Abstract
The objective of this study was to evaluate the ability of the phytohormone S-abscisic acid (S-ABA) to protect maize seedlings grown under drought stress and to measure their increased drought tolerance. The maize hybrids ‘Zhengdan 958’ (ZD958; drought tolerant) and ‘Xundan 20’ (XD20; drought sensitive) were treated with nutrient solutions of different concentrations (1, 2, 4, 8, and 10 mg/kg) of S-ABA under polyethylene glycol (PEG, 15% w/v, MW 6000) simulated drought stress. Optimal concentrations of S-ABA were designed to be sprayed onto the leaves of seedlings, and their effect on endogenous ABA, malondialdehyde (MDA), osmotic substances, antioxidant enzyme activities, and Asr1 gene expression in seedlings were studied. Results indicated that, under drought stress, S-ABA treatment significantly improved maize seed germination rate (GR), germination energy (GE), and seedling biomass (p < 0.05). After spraying 4 mg/kg S-ABA onto leaves, the endogenous hormone ABA, osmotic substances, antioxidant enzyme activities, and expressive quantity of the Asr1 gene were extended and MDA content dropped significantly (p < 0.05). Moreover, ZD 958 endogenous ABA content, osmotic substances content, antioxidant enzyme activity and Asr1 gene expressive quantity were higher than that of XD 20 (p < 0.05). In conclusion, S-ABA treatment increased the content of endogenous ABA, induced an increase in antioxidant enzyme activity and Asr1 gene expression level, reduced the oxidative damage caused by drought to maize leaves, and improved the adaptability of maize seedlings to withstand drought stress. The promoting effect of S-ABA on the drought-tolerant variety ZD 958 was more obvious (p < 0.05). These results serve as a reference for the use of S-ABA in mitigating drought stress in maize.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is an important topic that urgently needs to be addressed in the light of rising global temperatures. Only four-fifths of global arable land has natural precipitation as its main source of water (Turner 2004) and of this, more than 30% falls in arid or semi-arid areas, spread over many countries and regions (Zhao et al. 2007). It is one of the major constraints to the cultivation of maize (Zea mays L.) crops.
In China, arid and semi-arid areas account for about half of the country’s land area and these face the threat of periodic drought (Leng et al. 2015). Maize is one of the most important cereals grown globally. Normally, the crop needs 500–800 mm of water during its life cycle (80–110 days). The consequences of drought stress in crop production systems are perhaps more deleterious than abiotic stress under changing climatic scenarios, which seriously restrict maize yield (Badu-Apraku et al. 2013). The Huang-Huai-hai area is an important region for the planting of summer maize, and here the occurrence of drought stress during the growing period may hamper water-use efficiency leading to significant yield losses.
Throughout evolution, plants have established complicated self-defense mechanisms, including antioxidant defense systems such as superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), peroxidase (POD, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11), polyphenol oxidase (PPO, EC 1.10.3.2), and phenylalanine ammonia lyase (PAL, EC 4.3.1.5), and non-antioxidant defense systems such as Pro, soluble protein, and so on (Ge et al. 2006; Hawrylak-Nowak et al. 2018; Tan et al. 2011). Many studies have demonstrated a correlation between the activity of these protective enzymes and water stress (Srivalli et al. 2010). At the same time, drought stress induces the expression of related genes in plants. Abscisic acid-stress-ripening-induced (ASR) proteins are a group of plant-specific proteins that are hydrophilic with a low molecular weight and are considered to be transcription factors that were first discovered in tomato plants. Genes of the Asr family were characterized by their strong induction under abiotic stress and ABA signaling. Their members harbor the ABA/WDS (abscisic acid/water deficit stress) domain as a common denominator (Virlouvet et al. 2011). The function of the Asr gene is mainly to regulate plant growth and development, senescence, fruit ripening, glucose metabolism, and response to stress (Dominguez et al. 2013; Iusem et al. 1993; Yang et al. 2008). Under water stress, Asr1 overexpression in maize plants increased yield, which suggests that Asr1 participates in plant growth through the regulation of kind of metabolites (Virlouvet et al. 2011). In a wide variety of species including lily, maize, rice, tobacco, and banana, the expression of Asr1 can be induced by ABA, thereby enhancing crop drought tolerance (Hu et al. 2013; Joo et al. 2013; Virlouvet et al. 2011; Yang et al. 2005). S-Abscisic acid (S-ABA; scientific name: abscisic acid) is an endogenous plant hormone with important physiological activities. Recent research has found that S-ABA is an “anti-hard environments inducing factor” that can induce a resistance in plants against harsh environments, such as drought and other stressful situations.
To increase crop yields in water-deficit areas, it is crucially important to induce drought tolerance in plants and several agronomical and physiological practices have been developed to achieve this goal. The effects of drought stress on growth and development, eco-physiological characteristics, and the expression levels of various genes in maize have been studied by many scholars in the recent years. Du et al. (2013) indicate that the application of ABA can reduce the rate of transpiration loss and reduce production. The use of S-ABA hormone before heading and flowering to alleviate heat damage to rice under drought conditions is reported by Gao et al. (2015). Li et al. (2010) show that the application of ABA can increase antioxidant enzyme activity in sugarcane leaves, reduce the content of H2O2 and MDA, reduce membrane lipid peroxidation, and then enhance the antioxidant defense system of sugarcane and improve drought resistance. Ghosh et al. (2015) show that the ASR gene is present in the potato and its expression is induced by the stress of dehydration and abscisic acid. Sugiharto et al. (2002) report the cloning of the ASR gene in sugarcane, and their results show that the expression of this gene is induced by ABA treatment. The ability of S-ABA to increase tolerance to drought stress has been reported in rice, cotton, and wheat (Li et al. 2017; Travaglia et al. 2007; Yan et al. 2014). In spite of this, there have been relatively few studies on the regulation of drought resistance of maize by S-ABA and very little is known about the interaction between S-ABA and drought-tolerant maize genotypes or drought-sensitive maize genotypes.
In this context, the objective of this study was to evaluate the possible roles of the exogenous application of ABA for increasing drought tolerance in drought-tolerant and drought-sensitive maize hybrids based on Asr1 gene-expressive quantity and physiological changes such as endogenous ABA and antioxidant enzyme activity.
Materials and Methods
The concentrated (98%) S-ABA pesticide used in this study was obtained from Sichuan Dragon Python Fusheng Technology Co. Ltd. Aliquots of 0.5102 g of 98% S-ABA were dissolved in acetone to a constant volume of 50 ml in a volumetric flask to obtain the mother liquor A at a concentration of 10,000 mg/kg. Volumes of 1 ml of mother liquor A were placed into 100 ml volumetric flasks using a pipette gun (Eppendrof), and the volume was kept constant by the addition of 0.1% Tween 80. Finally, this solution was diluted to obtain 1, 2, 4, 8, 10 mg/kg of S-ABA.
Experimental Setup and Stress Conditions
Maize (Zea mays L.) seeds were surface-sterilized with 10% NaClO for 10 min and rinsed thoroughly with distilled water then placed on neutral filter paper to dry. Under the conditions simulated in 15% PEG-6000 experiments, 1, 2, 4, 8, and 10 mg/kg (active principle) concentrations of the commercial raw pesticide S-ABA were prepared in which to soak the seeds. After soaking for 10 h, the seeds were removed and placed into a 15 cm Petri dish with two layers of filter paper, setting blank controls for drought and using distilled water; these were named CK2 and CK1, respectively. The seeds were grown in a 28 °C constant temperature incubator. For this purpose, an experiment was carried out with three replications and 30 seeds in each petri dish. The number of seeds that germinated from the third day was counted, and the germination energy (GE) and germination rate (GR) were calculated. At the end of the eighth day of the germination test, ten plants were sampled randomly to measure the root length, shoot length, and the dry and fresh weight of roots and shoots. Before recording the plant dry biomass, harvested plants were cut into pieces and placed in an oven at 80 °C until a constant weight was achieved.
To study the physiological effects of S-ABA on maize seedlings under drought conditions, a sand culture was incubated in a constant-temperature incubator at 28 °C, and Hoagland nutrient solution was used during the process (Hoagland 1950). When the third leaves of maize plants were fully expanded, hydroponics models were constructed using three groups: group A (Hoagland), group B (Hoagland + PEG), group C (Hoagland + PEG + 4 mg/kg S-ABA). Samples were taken at 2, 4, 8, 12, and 24 h after treatment; they were washed with double-distilled water and then frozen in liquid nitrogen for 2 min and vacuum freeze-dried before being stored at − 80 °C until analysis.
Extraction and Quantification of Leaf ABA
The method for the extraction of leaf ABA was adapted from the study of Wang et al. (1998). Five hundred milligrams of leaf tissue were macerated in liquid nitrogen and mixed with 5 ml 80% (v/v) methanol containing 0.1% butylated hydroxytoluene (BHT) as the antioxidant. The extract was incubated at 4 °C for 4 h and subsequently centrifuged for 15 min at 12,000×g at the same temperature. The supernatant fluid was collected, and an immunoassay was carried out according to the ELISA kit procedure (the ABA Enzyme Immunoassay Test Kit was provided by China Agricultural University).
Determination of Proline Content
Free proline content was measured using ninhydrin colorimetry (Abraham et al. 2010). Fresh maize leaf material (0.5 g) from each replicate within each treatment was triturated in 5 ml of 3% sulfosalicylic acid solution. Two milliliters of the filtrate was reacted with 3 ml acid ninhydrin solution and 3 ml of glacial acetic acid in a test tube; the sample mixture was then incubated at 100 °C for 60 min. After cooling, 4 ml of toluene was added to the mixture and it was mixed vigorously for 1–2 min. The absorbance of the colored portion containing toluene was read at 520 nm using toluene as the blank.
Estimation of Soluble Sugar Content
Leaf total soluble sugar concentrations were evaluated at 620 nm using a spectrophotometer according to the anthrone method (Fales 1951). Fresh leaves (0.5 g) were put into 15 ml of distilled water and boiled in a water bath for 20 min. After cooling, 5 ml of anthrone was added to 0.1 ml of boiled sample. Then, 3 ml of boiled sample was transferred to a cuvette and the absorbance was read. Finally, the amount of total soluble sugar in the samples was quantified using a standard glucose curve.
Determination of Hydrogen Peroxide Content
Hydrogen peroxide (H2O2) content in leaves was determined according to the method of Prasad et al. (1994). Samples (0.5 g) were homogenized in an ice bath with 2 ml of 0.1% (w/v) trichloroacetic acid. The homogenate was centrifuged at 10,000×g for 10 min, and 1 ml of the supernatant was added to 1 ml of 10 mM potassium phosphate buffer (pH 7.0) and 2 ml of 1 M KI. The absorbance of the supernatant was measured at 390 nm.
Estimation of Malondialdehyde Content
Malondialdehyde (MDA) content of the maize leaves was analyzed using the thiobarbituric acid (TBA) method (Cakmak and Horst 2006). Fresh leaf samples (0.2 g) were triturated in a 10 ml solution of 0.1% TCA. The sample was then centrifuged at 12,000×g for 10 min, and the supernatant was separated. To 1 ml of the supernatant, 4 ml of 20% TCA solution was added, which contained 0.5% TBA solution. Subsequently, the sample was heated in a water bath at 95 °C for 40 min and then cooled immediately on ice for 5 min. The cooled samples were centrifuged for 15 min at 3000×g at 4 °C, and then the absorbance of the supernatant was read at 532 nm, 600 nm, and 450 nm.
Enzyme Extraction and Activity
Enzyme extraction was carried out by immersing 0.5 g of leaves in liquid nitrogen, to which was added 10 ml of extraction buffer made up of 0.05 M potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 0.3% Triton-X100, and 4% polyvinylpyrrolidone (PVP); this was repeated three times. The extract was centrifuged at 15,000×g for 20 min at 4 °C, and the supernatant was collected and stored at − 80 °C during the analysis period. The collected supernatants were used in all enzyme analyses. The activity of enzymes was expressed in milligrams (mg) of proteins, which were determined by the Bradford (1976) method, using the standard curve of bovine serum albumin (BSA).
The activity of superoxide dismutase (SOD, EC 1.15.1.1) was evaluated by its ability to inhibit the photoreduction of nitroblue tetrazolium (NBT), using the method of Giannopolitis and Ries (1977). Measurements were taken at 560 nm and one unit of SOD corresponded to the amount of enzyme capable of inhibiting 50% of NBT photoreduction under experimental conditions. Catalase activity (CAT, EC 1.11.1.6) was determined by H2O2 consumption at 240 nm during 3 min (Havir and McHale 1987). Peroxidase (POD, EC 1.11.1.7) activity was determined by the guaiacol method (Lin and Wang 2002). The absorbance of the reaction was read at 420 nm with a 30 s interval up to 2 min; this used the absorbance change 0.01 as a POD activity. Ascorbate peroxidase activity (APX EC 1.11.1.11) was assayed according to Nakano and Asada (1981). The change in absorbance after adding H2O2 was read at 290 nm for 2 min at 30 s intervals. Polyphenol oxidase activity (PPO, EC 1.10.3.2) was assessed through the method proposed by Mohammadi and Kazemi (2002) with some modifications. The activity of phenylalanine ammonia lyase (PAL, EC 4.3.1.5) was determined by the formation of cinnamic acid at 290 nm (Cahill and McComb 1992).
RT-qPCR
Total RNA was extracted using the Ominiplant Plant Kit (DNase I) (CWBIO) from samples homogenized in liquid nitrogen using a pestle and mortar (2 × 3 repetitions). cDNA was synthesized using a HiFiScript cDNA Synthesis Kit (CWBIO). cDNA (20× diluted) was mixed with the UltraSYBR Mixture (Low ROX; CWBIO) and 200 nM of respective primers to a final volume of 25 µl. The RT-qPCR was performed using a LightCycler® 96 (Roche Applied Science). The qPCR program was set to an initial denaturation (10 min, 95 °C), followed by 40 cycles of primer denaturation (15 s, 95 °C), annealing, and elongation (60 s, 60 °C). The relative content of RNA was calculated according to the method of Hellemans et al. (2007). EF1α was used as the reference gene. Primers were designed according to sequences retrieved from the NCBI database (Lamesch et al. 2012) using the Primer3 program (Untergasser et al. 2007). Primer sequences are shown in Table S1.
Statistical Analysis
Data were analyzed using the Microsoft Excel 2010 and SPSS 20.0 statistical packages. Origin 2018 was used for graphical presentation of the data. A factorial experiment based on randomized complete block design was carried out with three replicates (n = 3). Duncan’s multiple range test (p < 0.05) was used to compare the means.
Results
Effects of S-ABA Seed Soaking on the Biological Character of Maize Seeds Under Drought Stress
Compared with CK1, drought stress significantly inhibited germination in the two maize varieties. The GR and GE of XD20 and ZD958 decreased by 23.19%, 18.81%, 41.96%, and 22.09%, respectively. Under drought conditions, seed soaking with different concentrations of S-ABA could increase the GR and GE of seeds; furthermore, with an increase in soaking concentration, the trend first increased then decreased (Fig. 1). For GR, all treatments (except the 10 mg/kg treatment) of ZD958 reached significant levels compared to CK2, whereas XD20 reached significant levels only at concentrations of 1, 2 and 4 mg/kg (p < 0.05). These results suggest that soaking seeds with suitable concentrations of S-ABA can effectively mitigate the effect of drought stress on the germination of maize seeds, with the concentration of 4 mg/kg being optimum (p < 0.05; Fig. 1).
Effects of S-ABA seed soaking on a germination rate and b germination energy of maize seeds under drought stress. Means followed by the same letters for the treatments do not differ according to Duncan’s test at 5% significance (p < 0.05). Capped bars above means represent ± SE of three replicates. Small alphabetical letters above means denote the significant differences among treatment within two maize hybrids at p < 0.05. CK1, control (irrigate); CK2, control (drought)
Effects of S-ABA Treatment on the Growth of Maize Seedlings Under Drought Stress
Regarding water stress, root and bud growth of both varieties was drastically reduced; this was expressed as a sharp decrease in root and shoot fresh and dry weight, although the drought stress on ZD958 was less evident (p < 0.05). Under drought stress, the root length, bud length, lateral root number, root fresh weight, bud fresh weight, root dry weight, and bud dry weight of the two maize varieties when soaked with S-ABA were significantly increased (p < 0.05). Analysis of variance results showed that soaking the seeds in different concentrations of S-ABA mitigated the effect of drought stress on maize seedlings to varying degrees. When compared with the drought control, the treatment of S-ABA at the concentration of 4 mg/kg was most significant (p < 0.05) (Table 1). Meanwhile, the results showed that 4 mg/kg of S-ABA was most effective for alleviating drought (p < 0.05). Compared to CK2, the average root length, bud length, lateral root number, root fresh weight, bud fresh weight, root dry weight, and bud dry weight of ZD958 increased 48.97%, 82.41%, 38.62%, 44.14%, 73.96%, 16.18%, and 73.68%, respectively; whereas those of XD20 increased 35.62%, 77.63%, 31.18%, 11.81%, 23.37%, 14.28%, and 35.29%, respectively. Moreover, the results from ZD958 were markedly superior to those of XD20 (p < 0.05).
Effects of S-ABA Treatment on Endogenous ABA Content in Maize Leaves Under Drought Stress
In all treatments, analysis of variance showed that there was essentially no difference in endogenous ABA content between the two cultivars under the normal watering treatment, whereas endogenous ABA content in the leaves of the two maize cultivars treated with PEG and PEG + ABA presented a sharp rise (p < 0.05). After treatment for 8 h, the ABA content of endogenous hormone in ZD958 reached its maximum, which was 84.59% higher than that in the drought control. The ABA content of endogenous hormone in XD20 reached a maximum after treatment for 24 h, which was an increase of 75.80% (Table 2).
Effects of S-ABA Treatment on Hydrogen Peroxide (H2O2) Content in Maize Leaves Under Drought Stress
Compared with the control, the treatment with PEG and PEG + ABA significantly increased the H2O2 content; the PEG + ABA treatment showed the highest H2O2 content, and the treatment of XD20 showed greater sensitivity (p < 0.05). After 12 h of stress, XD20 + PEG + ABA showed the highest content of H2O2 (Table 3). The ZD958 + PEG + ABA exhibited significantly higher H2O2 content at 8 h under water stress, followed by XD20 + PEG, and ZD958 + PEG. There was no difference among the irrigation treatments (p < 0.05).
Effects of S-ABA Treatment on MDA Content of Maize Leaves Under Drought Stress
Malondialdehyde level in maize leaves showed a significant tendency to increase in both the PEG and PEG + ABA treatments compared with the control group (p < 0.05). However, MDA in XD20 leaves increased faster than that in ZD958 leaves (p < 0.05). The MDA of maize in the PEG and PEG + ABA groups was higher than that in the control group in the 2 h treatment, but the difference was insignificant (p < 0.05). From 4 to 24 h, there was a significant difference (elevation) in MDA content between maize leaves treated with drought and normal growth; however, the rate of increment was higher in XD20 at 12 h (p < 0.05). Results also showed that MDA content reached a maximum at 24 h. After ABA treatment, the decrease in MDA content in leaves of the two maize cultivars also reached a significant level after 12 h and 24 h of stress (p < 0.05; Table 4).
Effects of S-ABA Treatment on Osmotic Substance Content in Maize Leaves Under Drought Stress
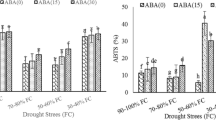
As a result, the proline content showed an increasing tendency with stress time and the difference was significant in each time period (p < 0.05). After treatment with S-ABA for 4 h, the difference in treatment between PEG + ABA and PEG was significant. The proline content of ZD958 was higher than that of XD20 in all treatments (p < 0.05; Fig. 2a). After treatment for 24 h, the content of proline in the PEG + ABA treatment reached a maximum; ZD 958 increased by 28.23% compared with the PEG treatment, and XD20 increased by 27.41%.
Effects of S-ABA treatment on a proline and b soluble sugar content in maize leaves under drought stress. Means followed by the same letters for the treatments do not differ according to Duncan’s test at 5% significance (p < 0.05). Capped bars above means represent ± SE of three replicates. Small alphabetical letters above means denote the significant differences among treatment with in two maize hybrids at p < 0.05
Analysis of variance showed that drought stress increased the soluble sugar content of maize leaves, and the application of exogenous ABA further increased the soluble sugar content. There were no significant differences between all experiments after 2 h of treatment (p < 0.05). After 8 h of stress, the soluble sugar content of XD20 + PEG + ABA treatment peaked. After 12 h of stress, ZD958 + PEG + ABA exhibited the highest soluble sugar contents followed by the treatments XD20 + PEG, ZD958 + PEG, and XD958 + PEG + ABA. With increasing time (24 h), there was an inversion in the results, with XD20 + PEG + ABA showing a significantly higher content, followed by XD20 + PEG (Fig. 2b).
Effects of S-ABA Treatment on Enzyme Activity in Maize Leaves Under Drought Stress
The increase in drought stress time resulted in higher enzyme activity in both ZD958 and XD20. However, with normal watering there was no significant change (p < 0.05). With increasing stress time, the activity of protective enzymes (including SOD, CAT, POD, APX, PPO, and PAL) in the leaves initially rose and then declined (Figs. 3, 4).
Effects of S-ABA treatment on the activities of a superoxide dismutase (SOD), b catalase (CAT), c peroxidase (POD) and d ascorbate peroxidase (APX) maize leaves under drought stress. Means followed by the same letters for the treatments do not differ according to Duncan’s test at 5% significance (p < 0.05). Capped bars above means represent ± SE of three replicates. Small alphabetical letters above means denote the significant differences among treatment with in two maize hybrids at p < 0.05
Effects of S-ABA treatment on the activities of a polyphenol oxidase (PPO) and b phenylalanine ammonia lyase (PAL) maize leaves under drought stress. Means followed by the same letters for the treatments do not differ according to Duncan’s test at 5% significance (p < 0.05). Capped bars above means represent ± SE of three replicates. Small alphabetical letters above means denote the significant differences among treatment with in two maize hybrids at p < 0.05
No significant differences were observed in the analysis of SOD activity among treatments during the first 2 h of stress (Fig. 3a). After the fourth hour of stress, there was a significant increase in SOD activity in the PEG and PEG + ABA treatments of ZD958 (p < 0.05). Furthermore, the activity of SOD peaked at 12 h, and was 19.71% higher than that of the PEG control. However, XD20 showed a significant difference after 12 h of treatment; SOD activity peaked after 8 h of treatment, which was a 12.74% increase compared with the PEG control (p < 0.05; Fig. 3a).
Under 2 h of drought stress, S-ABA application led to an increase in CAT activity in the leaves of ZD958 under water stress conditions; CAT activity was higher in ZD958 than in XD20 (p < 0.05). The CAT activity of ZD958 gradually increased and peaked at 12 h, which was an increase of 91.47% over the drought control (p < 0.05). However, the CAT activity of XD20 + PEG + ABA was lower than that of XD20 + PEG in the first 4 h stress treatment. XD20 showed a significant difference only after 8 h and 24 h. CAT activity reached a maximum at 24 h of stress, which was an increase of 307.42% compared with the XD20 + PEG treatment. After 24 h of stress, ZD958 + PEG + ABA increased by 401.40% compared with ZD958 + PEG. Furthermore, the CAT activity of XD20 initially increased and then decreased and finally increased again after treatment with S-ABA (p < 0.05; Fig. 3b).
In the evaluation of POD enzyme activity, the treatments showed no obvious differences at 4 h of stress (Fig. 3c). At 12 h in the stressed treatment, ZD958 showed superior POD activity with S-ABA application and an increase of 52.89% compared to the PEG + ZD958 treatment (p < 0.05). After 24 h of stress, S-ABA application also elevated the POD enzyme activity of XD20 + PEG + ABA to values similar to those of ZD958 + PEG + ABA. In addition, the treatment XD20 + PEG + ABA showed a POD activity increase of 35.02% compared with XD20 + PEG (Fig. 3c).
After 2 h of stress, the treatments PEG and PEG + ABA of the two maize cultivars had greater activity of APX enzyme than under the remaining treatments. In addition, the PEG + ABA treatment differed significantly (p < 0.05). ZD958 had the highest activity of APX enzyme after treatment for 12 h, which was 72.22% higher than that of the drought treatment. These figures for XD20 peaked after treatment for 4 h, an increase of 53.33%.
After stress treatment for 12 h, the stressing treatments (PEG and PEG with ABA application) for both hybrids produced significantly more pronounced PPO activity than the irrigated treatments (p < 0.05). After 2 h of stress treatment, the difference between the treatments was not significant. After 4 h of stress treatment, the application of S-ABA significantly increased the PPO enzyme activity of the two maize varieties. After 24 h of stress, XD20 + PEG + ABA and ZD958 + PEG + ABA showed greatly elevated means; compared with the PEG treatment, they increased by 32.27% and 29.41%, respectively (Fig. 4a).
Regarding PAL means activity, there were no significant differences among the treatments given 2 h of water stress (p < 0.05). After 4 h of stress treatment, the application of S-ABA showed significant differences. ZD958 + PEG + ABA had the highest activity of PAL enzyme at 12 h, which was 64.54% higher than that in the PEG treatment (p < 0.05). However, XD20 + PEG + ABA had the highest activity of PAL enzyme at 8 h, which was 29.98% higher than that in the PEG treatment (p < 0.05; Fig. 4b).
Effects of S-ABA Treatment on Asr1 Gene-Expressive Level in Maize Leaves Under Drought Stress
Water stress increased the gene expressive level of Asr1 in both cultivars. Compared with drought stress, the expression levels of Asr1 in the two varieties after exogenous application of S-ABA were up-regulated at different time periods (p < 0.05). Among them, the gene expressive level of XD20 Asr1 was the highest after the 12-h treatment with S-ABA, whereas that of ZD958 reached a maximum at 24 h (p < 0.05). In contrast, the increase of Asr1 expression level in ZD958 was greater than that in XD20 (Fig. 5).
Effects of S-ABA treatment on Asr1 gene-expressive quantity in maize leaves under drought stress. Means followed by the same letters for the treatments do not differ according to Duncan’s test at 5% significance (p < 0.05). Capped bars above means represent ± SE of three replicates. Small alphabetical letters above means denote the significant differences among treatment with in two maize hybrids at p < 0.05
Discussion
Water stress is one of the most serious environmental factors limiting plant growth and development. The relationship between ABA accumulation and stress resistance under stress conditions has become an important area of stress research. Our results indicated that the GE, GR, and seedling biomass of the two maize varieties decreased significantly under drought stress (p < 0.05). This observation agrees well with the results of Yongsheng et al. (2016). Abscisic acid is an important phytohormone, not only because of its regulatory functions in physiological and developmental processes but also because it is the main regulator in adaptive responses to drought stress (Jiang and Zhang 2002; Lou et al. 2017). The results showed that the soaking of seedlings with S-ABA significantly promoted maize seed germination under drought stress, but there was a significant inhibitory effect at high concentrations. Among the treatments tested, 4 mg/kg S-ABA had the best effect (p < 0.05; Fig. 1). These results suggest that the expression of maize drought resistance-related genes can be activated by 4 mg/kg of S-ABA, thereby alleviating the inhibition of drought on maize seed germination and seedling biomass accumulation, and increasing the germination rate of maize seeds under drought stress. This is consistent with the results reported by Gang et al. (2017), who reported that S-ABA spray can alleviate the inhibition of drought on the growth of maize seedlings. However, the different maize varieties had different responses to drought stress with XD20 being more sensitive than ZD958 (Table 1).
ABA is a major regulator of drought stress, and some studies report an increase in endogenous ABA in drought-tolerant maize under drought stress (De Souza et al. 2014). ABA balance between the roots and leaves has also been reported to affect plant moisture status. Water stress increases plant-endogenous ABA content, and the external application of ABA further promotes the synthesis of endogenous ABA to increase total levels; this has been confirmed in wheat, sugarcane, and other crops (Li et al. 2017; Ma et al. 2016; Virlouvet et al. 2011). Our results showed that endogenous ABA content increased significantly with the prolongation of water stress (p < 0.05). Similarly, Alvarez et al. (2014) report that in wheat, lower ABA accumulation can be found in drought-tolerant genotypes towards the end of the stress treatment. Compared with the drought treatment, exogenous application of S-ABA increased ABA content, in agreement with the results of Kizis and Pagès (2002) (Table 2). Furthermore, Zhang and Xie (2002) show that ABA positively regulates the content of endogenous hormones; this may be an important reason why ABA can improve drought resistance in maize; however, further research is needed to confirm this finding.
A noticeable fact concerning hydrogen peroxide (H2O2) was that, with the increase of drought stress time, PEG and PEG + ABA treatments showed higher H2O2 content; with ZD958 under this same treatment, a significantly lower content was observed compared to the stressed treatments and ABA application seemed to have increased this situation (Table 3). Conversely, under 24 h of stress, the plants showed a different behavior; there was a noticeable decrease when compared to the other treatments. In relation to the increase of H2O2 content following stress in the two maize hybrids, with ABA application, similar results were found by Kellos et al. (2008), where a tolerant maize lineage showed higher H2O2 content when ABA was applied. The increase of H2O2 at the beginning of stress can be explained by its role as a potent stress-signaling molecule (Møller et al. 2007). With increasing water stress, H2O2 participation in the Haber–Weiss/Fenton reaction as a free radical attacking the cell membranes is noticeable (Queval et al. 2008). Exogenous treatment with ABA can increase internal ABA content (Table 2) and this can lead to an accumulation of H2O2, a signaling molecule for the activation of antioxidant enzymes. Such an explanation was previously demonstrated in maize (evaluating CAT activity and other enzymes; Jiang and Zhang 2002).
Drought stress causes a dramatic increase in reactive oxygen species (ROS) in plant cells. The accumulation of ROS causes membrane lipid peroxidation. MDA is a product of peroxidation of unsaturated fatty acids in phospholipids, and lipid peroxidation is responsible for cell membrane damage. ABA can cause the reduction of lipid peroxidation as expressed by MDA production in plant cells (Bhaskara et al. 2017; Kaya et al. 2018). The results showed that with the prolongation of drought stress, MDA content in the two varieties (ZD958 and XD20) accumulated greatly. Exogenous application of S-ABA led to a reduction in MDA content compared with no application, which was remarkable in a tolerant cultivar (p < 0.05; Table 1). The latter may be related to the increase of antioxidant enzyme activity in ZD958 leaves and the better protection against oxidative stress. The current study confirmed that exogenous administration of S-ABA can alleviate damage caused by membrane lipid peroxidation in plants under water stress (p < 0.05; Tables 3, 4). Our findings are in agreement with those of Ma et al. (2016), who show that the application of S-ABA reduces MDA content in wheat. The decrease of MDA and H2O2 contents under PEG + S-ABA treatment, which can be due to the increase of antioxidant enzyme activity in leaves, promotes a better protection against oxidative stress (Moussa and Abdel-Aziz 2008).
Osmotic adjustment is an innate behavior of plants that helps to maintain water balance and adaptation to adverse conditions through the synthesis of different osmotic substances. The accumulation of osmotic substances under drought stress contributes to the regulation of plant physiological processes to adapt to adverse conditions. Generally, proline accumulation increases under stress conditions, which not only helps in maintaining cell turgor but also involves quenching-free radicals, maintaining sub-cellular structures, and buffering cellular redox potential. The current study showed that proline and soluble sugar accumulated rapidly in maize leaves after drought stress. Exogenous use of S-ABA hormone further increased the accumulation of proline and soluble sugar content, with ZD958 having a more significant response. Srivastava et al. (2009) showed that the application of exogenous ABA under water stress can increase the content of proline and soluble sugar in sugarcane seedlings (p < 0.05) so, in maize, a similar response could be conducive to improving drought resistance. Under water stress, the use of S-ABA increases the content of proline and soluble sugar in maize leaves and reduces crop damage (Fig. 2).
A large number of experimental studies show that plants possess a well-defied antioxidant defense mechanism that eliminates hazardous free radicals (Moussa and Abdel-Aziz 2008; Suzuki and Katano 2018). A crop’s protective enzymes (SOD, POD, CAT and APX) play an important role in the removal of peroxides and reactive oxygen species. Previous studies prove that higher enzyme activity or levels are important for inducing drought tolerance. The contribution of enzymatic antioxidants may ensure stress tolerance in plants subjected to long-term drought stress (De Souza et al. 2014; Gang et al. 2017). Jiang and Zhang (2002) reported that the application of exogenous ABA under stress conditions can induce the expression of related antioxidase genes and increase the activity of related antioxidant defense enzymes. Plant antioxidant systems are induced and regulated by ABA signaling (Wang et al. 2016). In our study, the activity of antioxidant enzymes (including superoxide dismutase, catalase, peroxidase, ascorbate peroxidase, polyphenol oxidase, and phenylalanine ammonia-lyase) in maize leaves under drought stress increased rapidly (p < 0.05). This was related to the rapid accumulation of endogenous ABA content under drought stress; however, the antioxidant enzyme activity decreased to different degrees after reaching a peak during different periods. An assumption was made that the equilibrium was destroyed due to the accumulation of large amounts of ROS. Our results are similar to the findings of Huang et al. (2013), who report that ABA spray causes an increase in related enzyme activity over that observed in untreated sugarcane. Exogenous administration of S-ABA compared with PEG treatment further increased the activity of antioxidant protective enzymes. Our results indicated that antioxidant enzyme content was higher in the tolerant cultivar than in the susceptible cultivar in all treatments (p < 0.05; Figs. 3, 4). Anjum et al. (2017) demonstrate that the antioxidant response of maize under drought conditions is closely related to tolerance in maize varieties. Polyphenol oxidase (PPO), together with guaiacol peroxidase (POD), can act in diphenols and phenols to produce other phenolic compounds, such as flavonoids, which are important in scavenging free radicals (Pourcel et al. 2007). Whereas the Fazeli et al. (2007) study evidenced higher PPO activity in tolerant genotypes. Enhancement of l-phenylalanine ammonia lyase (PAL) activity was verified in drought stress. These data resemble those found by De Souza et al. (2014), where maize tolerant genotypes showed higher PAL activity. High PAL activity at the beginning of stress can also be important for a quick production of phenolic compounds, enhancing the protection against oxidative stress (Gholizadeh 2010; Hura et al. 2008).
The Asr gene family has drawn wide attention due to its excellent ability to relieve osmotic stress (Cakir et al. 2003). Much research indicates that after plants are subjected to stress (such as drought, low temperature, salt stress, etc.), the Asr gene responds to ABA regulation to alleviate any damage caused by adversity (Jia et al. 2016; Li et al. 2017; Shinozaki et al. 2003). Later studies reveal that the heterologous over-expression of plantain Asr1 in Arabidopsis increases total soluble sugar levels in leaves (Dai et al. 2011). In addition, Asr1 overexpression in maize plants increases yield and reduces sugar and amino acid levels; which suggests that Asr1 also participates in plant growth through the regulation of this type of metabolite (Virlouvet et al. 2011). There is evidence suggesting that Asr1 has a role in cell antioxidant activity. For instance, the overexpression of wheat Asr1 in tobacco enhances the expression of ROS-related and stress-responsive genes under osmotic stress (Hu et al. 2013). Our results showed that the gene expressive level of Asr1 in maize leaves increased under drought stress. It was speculated that the maize Asr1 gene may play a role in signal transduction during drought resistance and then transmit more signals to induce downstream gene expression, thereby alleviating drought damage in maize (p < 0.05). The expression of Asr1 in exogenous S-ABA maize was significantly induced, and drought resistance was increased. This indicates that expression of the Asr1 gene is regulated by S-ABA, which is consistent with the results of physiological indicators. Recently there have been similar reports for rice. With the application of exogenous S-ABA, the expression of the Asr1 gene was significantly induced and the drought resistance of maize was increased (p < 0.05; Fig. 5). This indicates that Asr1 gene expression is regulated by S-ABA, and this is consistent with the physiological index results. Recently there have been similar reports in rice (Arenhart et al. 2016).
Conclusion
In summary, under drought stress, maize became seriously damaged during the seed germination and the seedling stages. Seed soaking with 4 mg/kg S-ABA significantly enhanced the germination rate and mitigated the inhibitory effect of drought on seed germination. Foliar application of exogenous S-ABA increased endogenous ABA content, free proline, soluble sugar content, and Asr1 level of gene expression; it also induced the enhancement of antioxidant enzyme activity (including SOD, CAT, POD, APX, PPO, and PAL), and reduced the accumulation of MDA, thereby improving drought resistance in maize. There were genotypic differences between the two maize varieties. Under drought stress, endogenous ABA content, osmotic substance content, antioxidant enzyme activity, and Asr1 gene expression level were higher in the drought-resistant ZD958 variety. These results provide a basis for further study of the mechanism of S-ABA in mitigating damage caused by drought stress to maize seedlings; in the meantime they provide a theoretical reference for the screening of maize varieties.
References
Abraham E, Hourton-Cabassa C, Erdei L, Szabados L (2010) Methods for determination of proline in plants. Methods Mol Biol (Clifton NJ) 639:317–331. https://doi.org/10.1007/978-1-60761-702-0_20
Alvarez S, Roy Choudhury S, Pandey S (2014) Comparative quantitative proteomics analysis of the ABA response of roots of drought-sensitive and drought-tolerant wheat varieties identifies proteomic signatures of drought adaptability. J Proteome Res 13:1688–1701. https://doi.org/10.1021/pr401165b
Anjum SA et al (2017) Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00069
Arenhart RA, Schunemann M, Bucker Neto L, Margis R, Wang ZY, Margis-Pinheiro M (2016) Rice ASR1 and ASR5 are complementary transcription factors regulating aluminium responsive genes. Plant Cell Environ 39:645–651. https://doi.org/10.1111/pce.12655
Badu-Apraku B et al (2013) Assessing the representativeness and repeatability of testing sites for drought-tolerant maize in West Africa Canadian. J Plant Sci 93:699–714. https://doi.org/10.4141/Cjps2012-136
Bhaskara GB, Nguyen TT, Yang T-H, Verslues PE (2017) Comparative analysis of phosphoproteome remodeling after short term water stress and ABA treatments versus longer term water stress acclimation. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00523
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cahill DM, McComb JA (1992) A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol Mol Plant Pathol 40:315–332. https://doi.org/10.1016/0885-5765(92)90014-M
Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15:2165–2180. https://doi.org/10.1105/tpc.013854
Cakmak I, Horst W (2006) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycinemax). Physiol Plant. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x
Dai J-R et al (2011) MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep 30:1219–1230. https://doi.org/10.1007/s00299-011-1030-1
de Souza TC, Magalhaes PC, de Castro EM, Carneiro NP, Padilha FA, Gomes CC (2014) ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul 73:205–217. https://doi.org/10.1007/s10725-013-9881-9
Dominguez PG, Frankel N, Mazuch J, Balbo I, Iusem N, Fernie AR, Carrari F (2013) ASR1 mediates glucose-hormone cross talk by affecting sugar trafficking in tobacco plants. Plant Physiol 161:1486–1500. https://doi.org/10.1104/pp.112.208199
Du Y-L, Wang Z-Y, Fan J-W, Turner NC, He J, Wang T, Li F-M (2013) Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct Plant Biol 40:494–506. https://doi.org/10.1071/FP12250
Fales FW (1951) The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem 193:113–124
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51:98–103. https://doi.org/10.1007/s10535-007-0020-1
Gang LI et al (2017) Effect of S-abscisic acid on growth and physiological function of maize (Zea mays) seedling under drought stress. Plant Physiol J. https://doi.org/10.13592/j.cnki.ppj.2017.0120
Gao Y et al (2015) A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice. Plant Mol Biol 87:413–428. https://doi.org/10.1007/s11103-015-0288-z
Ge T, Suo F, Bai L, Lu Y, Zhou G (2006) Effects of water stress on the protective enzyme activities and lipid peroxidation in roots and leaves of summer maize. Agric Sci China 5:291–298
Gholizadeh A (2010) Anti-oxidation profile in the leaves of maize inbreds: elevation in the activity of phenylalanine ammonia lyase under drought stress. J Plant Sci. https://doi.org/10.3923/jps.2010.137.145
Ghosh S, Majumdar S, Sarkar D, Datta K (2015) An efficient adventitious shoot regeneration system for potato (Solanum tuberosum L.) using leaf discs. J Plant Biochem Biotechnol 24:298–304. https://doi.org/10.1007/s13562-014-0273-7
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455. https://doi.org/10.1104/pp.84.2.450
Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R, Woch W, Hasanuzzaman M (2018) Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol Biochem 127:446–456. https://doi.org/10.1016/j.plaphy.2018.04.018
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19 https://doi.org/10.1186/gb-2007-8-2-r19
Hoagland R (1950) The water culture methods for growing plants without soil. Circ Calif Agric Exp Sect 347(2):32
Hu W et al (2013) TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ 36:1449–1464. https://doi.org/10.1111/pce.12074
Huang X, Zhang BQ, Yang rui LI (2013) Effects of exogenous abscisic acid on cell membrane and endogenous hormones contents in leaves of sugarcane seedling under cold stress. J Huazhong Agric Univ 17:59–64. https://doi.org/10.13300/j.cnki.hnlkxb.2013.04.016
Hura T, Hura K, Grzesiak S (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112. https://doi.org/10.1111/j.1439-037X.2008.00297.x
Iusem ND, Bartholomew DM, Hitz WD, Scolnik PA (1993) Tomato (Lycopersicon esculentum) transcript induced by water deficit and ripening. Plant Physiol 102:1353–1354. https://doi.org/10.1104/pp.102.4.1353
Jia H et al (2016) Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol J 14:2045–2065. https://doi.org/10.1111/pbi.12563
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410. https://doi.org/10.1093/jxb/erf090
Joo J, Lee YH, Kim Y-K, Nahm BH, Song SI (2013) Abiotic stress responsive rice ASR1 and ASR3 exhibit different tissue-dependent sugar and hormone-sensitivities. Mol Cells 35:421–435. https://doi.org/10.1007/s10059-013-0036-7
Kaya C, Akram NA, Ashraf M (2018) Kinetin and indole acetic acid promote antioxidant defense system and reduce oxidative stress in maize (Zea mays L.) plants grown at boron toxicity. J Plant Growth Regul 37:1258–1266. https://doi.org/10.1007/s00344-018-9827-6
Kellos T, Timar I, Szilagyi V, Szalai G, Galiba G, Kocsy G (2008) Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol (Stuttgart Germany) 10:563–572. https://doi.org/10.1111/j.1438-8677.2008.00071.x
Kizis D, Pagès M (2002) Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J 30:679–689. https://doi.org/10.1046/j.1365-313x.2002.01325.x
Lamesch P et al (2012) The Arabidopsis information resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40:D1202–D1210 https://doi.org/10.1093/nar/gkr1090
Leng G, Tang Q, Rayburg S (2015) Climate change impacts on meteorological, agricultural and hydrological droughts in China. Global Planet Change 126:23–34. https://doi.org/10.1016/j.gloplacha.2015.01.003
Li CN, Srivastava MK, Nong Q, Li YR (2010) Mechanism of tolerance to drought in sugarcane plant enhanced by foliage dressing of abscisic acid under water stress. Acta Agron Sin 36:863–870. https://doi.org/10.3724/SP.J.1006.2010.00863
Li J et al (2017) OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signalling in rice. Plant Biotechnol J 15:183–196. https://doi.org/10.1111/pbi.12601
Lin J-S, Wang G-X (2002) Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci 163:627–637. https://doi.org/10.1016/S0168-9452(02)00173-5
Lou D, Wang H, Liang G, Yu D (2017) OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00993
Ma D et al (2016) Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 11:e0163082. https://doi.org/10.1371/journal.pone.0163082
Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498. https://doi.org/10.1016/S0168-9452(01)00538-6
Møller I, Jensen P, Hansson A (2007) Oxidative modifications to cellular components in plants. Ann Rev Plant Biol 58:459–481. https://doi.org/10.1146/annurev.arplant.58.032806.103946
Moussa HR, Abdel-Aziz SM (2008) Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust J Crop Sci 1(1):31–36
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Pourcel L, Routaboul J-M, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36. https://doi.org/10.1016/j.tplants.2006.11.006
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant cell 6:65–74. https://doi.org/10.2307/3869675
Queval G, Hager J, Gakière B, Noctor G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in quantitative assays of leaf extracts. J Exp Bot. https://doi.org/10.1093/jxb/erm193
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417. https://doi.org/10.1016/s1369-5266(03)00092-x
Srivalli B, Sharma G, Khanna-Chopra R (2010) Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiol Plant 119:503–512
Srivastava MK, Li CN, Nong Q, Li YR (2009) Effect of exogenous ABA application on chlorophyll fluorescence in sugarcane under water stress conditions. Guangxi Agric Sci 40:1411–1417
Sugiharto B et al (2002) Identification and characterization of a gene encoding drought-inducible protein localizing in the bundle sheath cell of sugarcane. Plant Cell Physiol 43:350–354. https://doi.org/10.1093/pcp/pcf039
Suzuki N, Katano K (2018) Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00490
Tan M, Lu J, Zhang A, Hu B, Zhu X, Li W (2011) The distribution and cooperation of antioxidant (Iso)enzymes and antioxidants in different subcellular compartments in maize leaves during water stress. J Plant Growth Regul 30:255–271. https://doi.org/10.1007/s00344-010-9189-1
Travaglia C, Cohen AC, Reinoso H, Castillo C, Bottini R (2007) Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J Plant Growth Regul 26:285–289. https://doi.org/10.1007/s00344-007-9018-3
Turner NC (2004) Agronomic options for improving rainfall-use efficiency of crops in dryland farming systems. J Exp Bot 55:2413–2425. https://doi.org/10.1093/jxb/erh154
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71–W74. https://doi.org/10.1093/nar/gkm306
Virlouvet L et al (2011) The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol 157:917–936. https://doi.org/10.1104/pp.111.176818
Wang M, Meulen R, Visser K, Schaik H, Duijn B, Boer A (1998) Effects of dormancy-breaking chemicals on ABA levels in barley grain embryos. Seed Sci Res 8:129–137
Wang J et al (2016) Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol Environ Saf 133:146–156. https://doi.org/10.1016/j.ecoenv.2016.07.002
Yan H, Jia H, Chen X, Hao L, An H, Guo X (2014) The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol 55:2060–2076. https://doi.org/10.1093/pcp/pcu133
Yang C-Y, Chen Y-C, Jauh GY, Wang C-S (2005) A Lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol 139:836–846. https://doi.org/10.1104/pp.105.065458
Yang C-Y, Wu C-H, Jauh GY, Huang J-C, Lin C-C, Wang C-S (2008) The LLA23 protein translocates into nuclei shortly before desiccation in developing pollen grains and regulates gene expression in. Arabidopsis. Protoplasma 233:241–254. https://doi.org/10.1007/s00709-008-0016-5
Yongsheng LI et al (2016) Effects of exogenous hydrogen sulfide on seed germination and seedling growth under PEG stimulated drought stress in maize. J Nucl Agric Sci. https://doi.org/10.11869/j.issn.100-8551.2016.04.0813
Zhang M, Xie B (2002) Relationship between changes on endogenous hormone of sweet potato under water stress and drought resistance. Agri Sci China 1(6):626–630
Zhao H, Wang RY, Wang HL, Yang QG, Deng ZY, Xiao GJ (2007) Regional diversity in response of spring wheat growth on climate change in arid or semi-arid areas. Adv Earth Sci 22:636–641. https://doi.org/10.3321/j.issn:1001-8166.2007.06.011
Acknowledgements
This study received funding from the Technology Research and Demonstration on Reduction of Chemical Fertilizers and Pesticides in Summer Corn in Huang-Huai-Hai Region (Grant no. 2018YFD0200604), the National Modern Agricultural Technology & Industry System (Grant no. CARS-02-18), the Provincial Major Technological Innovation Program of Agricultural Application in Shandong, and the Shandong “double first-class” award (Grant no. SYL2017-XTTD11).
Author information
Authors and Affiliations
Contributions
CY and FZ carried out the measurements, data analysis, and drafted the manuscript. XJ designed the experiments. XS, DS, and FH performed the experiment, XS, DS, FH, and XJ made substantial contributions to conception, and critically revised the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, C., Zhang, F., Sun, X. et al. Effects of S-Abscisic Acid (S-ABA) on Seed Germination, Seedling Growth, and Asr1 Gene Expression Under Drought Stress in Maize. J Plant Growth Regul 38, 1300–1313 (2019). https://doi.org/10.1007/s00344-019-09934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09934-9