Abstract
Drought is a major limiting factor to maize (Zea mays L.) yield. Plant hormones, including gibberellins (GAs), play important roles in plant response to drought stress. In previous studies, significant reductions in GAs levels have been reported under drought stress. In maize, GA content is correlated to drought tolerance, but the molecular mechanism remains unclear. In the present study, AtG2ox1, a member of the GA2ox family with a clear function, was used to create GA deficiency maize. The transgenic maize had a higher chlorophyll content and faster growth rate, when compared to the wild type (WT) plants, under drought stress in a greenhouse. The physiological and biochemical test results revealed that transgenic maize had decreased levels of GA1 and malondialdehyde (MDA), and increased content of proline and soluble sugars, and antioxidant enzyme activities, when compared to the WT. Furthermore, the transcriptomic analysis revealed that some differentially expressed genes involved in transcription factors correlated to drought stress and abiotic stress responses, and that signaling was enriched. All these results reveal the possible molecular mechanism of GA regulation in drought tolerance, in which the overexpression of AtGA2ox1 altered the expression of multiple genes correlated to the internal antioxidant system and maintenance of cell osmotic potential. The present study demonstrates that the overexpression of AtGA2ox1 could control GA content and improve drought tolerance in transgenic maize. Furthermore, this strategy represents a novel approach to address drought tolerance in maize breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize is one of the most widely consumed and valuable staple food worldwide. Drought is presently one of the major factors that severely limit maize growth and productivity (Edmeades et al. 2000), causing 15–20% loss of production annually. As global climate change progresses, drought due to severely arid climates has become a severe threat (FAOSTAT 2010), particularly in regions that rely on in-season rainfall. Therefore, improving drought tolerance in maize has been the most daunting challenge.
Plant hormones, including gibberellins (GAs), have long been known to be highly involved in the regulation of plant growth and development. Previous studies have extensively revealed GA metabolic patterns and mechanisms of action (Yamaguchi 2008), and further cloned the majority of GA-associated genes from Arabidopsis thaliana and rice (Thomas et al. 1999; Hedden and Phillips 2000; Sakamoto et al. 2004).
Abiotic stresses, including drought, often elicit changes in GA metabolic pathways, leading to the promotion of specific protective mechanisms in plants. For instance, drought results in the rapid decrease in levels of GAs in maize leaves (Wang et al. 2008; Nelissen et al. 2018). Previous studies have revealed the enhancement of salt tolerance in plants by reducing the level of bioactive GAs. Magome et al. (2004) reported the higher survival rate (92.7%) of GA-deficient Arabidopsis mutant, ga1-3, in high-salinity stress conditions, when compared to wild-type (WT) plants (52.7%). Paclobutrazol (PBZ) is a GA inhibitor that is responsible for inducing stress tolerance in plants by increasing antioxidant enzyme activity (Somasundaram et al. 2009). Conversely, the external application of GAs would increase plant sensitivity to abiotic stress. Furthermore, the survival rate of Arabidopsisga1-3 mutants sprayed with exogenous GAs decreased by 54.5% under high-salinity stress (Magome et al. 2004). These studies suggest the important role for GAs in plant response to stress conditions (Colebrook et al. 2014).
The overexpression of the GA 2-oxidase (AtGA2ox) gene has been proven to offer a direct approach to decrease endogenous levels of GAs in the model plant A. thaliana (Schomburg et al. 2003; Biemelt et al. 2004; Lee and Zeevaart 2005; Dijkstra et al. 2008; Huang et al. 2010a, b). Furthermore, previous studies have revealed the critical role of the GA2ox gene family in the GA metabolic pathway by metabolizing bioactive GAs into non-bioactive GAs in higher plants (Yamaguchi 2008; Han and Zhu 2011). Achard et al. (2008a, b) revealed that the overexpression of AtGA2ox increased the expression of genes related to reactive oxygen species (ROS)-detoxification enzymes, resulting in improved stress tolerance in A. thaliana. Shan et al. (2013) reported a significant increase in GA2ox expression level under drought stress in maize inbred line Zheng58, suggesting the active regulation of adaptation to drought by bioactive GAs (Biemelt et al. 2004; Lee and Zeevaart 2005; Dijkstra et al. 2008; Huang et al. 2010a, b). Furthermore, the expression of Oryza sativa GA 2-oxidase 5 (OsGA2ox5) in transgenic Arabidopsis and rice lines resulted in higher tolerance to high-salinity stress (Shan et al. 2014), indicating that the GA2ox gene is involved in abiotic stress resistance. Although many studies have shown that GA is closely correlated to drought, the molecular mechanisms remain unclear, in terms of whether the method of regulating endogenous GA content can affect the drought tolerance of maize.
In maize, 10 ZmGA2ox family members were found by bioinformatic analysis, and the expression patterns during seed germination were reported in a previous study (Song et al. 2011). To the best of our knowledge, none of ZmGA2ox’s functions were analyzed in detail, and it remains unknown which function controls the bioactive GA levels. In the present study, GA deficiency model was created in maize by expressing a heterologous GA oxidase, AtGA2ox1, which is known to decrease endogenous GAs and regulate the architecture in A. thaliana. The molecular mechanisms of the endogenous GA content in conferring maize drought tolerance were further explored through a series of physiological and biochemical experiments, and differential transcriptome analysis. The present study provides a method for producing a GA-deficiency model in maize through the exogenous expression GA 2-oxidase, supporting the notion that the overexpressed AtGA2ox gene can substantially improve plant architecture and drought tolerance in maize. In addition, the possible molecular mechanisms of AtGA2ox1 for improving maize drought resistance were also proposed in the present study.
Materials and methods
Construction of plant expression vectors and Agrobacterium-mediated transformation
The AtGA2ox1 gene (GenBank accession no. AT1g78440) was cloned into the monocot expression vector pCAM3300, which was named as pCAM3300-GA2ox. The ubiquitin promoter was used to drive the expression of the AtGA2ox1 gene (Fig. 1a). Transgenic maize plants were produced by using Agrobacterium-mediated transformation, as described by Ishida et al. (2007).
Overexpression of AtGA2ox1 in maize. a The map of pCAM3300-GA2ox vector, b expression levels of AtGA2ox1 in different transgenic lines detected by qRT-PCR. All reactions were performed in three biological duplications, and each biological duplication had three technical replicates, c the GA1 content in 5-week-old WT and T2 generation transgenic plants, d the plant heights of WT and different transgenic lines at flowering stage, and e the plant phenotype at flowering stage. Bar = 10 cm. The data were presented as mean ± SE. The asterisks indicate the significant differences between transgenic lines (#1, #2 and #4) and WT plants (t-test, ***P < 0.001)
Measurement of GA1 content
GA1 was extracted and purified according to the protocol of Yang et al. (2014). Approximately 1 g of leaf tissues were sampled from 5-week-old seedlings. Then, these were homogenized with ice-cold 80% methanol at 4 °C for 12 h to extract the GAs. Next, the extracts were centrifuged at 8000 × g for 10 min at 4 °C. Then, the supernatants were allowed to pass through the Sep-Pak C18 columns (Waters Corporation) preconditioned with 100% and 80% methanol. Afterwards, the eluant was collected, dried and re-dissolved in a mixture of methanol–1% acetic acid (2:3, v/v) for the high-performance liquid chromatography analysis. The GA contents were assayed using the method of Urbanová et al. (2013). Three biological replicates were analyzed for each sample.
Phenotyping and relative chlorophyll content analysis
Plant height, which was defined as the length from the first internode to the top of the first leaf, was measured with five replicates from 3-week-old seedlings in the greenhouse. Relative chlorophyll content was estimated from the third expanded leaf with three replicates using SPAD-502 (Konica Minolta, Japan).
Drought treatment for transgenic maize plants
T2 generation transgenic lines (#1, #2 and #4) and WT plants were grown in pots (10 × 10 cm) containing peat-vermiculite (5:1, v/v) medium in a greenhouse at 27 ± 2 °C under natural light. PCR analysis was used to screen for positive transformants, from which watering was suspended for 10 plants from each line at 21 days old for 9 days for drought treatment. Then, the leaves were collected from each line at the end of treatment, and stored at − 80 °C for further analysis. The amount of substrate and water for each of the experimental materials were applied at equal levels.
Physiological and biochemical analysis
Proline content was analyzed by ninhydrin colorimetric assay (Bates et al. 1973). The soluble sugar content was measured by anthrone colorimetric assay, as previously described (Yemm and Willis 1954). Malondialdehyde (MDA) content was estimated following a previously described protocol (Gilbert et al. 1984). SOD, CAT and POD activity was measured, as previously described by Hodges et al. (1999), Beers and Sizer (1952), and Upadhyaya et al. (1985), respectively. For all the above measurements, three replicates per line were used. Statistical differences were determined using Student’s two-tailed t test.
Statistical analysis
In experiments for the GA1 measurement, phenotyping and relative chlorophyll content analysis, drought treatment, and physiological/biochemical analysis, the data were statistically analyzed, and the means of each transgenic line (#1, #2 and #4) and WT were compared using t-test, and were calculated using the statistical software GraphPad version 7.0 (San Diego, CA, USA). The data were presented as the mean and standard error (SE) of the mean. All tests had three replicates.
RNA-seq analysis
RNA sequencing was performed on T2 plants from transgenic line #1, and WT plants were used as a control. Leaf tissues were harvested in plants at stages V5 (Vegetative 5, five fully extended leaves) for T2 plants in a controlled chamber. The RNAs were pooled from 10 transgenic plants and 10 control plants, and these were randomly divided into three groups as three biological replicates in both the transgenic plants and control. The sequencing libraries were generated using a NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, USA), according to manufacturer’s recommendations, and index codes were added to attribute the sequences to each of the samples. Clean reads (http://www.ncbi.nlm.nih.gov/bioproject/359734) were generated by removing the adaptor, low-quality sequence reads were obtained from the raw datasets, and these were mapped to the maize reference genome sequence (http//ftp.ensemblgenomes.org/pub/plants/release-32/fasta/zea_mays/) using Tophat2 software tools. Reads with only a perfect match or one mismatch with the reference genome were further analyzed and annotated based on the following databases: KEGG Ortholog (KO) database (https://www.kegg.jp/kegg/ko.html), and Gene Ontology (GO) database (http://www.geneontology.org). The gene expression levels were quantified to fragments per kilobase of the transcript per million fragments mapped. The read counts for each sequenced library were adjusted in the edgeR program package by one scaling normalized factor, and applied to the differential expression analysis using the DEGseq (2010) R package. The P-value was adjusted using the q-value (Storey and Tibshirani 2003). A Q-value of < 0.005 and a log2 (fold change) of ≥ 1 were set as the thresholds for significant differential expression.
Real-time PCR (qRT-PCR) analysis
Total RNA from T2 transgenic event #1 was isolated from 0.1 g of flash-frozen, tissue samples using an EasyPure Plant RNA Kit (TransGen Biotech, China). Then, a TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, China) was used to synthesize the cDNA. The quantitative real-time gene expression was determined through gene-specific primers using TB Green™ Premix Ex Taq™ (Tli RNaseH Plus), ROX plus (Takara, China) on an ABI7900HT system (Applied Biosystems, USA). All reactions were performed in three biological duplications. The ZmGAPDH gene (GenBank accession no. X07157) was used as an internal control. Statistical analysis was performed using the 2−ΔΔCT method (Livak and Schmittgen 2001). The gene-specific primers are listed in Table S1.
Results
AtGA2ox1 gene overexpression results in the GA deficient phenotype in maize
GA deficiency may induce plant stress response. In order to determine whether reducing the bioactive GA content can improve the drought tolerance of maize, GA-deficient maize was created by overexpressing AtGA2ox1 (Fig. 1a). Three individual transgenic events with different increased AtGA2ox1 expression levels were identified (Fig. 1b). Among these, transgenic line #1 had the highest expression level of AtGA2ox1, followed by transgenic line #2 and #4. In order to further determine whether the overexpression of the AtGA2ox1 gene affected the GA content in maize, GA1 content was tested among the three independent transgenic events. Compared with the WT plants, the GA1 content was reduced by 49.5–74% in transgenic maize (Fig. 1c). Compared to the WT, the activation of AtGA2ox1 in transgenic maize created a dominant semi-dwarf phenotype, and there was a dramatic change in plant height from the jointing stage to the mature stage (Fig. 1d, e). Our results support the notion that the overexpression of AtGA2ox1 could create a GA-deficient phenotype in maize.
AtGA2ox1 gene overexpression enhances tolerance to drought
In order to determine the effects of AtGA2ox1 overexpression on drought tolerance, three T2 transgenic maize lines (#1, #2 and #4) were evaluated in drought stressed conditions. After 3 weeks in the greenhouse under normal conditions, the plant height was found to be slightly different, and there were no other visible differences in phenotype between the transgenic plants and control plants (Fig. 2a). When water was withheld for 9 days, the control plants exhibited a visibly wilting phenotype, while plants of the three transgenic events suffered less damage (Fig. 2b). In drought stress conditions, the chlorophyll content and plant growth rate were dynamically detected. Before drought stress, the transgenic maize had a higher chlorophyll content and shorter plant height, when compared to the WT. When water was withheld, the WT plants grew slower than the transgenic maize under drought stress (Fig. 2c), and the rate of decline in chlorophyll content was significantly slower than the WT plants (Fig. 2d). These results reveal that the transgenic maize had less impact on growth and development under drought stress, when compared to the WT.
The performance of transgenic maize with overexpressed AtGA2ox1 under drought treatment. a The growth performance of WT and transgenic lines (#1, #2 and #4) before drought stress, b the growth performance of WT and transgenic lines (#1, #2 and #4) after 9 days drought stress, c the growth rate of WT and transgenic lines (#1, #2 and #4), and d chlorophyll SPAD values of the third expanded leaves of WT and transgenic lines (#1, #2 and #4). WT and T2 generation transgenic plants were grown in a greenhouse for 21 days and then stopped to water for 9 days. The values were measured during the 9 days of drought treatment. Bar = 10 cm. The data were presented as mean ± SE (n = 3)
AtGA2ox1 gene overexpression increases proline and soluble sugar content, reduces MDA content, and enhances antioxidant enzyme activities under drought stress
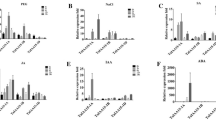
Amino acid proline, soluble sugar, MDA and antioxidant enzymes have been identified as crucial indicators in many plant species in response to environmental stresses, and these have been proposed to play an important role in the adjustment of osmotic and oxidative stresses caused by drought tolerance. In the present study, these indicators were tested. As shown in Fig. 3a, b, all stressed plants contained higher concentrations of proline and soluble sugars, and the levels in the transgenic lines were 1.54–2.58- and 1.4–1.79-fold of those in control plants, respectively. These results indicate that the overexpression of AtGA2ox1 could increase the accumulation capacity of proline and soluble sugars in transgenic maize. The MDA content of transgenic maize after 9 days of drought stress treatment was reduced by 20.7–38.3%, when compared to the WT (Fig. 3c), leading to high levels of tolerance to drought. Antioxidant reductases, including SOD, CAT and POD, have been reported to be associated with the elimination of ROS. In the present study, compared to the WT, the activity levels of SOD, CAT and POD in transgenic maize increased to 1.98–2.49-fold (Fig. 3d), 1.29–2.22-fold (Fig. 3e), and 1.68–2.27-fold (Fig. 3f), respectively, when under drought stress conditions. The present result supports the notion that AtGA2ox1-OE increases antioxidant enzyme activity, reduces the accumulation of ROS, and decreases oxidative damage to the cell membrane in maize.
Comparisons of solute content and antioxidant enzyme activity in transgenic maize and the WT under drought treatment. WT and T2 generation transgenic plants were grown in a greenhouse for 21 days and then stopped to water for 9 days. The proline contents (a), soluble sugar contents (b), MDA (c), SOD (d), CAT (e), and POD (f) were measured in WT and transgenic plants after 9 days drought treatment. The data were presented as the mean ± SE (n = 3). The asterisks indicate the significant differences between transgenic lines (#1, #2 and #4) and the WT (t-test, ***P < 0.001)
Differential transcriptome analysis between transgenic maize and control plants
GA is an essential hormone that regulates growth and development throughout the entire life cycle of plants (Yamaguchi 2008). Altered GA content may impact many aspects of plant growth and developmental processes. In order to better understand how GA1 deficiency improves maize drought tolerance, a whole genomic transcriptomics profiling was performed for the leaf of transgenic line #1 and the WT plants as a control. Then, the RNA-seq data were further analyzed, and the differentially expressed genes (DEGs) were identified. The consistency was observed between three replications, and the large transcriptional differences were found between transgenic and WT plants. Based on stringent statistics and filtering, the expression of 3064 genes changed due to the ectopic expression of AtGA2ox1, with a total 1943 and 1121 genes being upregulated and downregulated at the V5 leaf stages in transgenic maize. In order to confirm the transcriptomic data, 16 genes, including eight upregulated genes and eight downregulated genes, were selected (Table S4), and the expression was validated by qRT-PCR. The expression patterns of these 16 genes were consistent with the trend of the transcriptome data, proving that the transcriptome data was accurate (Fig. S1).
GO functional enrichment analysis was performed with the DEGs from the comparisons between transgenic line #1 and the WT (Table S2). A total of 2464 DEGs were annotated, and 35 pathways associated with drought stress in the GO biological processes (Fig. 4), including response to stress (GO:0006950), response to heat (GO:0009408), response to hydrogen peroxide (GO:0042542), fatty acid beta-oxidation (GO:0006635), toxin catabolic process (GO:0009407), cellular response to water deprivation (GO:0042631), heat acclimation (GO:0010286), response to ROS (GO:0000302), response to water (GO:0009415) and GA catabolic process (GO:0045487), were significantly upregulated. However, the gibberellic acid mediated signaling pathway (GO:0009740) and GA biosynthetic process (GO:0009686) were significantly downregulated. In addition, a KEGG analysis was performed in the present study. It was revealed that the enrichment of 333 DEGs in 24 pathways were combined with drought stress, and more than two-thirds of these DEGs were enriched in the pathway of protein processing, endoplasmic reticulum, plant hormone signal transduction, biosynthesis of amino acids, starch and sucrose metabolism, glutathione metabolism, glycerophospholipid metabolism, and carbon metabolism (Table 1). Furthermore, five of these pathways, including protein processing in the endoplasmic reticulum, galactose metabolism, fatty acid metabolism, fatty acid biosynthesis, and biosynthesis of unsaturated fatty acids, were upregulated.
The enrichment of DEGs in the biological process GO terms. The enrichment of DEGs in the biological process GO terms: (GO:0055114): oxidation–reduction process, (GO:0009408): response to heat, (GO:0042542): response to hydrogen peroxide, (GO:0009414): response to water deprivation, (GO:0006950): response to stress, (GO:0006952): defense response, (GO:0006979): response to oxidative stress, (GO:0045454): cell redox homeostasis, (GO:0009723): response to ethylene, (GO:0009867): jasmonic acid mediated signaling pathway, (GO:0031348): negative regulation of defense response, (GO:0009753): response to jasmonic acid, (GO:0010286): heat acclimation, (GO:0000165): MAPK cascade, (GO:0006635): fatty acid beta-oxidation, (GO:0006633): fatty acid biosynthetic process, (GO:0009739): response to GA, (GO:0042744): hydrogen peroxide catabolic process, (GO:0010193): response to ozone, (GO:0009407): toxin catabolic process, (GO:0009740): gibberellic acid mediated signaling pathway, (GO:0050896): response to stimulus, (GO:0009628): response to abiotic stimulus, (GO:0042631): cellular response to water deprivation, (GO:0015824): proline transport, (GO:0080170): hydrogen peroxide transmembrane transport, (GO:0000303): response to superoxide, (GO:0009415): response to water, (GO:0009685): GA metabolic process, (GO:0000302): response to reactive oxygen species, (GO:0045487): GA catabolic process, and (GO:0009686): GA biosynthetic process
Molecular mechanisms of AtGA2ox1 created GA-deficit phenotype and improved maize drought resistance
AtGA2ox1-OE creates a semi-dwarf and drought tolerance enhance phenotype, and the possible molecular mechanisms are proposed in Fig. 6 based on the physiological and biochemical indicators and transcriptome analysis. The qRT-PCR analysis verified that two GA2ox family members, Zm00001d039394 and Zm00001d043411, were upregulated in the AtGA2ox1-OE maize plant, while the GA20ox family member, Zm00001d042611, was significantly downregulated (Fig. 5a; Table S3). These revealed that AtGA2ox1-OE produces a GA-deficit phenotype not only through the deactivation of bioactive GA pathway, but also through the inhibition of the endogenous GA biosynthesis pathway.
The expression pattern of DEGs involved in GA, ROS, Pro and TFs identified by RNA-seq in transgenic maize line #1. a The relative expression of DEGs in the GA metabolism pathway. Zm00001d043411 (GA2ox3), Zm00001d039394 (GA2ox13), Zm00001d042611 (GA20ox3). b The relative expression of DEGs in the ROS scavenging and Pro metabolism pathway. Zm00001d034760 (Fdx3), Zm00001d017240 (unannotated), Zm00001d028992 (unannotated), Zm00001d033079 (unannotated), Zm00001d014848 (CAT1). c The relative expression of DEGs in TFs. Zm00001d034433 (HSFTF11), Zm00001d043877 (NACTF94), Zm00001d009103 (EREB148), Zm00001d013130 (bHLH60), Zm00001d012379 (MYB36), Zm00001d039658 (bZIP6), Zm00001d023332 (WRKY63). All reactions were performed in three biological duplications, and each biological duplication had three technical replicates. Statistical analysis was performed using the 2−ΔΔCT method. The error bars are presented as mean ± standard error (SE)
In addition, other DEGs involved in ROS signaling and related to proline metabolism were also enriched, such as CAT, glutaredoxins (Grxs), thioredoxins (Trx), ferredoxins (Fds) and proline-rich protein (Table S3). These genes are involved in ROS removal, and improve the stability of the plasma membrane biochemical process. Zm00001d014848, which is a catalase isozyme gene, Zm00001d017240, which is a Grx-like gene, Zm00001d028992, which is a Trx family member, Zm00001d034760, which is a member of the Fds family, and Zm00001d033079, which is a proline-rich protein gene, are the five DEGs selected for qPCR verification. These were all upregulated in the AtGA2ox1-OE maize, and the results were consistent with those in the transcriptome data (Fig. 5b; Table S3). Furthermore, these results were in accordance with the physiological and biochemical findings of transgenic maize under drought stress, suggesting that these DEGs may play an important role in ROS detoxification enzyme activity, proline content increase, plasma membrane stability, and osmotic balance of transgenic maize under drought stress.
Next, it was also found that the DEGs of various transcription factor (TF) families were enriched, including HSF, NAC, WRKY, AP2/ERF, bHLH, MYB, and bZIP (Tables 2, S3). In the present study, seven DEGs were selected from each TF family, and the expression patterns were also verified by qRT-PCR. Zm00001d034433 (HSF), Zm00001d043877 (NAC), Zm00001d009103 (AP2/ERF), Zm00001d013130 (bHLH), Zm00001d012379 (MYB) and Zm00001d039658 (bZIP) were upregulated in AtGA2ox1-OE maize, while Zm00001d023332 (WRKY) was downregulated in AtGA2ox1-OE maize. These results were consistent with those of the transcriptome data (Fig. 5c; Table 2). Therefore, this indicates that these TFs play an important role in the tolerance of transgenic maize to drought stress.
Discussion
GAs have been proven to be important components of stress tolerance in plants (Vettakkorumakankav et al. 1999). To date, some genes in the GA2ox family have been reported to be involved in stress responses in different plant species (Achard et al. 2008a, b; Magome et al. 2004, 2008). In order to explore the potential molecular mechanism, and verify whether the strategy of improving drought resistance of maize by accelerating the inactivation of bioactive GA into inactive GA is feasible, AtGA2ox1 was overexpressed in maize, and the transgenic maize presented a GA deficiency phenotype (Fig. 1e). AtGA2ox1 has a clear function (Thomas et al. 1999; Yamaguchi 2008), and has been validated in Bahiagrass to obtain a GA deficient transgenic plant (Agharkar et al. 2007). In addition, the results of nucleotide sequence alignments and phylogenetic analysis (data from the database of MaizeGDB) indicated no gene directly homologous to AtGA2ox1 in maize. Hence, AtGA2ox1 was preliminarily selected to generate the GA-deficient transgenic maize. The present results have proven the contribution of reduced levels of bioactive GAs in improving drought tolerance, strengthening the understanding of the molecular mechanism underlying drought tolerance in maize.
The physiological and biochemical indicators and transcriptome analysis revealed that the remarkable role of AtGA2ox1 overexpression in transgenic maize for the enhancement of drought tolerance were via two pathways (Fig. 6). The first pathway was the ROS-detoxification pathway, which enhances antioxidant enzyme activities in transgenic maize under drought stress conditions. In the present study, SOD, CAT and POD enzyme activities were enhanced in transgenic maize plants (Fig. 3d–f), resulting in the reduction of the accumulation of ROS to inhibit membrane lipid peroxidation under drought stress conditions. The transcriptome analysis revealed that the DEGs were significantly enriched in the pathways of the oxidation–reduction process (Fig. 4; Tables 1, S3). Furthermore, these DEGs potentially enhanced the ability of ROS-detoxification in transgenic maize. Under drought stress, reductions in carbon assimilation resulted in an imbalance between electron excitation and utilization through photosynthesis, which lead to the production and accumulation of ROS. Then, ROS damages cell membranes, proteins and nucleic acids, causing oxidative stress (Sharma et al. 2012). The present study confirms that the reduced GA content in transgenic maize could activate internal antioxidant systems to eliminate ROS during drought stress. This support the results of previous studies (Achard et al. 2008a, b; Zhong et al. 2014; Lo et al. 2017), which reported the improvement of stress tolerance by reducing GA content to enhance the activity of ROS-detoxification enzymes for decreasing the levels of ROS. Due to the ROS-detoxification, the MDA content was reduced in transgenic maize (Fig. 3c). MDA is the catabolic end product of membrane lipid peroxidation, which serves as an indicator of the degree of abiotic stress-induced damage in plants. The functions of MDA were altered by reacting with proteins and nucleic acids, weakening the intermolecular bridges and bonds between cellulose molecules, and inhibiting protein synthesis (Moore and Roberts 1998).
Pathways that lead to increased drought stress tolerance. The solid arrowheads indicate the upregulation and downregulation of events. The open arrowheads indicate the suggested sequence of events. The upregulated and downregulated clustered genes are marked in red and green, respectively. The detailed list of genes in each group and the extent of changes are illustrated in Table S3. (Color figure online)
The second pathway is involved in the drought-induced increase in proline and soluble sugar content in transgenic maize (Fig. 3a, b). The transcriptome analysis revealed that some DEGs involved in the proline metabolic pathway were also enriched (Table S3). Proline is an essential osmolyte that allows plants to maintain the cell osmotic potential, cellular protection, and osmotic balance (Sperdouli and Moustakas 2012). The present study confirmed that the activation of the internal antioxidant system increased the accumulation of osmolytes, which mediated osmotic adjustment by reducing GA content, leading to the enhancement of tolerance to drought.
Furthermore, some TFs involved in abiotic stress responses and signaling were highly enriched, such as NAC, HSF, WRKY, AP2/ERF, bHLH, MYB and bZIP (Tables 2, S3). The TF family has been reported to join in the osmotic equilibrium, ROS scavenge, and protein–protein interaction between TFs. AtJUB1 (JUNGBRUNNEN 1) is a hydrogen peroxide (H2O2)-induced NAC TF. AtJUB1-OE plants have been identified to have higher proline content, with improved drought tolerance in Arabidopsis and tomato (Wu et al. 2012; Thirumalaikumar et al. 2018). Interestingly, Zm00001d043877, the homology of AtJUB1, was also found to be upregulated in transgenic maize (Table 2). AtERF71/HRE2-overexpressing transgenic plants exhibited tolerance to salt stress, and had lower levels of ROS under high salt treatment (Park et al. 2011). A similar phenomenon was also found, in which Zm00001d009103, a transcriptional factor AP2/ERF, increased its expression in transgenic maize. In the present study, Zm00001d012379, as a member of the MYB TF family, was upregulated in transgenic maize (Table 2). A previous study revealed that in AtMYB44 overexpressed plants, the WRKY70 and PR genes were upregulated, and this lead to the enhanced resistance to biotic stress in transgenic tomato (Shim et al. 2013). Zm00001d039658, as a HY5 and a member of the bZIP TF family, was upregulated in transgenic maize (Table 2). Related studies have revealed that HY5 binds to the promoters of many WRKY genes that encode for TFs involved in defense responses (Lee et al. 2007). HSF is an important TF family, which is involved in various abiotic stresses in plants (Guo et al. 2016). In HSP-related genes and cloning from Arabidopsis, chickpea and sunflower, these were overexpressed in various plants, and significantly improved heat, salt and drought tolerance (Wu et al. 2012; Yokotani et al. 2008; Liu et al. 2011; Ma et al. 2016; Ogawa et al. 2007). In the present study, it was observed that HSF and HSP had the most significant number among all groups (Table S2), and that these might have had a considerable contribution to the drought tolerance of transgenic maize. Zm00001d034433 is a member of HSFs, and is upregulated in transgenic maize (Table 2). Some of the reported TFs observed to have a decreased expression under drought stress were also found in the present. Furthermore, Zm00001d023332 was annotated as a WRKY, and was downregulated in transgenic maize (Table 2). The homology of Zm00001d023332 in Arabidopsis was identified to be correlated to drought tolerance. WRKY54 negatively regulates drought tolerance by inhibiting dehydration-inducible gene expression, and the wrky46/wrky54/wrky76 triple mutant had a stronger drought-resistant phenotype, when compared to the WT (Chen et al. 2017). In previous studies, reduced endogenous GA levels were found to directly or indirectly induce the expression of a series of abiotic stress-related TFs (Gallego-Bartolome et al. 2011; Qi et al. 2014). PIF5, a bHLH transcriptional factor, inhibits GA metabolism in seed germination by indirectly repressing GA synthetic genes GA3ox1 and GA3ox2, and activating the GA catabolic gene GA2ox2, increasing ABA levels (Oh et al. 2007). Zm00001d013130 and PHYTOCHROME-INTERACTING FACTOR3-LIKE5 (PIF5) were also found to be upregulated in OE maize plants (Table 2). This supports that the change in GA content and regulation of the GA pathway metabolism could improve drought tolerance in maize. The present study reports the improvement of drought tolerance by overexpressing the AtGA2ox1 gene to reduce the content of bioactive GAs in maize. Combined with previous studies, it was further confirmed that the improvement of drought resistance of genetically modified maize was through the enhancement of antioxidant enzyme activities and accumulation of osmolytes.
Although the regulated GA pathway can improve drought tolerance, one problem that should be noted is that it usually produces a dwarf phenotype, which affects the yield. The change in GAs content in plants could affect plant height, and the overexpression of the GA2ox gene would reduce plant height. However, the degree of plant height reduction is often uncertain, resulting in extreme or semi-dwarfing traits (Lo et al. 2008). In a previous study, it was found that drought tolerance can be enhanced through the application of a growth regulator and chlormequat chloride, limiting GA synthesis and production in maize, without producing an extreme-dwarfing phenotype (Spitzer et al. 2015). It is well-known that an appropriate GA content is very important to keep the balance among plant height, stress resistance and yield. The present study provides a new strategy to improve drought tolerance in maize by AtGA2ox1-OE biotechnology. Fortunately, an extreme dwarfing phenotype of maize was not obtained, but a semi-dwarfing phenotype. This provides a new approach for the biotechnological improvement of drought tolerance in maize. However, it should be noted that the influence of the overexpression of AtGA2ox1 on the other growth and development pathways of transgenic maize remains unclear, especially the impact on yield and other traits with the reduction of endogenous bioactive GAs and plant height, which needs to be further researched. A greater tissue specificity of the GA2ox gene transgene expression would be needed to maximize the benefits, while avoiding negative impacts on maize development. Moreover, the genes in the GA metabolism pathways can also be mutated by gene editing and other techniques, in order to obtain the degradation pathway with a weakened function. It is hoped that this work may provide new insight for future studies on the roles of the GA2ox family members in maize.
References
Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P (2008a) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18(9):656–660
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008b) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20(8):2117–2129
Agharkar M, Lomba P, Altpeter F, Zhang H, Lange T (2007) Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol J 5(6):791–801
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Biemelt S, Tschiersch H, Sonnewald U (2004) Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol 135(1):254–265
Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P et al (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29(6):1425–1439
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217(1):67–75
Dijkstra C, Adams E, Bhattacharya A, Page AF, Anthony P, Kourmpetli S et al (2008) Over-expression of a gibberellin 2-oxidase gene from Phaseolus coccineus L. enhances gibberellin inactivation and induces dwarfism in Solanum species. Plant Cell Rep 27(3):463–470
Edmeades GO, Cooper M, Lafitte R, Zinselmeier C, Ribaut JM, Habben JE et al (2000) Abiotic stresses and staple crops. In: Crop science: progress and prospects (no. CIS-4429). CIMMYT
FAO (2010) FAOSTAT statistical database of the Food and Agriculture Organization of the United Nations. FAO, Rome. http://faostat.fao.org. Accessed 14 July 2011
Gallego-Bartolome J, Alabadi D, Blazquez MA (2011) DELLA-induced early transcriptional changes during etiolated development in Arabidopsis thaliana. PLoS ONE 6:e23918
Gilbert HS, Stump DD, Roth EF Jr (1984) A method to correct for errors caused by generation of interfering compounds during erythrocyte lipid peroxidation. Anal Biochem 137(2):282–286
Guo M, Liu JH, Ma X, Luo DX, Gong ZH, Lu MH (2016) The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci 7:114
Han F, Zhu B (2011) Evolutionary analysis of three gibberellin oxidase genes in rice, Arabidopsis, and soybean. Gene 473(1):23–35
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5(12):523–530
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4):604–611
Huang J, Tang D, Shen Y, Qin B, Hong L, You A, Cheng Z (2010a) Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). J Genet Genomics 37(1):23–36
Huang XS, Liu JH, Chen XJ (2010b) Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol 10(1):230
Ishida Y, Hiei Y, Komari T (2007) Agrobacterium-mediated transformation of maize. Nat Protoc 2(7):1614
Lee DJ, Zeevaart JA (2005) Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol 138(1):243–254
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Liu HC, Liao HT, Charng YY (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34(5):738–751
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Lo SF, Yang SY, Chen KT, Hsing YI, Zeevaart JA, Chen LJ, Yu SM (2008) A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20(10):2603–2618
Lo SF, Ho THD, Liu YL, Jiang MJ, Hsieh KT, Chen KT, Yu LC et al (2017) Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol J 15(7):850–864
Ma H, Wang C, Yang B, Cheng H, Wang Z, Mijiti A et al (2016) CarHSFB2, a class B heat shock transcription factor, is involved in different developmental processes and various stress responses in chickpea (Cicer arietinum L.). Plant Mol Biol Report 34(1):1–14
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2004) Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37(5):720–729
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56(4):613–626
Moore K, Roberts LJ (1998) Measurement of lipid peroxidation. Free Radic Res 28(6):659–671
Nelissen H, Sun XH, Rymen B, Jikumaru Y, Kojima M, Takebayashi Y et al (2018) The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol J 16(2):615–627
Ogawa D, Yamaguchi K, Nishiuchi T (2007) High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased thermotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J Exp Bot 58(12):3373–3383
Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I et al (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19(4):1192–1208
Park HY, Seok HY, Woo DH, Lee SY, Tarte VN, Lee EH et al (2011) AtERF71/HRE2 transcription factor mediates osmotic stress response as well as hypoxia response in Arabidopsis. Biochem Biophys Res Commun 414(1):135–141
Qi T, Huang H, Wu D, Yan J, Qi Y, Song S, Xie D (2014) Arabidopsis DELLA and JAZ proteins bind the WD-Repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 26:1118–1133
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K et al (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134(4):1642–1653
Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JA, Amasino RM (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15(1):151–163
Shan X, Li Y, Jiang Y, Jiang Z, Hao W, Yuan Y (2013) Transcriptome profile analysis of maize seedlings in response to high-salinity, drought and cold stresses by deep sequencing. Plant Mol Biol Report 31(6):1485–1491
Shan C, Mei Z, Duan J, Chen H, Feng H, Cai W (2014) OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS ONE 9(1):e87110
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Shim JS, Jung C, Lee S, Min K, Lee YW, Choi Y et al (2013) AtMYB 44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J 73(3):483–495
Somasundaram R, Jaleel CA, Abraham SS, Azooz MM, Panneerselvam R (2009) Role of paclobutrazol and ABA in drought stress amelioration in Sesamum indicum L. Glob J Mol Sci 4(2):56–62
Song J, Guo B, Song F, Peng H, Yao Y, Zhang Y, Sun Q, Ni Z (2011) Genome-wide identification of gibberellins metabolic enzyme genes and expression profiling analysis during seed germination in maize. Gene 482(1–2):34–42
Sperdouli I, Moustakas M (2012) Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. Plant Physiol 169:577–585
Spitzer T, Míša P, Bílovský J, Kazda J (2015) Management of maize stand height using growth regulators. Plant Prot Sci 51(4):223–230
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100(16):9440–9445
Thirumalaikumar VP, Devkar V, Mehterov N, Ali S, Ozgur R, Turkan I et al (2018) NAC transcription factor JUNGBRUNNEN 1 enhances drought tolerance in tomato. Plant Biotechnol J 16(2):354–366
Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96(8):4698–4703
Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith BN (1985) Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol 121(5):453–461
Urbanová T, Tarkowská D, Novák O, Hedden P, Strnad M (2013) Analysis of gibberellins as free acids by ultra performance liquid chromatography–tandem mass spectrometry. Talanta 112:85–94
Vettakkorumakankav NN, Falk D, Saxena P, Fletcher RA (1999) A crucial role for gibberellins in stress protection of plants. Plant Cell Physiol 40(5):542–548
Wang C, Yang A, Yin H, Zhang J (2008) Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol 50(4):427–434
Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI et al (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24(2):482–506
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang D, Wang N, Yan X, Shi J, Zhang M, Wang Z, Yuan H (2014) Microencapsulation of seed-coating tebuconazole and its effects on physiology and biochemistry of maize seedlings. Colloids Surf B 114:241–246
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508
Yokotani N, Ichikawa T, Kondou Y, Matsui M, Hirochika H, Iwabuchi M, Oda K (2008) Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 227(5):957–967
Zhong T, Zhang L, Sun S, Zeng H, Han L (2014) Effect of localized reduction of gibberellins in different tobacco organs on drought stress tolerance and recovery. Plant Biotechnol Rep 8(5):399–408
Acknowledgements
This work was supported by the Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2017ZY026) and the National Natural Science Foundation of China (No. 31771879).
Author information
Authors and Affiliations
Contributions
ZC and YL contributed equally to the study. CG and XL designed the experiments. ZC, YY, YL, QL, NL, WH and DH performed the experiments. ZC and XL analyzed the data. ZC and XL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Liu, Y., Yin, Y. et al. Expression of AtGA2ox1 enhances drought tolerance in maize. Plant Growth Regul 89, 203–215 (2019). https://doi.org/10.1007/s10725-019-00526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00526-x