further revealing the mechanism of exogenous ABA in regulating plant growth under non-stress environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abscisic acid (ABA) is an endogenous plant hormone that was first described in the 1960s. Much of the research on ABA has focused on enhancing stress tolerance in plants (Yao et al. 2019; Dong et al. 2020; Etehadnia et al. 2008; Veselov et al. 2008). Its physiological activity can promote plant leaf aging and shedding, organ aging, and other processes (Addicott et al. 1983; Osborne et al. 1989). ABA seems to inhibit plant growth compared with other endogenous hormones, but recent study results (Teng et al. 2021; Shu et al. 2013, 2016a, b; Shu et al. 2016a) show that the above understanding is not comprehensive. ABA can both promote and inhibit growth under different treatment concentrations and times (Skriver et al. 1990; Tari et al. 2010; Fukaki et al. 2009; Anandan et al. 2012). As a strong growth inhibitor, a high concentration of ABA mainly restricts cell elongation and keeps cells in the G1 phase of the cell cycle to inhibit cell division (Su. 2011). Cui et al. (2012) found that, in the early growth stage of bamboo, the three parts of the stem segment (base, middle, and top) maintained a high ABA concentration, which inhibited its growth. Yang et al. (2010) found that 10.0 mol/g ABA treatment severely inhibited the elongation and growth of the aerial parts of rice, and the chlorophyll content in the leaves of five rice varieties showed a downward trend, while cell membrane permeability significantly increased. However, Ghassemian et al. (2000) found that ABA promoted root growth when its exogenous concentration was 0.1 mmol/L. When its concentration reached 1.0 mmol/L, root elongation and growth were inhibited. Li et al. (2012) found that different exogenous ABA applications had a significant impact on the photosynthesis and yield of rapeseed. Treatment with high-concentration ABA reduced photosynthesis, water use efficiency (WUE), and yield of rapeseed, while these indicators increased significantly after low-concentration ABA treatment. Previous studies (Su et al. 2011) have found that ABA-deficient plants have a short stature even under good growth conditions, which may imply that lower concentrations of endogenous ABA can promote growth when plants are not stressed. In summary, these results suggest that high ABA concentrations inhibit plant growth, while low ABA concentrations promote it.
This study explored whether exogenous ABA is able to promote growth, and how it is promoted in the early stage of plant growth. We chose the model plant tobacco variety CF965 because of its rapid growth, large leaves, and simple laboratory culture, as well as its large cells that are susceptible to external factors. In addition tobacco provides a large amount of plant physiology information, which enabled us to collect more detailed experimental results. At the same time, as an important cash crop, the yield and quality of tobacco have a critical impact on its economic value. Therefore, the cultivation of tobacco seedlings at the seedling stage can: (1) shorten the growth period of the field and make economic use of land. (2) Environmental conditions are easy to meet, conducive to the cultivation of strong seedlings. (3) Using the independent stage, can improve the uniformity of tobacco seedlings. (4) Overcome the restriction of short frost-free period on tobacco. This has economic and practical significance in production.
Materials and methods
Plant materials and growth conditions
The tobacco variety CF965 (from The College of Tobacco, Shandong Agricultural University) was selected in this study. Tobacco seeds were washed in 75% ethanol for 45 s, soaked in 5% sodium hypochlorite for 8 min, and washed in sterile water three times. Appropriate amounts of perlite, peat soil, and horticultural vermiculite were prepared, and then mixed in a ratio of 1:1:1 to form a matrix, which was sterilized under high temperature and high humidity for tobacco cultivation. Plants were cultured in a greenhouse at 27 oC, 16/8 h light/dark cycle, and 70% humidity. When seedlings in the suspension tray grew to have four leaves, healthy looking similar sized plants were transplanted into a small 90 mm x 110 mm pot where they were raised in a nutrient rich soil. In the experiments, tobacco plants treated with different concentrations of exogenous ABA spray were selected as the experimental material, and tobacco plants treated with water spray was used as blank control (CK), with three replicates in each treatment and 10 plants in each replicate.
Experimental design
-
1)
ABA was dissolved in acetone and deionized water at a constant volume of 100 mL to prepare 1000 mg/L ABA solution. When the seedlings grew to the four-leaf stage, healthy seedlings of similar size were selected and transplanted into small pots (90 mm×110 mm), cultivated with nutrient soil, and transplanted for a week. Through a pre-test screening in the early stage, we established this experiment with four ABA groups and one control group, with three replicates in each group, and 10 tobacco plants in each replicate. The 1000 mg/L ABA solution was diluted with water to 50 mg/L, 10 mg/L, 5 mg/L and 0.5 mg/L. The control group was treated with water. The solutions were applied evenly to the leaves with a watering can. The treatment was applied only once in the whole experimental cycle, and each tobacco plant was sprayed with about 15 mL of solution.
-
2)
When the tobacco grew to the 6–7-leaf stage, the photographic comparison, phenotypic investigation, and fresh and dry weight determination were carried out.
-
3)
When the tobacco grew to the eight-leaf stage (seedling stage), we selected the third tobacco leaf (from the top down) as the experimental object. The chlorophyll fluorescence parameters of tobacco leaves were determined using a cirAS-3 portable photosynthetic fluorescence system. Chlorophyll content was determined by spectrophotometry.
-
4)
When the tobacco grew to eight leaves (seedling stage), we choose the third leaf (top to bottom) as the experimental subject. Samples were taken from the middle of a vein and were 1.5 mm × 20 mm × 1.5 mm in size. PB quickly rinsed the tissue and it was immediately placed in 3% glutaraldehyde fixative solution (pH 7.4). Transmission electron microscopy was performed.
-
5)
When tobacco grew to the eight-leaf stage (seedling stage), the treatment with the largest phenotypic difference was selected. The third leaf (from top to bottom) was used as the experimental material, wrapped in tin foil and immediately stored in liquid nitrogen at -80℃ for transcriptome and metabolome analysis.
Determination of plant body size and biomass
Leaf length and width were measured using a ruler. For the fresh weight measurement, the plant material was quickly cut, placed into a container (or plastic bag) of known weight, taken indoors, and placed in an analytical balance for fresh weight (FW) measurement. For dry weight (DW) measurement, we turned on the oven in advance and let the temperature rise to 100–105 ℃. The fresh-weighed plant material was placed into a paper bag, placed in the oven at 100–105 ℃ for 10 min, then the temperature was reduce to about 70 ~ 80 ℃ where it was baked until it had a constant weight. The samples were cooled in a dryer to room temperature and the dry weight (DW) was recorded.
Determination of chlorophyll fluorescence parameters and chlorophyll determination
The portable photosynthetic apparatus is simple, fast, and accurate, but a change in environmental factors may have a greater impact on the results. Therefore, the data were measured in a greenhouse with good fresh-air ventilation. The third leaf was used as the test sample. Chlorophyll content was determined by ultraviolet spectrophotometry. Three tobacco plants were randomly selected from each treatment and 2 g of material were obtained from each leaf. The third leaf was used as the test sample. Chlorophyll content was calculated according to: (chlorophyll content at equal mass) × (ratio of leaf area between treatmenup and control group).
Observation on microstructure of mesophyll cells
When the tobacco grew to the eight-leaf stage (seedling stage), we selected the third tobacco leaf (from the top down) as the experimental object. Samples were taken from the middle of the veins, with a size of 1.5 mm × 20 mm × 1.5 mm. The tissues were quickly rinsed with PB and immediately placed in 3% glutaraldehyde fixative solution (pH 7.4). The sample block was dressed to 1 mm × 1 mm × 3 mm, and then rinsed, immobilized with 1% osmium tetroxide, rinsed, dehydrated, soaked, and embedded with Epon812 according to the conventional TEM sample preparation method. After positioning the semi-thin section, the 70 ~ 100 nm ultra-thin sectioning was carried out with an LKB-V ultra-thin slicer. Electron staining of lead citrate and uranium acetate was observed using a JEOL-1200E transmission electron microscope, and recorded with a Morada-G2.
Data analysis and collation
The above data were processed using a DPS data processing system; significant differences were analyzed by Duncan’s new rich range method, and the Origin system was used for mapping.
Transcriptome sequencing
When tobacco reached the eight-leaf stage, the treatments with the largest phenotypic differences were used as experimental materials, wrapped with tin foil, immediately frozen in liquid nitrogen, and used for transcriptome sequencing. Total RNA was extracted with Trizol reagent (Invitrogen) with a kit (Evo M-MLV AG11728) to form cDNA. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100.
Library preparation
A total amount of 1 µg RNA per sample was used as input material for the RNA sample preparations. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in First Strand Synthesis Reaction Buffer (5X). Firststrand cDNA wassynthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-). Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, Adaptor with hairpin loop structure were ligated to prepare for hybridization. In order to select cDNA fragments of preferentially 370 ~ 420 bp in length, the library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, USA). Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system.
Data processing and differential expression analysis
To ensure the quality and reliability of our data analysis, raw data was filtered to remove reads with joints (adapter); remove reads with N (N means uncertain base information); remove low quality reads. After raw data filtering, sequencing error rate checking, and GC content distribution checking, the clean reads system (Agilent Technologies, CA, USA) used for subsequent analysis was obtained. The sequencing fragment was randomly interrupted by mRNA. To determine which genes were transcribed, the quality control clean reads were aligned to the reference genome. A fast and accurate alignment of Clean Reads to the reference genome was achieved using HISAT2 software to obtain the mapping information of Reads on the reference genome.
After the assembly of the new transcripts, they were annotated with Pfam, SUPERFAMILY, GO, KEGG databases, and the differentially expressed genes (DEGs) general reference | log2 (FoldChange) |> 1 & padj < = 0.05, to identify the DEGs between the two samples and the enrichment analysis of gene function.
qRT-PCR analysis of the expression levels of DEGs
Candidate DEGs were detected by RT-qPCR. The sequences of the primer pairs designed in this experiment were selected in NCBI, using the ACTIN gene as the internal reference gene. Each sample consisted of three biological and technical replicates. The 2−ΔΔCt method was used to calculate the relative gene expression.
Metabolomics study
Liquid mass linking (LC-MS) technology was used in this study (Dunn et al. 2011; Want et al. 2010). A class-targeted metabolomics study based on the high-sensitivity SCIEX QTRAP® 6500 + mass spectrometry platform, with SCIEX OS V1.4 software, was used to open the underlying mass spectrometry file (.wiff). The integration and calibration of chromatographic peaks were carried out, and the peak Area (Area) of each chromatographic peak represented the relative quantitative value of the corresponding substance. Finally, the integral data of all chromatographic peak areas were derived. The quality control (QC) of QC samples was used to ensure the accuracy and reliability of data results. Next, a multivariate statistical analysis of metabolites, including principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), were conducted to reveal differences in metabolic patterns of different groups. Hierarchical clustering (HCA) and metabolite correlation analysis were used to reveal the relationship between samples and metabolites. Finally, the biological significance of metabolite correlation was explained by functional analysis such as that of metabolic pathways.
Screening of the differential metabolites
Partial Least Squares Discrimination Analysis (PLS-DA) is a supervised statistical method for discriminant analysis that uses partial least squares regression (Boulesteix et al. 2007). The relationship between the relative quantitative value of metabolites and sample category was modeled to achieve the prediction of sample category. PLS-DA models were established for each comparison group, and 7-fold cross-validation was performed. The model evaluation parameters (R2, Q2) were obtained when the biological repetition number of samples n ≤ 3, and k = 2n, respectively. If the values of R2 and Q2 were closer to 1, the model was more stable and reliable.
Results
Effects of exogenous application of ABA on tobacco seedling biomass
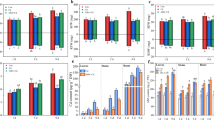
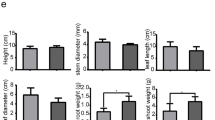
It can be seen from Fig. 1 that the plant body size of the CK group was smaller than that of the ABA treatment group. Compared with those in CK, the leaf length and width in the 0.5 mg/L treatment increased by 52.86% and 33.95%, respectively (Fig. 2a). Fresh weights of aerial and underground parts increased by 34.91% and 46.47%, respectively (Fig. 2b and d), while the dry weight of aerial and underground parts increased by 86.09% and 84.09% (Fig. 2b and d). Leaf length and width increased in plants with ABA treatment. However, such increase was negatively related with ABA concentration. Of all the treatments, the most significant increase in biomass was seen with the 0.5 mg/L ABA treatment.
Effects of different ABA concentrations on the biomass of tobacco seedlings after 8 days. a, leaf length diameter and leaf cross diameter of tobacco seedlings treated with different ABA concentrations (A: leaf length diameter, B: leaf cross diameter). b, Aerial biomass of tobacco seedlings (A: Fresh weight of aerial part B: dry weight of aerial part). c, Leaf-number and stem-length of tobacco seedlings (A: leaf-number, B: stem-length). d, underground biomass of tobacco seedlings (A: fresh underground weight, B: dry underground weight). Duncan’s test showed that there were significant differences among groups (P < 0.05). The bar chart above the mean represents the standard deviation of ± 5 repetitions. The above lowercase letters indicate significant differences between treatments
Effects of exogenous ABA on chlorophyll and chlorophyll fluorescence parameters in tobacco seedlings
As can be seen from Fig. 3, chlorophyll A, chlorophyll B, and carotenoids of tobacco seedlings treated with all ABA concentrations increased to varying degrees compared with CK. In this experiment, exogenous ABA had almost no effect on Fv/Fm of tobacco seedlings (Fig. 5), but PIabs value was higher than for CK. However, such increase was negatively related-with ABA concentration. (Fig. 4). After ABA treatment, Phi (Po) was only slightly increased compared with CK after 0.5 mg/L treatment, and the remainder showed no significant change (Fig. 4). Compared with CK, ABA significantly increased Phi (Eo) and Fm at 0.5 mg/L and 5 mg/L. As can be seen from (Fig. 5), compared with CK, Sm index of the treatment group increased significantly, and N index of the treatment group also increased to varying degrees. Meanwhile, Vj of the treatment group was significantly lower than that of CK.
Chlorophyll content of tobacco seedlings under different treatments (a: chlorophyll A; B: chlorophyll B; C: carotenoid). Duncan’s test showed that there were significant differences among groups (P < 0.05). The bar chart above the mean represents the standard deviation of ± 3 repetitions. The above lowercase letters indicate significant differences between treatments
Effects of exogenous ABA on PIabs, Fm, Phi (Eo), and Phi (Po) of tobacco seedlings. Duncan’s test showed that there were significant differences among groups (P < 0.05). The bar chart above the mean represents the standard deviation of ± 10 repetitions. The above lowercase letters indicate significant differences between treatments
Effects of exogenous ABA on Sm, N, Vj, and Fv/Fm of tobacco seedlings. Duncan’s test showed that there were significant differences among groups (P < 0.05). The bar chart above the mean represents the standard deviation of ± 10 repetitions. The above lowercase letters indicate significant differences between treatments
Electron microscopic observation on chloroplast ultrastructure of mesophyll cells of tobacco seedlings
As can be seen in Fig. 6, the number of osmiophilic granules in the form of black oil droplets increases significantly at the same magnification and under 0.5 mg/L ABA treatment. The black rectangle around the starch granule is called the grana lamella and the rest is called the stromal lamella. The grana lamella and stroma lamella make up the chloroplast stroma. Figure 6 shows that under 0.5 mg/L ABA treatment, the chloroplast stroma volume increased, the number of stromal lamella increased and the volume of stromal lamella increased.
Analysis of the effects of exogenous ABA application on tobacco seedlings on the transcriptome
The correlation of gene expression levels between samples is an important indicator for testing the reliability of the experiment and sample selection. The closer the correlation coefficient is to 1, the higher the similarity of expression patterns between the samples. If it is < 0.8, the experimental procedure needs to be repeated. Figure 7 A shows that the R2 of six samples in two treatments were all > 0.8, indicating the reliability of samples in this experiment. A total of 680 DEGs were found from this transcriptome (Fig. 7C), of which 501 genes were downregulated and 189 were upregulated. KEGG enrichment showed that (Fig. 7B) these genes were mainly involved in plant-pathogen interactions, the fatty acid chain extension system, MAPK signaling pathway, biosynthesis of stilbene, diarylheptane and gingerol, phosphatidylinositol signaling system, glutathione metabolism, biosynthesis of phenylalanine, tyrosine and tryptophan, ether lipid metabolism, nitrogen metabolism, and plant hormone signal transduction. We screened some genes for analysis and validation, these genes were involved in plant-pathogen interactions and plant hormone signal transduction pathways.
Transcriptome results of 0.5 mg/L ABA treatment and CK (0.5 mg/L: ZS CK: ZK). (a) Correlation between samples. The closer the correlation coefficient is to 1, the higher the similarity of expression patterns among samples. For specific project operations, we require R2 of at least 0.8 between biological duplicates. (b) From the KEGG enrichment results, the 20 most significant KEGG pathways were selected to draw scatter plots (the horizontal axis is the ratio of gene annotations to the total number of genes in the KEGG pathway; the vertical axis is the KEGG pathway; the size of points represent the number of gene annotations in the KEGG pathway; and the color from red to purple represents the significance of the enrichment). (c) Difference comparison, bar chart of the number of differentially expressed genes in combinations (blue and gray represent up-regulated and down-regulated differentially expressed genes, respectively, and the numbers on the columns represent the number of differentially expressed genes)
Effect of exogenous ABA on tobacco seedlings on the metabolome
To represent the differential significance level, we set a threshold of VIP (Variable Importance in the Projection. Represents the metabolite’s contribution to the group) > 1.0, difference multiple FC (Fold Change) > 1.2 or FC < 0.833, and P-value < 0.05. A total of 53 differentially expressed metabolites were selected (Fig. 8B), 24 of them were up-regulated, including deoxyadenosine triphosphate, alkaloids atropine, keraxanthin, flaproone, L-phenylalanine,inositol, cinnamic acid, hydroxycinnamon acid, feruate, and some nucleotides; and 29 metabolites were down-regulated, including nucleotides, alkaloids, amino acids, phenylpropanoids,and alcohols. (Fig. 8C) Using the hypergeometric test, pathway-enriched P-values were obtained, with a P-value of 0.05 used as the threshold; KEGG pathways satisfying this condition were defined as the KEGG pathways significantly enriched in differentially expressed metabolites. We obtained three significant KEGG pathways: biosynthesis of bases, piperidine and pyridine alkaloids, biosynthesis and metabolism of phenylpropane, including (upregulated) L-phenylalanine, N-acetyl-D-phenylalanine, ketone, (downregulated) 1,5-diaminamentane, adjacent hydroxycinnamic acid (coumaric acid), and coumaraldehyde.
Metabolomic results of 0.5 mg/L ABA treatment and CK (0.5 mg/L: XS CK: XK).a, Cluster analysis of group differentially expressed metabolites. Longitudinally shown is the cluster of samples, and transversely shown is the cluster of metabolites. The shorter the cluster branch is, the higher the similarity. b, Volcanic map of differentially expressed metabolites. The horizontal coordinate represents the difference multiple of metabolites in different groups (log2FC), and the vertical coordinate represents the significance level (-log10(p-value). Each point in the volcano map represents a metabolite, and the significantly up-regulated metabolites are represented by red dots, while significantly down-regulated metabolites are represented by green dots. The size of the dot represents the VIP value. c, KEGG enrichment bubble diagram (positive and negative). The horizontal coordinate in the figure is X/Y (the number of differentially expressed metabolites in the corresponding metabolic pathway/the total number of identified metabolites in this pathway). The larger the value, the higher the degree of accumulation of differential metabolites in this pathway. The color of the point represents the P-value value of the hypergeometric test. The smaller the value, the more reliable and statistically significant the test. The size of the point represents the number of differentially expressed metabolites in the corresponding pathway. The larger the point, the more differentially expressed metabolites in the pathway
RNA-seq of differentially expressed genes (DEGs) compared with qRT-PCR
We screened seven genes of interest in pathways related to disease resistance and endogenous hormones and evaluated their expression patterns by qRT-PCR. All seven genes showed similar patterns to those obtained in RNA-seq results, which demonstrated the reliability of this experiment. The results indicate that exogenous ABA affects the plant-pathogen interactions and plant hormone signal transduction in tobacco. In the next experiments, these DEGs can be subjected to functional validation of genes to further reveal the effects of exogenous ABA on tobacco growth.
Discussion
Physiological effects of exogenous ABA administration on tobacco seedlings
According to the results (Figs. 1 and 2), exogenous ABA significantly promoted the growth of tobacco seedlings, which meant that the treated seedlings exhibited stronger growth than the control seedlings at the seedling stage. In agricultural and forestry production, the transplantation of plants is often necessary, but transplanting plants from their accustomed growth environment to a strange soil and climate environment can be an adverse process (Parihar et al. 2015). At this time, plants are in a relatively weak physiological state and have weak resistance to adverse factors present in the external environment. Munns and Tester (2008) found that salt stress inhibited the growth of young leaves and accelerated the senescence of mature leaves. Drought, high temperature, and cold stress (Wang et al. 2004; Winfield et al. 2010) destroy protein conformation in plants, affect their normal functions, and eventually lead to physiological and anatomical damage. Therefore, improving growth at the seedling stage can increase survival rate and resistance to adversity after transplantation. As can be seen from our results (Fig. 3), the total chlorophyll content of tobacco seedlings in the treated group was higher than that in CK. Through its properties as a photosynthetic pigment, chlorophyll content can directly reflect the strength of the photosynthetic capacity of plants. When photosynthesis is weak (South et al. 2019; Nayak et al. 2022), plant biomass will be significantly reduced; when it is strong, it will increase the biomass of plants and promote growth. Therefore, photosynthesis is the most important physiological process in plants, and chlorophyll fluorescence parameters are an effective means of analyzing photosynthesis (Faseela et al. 2020). Fv/Fm reflects the maximum light energy conversion efficiency of the PS reaction center, and this value hardly changes under non-adverse conditions. In this experiment, exogenous ABA had almost no effect on Fv/Fm of tobacco seedlings (Fig. 5), but the PIabs value was higher than CK, and gradually decreased with the increase of concentration (Fig. 4). PIabs is based on light absorption. When the maximum light conversion efficiency of tobacco seedlings remained constant, PIabs increased, which improved the ability of seedling leaves to absorb light energy.
When visible light hits the plant, the antenna pigment molecules (most of chlorophyll a and all of chlorophyll b, carotene) are excited by the absorption of light quanta and cause a primary reaction (the process of absorption, conversion and utilisation of light energy by the chloroplast pigment molecules). The reaction centre is the most basic pigment-protein complex for primary reactions and includes primary electron acceptors, donors and secondary electron donors. In the presence of light, the primary reactions are carried out continuously, constituting a “source” and “library” of electrons, which are converted into electrons by absorbing light quanta, enabling the conversion of light energy into electrical energy and ultimately the production of water and ATP. In the PS II electron transport chain, the secondary electron donor provides electrons directly to the antenna pigment molecules and releases O2. The antenna pigment molecules also pass electrons to QA, which in turn passes electrons to QB, which constitutes a source-to-library and ultimately generates ATP. Phi (Po) reflects the maximum photochemical efficiency of plants after dark adaptation. Phi (Eo) refers to the quantum yield of photons absorbed by the reaction center for electron transport. Fm refers to the fluorescence when all reaction centers are completely closed; that is, the maximum fluorescence intensity after dark adaptation. Reflects electron transfer through PSII. The results showed that exogenous ABA enhanced photosynthesis of tobacco seedlings by increasing electron transport quantum yield. Sm reflects the energy required for QA to be completely reduced from 2 ms to tFm; that is, the size of QA’s downstream electron receptor library. The lower the proportion of electrons transferred from QA, the shorter the time required for the fluorescence signal to reach Fm, and the smaller Sm is. N reflects the REDOX times of QA from 2 ms to tFm. If N decreases, it indicates that QA’s ability to transfer electrons decreases and the openness of the reaction center decreases (Liu 2009). Vj refers to the relative variable fluorescence intensity of point J, which reflects the closing degree of the active reaction center under a 2 ms illumination, and its size reflects the balance between the excitation rate of the pigment in the PSII antenna and the forward electron transfer rate. The results showed that exogenous ABA increased the electron transfer capacity of the PSII receptor side, increased the openness of reaction center and forward electron transfer efficiency, and promoted the absorption of light energy and the transfer and utilization of excitation energy.
Effect of exogenous ABA on the transcriptome of tobacco seedlings
As per KEGG enrichment (Fig. 7B), of the 20 pathways with P-values < 0.05 as the threshold of significance for the enrichment, we selected three extremely significantly enriched pathways, namely “Plant-pathogen interaction”, “Fatty acid elongation”, and “MAPK signaling pathway-plant”. This phenomenon is interesting, as the three pathways are the main channels of tobacco defense against pathogen invasion. We analyzed the genes in all the pathways, screened and identified seven major differentially expressed genes, and found that they mainly encoded CNGC, CDPK, CaM/CML, FLS2, RIN4, AUX1, GID1, and other proteins at the protein level. They are concentrated in the plant-pathogen interaction pathway and plant signal transduction pathway.
Differentially expressed genes in the plant-pathogen interaction pathway
Currently, there is a great controversy regarding the effects of ABA on plant disease resistance. As a plant hormone to resist stress, ABA induces the production of reactive oxygen species (ROS) in plant tissues, that is, it induces self-defense (Muhammad et al. 2015; Almeselmani et al. 2006; Liu et al. 2000; Larkindale et al. 2004; Sairam et al. 2000; Bhaskara et al. 2017; Gao et al. 2021). However, ABA is also widely involved in the response of plants to various biological stressors caused by various plant pathogens as a negative regulator of disease resistance (Mauch-Mani et al. 2005; Curvers et al. 2010; Fan et al. 2009). It was found that applying exogenous ABA at appropriate concentrations could induce plant disease resistance. For example, lilium plants were treated with 1.0 mg/L ABA, and their tolerance to root rot was observed. After spraying, the activity of defense enzymes in plant leaves increased, plant body size and growth traits significantly improved, and disease index decreased (Fang et al. 2010). Treatment with an appropriate concentration of exogenous ABA induced downy mildew resistance and reduced the cucumber disease index, with 10.0 mg/L ABA treatment concentration having the best effect (Liu et al. 2012). In mutants lacking ABA biosynthesis, resistance to the biotrophic pathogen Hyaloperonospora parasitica is increased (Mohr et al. 2003), while resistance to the necrotizing pathogen A. Brassicola is impaired (Ton et al. 2009). Therefore, the effect of ABA on the defense system of plant pathogens is complex.
From the results of this experiment, among the CNGCs, CDPK, CAM / CML, FLS2, and RIN4 protein genes that are differentially expressed in plant pathogen interaction, FLS2 is a typical leucine-rich repeat receptor-like kinase, which starts immune signaling by heteromerization with its co-receptor BAK1 after recognizing bacterial flagella flg22 (Chinchilla et al. 2007). The down-regulation of the LRR receptor-like serine/threonine protein kinase coding gene in FLS2 protein may lead to the obstruction of the plant innate immune system.
Plant cyclic nucleotide-gated channels (CNGCs) are a large family of proteins (Mäser et al. 2001). The results of this study showed that NtGNGC1 protein gene expression was down-regulated. Previously, Arazi et al. (1999) revealed the function of its homologous gene NtCBP4 in tobacco. Studies have also shown that transgenic tobacco with overexpression of NtCBP4 could improve Ni2 + tolerance and sensitivity to Pb2+.
Calcium-dependent protein kinases, (CDPKs) are involved in the regulation of many physiological processes and stress responses, such as in the fruit development of strawberry and apple (Nishiyama et al. 1999; Llop-Tous et al. 2002) and root system elongation in cucumber (Lanteri et al. 2006). The transcription of MsCPK3 was induced in alfalfa after 30 min of heat shock at 37 ℃ (Davletova et al. 2001); and McCPK1 in Mesembryanrhemum crystallimum is induced by salt and drought (Patharkar et al. 2000); meanwhile, plants overexpressing AtCPK23 are more sensitive to drought and salt stress (Anil et al. 2003). These results demonstrate that CDPK is involved in signal transduction pathways transmitting information about adversity. Transcriptome results showed that NtCDPK1 gene expression was down-regulated. NtCDPK1 expression is known to be limited to young tissues with rapidly dividing cells (Lee et al. 2003), and is downregulated as these tissues mature and differentiate. It may also regulate the division, differentiation, and death of plant cells (Lee et al. 2003). Research shows that NtCDPK1 is an interesting scaffolding kinase that increases the specificity and efficiency of signaling by coupling catalysis with scaffolding on the same prote (Ito et al. 2014). Furthermore, gibberellin can negatively regulate NtCDPK1 (Ito et al. 2018). NtCDPK1 is involved in feedback regulation of the plant hormone gibberellin through the phosphorylation of the transcription factor, REPRESSION OF SHOOT GROWTH (RSG). In our transcriptome results, we also detected the upregulated gene expression of gibberellin receptor protein GID1-C. This may indicate an association where NtCDOK1 and GID1-C can interact to co-regulate the growth of tobacco seedling.
Much evidence indicates that the downregulation of CaM /CMLs gene expression or loss of CaM /CMLs function can seriously affect the immune function of plants. The overexpression of CaM1 in pepper can activate the production of ROS and NO and induce the expression of HR and related defense genes to increase its local resistance to pathogens (Choi et al. 2009). Studies have shown that the tobacco CML protein RGS-CAM (regulator of gene silencing) plays a role in plant defense responses by regulating RNA silencing (Nakahara et al. 2012). SCaM-4 and SCaM-5 are transcriptionally activated by plant pathogenic bacterial infection or fungal elicitor application, indicating that they are involved in defenses against pathogen attacks (Heo et al. 1999). These studies also provide evidence for the involvement of CaM subtypes and CMLs in plant immunity. However, studies of the NtCaM7 protein gene downregulated in this trial showed that it can interact with CNGC14 to regulate Arabidopsis root hair growth (Zeb et al. 2020). Furthermore, it can also regulate trauma-induced responses together with other CaM proteins (Yamakawa et al. 2001).
RIN4 is a conserved plant immunomodulator, where pathogen-associated molecular patterns (PAMPs) (such as flagellin in bacteria and chitin in fungi) are perceived by pattern recognition receptors (PRRs) on the plant cell surface. This process causes PAMP to trigger immunity (PTI), which constitutes the first layer of plant defense response (Cyril et al. 2009). RIN4 is a negative regulator of PTI processes, and RIN4 mutants exhibit enhanced defense responses, while the opposite occurs in plants overexpressing RIN4 (Kim et al. 2005). Transcriptome analysis showed that the expression of the RIN4 protein gene in tobacco was down-regulated, suggesting that exogenous ABA might affect the disease resistance of tobacco.
Taken together, ABA promotes resistance in some plant-pathogen interactions and increases susceptibility in others (Ton et al. 2009). Therefore, the regulatory effect of exogenous ABA on disease resistance in tobacco seedlings is also complex. The results of this study also support this idea and provide candidate genes for further research.
Differentially expressed genes in the plant hormone signal transduction pathway
In the transcriptomic results, the expression of the genes of JAZ, AUX1, GID1, and other proteins changed, and the NtTIFY 10 A gene of the JAZ protein was downregulated. The TIFY family is a novel plant-specific protein family. The TIFY gene plays an important role in the jasmonate (JA) signaling pathway (Qi et al. 2014; Van et al. 2013), plant growth and development (Oh et al. 2013; Toda et al. 2013; Hakata et al. 2012), and pathogen response (Song et al. 2017; Ishiga et al. 2013). It has been shown that TIFY 10 A protein positively regulates plant alkaline stress response and negatively regulates the JA signaling pathway (Dan et al. 2014). Furthermore, (Qi et al. 2014) used various aspects of development and defense to unravel the mechanism of GA and JA signaling. Both DELLAs and JAZs interact with the WD-repeat/bHLH/MYB complex, mediating the synergistic and interdependent roles of GA and JA signaling in the regulation of plant trichome development. DELLAs and JAZs interact with bHLH factors (EGL3 and GL3) and important components of the MYB factor (GL1) to inhibit their transcriptional activity; however, GA and JA induce the degradation of DELLAs and JAZs. Through transcriptomic results, upregulation of the GID1-C protein gene, GID1, was identified, which is one of the three receptors for Arabidopsis gibberellin; it has special functions in both proteolytic and non-protein pathways (Hauvermale et al. 2014). DELLA protein plays a negative regulatory role in gibberellin signaling and degrades DELLA protein to promote plant growth and development. These results indicate that exogenous ABA may induce activation of JA signaling by downregulating gene expression of the NtTIFY 10 A protein, thus inducing the expression of plant defense genes. At the same time, the GID1-C gene was up-regulated, the DELLA protein was degraded, and the interaction with JA signal was used to jointly regulate plant growth.
The AUX1/LAX auxin influx vector is a transmembrane protein that is the main carrier of auxin influx and is involved in regulating key plant processes, including root and lateral root development, and root orientation (Swarup et al. 2019). Studies have shown that the rice auxin input vector gene OsAUX1 plays an important role in root development, and overexpression of OsAUX1 can promote lateral root development (Zhao et al. 2015). Meanwhile, overexpression of the MdAUX1 gene in tobacco can significantly promote plant growth (An et al. 2016). We hypothesized that exogenous ABA might regulate the growth of tobacco seedlings by upregulating the gene encoding the NtAUX1 protein.
Effects of exogenous ABA on the metabolome of tobacco seedlings
According to experimental results, after the application of exogenous ABA, the fresh and dry weight of tobacco seedlings above and underground were greatly increased compared with those in CK. From the metabolomic results, we detected L-phenylalanine, ketone; (toppinone), phenylalanine, and inositol phospholipase C 2-like, inositol. We first analyzed three metabolic pathways that showed significant differences upon ABA treatment. We detected the upregulation of scopolamine in the biosynthetic pathway of scopolamine, piperidine, and pyridine alkaloids. It has been reported (Rocha et al. 2013) that, in most cases, the leaves of transgenic plants overexpressing scopolamine show higher nicotine content than the leaves of control plants. We also detected the upregulation of L-phenylalanine in the phenylpropanine biosynthesis and metabolism pathway, as found by previous studies (Sewalt et al. 1997); the L-phenylalanine content was positively correlated with the content of lignin. In the metabolome results, the biosynthetic pathway of lignin monomers, including ferulic and eruinic acids, was found to be upregulated.
The alanine metabolic pathway is an important secondary metabolic pathway of flue-cured tobacco. Phenylalanine is also a precursor of the main polyphenol compounds in tobacco leaves. Polyphenols play an important role in tobacco growth and development and color, and are also an important factor for measuring tobacco quality. There are many factors affecting the synthesis of phenolic compounds, including variety and environmental conditions (Xu et al. 2003). The phenolic compounds produced by tobacco may also be a way to resist adverse environmental factors, with the increased activity of enzymes regulating phenolic synthesis in vivo and increased phenolic accumulation when tobacco is in an adverse environment (Zhou et al. 1996). Plant growth regulators also have a great impact on the synthesis and accumulation of polyphenols in tobacco. Studies have shown that spraying IAA, 2, 4-D, and GA on the surface of tobacco leaves can reduce the content of polyphenols in the upper leaves of flue-cured tobacco, while spraying ABA can increase the content of polyphenols (Xu et al. 2007). Amino acids are also one of the most important compounds in tobacco which affect the quality and flavor of tobacco leaves (Zhou et al. 1996; Yang et al. 1998). Some amino acids such as phenylalanine can be decomposed into benzyl methanol, phenylethanol, and other incense-causing precursor substances through chemical reactions (Hecht et al. 1977; Andersen et al. 1982; Gilchrist et al. 1980). Polyphenol compounds synthesized through the phenylalanine pathway can be oxidized by quinol oxidase to form quinone substances, and melanin substances can also be obtained by the Maillard reaction to affect the color of tobacco leaves (Xu et al. 2003). Therefore, from the metabolomic results, exogenous application of ABA may affect the synthesis of polyphenols in tobacco, leading to an increase in both the fresh and dry weight of tobacco, thus changing its quality and aroma.
Conclusions
Through a combined analysis of physiological, transcriptomics, and metabolomics data, the effects of different concentrations of exogenous ABA treatment on tobacco seedling growth were revealed. We found that exogenous ABA could change the content and size of chlorophyll in tobacco leaves and change chlorophyll fluorescence parameters, thus promoting the growth of tobacco seedlings. In transcriptome results, we found that exogenous ABA can regulate the growth of tobacco seedlings by affecting hormones. However, exogenous ABA may affect pathogen defense of tobacco seedling as well as resistance to abiotic stresses and may regulate the growth of tobacco seedling by affecting the synthesis of alkaloids and alkaloid precursors. To explore the effects of exogenous ABA on tobacco growth, this study screened some candidate genes and metabolic pathways, and verified seven candidate genes of interest. In qRT-PCR results, the seven genes showed similar patterns to those obtained by RNA-seq, demonstrating the reliability of this experiment. The collected data also provide a basis for future experiments.
References
Addicott FT (1983) Abscisic acid. Chin J Appl Chem 26(11):1297–1300
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant science: an international journal of experimental plant biology 171(3):382–388. https://doi.org/10.1016/j.plantsci.2006.04.009
An JP, Wang XF, Liu X, Hao-Hao LI, You CX, Hao YJ (2016) Molecular cloning and genetic transformation analysis of an apple auxin influx transporter gene MdAUX1 in Arabidopsis thaliana and tobacco. Bull Bot Res 36(6):902–908
Anandan R, Sudhakar D, Balasubramanian P et al (2012) In vitro somatic embryogenesis from suspensioncultures of Carica papaya L. Sci Hort 136:43–49. https://doi.org/10.1016/j.scienta.2012.01.003
Andersen RA, Kasperbauer MJ, Burton HR, Hamilton JL, Yoder EE (1982) Changes in chemical composition of homogenized leaf-cured and air-cured burley tobacco stored in controlled environments. J Agric Food Chem 30(4):663–668
Anil VS, Harmon AC, Rao KS (2003) Temporal association of ca(2+)-dependent protein kinase with oil bodies during seed development in Santalum album L.: its biochemical characterization and significance. Plant Cell Physiol 4367–376. https://doi.org/10.1093/pcp/pcg046
Arazi T, Sunkar R, Kaplan B, Fromm H (1999) A tobacco plasma membrane calmodulin-binding transporter confers Ni2 + tolerance and Pb2 + hypersensitivity in transgenic plants. Plant J 20(2):0960–7412. https://doi.org/10.1046/j.1365-313x.1999.00588.x
Bhaskara GB, Nguyen TT, Tsu-Hao Y, Verslues PE (2017) Comparative analysis of phosphor proteomeremodeling after short term water stress and aba treatments versus longer term water stress acclimation. Front Plant Sci 8(606). https://doi.org/10.3389/fpls.2017.00523
Boulesteix AL, Strimmer K (2007) Partial least squares: a versatile tool for the analysis of high-dimensional genomic data. Brief Bioinform 8(1):32–44. https://doi.org/10.1093/bib/bbl016
Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nuernberger T, Jones JDG et al (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448(7152):497–500. https://doi.org/10.1038/nature05999
Choi HW, Lee DH, Hwang BK (2009) The pepper calmodulin gene CaCaM1 is involved in reactive oxygen species and nitric oxide generation required for cell death and the defense response. Molecular plant-microbe interactions: MPMI. 111389–1400. https://doi.org/10.1094/MPMI-22-11-1389
Cui K, He CY, Zhang JG, Zeng YF (2012) Temporal and spatial profiling of internode elongation-associated protein expression in rapidly growing culms of bamboo. J Proteome Res 42492–2507. https://doi.org/10.1021/pr2011878
Curvers K, Seifi H, Mouille G, Rycke RD, Asselbergh B, Hecke AV et al (2010) Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistanceto botrytis cinerea. Plant Physiol 154(2):847–860. https://doi.org/10.1104/pp.110.158972
Cyril Zipfel (2009) Early molecular events in PAMP-triggered immunity. Current Opinion in Plant Biology(4):414 – 20. https://doi.org/10.1016/j.pbi.2009.06.003
Dan Z, Rongtian L, Xin L, Mingzhe S, Jing W, Ning Z et al (2014) The positive regulatory roles ofthe TIFY10 proteins in plant responses to alkaline stress. PLoS ONE 11e111984. https://doi.org/10.1371/journal.pone.0111984
Davletova S, Mészáros T, Miskolczi P, Oberschall A, Török K, Magyar Z, Dudits D, Deák M (2001) Auxin and heat shock activation of a novel member of the calmodulin like domain protein kinase gene family in cultured alfalfa cells. J Exp Bot 52(355):215–221
Dong H, Ma X, Zhang P, Wang H, Song CP (2020) Characterization of Arabidopsis thaliana root-related mutants reveals aba regulation of plant development and drought resistance. J Plant Growth Regul 1:1393–1401. https://doi.org/10.1007/s00344-020-10076-6
Dunn WB, Broadhurst D, Begley P, Goodacre (2011) Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 71060–1083. https://doi.org/10.1038/nprot.2011.335
Etehadnia M, Waterer DR, Tanino KK (2008) The method of ABA application affects salt stress responses in resistant and sensitive potato lines. J Plant Growth Regul 27(4):331–341. https://doi.org/10.1007/s00344-008-9060-9
Fan J, Hill L, Crooks C, Doerner P, Lamb C (2009) Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 150(4):1750–1761. https://doi.org/10.1104/pp.109.137943
Fang G, Wang YJ, Xie ZK, Gao YP, Guo ZH, Zhang YB et al (2010) The effects of inducers on growth, disease resistance and related enzymes activities of lily cut flower. Acta Horticulturae Sinica 37(5):763–768. https://doi.org/10.16420/j.issn.0513-353x.2010.05.027
Faseela P, Sinisha AK, Brestič M, Puthur JT (2020) Chlorophyll a fluore scence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 58:293–300
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 4:437–449. https://doi.org/10.1007/s11103-008-9417-2
Gao Z, Sun B, Chen Z, Zhai H, Yao Y, Du Y (2021) Phosphoproteomic analysis of ozone stress-responsive mechanisms in grapevine identifies KEG required for stress regulation. Plant Sci 311:111008. https://doi.org/10.1016/J.PLANTSCI.2021.111008
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12(7):1117–1126. https://doi.org/10.1105/tpc.12.7.1117
Gilchrist DG, Kosuge T (1980) Aromatic Amino Acid Biosynthesis and Its Regulation
Hakata M, Kuroda M, Ohsumi A, Hirose T, Nakamura H, Muramatsu M et al (2012) Over expressionof a rice tify gene increases grain size through enhanced accumulation of carbohydrates in the stem. Biosci Biotechnol Biochem 76(11):2129–2134. https://doi.org/10.1271/bbb.120545
Hauvermale AL, Ariizumi T, Steber CM (2014) The roles of the GA receptors GID1a, GID1b, and GID1c in sly1-independent GA signaling. Plant Signal Behav 2. https://doi.org/10.4161/PSB.28030
Hecht SS, Schmeltz I, D Hoffmann (1977) Nitrogenous compounds in cigarette smoke and their possible precursors. Rec. Adv Tob Sci 3:59–93
Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ, Park CY, Park HC, Choi JY, Cho MJ (1999) Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc Natl Acad Sci USA 96(2):766–771. https://doi.org/10.1073/pnas.96.2.766
Ishiga Y, Ishiga T, Uppalapati SR, Mysore KS (2013) Jasmonate ZIM-domain (JAZ) protein regulates host and nonhost pathogen-induced cell death in tomato and Nicotiana benthamiana. PLoS ONE 8(9):e75728. https://doi.org/10.1371/journal.pone.0075728
Ito T, Nakata M, Fukazawa J, Ishida S, Takahashi Y (2014) Scaffold function of Ca2+-Dependent protein kinase: Tobacco Ca2+-DEPENDENT PROTEIN KINASE1 transfers 14-3-3 to the substrate REPRESSION OF SHOOT GROWTH after Phosphorylation. Plant Physiol 165(4):1737–1750. https://doi.org/10.1104/pp.114.236448
Ito T, Ishida S, Takahashi Y (2018) Autophosphorylation of Ser-6 via an intermolecular mechanism is important for the rapid reduction of NtCDPK1 kinase activity for substrate RSG. PLoS ONE 13(4):e0196357. https://doi.org/10.1371/journal.pone.019635
Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL(2005). The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proceedings of the National Academy of Sciences of the United States of America 102(18):6496–6501. https://doi.org/10.1073/pnas.0500792102
Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57(6):1341–1351. https://doi.org/10.1093/jxb/erj109
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161(4):405–413. https://doi.org/10.1078/0176-1617-01239
Lee SS, Cho HS, Yoon GM, Ahn JW, Kim HH, Pai HS (2003) Interaction of NtCDPK1 calcium-dependent protein kinase with NtRpn3 regulatory subunit of the 26S proteasome in Nicotiana tabacum. The Plant journal: for cell and molecular biology 33(5):825–840. https://doi.org/10.1046/j.1365-313x.2003.01672.x
Li J, Zhang CL, Ma N et al (2012) Effects of ABA on photosynthetic characteristics of pods and Yieldof Brassicanapus. Agricultural Sci Technol 04760–762. https://doi.org/10.16175/j.cnki.1009-4229.2012.04.035
Liu YH (2009) Study on the photoprotective mechanisms in Limonium bicolor under salt stress (Master dissertation, Shandong Normal University). https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD2009&filename=2009125977.nh
Liu GF (2012) Efficiency of cucumber resistance induced by ABA to Pseudoperonospora cubensis. North Hortic. (02):146–148
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop ence 40(2):503–510. https://doi.org/10.2135/cropsci2000.402503x
Llop-Tous I, Domínguez-Puigjaner E, Vendrell M (2002) Characterization of a strawberry cDNA clone homologous to calcium-dependent protein kinases that is expressed during fruit ripening and affected by low temperature. J Exp Bot 53(378):2283–2285. https://doi.org/10.1093/jxb/erf103
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126(4):1646–1667. https://doi.org/10.1104/pp.126.4.1646
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8(4):409–414. https://doi.org/10.1016/j.pbi.2005.05.015
Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to pseudomonas Syringae pv tomato and peronospora parasitica. Funct Plant Biol 30(4):461–469. https://doi.org/10.1071/FP02231
Muhammad AA, Rizwan R, Iqbal H, Muhammad I, Muhammad ZH, Sadia P, Muhammad AS (2015) Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Arch Agron Soil Sci 61(4):507–523. https://doi.org/10.1080/03650340.2014.938644
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.0326
Nakahara KS, Masuta C, Yamada S, Uyeda I (2012) Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc Natl Acad Sci USA 109(25):10113–10118. https://doi.org/10.1073/pnas.1201628109
Nayak L, Panda D, Dash GK, Lal MK, Swain P, Baig MJ, Kumar A (2022) A chloroplast glycolate catabolic pathway bypassing the endogenous photorespiratory cycle enhances photosynthesis, biomass and yield in rice (Oryza sativa L). Plant Sci 314:111103. https://doi.org/10.1016/J.PLANTSCI.2021.111103
Nishiyama R, Mizuno H, Okada S, Ohyama K (1999) Two mRNA species encoding calcium-dependent protein kinases are differentially expressed in sexual organs of Marchantia polymorpha through alternative splicing. Plant Cell Physiol 40(2):205–212. https://doi.org/10.1093/oxfordjournals.pcp.a029529
Oh Y, Baldwin IT, Galis I (2013) A jasmonate zim-domain protein najazd regulates floral jasmonic acid levels and counteracts flower abscission in Nicotiana attenuata plants. PLoS ONE 8(2):e57868. https://doi.org/10.1371/journal.pone.0057868
Osborne DJ, Morgan PW (1989) Abscission. CRC Crit Rev Plant Sci 8(2):103–129
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollution Res 22(6):4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Patharkar OR, Cushman JC (2000) A stress-induced calcium‐dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two‐component pseudo‐response regulator. Plant J 24(5):679–691. https://doi.org/10.1046/j.1365-313x.2000.00912.x
Qi T, Huang H, Wu D, Yan J, Qi Y, Song S et al (2014) Arabidopsis della and jaz proteins bind the wd-repeat/bhlh/myb complex to modulate gibberellin and jasmonate signaling synergy. Plant cell. Plant Cell 26(3):1118–1133. https://doi.org/10.1105/tpc.113.121731
Rocha PS, Olaf P, Adrian W, Nicholas C, Paul D, Birgit LM (2013) Functional expression of tropinone reductase I (trI) and hyoscyamine-6 beta-hydroxylase (H6H) from Hyoscyamus niger in Nicotiana tabacum. Plant Science. 162:905-913.10.1016/S0168-9452(02)00033-X
Sairam R, Srivastava G, Saxena D (2000) Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biol Plant. 2https://doi.org/10.1023/A:1002756311146
Sewalt V, Ni W, Blount JW, Dixon RA (1997) Reduced lignin content and altered lignin composition in transgenic Tobacco Down-Regulated in expression of L-Phenylalanine Ammonia-lyaseor cinnamate 4-Hydroxylase. Plant Physiol 115(1):41–50. https://doi.org/10.1104/pp.115.1.41
Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S et al (2013) Abi4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9(6):e1003577. https://doi.org/10.1371/journal.pgen.1003577
Shu K, Chen Q, Wu Y, Liu R, Zhang H, Wang S, Tang S, Yang W, Xie Q (2016a) ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. J Exp Bot 67(1):195–205. https://doi.org/10.1093/jxb/erv459
Shu K, Liu XD, Xie Q, He ZH (2016b) Two faces of one seed: hormonal regulation of dormancy andgermination. Mol Plant 9(001):34–45. http://doi.org/10.1016/j.molp.2015.08.010
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2(6):503–512. https://doi.org/10.1105/tpc.2.6.503
Song S, Qi T, Fan M, Zhang X, Gao H, Huang H et al (2017) The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet 9(7):e1003653. https://doi.org/10.1371/journal.pgen.1003653
South PF, Cavanagh AP, Liu HW, DR Ort (2019) Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363(6422):45–45. https://doi.org/10.1126/science.aat
Su YX (2011) Molecular basis of ABA function in Arabidopsis somatic embryogenesis(Doctoral dissertation, Shandong Agricultural University).https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD2011&filename=1011097642.nh
Swarup R, Bhosale R (2019) Developmental roles of AUX1/LAX auxin influx carriers in plants. Front Plant Sci 10:1306. https://doi.org/10.3389/fpls.2019.01306
Tari I, Guóth A, Benyó D, Kovács J, Poór P, Wodala B (2010) The roleS of ABA, reactive oxygen species and nitric oxide in root growth during osmotic stress in wheat: comparison ofa tolerant and a sensitive variety. Acta Biol Hung 61:189–196. https://doi.org/10.1556/ABIOL.61.2010.SUPPL.18
Teng Y, Yu A, Tang YM, Jiang ZY, Guan WJ, Li ZS, Zou LY (2021) Visualization and quantification of cadmium accumulation, chelation and antioxidation during the process of vacuolar compartmentalization in the hyperaccumulator plant Solanum nigrum L. Plant Sci 310:110961. https://doi.org/10.1016/J.PLANTSCI.2021.110961
Toda Y, Tanaka M, Ogawa D, Kurata K, Takeda S (2013) Rice salt sensitive3 forms aternary complexwith jaz and class-c bhlh factors and regulates jasmonate-induced gene expression and root cell elongation. Plant Cell 25(5):1709–1725. https://doi.org/10.1105/tpc.113.112052
Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. The Plant journal: for cell and molecular biology 38(1):119–130. https://doi.org/10.1111/j.1365-313X.2004.02028.x
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of aba in disease resistance. Trends Plant Sci 14(6):310–317. https://doi.org/10.1016/j.tplants.2009.03.006
Van Leon-Reyes dDD, Koornneef A, Van Verk A, Rodenburg MC N, Pauwels L et al (2013) Salicylic acid suppresses jasmonic acid signaling downstream of scfcoi1-jaz by targeting gcc promoter motifs via transcription factor ora59. Plant Cell 25(2):744–761. https://doi.org/10.1105/tpc.112.108548
Veselov DS, Sharipova GV, Veselov SU, Kudoyarova GR (2008) The effects of NaCl treatment on water relations, growth, and ABA content in barley cultivars differing in drought tolerance. J Plant Growth Regul 27(4):380–386. https://doi.org/10.1007/s00344-008-9064-5
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperons in the abiotic stress response. Trends Plant Sci 9(5):244–252
Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, Holmes E, Nicholson JK (2010) Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc 5(6):1005–1018. https://doi.org/10.1038/nprot.2010.50
Winfield MO, Lu C, Wilson ID, Coghill JA, Edwards KJ (2010) Plant responses to cold: transcriptome analysis of wheat. Plant Biotechnol J 8(7):749–771. https://doi.org/10.1111/j.1467-7652.2010.00536.x
Xu X (2003) Synthesis of polyphenolic compounds and their effects on to bacco quality. Chin Tob Sci, (01):3–5
Xu X, Li WU, Zhang FL (2007) Effects of plant growth regulator on contents of endogenous hormones, nicotine, and polypheno in flue-cured tobacco. Acta Tabacaria Sinica, (03):61–63
Yamakawa H, Mitsuhara I, Ito N, Seo S, Kamada H, Ohashi Y (2001) Transcriptionally and post-transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus-induced cell death and wounding in tobacco plant. Eur J Biochem 268(14):3916–3929. https://doi.org/10.1046/j.1432-1327.2001.02301.x
Yang DL, Wang SS, Wang BH, Li XZ (1998) Research progress of amino acid changes in tobacco and its relationship with tobacco quality. Chin Tob Sci. (03):13–15
Yang YH, Zhang YD, Zhu Z, Zhao L, Chen T, Zhao QY et al (2010) Effects of gibberellic acid 3 and abscisic acid on growth, physiological characteristics and gene expression of ga20ox2 and ga3ox2 in different rice cultivars. Chin J Rice Sci 24(4):433–437
Yao C, Zhang F, Sun X et al (2019) Effects of S-Abscisic acid (S-ABA) on seed germination, Seedling Growth, and Asr1 gene expression under Drought stress in Maize. J Plant Growth Regul 438:1300–1313. https://doi.org/10.1007/s00344-019-09934-9
Zeb Q, Wang X, Hou C, Zhang X, Dong M, Zhang S, Zhang Q, Ren Z, Tian W, Zhu H, Li L, Liu L (2020) The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J Integr Plant Biol 62(7):887–896. https://doi.org/10.1111/jipb.12890
Zhao H, Ma T, Wang X, Deng Y, Ma H, Zhang R, Zhao J (2015) OsAUX1 controls lateral root initiation in rice (Oryza sativa†L). Plant Cell Environ 38(11):2208–2222. https://doi.org/10.1111/pce.12467
Zhou JH, Zhu XP, Wang YP et al (1996) Physiology and biochemistry of tobacco. University of Science and Technology of China Press
Acknowledgements
This research was supported by the Post-doctoral Innovation Project of Shandong Province and the Special Science and Technology Project of Guizhou (202309).
Author information
Authors and Affiliations
Contributions
DJH performed the experiment and analyzed the data, DJH and JQ drafted the manuscript. JXY, WY, LLT, DZL and WJ initiated the project, designed the experiments and reviewed the manuscript. JXY and ZFW instructed laboratory work. All authors read and approved the manuscript.
Corresponding author
Additional information
Communicated by Longbiao Guo.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, J., Jiao, Q., Wang, Y. et al. Transcriptomic and metabolomic effects of exogenous ABA application on tobacco seedling growth. Plant Growth Regul 101, 399–414 (2023). https://doi.org/10.1007/s10725-023-01026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01026-9