Abstract
To identify the crosstalk between gene expression and metabolism in response to cold, drought and salt stresses, digital gene expression (DGE) analysis was performed on maize (Zea mays L.) seedlings subjected to these stresses. A total of 103,953 (70.79 %), 111,130 (68.62 %), 94,435 (69.33 %) and 94,577 (68.92 %) tags were matched to reference genes. The most differentially regulated tags, with a log2 ratio ≥1 or ≤−1 (P < 0.01 and FDR ≤0.001), were further analysed. Many genes and biological pathways were affected by multiple abiotic stresses. In particular, expression changes for the gibberellin (GA) metabolic genes could improve understanding of the molecular basis of the response of the GA pathway to stress conditions. In addition, a large dataset of tag-mapped transcripts was obtained that provide a strong basis for future research on the response to abiotic stress in maize. And a new list of candidate targets for functional studies on genes involved in cold, drought and salt stresses has been generated. In this study, we revealed complex changes at the transcriptional level in maize seedlings under different abiotic stresses. Such studies could lead to a better understanding of the genetic basis of the maize response to different environmental stimuli and would be essential for improving the abiotic stress tolerance of maize.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is a crop grown worldwide and used for human consumption and animal feed. However, the growth and yield of maize can be severely limited by drought, high salinity, and low temperature. To adapt to abiotic stresses, plants must modulate various physiological and metabolic responses, such as stomatal closure, repression of cell growth and photosynthesis, and activation of respiration (Hull et al. 1990; Miedema 1982; Zorb et al. 2004). Various abiotic stresses have been shown to induce many different functional genes, including the bZIP transcription factors (Jia et al. 2009; Ying et al. 2012), dehydration-responsive element binding (DREB) factors (Qin et al. 2004; Wang et al. 2011), mitogen-activated protein kinase (MAPK) cascades (Pan et al. 2012; Wang et al. 2010; Yang et al. 2011a), and CBL-interacting protein kinases (CIPKs) (Chen et al. 2011; Zhao et al. 2009). The identification of these genes has demonstrated that there are interacting mechanisms that respond to different abiotic stresses in maize. Previous reports have studied individual genes or groups of genes under different stresses; to obtain a broader understanding of the response of maize to abiotic stresses, we have analysed maize global transcript profiles under different environmental conditions.
Recently, high-throughput or deep-sequencing technology has become a powerful tool for identifying specific genes and analysing transcriptome profiles. Driven by Solexa/Illumina technology, digital gene-expression (DGE) is a new approach for expression analysis and has identified a large number of genes involved in stress response and plant development (Hao et al. 2011; Wang et al. 2012). Compared to microarray technology, this approach has provided a more thorough qualitative and quantitative description of gene expression. In this study, we present mRNA expression profiles created using DGE sequencing of elite maize inbred line seedlings subjected to salt (150 mM NaCl), hyperosmotic (20 % PEG6000), and cold (4 °C) stress treatments. We have comprehensively compared the transcriptomes of the various treatments and provided useful information for further research.
Materials and Methods
Plant Material and Stress Treatments
The elite maize inbred line Zheng58 was used in this study. Seeds of Zheng58 were pre-germinated in distilled deionised water in plant growth chambers at 60 % humidity, a day/night temperature of 24/20 °C, 300 μmol m−2 s−1 light intensity and a photoperiod of 14:10 h (day:night). After a 72-h pre-germination period, geminated seeds were planted into a mixture of vermiculite and soil (2:1, v:v) in the same chamber and watered daily. When the third leaves were fully expanded, the plants were subjected to different abiotic stresses.
For drought and salt treatments, maize seedlings were watered every 4 h with either 20 % PEG 6000 solution or 150 mM NaCl solution. Plants were treated for 24 h in the same growth chambers under the same condition as before. For cold treatment, the seedlings were incubated at 4 °C for 24 h, and the other conditions were not changed. After treatment, the plants were harvested at the time indicated and immediately frozen in liquid nitrogen. Then, total RNA was prepared using the RNeasy Plant Mini Kit (Qiagen) in accordance with the manufacturer’s instructions. We first determined the robustness of the five replicates for each stress and control using qRT-PCR (data not shown). Then, these replicates were mixed to provide a pooled sample for the sequencing analysis and qRT-PCR confirmation as follows.
Solexa/Illumina Sequencing and DGE Analysis
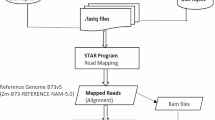
Solexa/Illumina sequencing was carried out by BGI-Shenzhen, China. The technological details are consistent with standard DGE methods (Shen et al. 2012), including sequencing; gene expression annotation, analysis and screening of differentially expressed genes (DEGs); gene ontology functional enrichment analysis; and pathway enrichment analysis for DEGs.
Real-time Quantitative RT-PCR (qRT-PCR) Analysis
Total RNA extracted from the seedlings was treated with DNase I to remove genomic DNA contamination. First-strand cDNA synthesis and qRT-PCR were carried out using the SuperScript™ III First-Strand Synthesis Kit (Invitrogen) and SYBR Green JumpStart™ Taq ReadyMix™ (Sigma), respectively. qRT-PCR was carried out in the ABI PRISM 7500 sequence detection system according to the manufacturer’s instructions. To validate the DGEs obtained from Solexa sequencing, 10 genes (Table 1) were subjected to quantitative real-time PCR. The maize actin gene was used as the endogenous control (forward primer CGATTGAGCATGGCATTGTCA and reverse primer CCCACTAGCGTACAACGAA). Each PCR reaction (20 μL) contained 10 μL 2× real-time PCR Mix, 0.2 μM of each primer and appropriately diluted cDNA. The thermal cycling conditions were 95 °C for 1 min, followed by 40 cycles of 5 s at 95 °C and 30 s at 60 °C. All reactions were performed in triplicate, including the non-template controls. Statistical analysis was performed using the 2−ΔΔCT method.
Results
Characterisation of the Sequenced Solexa/Illumina Libraries
To identify genes involved in the response to cold, drought and salt, four Solexa/Illumina libraries (CK (without any stress), Cold, Drought, and Salt) were constructed from maize seedlings treated with different conditions. Sequencing depths of 6,035,420, 6,203,607, 5,995,261, and 5,895,798 tags were achieved in the four libraries, including 368,168, 409,675, 343,279, and 340,338 distinct tags, respectively. To improve library quality, tags recorded only once were removed, leaving 146,855, 161,960, 136,206, and 137,226 clean tags that were detected multiple times in each library. The frequency of these tags is shown in Table 1 and Supplementary Fig. 1.
To identify the genes corresponding to the clean tags in each library, a sequence dataset containing 63,540 reference genes expressed in maize was used. From this group, 61,898 genes (97.42 %) possessed at least one CATG site, resulting in a total number of 183,943 (63.37 %) unambiguous reference tags. We observed that approximately 40 % of the tags from each library were perfectly matched to the reference genes (Table 1). Approximately 25 % of the tags in each library were mapped to more than 1 location (Supplementary Fig. 1). Additionally, approximately 16 % of the tags in the four libraries were mapped to the antisense strand, indicating that those regions might be bidirectionally transcribed. Regarding discrepancies between the reference and experimental tags, approximately 11 % of the tags detected in the four libraries contained 1-bp mismatches. As a result of the significant sequencing depth of Solexa technology and incomplete data, the percentage of unmatched tags ranged from 17.51 to 20.64 % in the four libraries (Supplementary Fig. 1).
Identification of Differentially Expressed Transcripts
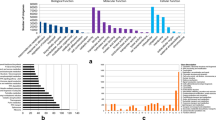
To investigate changes in gene expression, the gene expression level in response to different stress treatments was compared with the control group (CK). In total, 2,431, 1,448, and 1,264 differentially expressed genes (DEGs) were identified in the cold, drought, and salt treatment samples, respectively. Among the DEGs identified, 1,176, 558, and 377 genes were up-regulated in cold, drought, and salt stress treatment, respectively. Meanwhile, 1,255, 890, and 887 genes were down-regulated (Fig. 1; Supplementary Table 1; Supplementary Fig. 2).
We also performed cluster analysis of genes specifically expressed under different stress treatments (Supplementary Fig. 3). Gene expression levels in response to cold stress were somewhat different to the response to drought and salt stress. There were 6 genes up-regulated in response to cold that were down-regulated under drought and salt treatments. Twelve genes that were down-regulated in the cold group were up-regulated in the other two groups (Supplementary Table 2). In general, these results demonstrated that the levels of several transcripts were altered by more than one of the stresses, suggesting that these genes may interact through shared pathways in the biological responses to these stress conditions.
The DEGs that responded to different stresses were further analysed to determine their biological functions. Their functional categories were assembled from the metabolic and signalling pathways described in a range of public databases and in the literature, and we characterised significantly regulated pathways in detail (Q ≤ 0.05) (Supplementary Table 3). Pairwise analysis of the DGE data from the CK library and the other three stress libraries identified differentially expressed genes and the presence of distinct pathways under different stress conditions. Carotenoid biosynthesis, the biosynthesis of secondary metabolites, and diterpenoid biosynthesis, however, were affected in all the three stresses. Most of the differentially expressed genes in the diterpenoid biosynthesis pathways were involved in the regulation of gibberellin metabolism. These changes included gibberellin 2-oxidase (GA2ox) genes (GRMZM2G427618_T01, GRMZM2G022679_T01, and GRMZM2G051619_T01), which were up-regulated under salt and drought stresses, and gibberellin 3-oxidase (GA3ox) genes (GRMZM2G036340_T01, GRMZM2G046669_T01), which were down-regulated under these stresses, while the gibberellin 20-oxidase (GA20ox) gene (GRMZM2G368411_T01) was down-regulated by cold stress (Supplementary Table 4; Supplementary Fig. 4). These results imply that maize seedlings reduce their growth rate to cope with salt, drought or cold stress by reducing GA levels, as GA2ox encodes a GA-inactivating enzyme and GA3ox and GA20ox encode GA-biosynthetic enzymes.
In this study, we have focused on a group of genes involved in stress responses (Supplementary Table 5). These are well-characterised transcription factors, such as DREB, CBF, NAC, bZIP, MYB, and MYC that play major roles in abiotic stress. DREB/CBF-like genes (GRMZM2G124011_T01 and GRMZM2G069146_T01) were significantly up-regulated in response to all three stresses, while MYC factors (GRMZM2G049229_T01 and GRMZM2G001930_T01) were consistently down-regulated. On the other hand, the expression of the NAC, bZIP, and MYB genes were affected in different ways by the three stresses. The four CDPK genes (GRMZM2G112057_T01, GRMZM2G441511_T01, GRMZM2G115518_T01, and GRMZM2G115518_T03) were up-regulated only under cold treatment. Only one of the MAPK related genes, GRMZM2G174170_T01, was significantly induced by all three stresses. We also found that the expression of many functional genes, including the late embryogenesis abundant (LEA) gene, heat shock proteins (HSP), and reactive oxygen species (ROS)-related genes, were induced by stress. Among them, the LEA genes (GRMZM2G050607_T01 and GRMZM2G096475_T01) were similar to CDPK, which were up-regulated only in response to cold treatment. The other genes showed different express patterns in response to different stresses.
Quantitative, Real-time PCR (qRT-PCR) Confirmation
To evaluate the validity of Solexa analysis, nine known and one unknown transcript were selected for examination by real-time RT-PCR (qRT-PCR). Information for these genes and their gene-specific primers are showed in Table 2. The expression patterns determined using both qRT-PCR and DGE were consistent for all 10 genes (Fig. 2), suggesting that our transcriptome analyses were very reliable.
Confirmation of expression profiles by qRT-PCR with 10 selected differentially expressed genes (DEGs). Relative expression of the 10 genes in maize seedlings was detected by qRT-PCR under different conditions. Transcript levels were normalised to the expression level of actin. Error bars SE from three independent experiments
Discussion
Transcriptome analysis using sequencing technology has proven to be very useful for identifying stress-inducible genes (Hao et al. 2011; Wang et al. 2012; Yang et al. 2011b; Yu et al. 2012). The primary goal of the present study was to perform preliminarily expression analysis of transcripts involved in the physiological response of maize seedlings to cold, drought and salinity, as well as to provide the groundwork for investigating regulatory mechanisms in maize. The most differentially expressed genes with a log2 ratio ≥1 or ≤−1 participated in a number of biological pathways, including environmental response, signal transduction, hormone metabolism and transcriptional regulation. In the results from DGE sequencing, many of the genes that take part in these pathways were induced significantly by at least two of the types of stress studied here. Our results indicated that these pathways were generally involved in the plant seedling response to abiotic stress, and plant stress responses are dynamic and involve complex cross-talk between different pathways (Krasensky and Jonak 2012). Additionally, many stress-specific genes were significantly up- or down-regulated in response to just one stress, suggesting the existence of stress-specific pathways in addition to general response pathways. Some of these genes would be used to promote specific tolerance for maize breeding in the future.
In this study, many genes involved in hormone pathways were differently expressed in response to abiotic stress. Previous studies on Arabidopsis have suggested that phytohormones are involved in stress adaptation (Achard et al. 2006; Nishiyama et al. 2011; Vyroubalova et al. 2009). We have also found evidence that gibberellins (GAs) were involved in the response to stress. In maize, the expression of a number of genes involved in GA biosynthesis or inactivation were consistently induced by all three types of treatment (Supplementary Tables 3, 4; Supplementary Fig. 4). One Arabidopsis study revealed that salt caused a reduction in GA levels by activating genes encoding gibberellin 2-oxidase (GA2ox) (Achard et al. 2006). This enzyme converts bioactive and intermediate forms of GA to inactive forms by 2β-hydroxylation and is the primary enzyme known to deactivate bioactive GAs (Yamaguchi 2008). In our results, not only were the three GA2ox genes studied (GRMZM2G427618_T01, GRMZM2G022679_T01, and GRMZM2G051619_T01) strongly up-regulated but two GA3ox genes (GRMZM2G036340_T01, GRMZM2G046669_T01) were also down-regulated by all three treatments. GA3ox catalyses the final step in the synthesis of bioactive gibberellins (GA). Therefore, cold, drought, and salt can all reduce bioactive GA abundance, and thus increase the accumulation of DELLAs, producing growth inhibition. These physiological responses induce transient growth arrest, and thus allow the seedlings to survive periods of adversity. Some other GA-related genes were also induced by stress. The ent-kaurene synthase (KS) genes (GRMZM2G093603_T01, GRMZM2G016922_T01) and GA20ox gene (GRMZM2G368411_T01) genes were down-regulated by cold treatment, the ent-kaurenoic acid oxidase (KAO) gene (GRMZM2G042543_T01) was down-regulated by drought, and the KS (AC214360.3_FGT001, GRMZM2G016922_T01) and KAO (GRMZM2G042543_T01) genes were down-regulated by salt stress, all of which could influence GA biosynthesis and catabolism in maize plants. These results showed that salt stress induced GA metabolic genes more readily than did cold and drought stress and thus may reduce bioactive GA abundance more rapidly. In maize and other crops, it is largely unknown how GAs are regulated at the molecular level upon stress adaptation. Our study could lead to a better understanding of the molecular basis of the GA pathway response in maize under stress conditions.
Transcription factors (TFs) typically regulate the expression of multiple genes in a metabolic pathway, and TFs analysis has played a very important part in stress response research. In this study, we analysed the gene expression of a few important transcript factors (DREB, CBF, NAC, bZIP, MYB, and MYC) that were known to be involved in stress tolerance in plants. We found that only the CBF (GRMZM2G124011_T01, GRMZM2G069146_T01) and MYC (GRMZM2G049229_T01, GRMZM2G001930_T01) genes exhibited similar changes in expression in response to salt, drought and cold stresses. However, some TFs (e.g. NAC, MYB, and bZIP) responded differently under different stress conditions. NAC (GRMZM2G018436_T01), for example, was up-regulated in response to cold stress but down-regulated under salt stress conditions. The essential role of the CBF/DREB1 gene in response to cold, drought, and salt stress has been demonstrated in several previous studies (Krasensky and Jonak 2012; Lata and Prasad 2011; Yamaguchi-Shinozaki and Shinozaki 2006). Increased expression of the DREB2A genes has also been found to play an important role in the plant response and adaptation to abiotic stress (Qin et al. 2004, 2006, 2007). The ZmDREB1A and ZmDREB2A genes have been cloned form Zea mays, and the ZmDREB2A transcription factor was shown to be involved in the response of maize to various stress conditions, including cold, drought, salt and high temperature (Qin et al. 2006, 2007). In our DEG data, the DREB genes (GRMZM5G806839_T01 and GRMZM2G380377_T01) were up-regulated by cold and drought treatment, in accordance with previous studies (Qin et al. 2004). In addition, the expression of the CBF genes (GRMZM2G124011_T01 and GRMZM2G069146_T01) were up-regulated by all three stresses. These results might suggest that stress-related CBF/DREB-like genes have not yet been identified in Zea mays, and their functions may be different from the known CBF/DREB1 genes (Krasensky and Jonak 2012; Lata and Prasad 2011; Yamaguchi-Shinozaki and Shinozaki 2006).
In addition to TFs, a number of additional signalling components such as kinases and functional proteins are also important for the acclimatisation of plants to environmental stress. In this study, members of the MAPK, CIPK, CDPK, LEA, HSP and ROS gene families displayed differential induction by stresses. Most of these genes were expressed differently under different stresses. However, the CDPK (GRMZM2G112057_T01, GRMZM2G441511_T01, GRMZM2G115518_T01 and GRMZM2G115518_T03) and LEA (GRMZM2G050607_T01 and GRMZM2G096475_T01) genes were only up-regulated in maize seedlings in response to cold stress. Previous studies have reported that the LEA genes responded to low temperature, drought, and high salt in different plant species (Hundertmark and Hincha 2008; Vaseva et al. 2010; Yue et al. 2008). Some researchers have reported that the LEA genes are up-regulated in transgenic plants overexpressing ZmDREB2A (Qin et al. 2007). The LEA and CDPK genes were also identified in our study, which identified specific cold response genes that should be studied further. Signalling through the MAP kinase cascade is involved in the response to various stresses. The transcript level of ZmMPK3 increased within half an hour and remained high for a period (Wang et al. 2010). Cold stress also induced the expression and activity of ZmMAPK5 (Berberich et al. 1999). In our study, four MAPK genes (GRMZM2G174170_T01, GRMZM5G834697_T01, GRMZM2G163709_T01, and GRMZM2G020216_T01) were detected. The transcription level of one of these genes was increased under cold, drought and salt stress, while the other genes were not significantly induced. We suggest that the induced gene (GRMZM2G174170_T01) is a new maize MAPK gene associated with abiotic stress tolerance.
Conclusion
This study has demonstrated the utility of the digital gene expression (DGE) approach to identify differentially expression genes in maize seedlings in response to different abiotic stresses. A large dataset of tag-mapped transcripts was obtained that provide a strong basis for future research on the response to abiotic stress in maize. In addition, a new list of candidate targets for functional studies on genes involved in cold, drought and salt stresses has been generated. Further work should focus on characterising these genes. Such studies could lead to a better understanding of the genetic basis of the maize response to different environmental stimuli and would be essential for improving the abiotic stress tolerance of maize.
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Berberich T, Sano H, Kusano T (1999) Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Mol Gen Genet 262:534–542
Chen XF, Gu ZM, Xin DD, Hao LA, Liu CJ, Huang J, Ma BJ, Zhang HS (2011) Identification and characterization of putative CIPK genes in maize. J Gen Genomics 38:77–87
Hao QN, Zhou XA, Sha AH, Wang C, Zhou R, Chen SL (2011) Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes. BMC Genomics 12:525
Hull MR, Long SP, Raines CR (1990) The effects of low-temperature on activities of carbon metabolism enzymes in Zea-Mays L seedlings. Curr Res Photosynth 1–4:D675–D678
Hundertmark M, Hincha DK (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:118
Jia ZW, Lian Y, Zhu Y, He JG, Cao ZP, Wang GY (2009) Cloning and characterization of a putative transcription factor induced by abiotic stress in Zea mays. Afr J Biotechnol 8:6764–6771
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Lata C, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot 62:4731–4748
Miedema P (1982) The effects of low-temperature on Zea-Mays. Adv Agron 35:93–128
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmulling T, Tran LSP (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Pan J, Zhang M, Kong X, Xing X, Liu Y, Zhou Y, Sun L, Li D (2012) ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 235:661–676
Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Shinozaki K, Yamauchi-Shinozaki K (2006) Regulation and functional analysis of ZmDREB2A in response to drought and high temperature stress from Zea mays L. Plant Cell Physiol 47:S225–S225
Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Tran LSP, Shinozaki K, Yamaguchi-Shinozaki K (2007) Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J 50:54–69
Qin F, Sakuma Y, Li J, Liu Q, Li YQ, Shinozaki K, Yamagushi-Shinozaki KY (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45:1042–1052
Shen Y, Jiang Z, Yao X, Zhang Z, Lin H, Zhao M, Liu H, Peng H, Li S, Pan G (2012) Genome expression profile analysis of the immature maize embryo during dedifferentiation. PLoS ONE 7:e32237
Vaseva II, Grigorova BS, Simova-Stoilova LP, Demirevska KN, Feller U (2010) Abscisic acid and late embryogenesis abundant protein profile changes in winter wheat under progressive drought stress. Plant Biol 12:698–707
Vyroubalova S, Vaclavikova K, Tureckova V, Novak O, Smehilova M, Hluska T, Ohnoutkova L, Frebort I, Galuszka P (2009) Characterization of new maize genes putatively involved in cytokinin metabolism and their expression during osmotic stress in relation to cytokinin levels. Plant Physiol 151:433–447
Wang CT, Yang QA, Yang YM (2011) Characterization of the ZmDBP4 gene encoding a CRT/DRE-binding protein responsive to drought and cold stress in maize. Acta Physiol Plant 33:575–583
Wang G, Zhu QG, Meng QW, Wu CA (2012) Transcript profiling during salt stress of young cotton (Gossypium hirsutum) seedlings via Solexa sequencing. Acta Physiol Plant 34:107–115
Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M (2010) A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J Integr Plant Biol 52:442–452
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang G, Zou HD, Wu Y, Liu HK, Yuan YP (2011a) Identification and characterisation of candidate genes involved in chilling responses in maize (Zea mays L.). Plant Cell Tiss Org 106:127–141
Yang YH, Chen XJ, Chen JY, Xu HX, Li J, Zhang ZY (2011b) Identification of novel and conserved microRNAs in Rehmannia glutinosa L. by Solexa sequencing. Plant Mol Biol Rep 29:986–996
Ying S, Zhang DF, Fu J, Shi YS, Song YC, Wang TY, Li Y (2012) Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 235:253–266
Yu SC, Zhang FL, Yu YJ, Zhang DS, Zhao XY, Wang WH (2012) Transcriptome profiling of dehydration stress in the Chinese cabbage (Brassica rapa L. ssp. pekinensis) by tag sequencing. Plant Mol Biol Rep 30:17–28
Yue G, Zhuang Y, Li Z, Sun L, Zhang J (2008) Differential gene expression analysis of maize leaf at heading stage in response to water-deficit stress. Biosci Rep 28:125–134
Zhao J, Sun Z, Zheng J, Guo X, Dong Z, Huai J, Gou M, He J, Jin Y, Wang J, Wang G (2009) Cloning and characterization of a novel CBL-interacting protein kinase from maize. Plant Mol Biol 69:661–674
Zorb C, Schmitt S, Neeb A, Karl S, Linder M, Schubert S (2004) The biochemical reaction of maize (Zea mays L.) to salt stress is characterized by a mitigation of symptoms and not by a specific adaptation. Plant Sci 167:91–100
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31100192 and No. 31100242) and the Science Development Planning of Jilin Province (No. 20110752).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Statistics of distinct clean tag alignment (JPEG 59 kb)
Figure S2
Statistics of number of Differentially Expressed Genes (DEGs) under different stress treatments (JPEG 10 kb)
Figure S3
Clustering Analysis of Differentially Expressed Genes (DEGs) under different stress treatments (JPEG 1448 kb)
Figure S4
DEGs enriched in maize GA metabolism pathway under different stress treatments. Red and green boxes represent the up- and down-regulated genes respectively. 4.2.3.19, 1.14.13.79, 1.14.11.12, 1.14.11.15 and 1.14.11.13 represent KS (ent-kaurene synthase), KAO (ent-kaurenoic acid oxidase), GA20ox (GA 20-oxidase), GA3ox (GA 3-oxidase), and GA2ox (GA 2-oxidase) respectively. (JPEG 40 kb)
Supplementary Table 1
(XLS 120 kb)
Supplementary Table 2
Different gene expression under cold treatment from the other two stress treatments. (DOCX 14.9 kb)
Supplementary Table 3
Enriched pathways involved in different stress reactions. (DOCX 14.7 kb)
Supplementary Table 4
GA metabolic gene expression alteration under cold, drought, and salt stress. (DOCX 13.8 kb)
Supplementary Table 5
Differentially expressed genes in transcription factor families and stress related gene families in response to different stress treatments. (DOCX 22.3 kb)
Rights and permissions
About this article
Cite this article
Shan, X., Li, Y., Jiang, Y. et al. Transcriptome Profile Analysis of Maize Seedlings in Response to High-salinity, Drought and Cold Stresses by Deep Sequencing. Plant Mol Biol Rep 31, 1485–1491 (2013). https://doi.org/10.1007/s11105-013-0622-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-013-0622-z