Abstract
Soil salinity is a critical abiotic stress that poses threat to global agricultural production. Salinity induces osmotic and ionic stresses, which negatively affect the growth and development of plants. Exogenous applications of trace elements have demonstrated their possible role in overcoming the adverse effects of salt stress in plants. Selenium (Se) is one such candidate with tremendous potential of salt stresses alleviation in plants. In this context, the present study was conducted to evaluate the role of exogenous Se in mitigating salt stress in proso millet. Proso millet plants were subjected to salinity stress (150 mM NaCl) and three concentrations of Se (1 µM, 5 µM and 10 µM) as sodium selenite (Na2SeO3) was applied for mitigation in three increments within 2 weeks. Se (1 µM) improved the fresh weight by 44.14%, biomass accumulation by 46.78%, shoot length by 95.42%, and root length by 19.45%, of proso millet plants under 150 mM NaCl stress. Se enhanced the activity of antioxidant enzymes like superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidases, glutathione-S-transferases and glutathione reductase by 24.37%, 28.59%, 18.99%, 45.46%, 24.5% and 11.37%, respectively. It also improved levels of osmolytes like proline, glycine betaine and total soluble sugar by 37.98%, 17.33% and 22.31% respectively and decreased H2O2 content by 20.68%. Se treatment increased the expression of SOS1, SOS2, SOS3, HKT and NHX transporters by 3.1, 6.1, 3.4, 3.1 and 2.8-fold respectively. In conclusion, Se in low doses improved the plant’s defense mechanisms by boosting the antioxidant machinery and regulating Na+ transporters, thereby mitigated the adverse effects of salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different abiotic stresses like drought, cold, salinity, floods and high temperature negatively impact worldwide agricultural efficiency. Salinity is the most detrimental environmental stress, affecting over 100 countries and resulting in decreased crop quality and productivity (Dey et al. 2021; Sabagh et al. 2020; FAO 2019). It has been reported that around 20% of the world’s cultivated agricultural land is influenced by salt stress (Kirova 2020; Sairam and Tyagi 2004). Under salt stress, undue uptake of sodium (Na+) and chloride (Cl−) ions causes ionic stress and cytotoxicity, hampering plant growth and development (Munns and Tester 2008; Shah et al. 2021). Persistent salt stress is characteristically followed by oxidative stress induced by excessive reactive oxygen species (ROS) generation, eventually leading to plant death (Qin and Huang 2020; Hernández et al. 2001; Isayenkov 2012). To cope with these stress conditions, plants employ various strategies and undergo developmental, morphological, physiological and biochemical changes to attain homeostasis (Taji et al. 2004; Acosta et al. 2015). Various genes have been identified and characterized, which are involved in the transport and translocation of ions across the plasma membrane (Inan et al. 2004; Zhang et al. 2008). Strategies and methods have been employed to enhance the defense capabilities of plants against salinity. For example, the exogenous application of melatonin, polyamines, silicon, and selenium have been shown to enhance plant capabilities against salt stress (Jiang et al. 2016; Wei et al. 2015; Shu et al. 2015; Gengmao et al. 2015; Diao et al. 2014; Feng et al. 2013; Hawrylak-Nowak 2009). Selenium (Se) is a vital micronutrient beneficial for living systems (Tsuji and Hatfield 2022; Fakhrolmobasheri et al. 2021; Ferreira et al. 2021; Feng et al. 2013; Zhu et al. 2009). Exogenous applications of Se have improved growth and developmental features of plants under salt stresses (Rasool et al. 2022). Se plays a vital role in the activity of the enzyme glutathione peroxidase (GPX) (Lobanov et al. 2008), improves osmolytes production (Hawrylak-Nowak 2009; Shah et al. 2020), and activates the antioxidative detoxification systems (Iqbal et al. 2015; Hasanuzzaman and Fujita 2011). Se also improves photosynthesis and mitigates the negative impacts of salt on the photosystem II (PSII) (Rasool et al. 2020; Diao et al. 2014; Kong et al. 2005).
Due to the fact that salinized soils on the earth are increasing at a rate of 10% each year, it is expected that by 2050, more than half of the world’s arable land will be salinized (Kumar and Sharma 2020). The goal of this study was to minimise the adverse effects of salt stress on plants for long-term food security and sustainable agricultural development. In this context, rejuvenation of millets as a cereal crop is a viable alternative. Millets are a variety of small seeded C4 grasses in the Poaceae family that are used as a food, fodder, and bioenergy crop. Millets are becoming increasingly popular in Asia and Africa’s semiarid tropics (McDonough et al. 2000). They are drought/pest/disease resistant, have fewer irrigational necessities, show enhanced growth/yield in low nutrient input conditions and have little dependence on synthetic manures (Devi et al. 2014; Kole et al. 2015). Proso millet stands out among the group because of its short life cycle (45 days), making it an ideal choice for sustainable agriculture. It may be easily utilised on marginal soils because of its low agricultural requirements (Amadou et al. 2013). With these considerations in mind, we investigated the role of exogenous selenium in reducing the detrimental effects of salt stress in proso millet. We studied the effects of Se on several growth metrics and antioxidant enzyme activities. The main aim of the current study was to evaluate the regulation of three main sodium transporters (SOS1, NHX and HKT) in salt stressed proso millet under Se supplementation responsible for ion homeostasis.
Materials and methods
Experimental design
The proso millet (Panicum miliaceum L.) seeds were collected from the Gurez valley of Jammu and Kashmir, India and were identified at Centre for Biodiversity and Taxonomy, University of Kashmir. Seeds were surface disinfected with 70% (v/v) ethanol for 1 min and washed twice with double-distilled water (ddH2O). HgCl2 (Merck, India) was used for surface sterilization, and then seeds were washed three times with dd H2O. Controlled environmental conditions were maintained (temperature 26 ± 1 °C and 16-h photoperiod). The 30 seeds were sown in pots (diameter = 9.5 cm, height = 9 cm) containing 500 g of autoclaved sand. After germination pots were thinned to equal number of plants. The complete randomized design was followed, where each treatment had three replicates, i.e., three pots for each treatment. The treatment was initiated after the 14th day of germination, which include Sodium Chloride (150 mM NaCl) and selenium in form of sodium selenite (Na2SeO3) (1 µM, 5 µM and 10 µM) dissolved in Hoagland’s nutrient medium (pH 6.5). The eight treatments include Control, 1 µM Se, 5 µM Se, 10 µM Se, 150 mM NaCl, 150 mM NaCl + 1 µM Se, 150 mM NaCl + 5 µM Se and150 mM NaCl + 10 µM Se with three replicates each. The treatment was given for 2 weeks with a gap of 3 days between treatments. The plants were harvested and stored at – 80 °C till further analysis.
Determination of biomass accumulation (BA), relative water content (RWC) and electrolyte leakage (EL)
Ten plants were dried at 70 °C in an incubator for 48 h for determination of biomass accumulation (BA). After achieving constant weight, the plants were weighed and expressed as g/seedling. The fresh weight (FW) of leaves was taken to determine RWC. The leaves were immersed in de-ionized water overnight, then briefly air-dried and weighed (SW). Then the leaves were oven-dried at 70 °C for 48 h, and dry weights (DW) were measured using a reformed method of Barrs and Weatherley (Barrs and Weatherley 1962). Relative water content (RWC) was analysed using the formulae:
The electrolyte leakage (EL) was determined by the method of Tabot and Adams (Tabot and Adams 2013). It was measured as:
where EI: Electrolytes leaked from cells; ET: Total electrolyte pool.
Determination of thiobarbituric acid reactive species (TBARS)and in-situ detection of hydrogen peroxide (H2O2) content
The concentration of Malondialdehyde (MDA) was measured according to the method of Cakmak and Horst (1991), and H2O2 content was determined by following the protocol of Monteiro et al. (2011). The MDA concentration was calculated using the following equation:
where ε: The specific extinction coefficient (155 mM/cm); V: The volume of crushing medium; W: The fresh weight of leaf; A600: The absorbance at 600 nm wavelength; A532: The absorbance at 532 nm wavelength.
Determination of photosynthetic pigment content
Chlorophyll a, b, and carotenoid content were determined using the method of Lichtenthaler (1987). 100 mg of fresh leaves were taken from treated plants. These leaves were then finely grounded and extracted in 5 ml absolute ethanol (99%). Extracts were filtered, and the absorbance was recorded with a UV–vis spectrophotometer (UV-2450, Shimadzu Analytical, Japan) at 663 nm, 645 nm, and 480 nm. Anthocyanin was determined by the method given by Mancinelli (1990). The samples were extracted in 3 ml methanol-HCl (1% HCl, v/v); the samples were then refrigerated at 4 °C for 2 days. Then the extracts were filtered. A UV visible spectrophotometer measured the total anthocyanin content as the difference between the absorbance at 530 and 657 nm wavelength and placed in the A530–A657 formula to eliminate the chlorophyll content in the extract, defined quantitatively as OD 530 g−1 fresh weight.
Anthocyanin content (units/g FW) = A530 – 0.25*A657
Estimation of osmolytes and total soluble protein
The total soluble sugar (TSS) and free proline was estimated by following the method given by Dey (1990), and glycine betaine (GB) content was estimated by the method given by Grieve and Grattan (1983). The protein content was determined using the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
Evaluation of antioxidant enzyme activity
The superoxide dismutase (SOD) activity was estimated by the method devised by Beyer and Fridovich (1987) with slight modifications. Catalase (CAT) activity was determined by the method of Aebi (1984). Ascorbate peroxidase (APX) activity was measured according to the method of Nakano and Asada (1981). Guaiacol peroxidase (POD) activity was determined by the method of Whitaker and Bernhard (1972). Glutathione-s-transferase (GST) activity was determined according to the method of Habig and Jakoby (1981), and estimation of glutathione reductase (GR) was estimated using the method devised by Foyer and Halliwell (1976).
Determination of selenium (Se), sodium (Na), chloride (Cl−)and potassium (K) content
The leaf samples were extracted with 1 M HNO3to determine the K+ and Na+ content as described by Storey (1995), and Cl−contents were determined according to the method of Jiang et al. (2014). The total Se concentration was determined by the method given by Jiang et al. (2015) using inductively coupled plasma mass spectrometry (ICP–MS).
Transcriptome analysis
Total RNA extraction
For the transcriptome sequencing analysis, the samples were ground into powder in a mortar using liquid nitrogen. Total RNA was extracted from samples using a Trizol reagent (Invitrogen). RNase-free DNase (Promega, Madison, Wisconsin, USA) was added to the RNA samples to remove the genomic DNA contamination. The quality of the RNAs was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). DNA library construction and RNA-Seq high-throughput sequencing were commercially performed by Biokart Pvt limited (Bangalore, India).
Data processing and gene annotation
Raw single reads in FASTQ format were subjected to sequence quality control using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). FastQC performed a series of analysis modules on raw data and created a report with statistics for the data analysed. FastQC showed high per base sequence quality for each library, exceeding 30 on the Phred scale (less than 1/1000 chance of a base being wrong) and detected adapter contamination, matching the reads to known adapter sequences. The program HISAT2 (Kim et al. 2015) aligned sequencing reads to the reference genome using a minimum intron length of 60 and 2000. Cufflinks v2.0.2 (Trapnell et al. 2010) was used for de novo transcript identification and to get the read counts for each gene and find the genes expressions in all the samples.
cDNA synthesis, Primer designing and validation of genes
cDNA was synthesized from the RNAa using a revert-aid cDNA synthesis kit (Thermo Scientific), following the manufacturer’s protocol. Primers of the genes of interest (SOS1, SOS2, SOS3, NHX, HKT) were designed using the Primer3Plus software according to parameters established to get amplicons of 301 to 400 bp with a Tm of 55 °C to 65 °C, length 20 to 22 bp and GC 40 to 65%. The primers were obtained from IDT (Bangalore, India) and are listed in Table 1. For PCR, a master mix of 25 μl including 13.5 μl of H2O, 2.5 μl of 10 × Taq buffer, 2.5 μl DNTP, 3 μl of 25 mM MgCl2, 0.3 μl Taq, 1.5 μl R-Primer and 1.5 μl F-Primer and 2.5 μl cDNA template was used. The cycle parameters consisted of one cycle of 1 min at 95 °C for denaturation and then 35 cycles of 5 s at 95 °C for denaturation followed by X °C (X = annealing temperature) for annealing and 1 min at 70 °C for an extension. Lastly, 10 min of 70 °C for elongation and then hold at 4 °C for 5 min. The amplified products were gel electrophoresed and quantitated relative to their expression in the control sample.
Statistical analysis
All experiments were carried out in triplicates (n = 3) except for growth parameters like root and shoot length, BA, TI, and RWC, where n = 10. All data were subjected to two-way ANOVA using GraphPad Prism 8.4.3 software. Results were represented as the arithmetic mean ± standard error (SE). Tukey’s post hoc test was done to identify statistical differences between pairs of means at the 0.05 probability level.
Results
Influence of Se application on various morphological characters
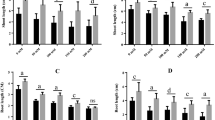
Salinity-stressed plants exhibited chlorosis, necrosis, and leaf burning. It was found that the shoot length of the plants decreased significantly with salt treatments by 43.12% compared to control. When Se followed salt treatment, there was a significant increase in shoot length. The increase was 95.42% at 1 µM; however, at 5 µM and 10 µM, it decreased by 0.98% and 30.71% respectively, to salt-treated plants. The root length of the plants decreased with salt treatments by 46.22% to control. When Se followed salt treatment, there was a significant increase in root length. The increase was 19.45% at 1 µM, 1.62% at 5 µM; however, at 10 µM, it decreased by 31.89%to salt-treated plants. The fresh weight of the plants decreased with salt treatments by 50.22% to control. When Se followed salt treatment, there was an increase in fresh weight. The increase was 44.14% at 1 µM; however, at 5 µM and 10 µM, it decreased by 14.41% and 76.57%, respectively, to salt-treated plants. Biomass accumulation of the plants decreased with salt treatments by 49.40% to control. When Se followed salt treatment, there was a significant increase in biomass accumulation. The increase was 46.78% at 1 µM; however, at5µM and 10 µM, it decreased by 2.14% and 24.27%, respectively, to salt-treated plants (Fig. 1).
A Shoot length, B Fresh weight, C Root length, and D Biomass accumulation of non-stressed and salt-stressed proso millet. The abbreviations 1 Se, 5 Se and 10 Se, indicate 1 μM Na2SeO3, 5 μM Na2SeO3 and 10 μM Na2SeO3, respectively and NaCl indicates 150 mM NaCl. Data are presented as means ± SD (n = 10). Different letters on bars represent significant differences [a = ****, b = ***, c = **, d = * and ns = not significant (p < 0.05)]
Influence of Se on electrolyte leakage (EL), relative water content (RWC), thiobarbituric acid reactive species (TBARS) and hydrogen peroxide (H2O2)
EL of the plants increased with salt treatments by 40.88% to control. When Se followed salt treatment, there was a significant decrease in EL. The reduction was 15.62% at 1 µM, 4.46% at 5 µM; however, at 10 µM, it increased by 7.5% to salt-treated plants. RWC of the plants decreased with salt treatments by 24.66% to control. When Se followed salt treatment, there was a significant increase in RWC. The increase was 16.40% at 1 µM, 1.32% at 5 µM; however, at 10 µM, it increased by 8.15% to salt-treated plants. TBARS of the plants increased with salt treatments by 85.93% to control. When Se followed salt treatment, there was a significant decrease in TBARS. The drop was 32.68% at 1 µM, 5.65% at 5 µM; however, at 10 µM, it increased by 10.14%to salt-treated plants. H2O2 of the plants increased with salt treatments by 38.52% to control. When Se followed salt treatment, there was a significant decrease in H2O2. The reduction was 20.68% at 1 µM,
16.63% at 5 µM; however, at 10 µM, it increased by 19.35% to salt-treated plants (Table 2).
Influence of Se on photosynthetic pigments
Chlorophyll-a content of the plants decreased with salt treatments by 85.68% to control. When Se followed salt treatment, there was a significant increase in chlorophyll-a. The increase was 85.71% at 1 µM; however, at 5 µM and 10 µM, it decreased by 4.76% and 61.90%, respectively, to salt-treated plants. Chlorophyll-b content of the plants decreased with salt treatments by 88.86% to control. When Se followed salt treatment, there was a significant increase in chlorophyll-b. The increase was 74.31% at 1 µM, 31.19% at 5 µM; however, at 10 µM, it decreased by 16.51% to salt-treated plants. The total chlorophyll of the plants decreased with salt treatments by 86.95% to control. When Se followed salt treatment, there was a significant increase in total chlorophyll. The increase was 81.81% at 1 µM, 7.52% at 5 µM; however, at 10 µM, it decreased by 46.39% to salt-treated plants. Anthocyanin of the plants decreased with salt treatments by 62.5% to control. When Se followed salt treatment, there was a significant increase in anthocyanin. The increase was one-fold at 1 µM, 0.4-fold at 5 µM, and 0.1-fold at 10 µM to salt-treated plants. Carotenoid of the plants decreased with salt treatments by 40.19% to control. When Se followed salt treatment, there was a significant increase in anthocyanin. The increase was 43.05% at 1 µM, 15.27% at 5 µM; however, at 10 µM, it decreased by 11.11% to salt-treated plants (Table3).
Influence of Se on total soluble sugar (TSS), total soluble protein (TSP), proline, and glycine betaine
TSS of the plants increased with salt treatments by 82.83% to control. When Se followed salt treatment, there was a significant increase in TSS. The increase was 22.31% at 1 µM; however, at 5 µM and 10 µM, it decreased by 4.32% and 10.01%, respectively, to salt-treated plants. TSP of the plants increased with salt treatments by 1.2-fold to control. When Se followed salt treatment, there was a significant increase in TSP. The increase was 12.56% at 1 µM, 8.81% at 5 µM; however, at 10 µM, it decreased by 10.93% to salt-treated plants. Proline of the plants increased with salt treatments by 2.4-fold to control. When Se followed salt treatment, there was a significant increase in proline. The increase was 37.98% at 1 µM, 11.39% at 5 µM; however, at 10 µM, it decreased by 41.16% to salt-treated plants. Glycine betaine of the plants increased with salt treatments by threefold to control. When Se followed salt treatment, there was a significant increase in glycine betaine. The increase was 17.33% at 1 µM, 44.67% at 5 µM; however, at 10 µM, it decreased by 16.12% to salt-treated plants (Fig. 2).
A Total soluble sugar, B Proline, C Total soluble protein, and D Glycine betaine of salt treated, Se treated and salt with Se treated proso millet. The abbreviations 1 Se, 5 Se and 10 Se, indicate 1 μM Na2SeO3, 5 μM Na2SeO3 and 10 μM Na2SeO3, respectively and NaCl indicates 150 mM NaCl. Data are presented as means ± SDs (n = 3). Different letters on bars represent significant differences [a = ****, b = ***, c = **, d = * and ns = not significant (p < 0.05)]
Influence of Se on various antioxidant enzymes
SOD activity of the plants increased with salt treatments by 90.47% to control. When Se followed salt treatment, there was a further significant increase in SOD activity. The increase was 24.37% at 1 µM, 11.87% at 5 µM; however, at 10 µM, it decreased by 18.75% to salt-treated plants. CAT activity of the plants increased with salt treatments by threefold to control. When Se followed salt treatment, there was a further significant increase in CAT activity. The increase was 28.59% at 1 µM, 11.42% at 5 µM; however, at 10 µM, it decreased by 36.86% to salt-treated plants. APX activity of the plants increased with salt treatments by 1.5-fold to control. When Se followed salt treatment, there was a further significant increase in APX activity. The increase was 18.99% at 1 µM, 20.99% at 5 µM; however, at 10 µM, it decreased by 37.08% to salt-treated plants.
GPX activity of the plants increased with salt treatments by 1.8-fold to control. When Se followed salt treatment, there was a further significant increase in GPX activity. The increase was 45.46% at 1 µM; however, at 5 µM and 10 µM, it decreased by 12.73% and 32.32%, respectively, to salt-treated plants. GST activity of the plants increased with salt treatments by 1.5-fold to control. When Se followed salt treatment, there was a further significant increase in GST activity. The increase was 24.5% at 1 µM, 8.5% at 5 µM; however, at 10 µM, it decreased by 11.5% to salt-treated plants. GR activity of the plants increased with salt treatments by 1.6-fold to control. When Se followed salt treatment, there was a further significant increase in GR activity. The increase was 11.37% at 1 µM, 0.51% at 5 µM; however, at 10 µM, it decreased by 32.51% to salt-treated plants (Fig. 3).
A Superoxide Dismutase (SOD) activity, B Catalase (CAT) activity, C Ascorbate peroxidase (APX) activity D Glutathione reductase (GR) activity E Guaiacol peroxidase (POD) activity F Glutathione-S- transferase (GST) activity of salt treated, Se treated and salt with Se treated proso millet. The abbreviations 1 Se, 5 Se and 10 Se, indicate 1 μM Na2SeO3, 5 μM Na2SeO3 and 10 μM Na2SeO3, respectively and NaCl indicates 150 mM NaCl. Data are presented as means ± SD (n = 3). Different letters on bars represent significant differences [a = ****, b = ***, c = **, d = * and ns = not significant (p < 0.05)]
Influence of Se application in the accumulation of selenium, chloride, sodium, and potassium ions
The Se content in control and NaCl treated plants was 1.16 and 1.1 μg/g/dry weight respectively. The exogenous Se treatment significantly increased the Se content in 1 µM to 16.3 μg/g/dry weight, in 5 µM to 41.3 μg/g/dry weight, and in 10 µM to 150.3 μg/g/dry weight. When applied with NaCl, it ranged at 15.4 μg/g/dry weight in 1 µM plus 150 NaCl, in 5 µM plus 150 NaCl at 39.1 μg/g/dry weight, and in 10 µM plus 150 NaCl to 148.76 μg/g/dry weight. The exogenous Se supply influenced the Se content in the plants. A considerably higher Se content was observed in the plants that received Se compared to the control. Na+ in the plants increased with salt treatments by tenfold to control. However, when Se followed salt treatment, there was a decrease in Na+. The drop was 13.7% at 1 µM, 10.5% at 5 µM; however, at 10 µM, it increased by 6.33% to salt-treated plants. Cl− in the plants increased with salt treatments by 7.6-fold to control. However, when Se followed salt treatment, there was a decrease in Cl−. The decline was 86.17% at 1 µM, 7.44% at 5 µM, and 4.25% at 10 µM to salt-treated plants. K+ decreased with salt treatments by 30.87% to control. However, when Se followed salt treatment, there was an increase in K+. The increase was 23.4% at 1 µM, 2.4% at 5 µM; however, at 10 µM, it decreased by 10.29% to salt-treated plants. Na+/K+ ratio in the plants decreased with salt treatments by 93.90% to control. However, when Se followed salt treatment, there was an increase in the Na+/K+ ratio. The increase was 42.8% at 1 µM, 14.05% at 5 µM; however, at 10 µM, it decreased by 15.98% to salt-treated plants. (Table 4).
Transcriptomic analysis
In this study, we performed imperative transcriptomic analysis between salt-treated and Salt plus Se treated Panicum miliaceum L. plants using high throughput Illumina hiseq platform. We identified genes involved in the salt overly sensitive (SOS) signalling pathway to understand the molecular basis of salinity tolerance by selenium treatments using an RNA sequencing approach. Transcriptomic analysis showed a significant change in the expression of genes encoding the essential SOS signalling pathway and transporters like NHX and HKT (Fig. 4). The expression of SOS1 was significantly increased by salt and Se (1 µM). The expression of SOS1 in salt (150 NaCl) and salt plus Se (150 NaCl + 1 µM Se) increased by 1.19 and 2.25 folds, respectively, compared to control. Also, an increase of 3.1-fold was observed in salt plus Se (150 NaCl + 1 µM Se) plants compared to salt (150 NaCl) treated plants. Similar results were observed in the expression analysis of SOS2 and SOS3.The expression of SOS2 in salt (150 NaCl) and salt plus Se (150 NaCl + 1 µM Se) increased by 4.2 and 3.68 folds, respectively, compared to control. Also, an increase of 6.1-fold was observed in salt plus Se (150 NaCl + 1 µM Se) plants compared to salt (150 NaCl) treated plants. The expression of SOS3 in salt (150 NaCl) and salt plus Se (150 NaCl + 1 µM Se) increased by 1.29 and 2.6 folds, respectively, compared to control. Also, an increase of 3.4-fold was observed in salt plus Se (150 NaCl + 1 µM Se) plants compared to salt (150 NaCl) treated plants. Moreover, the expression of HKT and NHX showed a similar trend. The expression of HKT in salt (150 NaCl) and salt plus Se (150 NaCl + 1 µM Se) increased by 2.3- and 2.9-fold, respectively, compared to control. Also, an increase of 3.1-fold was observed in salt plus Se (150 NaCl + 1 µM Se) plants compared to salt (150 NaCl) treated plants and the expression of NHX in salt (150 NaCl) and salt plus Se (150 NaCl + 1 µM Se) increased by 3.21- and 7.98-fold respectively compared to control. Also, an increase of 2.8-fold was observed in salt plus Se (150 NaCl + 1 µM Se) plants compared to salt (150 NaCl) treated plants.
Influence of Se on PmSOS1, PmSOS2, PmSOS3, PmHKT and PmNHX expression
An alternative strategy to confirm the results obtained from transcriptomic analysis revealed similar gene expression profiling (Figs. 5 and 6). The mRNA profiles of the genes encoding SOS1, SOS2, SOS3, NHX, and HKT were significantly altered in proso millet leaves under both salt and salt with selenium treatments. SOS1 transcripts were more expressed in salt (150 mM) and salt plus Se [150 mM NaCl + Se (1 µM)] treated leaves. Also, SOS2 transcripts were more expressed in the salt plus Se [150 mM NaCl + Se (1 µM)] treated leaves followed by salt (150 mM) treated. Similarly, SOS3 transcripts were significantly expressed in Salt plus Se [150 mM NaCl + Se (1 µM)] treated leaves. Moreover, the transcripts of HKT and NHX were more expressed in salt plus Se [150 mM NaCl + Se (1 µM)], followed by salt (150 mM).
Gel images depicting the differential gene expression in terms of band intensities of salt overly sensitive (SOS) signalling pathway (SOS1,SOS2,SOS3), high-affinity potassium transporters [HKT] and Na+/H+ antiporters [NHX] transporters in leaves of proso millet treated with salt [150 mM] and salt [150 mM + Se (1 µM)] (1) control (2) Se (1 µM) (3) Salt (150 NaCl) (4) salt with Se [150 NaCl + Se (1 µM)]
Relative expression profiles of the genes salt overly sensitive 1 (SOS1), salt overly sensitive 2 (SOS2), salt overly sensitive 3 (SOS3), Na+/H+ antiporters (NHX), and high-affinity potassium transporters (HKT) under salt and selenium treatments. Expression profiles were estimated on the basis of intensities of bands using NIH Image J software. Error bars indicate ± SD (n = 3) with respect to the control
Discussion
Salt stress adversely affects plant growth, development and diminishes crop productivity (Tanveer et al. 2018; Munns and Tester 2008). In this study, our main aim was to evaluate the role of Se in alleviating salt stress by regulating sodium transporters and associated proteins like Salt Overly Sensitive 1 (SOS1), Salt Overly Sensitive 2 (SOS2), Salt Overly Sensitive 3 (SOS3), K+/H+ antiporter (NHX), and High-affinity Potassium Transporters (HKT) in proso millet. We evaluated the role of exogenously applied Se (1 μM, 5 μM and 10 μM) in alleviating the negative impacts of salt stress in this particular species. We observed a concentration-dependent effect of Se on the plant, where 1 μM Se proved to be more beneficial compared to 5 μM and 10 μM Se. The exogenous application of 1 μM Se mitigated the adverse effects of salt stress, thereby affirming its potential role in alleviating the salt stress in plants. We observed that Se improved fresh weight, dry weight, shoot and root length in salt stressed proso millet, thereby improving plant growth and development. Various investigations have demonstrated that Se improves plant salt tolerance in different plant species like cucumber and tomato (Rasool et al. 2022). Se stimulates plant development at low concentrations but is lethal to plant at higher levels, which is attributed to the excessive accumulation of seleno-protein and disturbances in the homeostasis of both reactive oxygen and nitrogen species result in selenium-induced nitro-oxidative stress, contributing to toxicity (Kolbert et al. 2016). Salt stress also affects plant water relations, in current study, relative water content (RWC) decreases by NaCl application while 1 μM Se alleviates the deleterious effects of salt stress. This decrease in RWC is due to osmotic stress generated by salt stress. Selenium is also been previously reported to enhance RWC in Zea mays, Trifolium repens, Phaseolus mungo, Beta vulgaris and Cucumis sativus L. under stress (Jóźwiak and Politycka 2019; Nawaz et al. 2016; Wang 2011). Salinity also poses deleterious effects on photosynthetic capacity of plants by destroying the chloroplast (Munns and Tester 2008). Studies have documented a decrease of these photosynthetic pigments under salt stress, due to chlorosis and disordering in chlorophyll synthesis in plants (Betzen et al. 2019). In current study, salt stress declines chlorophyll, anthocyanin, and carotenoid content, however, the low concentrations of Se (1 μM) enhanced the photosynthetic pigment content in salt stressed proso millet. It was observed in salt-treated maize plants that Se application restored the photosynthetic capacity, chlorophyll content and the chloroplast ultrastructure in and high Se concentration (25 μM) had a deleterious effect on pigment content because high Se aggravated drastic damage to the chloroplast ultrastructure and photosynthetic system (Jiang et al. 2017).
Salt stress also increased ROS production, which leads to oxidative stress (Babitha et al. 2015). Excess ROS production incites lipid peroxidation, protein, and DNA damage, leading to signal transduction inhibition and reduction in overall growth and cell death (Mittler 2002). Previous studies have contemplated that exogenous Se can enhance the antioxidant activity and, therefore, increase the plant tolerance to different types of environmental stresses (Diao et al. 2014; Hasanuzzaman et al. 2011). The antioxidant activity of plants in stress and the non-stressed condition is crucial for plants. The balance of these antioxidant enzymes in signaling and scavenging ROS is integral in converting the superoxide radical (O2−) and hydrogen peroxide (H2O2) into forms that are not harmful to plants and protect the plant from various environmental stress, including salt stress. The elevated levels of H2O2 may lead to cell shrinkage, protein degradation, DNA fragmentation and apoptosis (Zhang et al. 2021). An assortment of antioxidants (enzymatic and non-enzymatic) plays a potential role in the sequestration of H2O2 (Gill and Tuteja 2010). Here, we reported a substantially lower concentration of H2O2 in the plants treated with 1 µM Se. The lower levels of H2O2 were probably due to the increased levels of various antioxidative enzymes like APX and CAT. These results were in concurrence with some past reports, where Se pre-treated rapeseed, canola and black gram had lesser levels of H2O2 under salinity stress. (Hasanuzzaman and Fujita 2011; Hashem et al. 2013; Jawad Hassan et al., 2020). These ROS leads to electrolyte leakage due to lipid peroxidation. Electrolyte leakage (EL) is an indispensable index of cell membrane permeability. In this study, it was observed that salinity disrupted membranes and increases EL and 1 µM Se significantly reduced the EL in salt-treated plants. Se (1 μM) application minimized the harm by increasing the production of antioxidants, which in turn help in scavenging ROS and thus decrease lipid peroxidation. The decrease in peroxidation was also observed in sorghum, tomato seedlings by Se application (Djanaguiraman et al. 2010; Diao et al. 2014). These outcomes support that Se plays an essential role in protecting cell membrane structure and cell integrity under salt stress. Plants also accumulate various osmoprotectants like sugars, proline, glycine betaine under salt stress (Chelli-Chaabouni et al. 2010). Total soluble sugar (TSS) is an essential compound directly related to various critical physiological processes (Karimi et al. 2019; Kerepesi and Galiba 2000). Proso millet, when exposed to salt stress, showed an augmentation in its TSS content and 1 µM Se further increased it. The accumulation of soluble sugars in the cytosol may aid in water conservation and absorption from intercellular gaps, allowing salt-stressed tissues to function normally. To counteract salt stress, this procedure is known as osmoregulation and helps to protect the plants from cellular damage by maintaining membrane integrity of the cells. Soluble sugars acts as a counteractive way to maintain homeostasis within plant cells. Similar outcomes were observed in rice and wheat under Se treatment (Subramanyam et al. 2019; Das et al. 2018; Nawaz et al. 2015). Glycine betaine (GB) is another compound that helps in stress tolerance in plants. In the current study, GB increased in salt as well as in 1 µM Se treated proso millet. GB, regarded as the finest osmotic regulator is engaged not only in cell osmotic homeostasis but also in macromolecule stability. GB helped in safeguarding protein quaternary structure, subsequently kept the enzymes in an active form. It protects the main enzymes and terminal oxidases of the TCA (tricarboxylic acid) cycle, for example, and stabilises the light system’s peripheral peptides (Li et al. 2020). Comparable results were found in salt stressed wheat cultivars and heat stressed cucumber plants (Rao et al. 2013; Hawrylak-Nowak et al. 2018). Proline, an amino acid, has been found to be an osmotically active organic solute in the root area. In this study, the proline content increased in salt-stressed plants, application of 1 µM Se further increased the proline content however 10 µM Se decreased it. Proline is associated with membranes and proteins protection against the damaging effects of increased ion concentrations. It was reported that proline increased salt tolerance in Pancratium maritimum by up-regulating stress-protective proteins and shielding the protein turnover machinery from stress induced damage. Salt stress signals cause A’-pyrroline-5-carboxylate synthetase (P5CS; a crucial enzyme in proline biosynthesis) to lose its feedback inhibition, resulting in proline build-up. By coordinating osmotic changes and safeguarding stressed plant’s subcellular structures, proline plays a vital role in acclimatisation to salt stress (Xie et al. 2020). Selenium applications is also reported to regulate transcript levels of genes P5CS2 and PDH in fragrant rice (Luo et al. 2021). In present study, there was also an increase in protein content in salt stressed plants, which was further increased by 1 μM Se. Protein accumulations is important for cell survival under salt stress and causes membranes stabilization under salt stress. Plants make new proteins that help them to grow and develop under saline condition. Higher protein concentration is due to higher efficiency of osmotic regulation mechanism in plants which in turn decreases sodium toxicity in cytoplasm. The Se application increased protein content in rice and ryegrass (Wang et al. 2012; Hartikainen and Xue 1999). In the antioxidant defense system, key antioxidant enzymes are crucial for enhancing stress tolerance as well as in minimizing the stress-induced oxidative damages, by detoxification of the ROS. SOD is the central defense enzyme of plants against ROS by catalyzing superoxide radicals into H2O2 and molecular oxygen (Van Raamsdonk and Hekimi 2012). In the present study, salt treatment enhanced the SOD activity. The addition of exogenous 1 µM Se further increased the SOD activity; however, at 10 µM Se, it decreased. Our results agree with the study on tomato, mulberry, and chickpea, where SOD activity increased under salt stress (Gapińska et al. 2008; Harinasut et al. 2003; Kukreja et al. 2005). These observations agree with the findings in sorrel, maize, rapeseed, and tomato were, Se improved SOD activity under salt stress (Diao et al. 2014; Hasanuzzaman et al. 2011; Jiang et al. 2017; Kong et al. 2005). Catalases (CAT) are produced in peroxisomes by oxidases involved in β-oxidation of fatty acids, photorespiration, and purine catabolism. In the current study, a significant CAT activity was observed under salinity stress, as was observed in cucumber, soybean, mustard, and tobacco (Ahmad et al. 2012b; Bueno et al. 1998; Comba et al. 1997; Lechno et al. 1997). The Se (1 µM) supplementation further increased the CAT activity; however, at 10 µM Se, it decreased. Comparable results were observed in Se-treated wheat under cold stress, rapeseed under drought stress, and maize under salt stress (Chu et al. 2010; Hasanuzzaman et al. 2011; Jiang et al. 2017). SOD and CAT are basically metalloproteins which catalyzes the dismutation of ROS and plays a central role in the antioxidative metabolic network and selenium acts as co-factor and thereby might stimulate the activity of these enzymes. APX is another important ROS scavenging enzymes and is crucial in maintaining ascorbic acid homeostasis (Diaz-Vivancos et al., 2013; Hernández et al. 2001). In our study, APX activity increased by salt stress and the application of 1 µM Se further enhanced it. The APX assumes a vital role in catalyzing H2O2 into H2O, utilizing ascorbate as an electron donor in the ascorbate–glutathione cycle (Chen et al., 2012; Correa-Aragunde et al., 2013). APX is involved in direct ROS scavenging in chloroplast, as CAT is absent in chloroplast. Similarly, GR is also considered as major ROS detoxifier and maintain redox homeostasis. GR assumes a pivotal role in the protection of plants from different types of stresses. We observed that under salt stress, GR activity increases in proso millet, which were further enhanced by the application of 1 µM Se. The similar results were obtained in peas, lentil and clover (Ahmad et al. 2012a; Bandeoğlu et al. 2004). It has been observed that Se treatment maintains a high GSH/GSSG proportion which is useful for other GSH dependent antioxidant enzymes and thus enhanced salt resilience of plants (Hasanuzzaman et al. 2017). Glutathione-S-transferase (GSTs) is yet another enzyme with high antioxidant capacity, which plays a vital role in plant response to biotic and abiotic stresses (Djanaguiraman et al. 2005). GST activity increased upon salt treatment and 1 µM Se further enhanced its activity. GSTs quench reactive molecules and catalyze the conjugation of GSH to an array of hydrophobic and electrophilic substrates, thus, protecting the cell from oxidative burst. Studies suggest that GSTs safeguard the cells toxicity and provide tolerance by catalyzing S-conjugation between the thiol group of GSH and electrophilic moiety in the hydrophobic and toxic substrate (Kumar and Trivedi 2018). In this study we can anticipate GSTs might catalyze the Se-conjugation and infer tolerance against ROS and ions. Similar results were obtained in salt-stressed barley and arsenic-stressed mungbean, where Se application increased GST activity (Halušková et al. 2009; Mittler et al. 2004). Guaiacol peroxidase (GPOX/POD) aids in defense against abiotic stresses by H2O2 detoxification. In our study, salt-treated plants increased POD activity, and 1 µM Se further enhanced its activity. Similar results were found in salt-stressed mungbean and rice (Panda 2001; Yamane et al. 2009). A comparable effect of Se on GPOX activity was reported in the cold stressed wheat and potato (Seppänen et al. 2003; Chu et al. 2010). Another deleterious effect of salinity is ionic stress. Ion homeostasis plays a vital role under salt stress; K+ ion status determines the tolerance of plants toward salinity (Assaha et al. 2017). In this study, an apparent decrease in K+ and increased Na+ was observed in plants, which disturbed the K+/Na+ ratio, when exposed to salinity stress. However, the application of Se reversed the ionic imbalance caused by salinity stress and improved the K+/Na+ ratio.
The improved K+/Na+ ratio under Se can be attributed to its effect on the respective ion channels. Thus, we can assume the selenium regulate the ion channels in order to maintain the K+/Na+ ratio and protect the plant from ion toxicity. Similar results were obtained in rice, garlic, and dill, where Se improved the ratio of K+/Na+ under salinity stress (Subramanyam et al. 2019; Astaneh et al. 2018; Shekari et al. 2017; Jiang et al. 2017). The net Na+ efflux capacity under salt stress can determine the extent of salt tolerance. In contrast, the net uptake of Na+ depends on the loading and unloading of Na+ into the xylem and its recirculation in the phloem (Wu 2018). Non-selective cation channels (NSCCs) and high-affinity potassium transporter (HKT) are the primary transporters through which Na+ moves into cells (Ward et al. 2003). Also, SOS1 (Na+/H+ antiporter salt overly sensitive1) can squeeze out excessive Na+ into the apoplast through the plasma membrane and also loads Na+ into the xylem (Wang et al. 2019). The expression of genes PMSOS1, PMSOS2, PMSOS3, PMHKT, and PMNHX, was examined to investigate the Se mediated tolerance in proso millet plants. The expression of all the genes under consideration was significantly higher in Se-treated plants under NaCl stress. The SOS pathway mediates Na+ exclusion, restricts K+ efflux, maintains ion homeostasis and maintains the selectivity of the plasma membrane, thereby confers salt tolerance (Ji et al. 2013; Yang et al. 2015). Upon NaCl exposure, the elevated NaCl concentrations are sensed by SOS3 (through Ca2+), which activates SOS2 and stimulates the membrane-localized Na+/H+ antiporter SOS1 (Ji et al. 2013). SOS1 controls the long-distance transport of Na+ and extrudes Na+ from the roots (Olías et al. 2009; Shi et al. 2002). In this study, the expression of PMSOS1, PMSOS2, and PMSOS3, was elevated by salt treatments. Also, the Se application further enhanced the application of these genes, which corroborated the study in Populus euphratica, in which PeSOS1expression was upregulated fivefold to tenfold by applying 200 mM NaCl for 24 h relative to the untreated control leaves (Wu et al. 2007). Similarly, PabSOS1 expression was about five times higher after 12 h of NaCl treatment (Tang et al. 2010). Furthermore, we observed the upregulation of Pmhkt and Pmnhx under salinity and Se treatment. Various studies have reported that under Se application, genes like MAPK5 (mitogen-activated protein kinase 5), CPK11 (calcium-dependent protein kinase11), MAPK7(mitogen-activated protein kinase7), and NHX1 (Sodium/hydrogen exchanger1) were upregulated (Jiang et al. 2017; Shi et al. 2010; Subramanyam et al. 2019).
The application of low levels of Se (1 μM) alleviated the inhibitory effect of high salinity via different mechanisms. Low levels of Se activated the antioxidant defence system to alleviate ROS damage and ameliorated ion homeostasis in proso millet under salt stress by upregulating SOS1, SOS2, SOS3, NHX and HKT genes. Thus, this study provides evidence that low Se application can enhance plant salt tolerance, and our results increase the understanding of the precise role of Se in the response of plants to salinity.
Conclusion
Proso millet adapts to salt stress by maintaining suitable Na+/K+ ratios, accumulating osmolytes, activating antioxidative enzymes, and activating stress response signalling networks to relegate the toxic effects of high salinity. Our study provides a piece of extensive evidence that Se in low concentration enhances the tolerance of proso millet towards salinity. Our study adds to the understanding of the role of Se in salt tolerance, however complete understanding of the role of Se as well as detailed protective mechanisms would be helpful for developing salinity tolerance in plants. The Se transformations in the plants is still unclear. Therefore, future studies are required to explore the exact mechanisms involved in Se transformations. There is still a need to better understand its ameliorative roles in different plant species under various environmental factors for the confirmation of the Se-mediated amelioration of salt stress.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Acosta-Motos JR, Diaz-Vivancos P, Álvarez S, Fernández-García N, Sanchez-Blanco MJ, Hernández JA (2015) Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 242(4):829–846
Aebi H (1984) Catalase in vitro. Methods in enzymology, vol 105. Elsevier, Amsterdam, pp 121–126
Ahmad P, John R, Sarwat M, Umar S (2012) Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2(4):353–366
Ahmad P, Kumar A, Ashraf M, Akram NA (2012) Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol 11(11):2694
Amadou I, Gounga ME, Le GW (2013) Millets: Nutritional composition, some health benefits and processing—a review. Emir J Food Agric 25:501–508
Asada KJ (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Assaha DV, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Astaneh RK, Bolandnazar S, Nahandi FZ, Oustan SJ (2018) The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf Process Agric 5(1):156–161
Babitha K, Vemanna RS, Nataraja KN, U M (2015) Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE 10(9):e0137098
Bandeoğlu E, Eyidoğan F, Yücel M, Öktem HA (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul 42(1):69–77
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15(3):413–428
Betzen BM, Smart CM, Maricle KL MariCle BR (2019) Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Trans Kansas Acad Sci 122(1–2):49–58
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Bueno P, Piqueras A, Kurepa J, Savouré A, Verbruggen N, Van Montagu M, Inzé D (1998) Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci 138(1):27–34
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83(3):463–468
Chelli-Chaabouni A, Mosbah AB, Maalej M, Gargouri K, Gargouri-Bouzid R, Drira N (2010) In vitro salinity tolerance of two pistachio rootstocks: pistacia vera L. and P. atlantica Desf. Environ Exp Bot 69(3):302–312
Chen L, Song Y, Li S, Zhang L, Zou C, Yu D (2012) The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta Gene Regul Mech 1819(2):120–128
Correa-Aragunde N, Foresi N, Delledonne M, Lamattina L (2013) Auxin induces redox regulation of ascorbate peroxidase 1 activity by Sitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J Exp Bot 64(11):3339–3349
Chu J, Yao X, Zhang ZJ (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136(3):355–363
Comba M, Benavides M, Gallego S, Tomaro M (1997) Relationship between nitrogen fixation and oxidative stress induction in nodules of salt-treated soybean plants. Phyton
Das D, Das P, Biswas AK (2018) Regulation of growth and carbohydrate metabolism in rice (Oryza sativa L.) seedlings by selenium and sulphate. J Plant Stud 7(1):61
Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB (2014) Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol 51(6):1021–1040
Dey P (1990) Oligosaccharides. Methods in plant biochemistry, vol 2. Elsevier, Amsterdam, pp 189–218
Dey G, Banerjee P, Sharma RK, Maity JP, Etesami H, Shaw AK et al (2021) Management of phosphorus in salinity-stressed agriculture for sustainable crop production by salt-tolerant phosphate-solubilizing bacteria—a review. Agronomy 11(8):1552
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H-y (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33(3):671–682
Diaz-Vivancos P, Barba-Espín G, Hernández JA (2013) Elucidating hormonal/ROS networks during seed germination: insights and perspectives. Plant Cell Rep 32(10):1491–1502
Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U (2005) Selenium–an antioxidative protectant in soybean during senescence. Plant Soil 272(1–2):77–86
Djanaguiraman M, Prasad PV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48(12):999–1007
Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, Karimi F, Mozafari AM (2021) COVID-19 and selenium deficiency: a systematic review. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02997-4
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Ferreira RLU, Sena-Evangelista KCM, de Azevedo EP, Pinheiro FI, Cobucci RN, Pedrosa LFC (2021) Selenium in human health and gut microflora: bioavailability of selenocompounds and relationship with diseases. Front Nutr 8:292
Food and agricultural organization of the united nations—FAO (2019) Salt-affected soils. Rome, Italy. Available in http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/en/ Accessed on 15 Sep 2019
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133(1):21–25
Gapińska M, Skłodowska M, Gabara B (2008) Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30(1):11
Gengmao Z, Shihui L, Xing S, Yizhou W, Zipan CJ (2015) The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci Rep 5:12696
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70(2):303–307
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-Transferases. Methods in enzymology, vol 77. Elsevier, Amsterdam, pp 398–405
Halušková L, Valentovičová K, Huttová J, Mistrík I, Tamás L (2009) Effect of abiotic stresses on glutathione peroxidase and glutathione S-transferase activity in barley root tips. Plant Physiol Biochem 47(11–12):1069–1074
Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R (2003) Salinity effects on antioxidant enzymes in mulberry cultivar. Sci Asia 29(2):109–113
Hartikainen H, Xue T (1999) The promotive effect of selenium on plant growth as triggered by ultraviolet irradiation. J Environ Qual 28(4):1372–1375
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143(3):1758–1776
Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143(3):1704–1721
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23(2):249–268
Hashem HA, Hassanein RA, Bekheta MA, El-Kady FAJ (2013) Protective role of selenium in canola (Brassica napus L.) plant subjected to salt stress. Egypt J Exp Biol 9(2):199–211
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Elem Res 132(1–3):259–269
Hawrylak-Nowak B, Hasanuzzaman M, Matraszek-Gawron R (2018) Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 269–295
Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F (2001) Antioxidant systems and O.2−/H2O2 production in the apoplast of pea leaves Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127(3):817–831
Inan G, Zhang Q, Li P, Wang Z, Cao Z, Zhang H, Zhang C, Quist TM, Goodwin SM, Zhu J (2004) Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol 135(3):1718–1737
Iqbal M, Hussain I, Liaqat H, Ashraf MA, Rasheed R, Rehman AU (2015) Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol 94:95–103
Isayenkov S (2012) Physiological and molecular aspects of salt stress in plants. Cytol Genet 46(5):302–318
Jawad Hassan M, Ali Raza M, Khan I, Ahmad Meraj T, Ahmed M, Abbas Shah G et al (2020) Selenium and salt interactions in black gram (Vigna mungo L): ion uptake, antioxidant defense system, and photochemistry efficiency. Plants 9(4):467
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Mol Plant 6(2):275–286
Jiang CQ, Ling Tong Q, Feng S, Na Y, Chang Hai W, Xiao Ming Y, Zheng QS (2014) Distribution of mineral nutrients and active ingredients in Aloe vera irrigated with diluted seawater. Pedosphere 24(6):722–730
Jiang C, Zu C, Shen J, Shao F, Li T (2015) Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc Bot Pol 84(1):71–77
Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q (2016) Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol Plant 38(4):82
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li DJ (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039
Jóźwiak W, Politycka B (2019) Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 8(7):217
Karimi M, Ahmadi A, Hashemi J, Abbasi A, Tavarini S, Pompeiano A et al (2019) Plant growth retardants (PGRs) affect growth and secondary metabolite biosynthesis in Stevia rebaudiana Bertoni under drought stress. S Afr J Bot 121:394–401
Kerepesi I, Galiba GJ (2000) Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40(2):482–487
Kim SK, Nair RM, Lee J, Lee S-H (2015) Genomic resources in mungbean for future breeding programs. Front Plant Sci 6:626
Kirova E (2020) Effect of nitrogen nutrition source on antioxidant defense system of soybean plants subjected to salt stress. In: Comptes rendus de l’Académie bulgare des Sciences
Kolbert Z, Lehotai N, Molnár Á, Feigl G (2016) “The roots” of selenium toxicity: a new concept. Plant Signal Behav 11(10):e1241935
Kole C, Muthamilarasan M, Henry R, Edwards D, Sharma R, Abberton M, Batley J, Bentley A, Blakeney M, Bryant J (2015) Application of genomics-assisted breeding for generation of climate resilient crops: progress and prospects. Front Plant Sci 6:563
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45(2):155–163
Kukreja S, Nandwal A, Kumar N, Sharma S, Unvi V, Sharma P (2005) Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49(2):305–308
Kumar P, Sharma PK (2020) Soil salinity and food Security in India. Front Sustain Food Syst 4:174
Kumar S, Trivedi PK (2018) Glutathione S-transferases: role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci 9:751
Lechno S, Zamski E, Tel Or E (1997) Salt stress-induced responses in cucumber plants. J Plant Physiol 150(1–2):206–211
Li J, Cui J, Cheng D, DaiC LT, Wang C, Luo C (2020) iTRAQ protein profile analysis of sugar beet under salt stress: different coping mechanisms in leaves and roots. BMC Plant Biol 20(1):1–12
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in enzymology, vol 148. Elsevier, Amsterdam, pp 350–382
Lobanov AV, Hatfield DL, Gladyshev VN (2008) Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol 9(3):R62
Luo H, He L, Lai R, Liu J, Xing P, Tang X (2021) Selenium applications enhance 2-acetyl-1-pyrroline biosynthesis and yield formation of fragrant rice. Agron J 113(1):250–260
Mancinelli AL (1990) Interaction between light quality and light quantity in the photoregulation of anthocyanin production. Plant Physiol 92(4):1191–1195
McDonough CM, Rooney LW, Serna-Saldivar SO (2000) The millets. Food science technology. Marcel Dekker, New York, pp 177–202
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Monteiro CC, Carvalho RF, Gratão PL, Carvalho G, Tezotto T, Medici LO, Peres LE, Azevedo RA (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ Exp Bot 71(2):306–320
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nawaz F, Ashraf M, Ahmad R, Waraich E, Shabbir R, Bukhari MJ (2015) Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem 175:350–357
Nawaz F, Naeem M, Ashraf MY, Tahir MN, Zulfiqar B, Salahuddin M, Shabbir RN, Aslam MJ (2016) Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front Plant Sci 7:1438
Olías R, Eljakaoui Z, Li J, De Morales PA, Marín-manzano MC, Pardo JM, Belver A (2009) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant, Cell Environ 32(7):904–916
Panda S (2001) Oxidative response of green gram seeds under salinity stress. Indian J Plant Physiol 6(4):438–440
Qin H, Huang R (2020) The phytohormonal regulation of Na+/K+ and reactive oxygen species homeostasis in rice salt response. Mol Breeding 40(5):1–13
Rao A, Ahmad S, Sabir S, Awan S, Hameed A, Abbas S, Shehzad M, Khan M, Shafique S, Ahmad Z (2013) Detection of saline tolerant wheat cultivars (Triticum aestivum L.) using lipid peroxidation, antioxidant defense system, glycine-betaine and proline contents. J Anim Plant Sci 23:1742–1748
Rasool A, Shah WH, Tahir I, Alharby HF, Hakeem KR, Rehman RUL (2020) Exogenous application of selenium (Se) mitigates NaCl stress in proso and foxtail millets by improving their growth, physiology and biochemical parameters. Acta Physiol Plant 42(7):1–13
Rasool A, Shah WH, Mushtaq NU, Saleem S, Hakeem KR, Rehman RUL (2022) Amelioration of salinity induced damage in plants by selenium application: a review. S Afr J Bot 147:98–105
Sabagh AE, Hossain A, Barutçular C, Iqbal MA, Islam MS, Fahad S, Erman M (2020) Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: an outlook of arid and semi-arid regions. Environment, Climate, Plant and Vegetation Growth. Springer, Cham, pp 503–533
Sairam R, Tyagi AJ (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 83:407–421
Seppänen M, Turakainen M, Hartikainen H (2003) Selenium effects on oxidative stress in potato. Plant Sci 165(2):311–319
Shah WH, Rasool A, Tahir I, Rehman RU (2020) Exogenously applied selenium (Se) mitigates the impact of salt stress in Setaria italica L. and Panicum miliaceum L. Nucleus 63(3):327–339
Shah WH, Rasool A, Saleem S, Mushtaq NU, Tahir I, Hakeem KR, Rehman RU (2021) Understanding the integrated pathways and mechanisms of transporters, protein kinases, and transcription factors in plants under salt stress. Int J Genom 2021:1–16
Shekari F, Abbasi A, Mustafavi SH (2017) Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J Saudi Soc Agric Sci 16(4):367–374
Shi H, Quintero FJ, Pardo JM, Zhu J-KJ (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14(2):465–477
Shi J, An HL, Zhang L, Gao Z, Guo XQ (2010) GhMPK7, a novel multiple stress-responsive cotton group C MAPK gene, has a role in broad spectrum disease resistance and plant development. Plant Mol Biol 74(1–2):1–17
Shu S, Yuan Y, Chen J, Sun J, Zhang W, Tang Y, Zhong M, Guo SJ (2015) The role of putrescine in the regulation of proteins and fatty acids of thylakoid membranes under salt stress. Sci Rep 5:14390
Storey R (1995) Salt tolerance, ion relations and the effect of root medium on the response of citrus to salinity. Funct Plant Biol 22(1):101–114
Subramanyam K, Du Laing G, Van Damme EJJ (2019) Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front Plant Sci 10:116
Tabot P, Adams J (2013) Early responses of Bassia diffusa (Thunb.) Kuntze to submergence for different salinity treatments. S Afr J Bot 84:19–29
Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, Narusaka Y, Narusaka M, Zhu J-K, Shinozaki K (2004) Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol 135(3):1697–1709
Tang RJ, Liu H, Bao Y, Lv QD, Yang L, Zhang HX (2010) The woody plant poplar has a functionally conserved salt overly sensitive pathway in response to salinity stress. Plant Mol Biol 74(4–5):367–380
Tanveer M, Shahzad B, Sharma A, Biju S, Bhardwaj R (2018) 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant Physiol Biochem 130:69–79
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Tsuji PA, Hatfield DL (2022) Editorial to special issue molecular biology of selenium in health and disease. Int J Mol Sci 23(2):808
Van Raamsdonk JM, Hekimi S (2012) Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci 109(15):5785–5790
Wang CQ (2011) Water-stress mitigation by selenium in Trifolium repens L. J Plant Nutr Soil Sci 174(2):276–282
Wang YD, Wang X, Wong YS (2012) Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J Proteomics 75(6):1849–1866
Wang Q, Guan C, Wang P, Ma Q, Bao AK, Zhang JL, Wang SM (2019) The Effect of AtHKT1; 1 or AtSOS1 mutation on the expressions of Na+ or K+ transporter genes and ion homeostasis in Arabidopsis thaliana under salt stress. Int J Mol Sci 20(5):1085
Ward JM, Hirschi KD, Sze HJ (2003) Plants pass the salt. Trends Plant Sci 8(5):200–201
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66(3):695–707
Whitaker JR, Bernhard RA (1972) Experiments for: an introduction to enzymology. The Whiber Press, Davis, pp 12–14
Williams MM, Boydston RA, Davis AS (2007) Wild proso millet (Panicum miliaceum) suppressive ability among three sweet corn hybrids. Weed Sci 55(3):245–251
Wu HJ (2018) Plant salt tolerance and Na+ sensing and transport. Crop J 6(3):215–225
Wu Y, Ding N, Zhao X, Zhao M, Chang Z, Liu J, Zhang L (2007) Molecular characterization of PeSOS1: the putative Na+/H+ antiporter of Populus euphratica. Plant Mol Biol 65(1–2):1
Xie E, Wei X, Ding A, Zheng L, Wu X, Anderson B (2020) Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 12(2):569
Yamane K, Mitsuya S, Kawasaki M, Taniguchi M, Miyake H (2009) Antioxidant capacity and damages caused by salinity stress in apical and basal regions of rice leaf. Plant Prod Sci 12(3):319–326
Yang Y, Tang RJ, Jiang CM, Li B, Kang T, Liu H, Zhao N, Ma XJ, Yang L, Chen SL (2015) Overexpression of the P t SOS 2 gene improves tolerance to salt stress in transgenic poplar plants. Plant Biotechnol J 13(7):962–973
Zhang Y, Lai J, Sun S, Li Y, Liu Y, Liang L, Chen M, Xie Q (2008) Comparison analysis of transcripts from the halophyte Thellungiella halophila. J Integr Plant Biol 50(10):1327–1335
Zhang Y, Wang Y, Wen W, Shi Z, Gu Q, Ahammed GJ, Guo S (2021) Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 17(10):2876–2890
Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14(8):436–442
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. G:630-130-1441. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. G:630-130-1441. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author information
Authors and Affiliations
Contributions
AR, WS, SAP and RUR conceived and designed the experiments. AR, WS performed the experiments and collected the data. IT, RUR, HFA and KRH helped to perform the analysis and write up of this paper. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest among them.
Additional information
Communicated by Tariq Aftab.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasool, A., Hafiz Shah, W., Padder, S.A. et al. Exogenous selenium treatment alleviates salinity stress in Proso Millet (Panicum miliaceum L.) by enhancing the antioxidant defence system and regulation of ionic channels. Plant Growth Regul 100, 479–494 (2023). https://doi.org/10.1007/s10725-022-00826-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00826-9