Abstract
Bacterial isolates with the ability to tolerate salinity and plant growth-promoting features were isolated from the saline areas of Gujarat, India, that is, Bhavnagar and Khambat. A total of 176 strains of rhizobacteria were isolated out of which 62 bacterial strains were able to tolerate 1 M NaCl. These were then further studied for their potential plant growth-promoting rhizobacteria characteristics like phosphate solubilization, siderophore production, and IAA production. Twenty-eight isolates of the 62 strains showed good tricalcium phosphate solubilization in solid medium in the range of 9–22 mm and 15 isolates showed good phosphate solubilization in liquid medium in the range of 9–45 μg/ml. Siderophore production was checked in all 15 isolates, and 13 were screened out that produced the hydroxamate type of siderophore in the range of 11–50 mM. Among the 13 isolates, 10 were able to produce indole acetic acid in the range of 10–26 μg/ml after 72 h of incubation. Pot trials were carried out on chickpea under 300 mM NaCl stress using the best five isolates. Plants inoculated with MSC1 or MSC4 isolates showed an increase in the parameters that evaluate plant growth when compared to uninoculated controls. Strains MSC1 and MSC4 were identified as Pseudomonas putida and Pseudomonas pseudoalcaligens, respectively, according to sequence analysis of the 16S rRNA gene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a serious environmental problem that causes osmotic stress and reduction in plant growth and crop productivity in irrigated areas of arid and semiarid regions (Cicek and Cakirlar 2002). Salt stress affects many aspects of plant metabolism and, as a result, growth and yield are reduced. Excess salt in the soil solution may adversely affect plant growth through either osmotic inhibition of water uptake by roots or specific ion effects. Specific ion effects may cause direct toxicity, or, alternatively, the insolubility or competitive absorption of ions may affect the plant’s nutritional balance. Salinity was shown to increase the uptake of Na+ or decrease the uptake of Ca2+ and K+ (Yildirim and others 2006). Accumulation of excess Na+ may cause metabolic disturbances in processes where low Na+ and high K+ or Ca2+ are required for optimum function. Uptake and accumulation of Cl− may disrupt photosynthetic function through the inhibition of nitrate reductase activity (Xu and others 2000). In addition to the use of traditional breeding and transgenic plants, the utilization of plant growth-promoting rhizobacteria (PGPR) is useful in strategies to facilitate plant growth in saline soils (Tank and Saraf 2010).
Plant growth-promoting bacteria are soil and rhizosphere bacteria that can be of benefit to plant growth by several different mechanisms such as asymbiotic N2 fixation, ammonia production, solubilization of mineral phosphate and other essential nutrients, production of plant hormones, and control of phytopathogenic microorganisms (Tank and Saraf 2003). Indirect promotion of growth is possible by biological control of plant pathogens and deleterious microbes, through the production of antibiotics, cell wall-degrading enzymes, hydrogen cyanide and siderophores, or through competition for nutrients and space. All these increase seedling emergence, vigor, and yield (Autoun and Kleopper 2001). This mechanism is a recent indirect mechanism of action of PGPR. In addition, PGPR can protect plants from deleterious effects of environmental stress like flooding, drought, salinity, heavy metals, and so on.
Chickpea (Cicer arietinum L.) is a major food legume and an important source of protein in many countries. In addition, it is also widely used as fodder and green manure. Chickpea is one of the most important legume crops for human nutrition grown in arid and semiarid regions and is considered to be a salt-sensitive species (Ashraf and Waheed 1993). Cultivars grown in India are either native (desi) types characterized by smaller, angular, pigmented seeds, or Mediterranean (kabuli) types with larger, rounded seeds which lack pigmentation. Because the genus is indigenous to arid areas, some genotypes may have a degree of salt adaptation (Garg and Singla 2004). Hence, selection and implementation of biotechnological approaches can improve economic yield under saline conditions. The objective of this study was to screen out native strains of PGPR from the saline soils of Gujarat that are able to tolerate salinity and use their ability to promote the growth of chickpea.
Materials and Methods
Isolation of Rhizobacteria
Soil samples were collected from the marginal lands of Gujarat, that is, Bhavnagar and Khambat, showing sparse vegetation. Bacterial strains were isolated using four different media: nutrient agar (General), yeast extract mannitol agar (Rhizobium), Ashby’s agar (Azotobacter), and King’s B agar medium (Pseudomonas). After 48 h of incubation, morphological characteristics and cultural characteristics of well-isolated colonies were checked. These isolates were then subcultured on respective agar plates and maintained on respective agar slants.
Tolerance to Salinity
The tolerance of isolates to salinity was tested by growing the strains on nutrient agar plates supplemented with increasing concentrations of NaCl from 300 to 1,000 mM. Salt-tolerant strains were maintained on nutrient agar supplemented with 500 mM NaCl. Salt-tolerant isolates then were further studied for their PGP potential.
Characterization of Rhizobacteria for PGP Traits
Phosphate Solubilization
Phosphate solubilization was checked using tricalcium phosphate (TCP) as insoluble phosphate. Spot inoculation of the isolates was done in the center of the Pikovyskyay medium amended with bromophenol blue. These plates were then incubated at 37°C for 48–72 h (Subba Rao 1982). Phosphate solubilization was checked for by looking for the formation of a clear yellow halo around the colony, representing the production of organic acids as a possible mechanism of the phosphate solubilization. Phosphate solubilization was quantified in liquid Pikovskyaya’s medium in flasks for 21 days. The concentration of the soluble phosphate in the supernatant was estimated every third day by the stannous chloride (SnCl2·2H2O) method (Gaur 1990). A simultaneous change in the pH was also recorded in the supernatant by a Systronics digital pH meter.
Production of Indole Acetic Acid
Indole acetic acid (IAA) production was detected as described by Brick and others (1991). Bacterial cultures were grown for 48 h on their respective media at 36 ± 2°C. Fully grown cultures were centrifuged at 3,000 rpm for 30 min. The supernatant (2 ml) was mixed with two drops of orthophosphoric acid and 4 ml Salkowsky’s reagent (50 ml, 35% perchloric acid, 1 ml 0.5 M FeCl3 solution). Development of pink color was measured at 536 nm by a spectrophotometer.
Siderophore Production
Siderophore production was detected by the method of Schwyn and Neilands (1987) using blue agar plates containing the dye chrome azurol S (CAS). Orange halos around the colonies on blue agar were indicative for siderophore production. Quantitative estimation was carried out by inoculating 1 ml of actively growing isolates with 0.5 OD at 600 nm in 50 ml of succinic acid medium in 250-ml Erlenmeyer flasks. All flasks were incubated at 30°C for 30 h on an orbital shaker. After 30 h, all cultures were centrifuged at 5,000 rpm for 20 min. Supernatant was collected and tested for pH, fluorescence, and siderophore production. A simultaneous change in the growth pattern of the isolates was also carried out. A catecholate type of siderophore was checked by Arnow’s method (1937), and for hydroxymate-type siderophores the Gibson and Magrath method (1969) was used.
Growth Promotion of Chickpea Under Salinity Conditions
The pot study was carried out in triplicate on chickpea using 500 g of soil in 10 × 10-cm pots. Seeds were surface sterilized by gently shaking with 70% ethanol (5 min) and 20% sodium hypochlorite solution followed by three rinses in sterile distilled water. Thereafter, seeds were soaked overnight in sterile distilled water and germinated on sterile cotton-covered Petri dishes. After 3 days of germination, four seeds were planted in the pots supplemented with saline solution for three consecutive days, according to the method of Tank and Saraf (2010), so as to maintain the salinity level at 300 mM. Thirty milliliters of active culture with 108 cells/ml was added to each pot, which was watered with distilled water everyday. Two sets of controls were kept: one was a negative control that was uninoculated and did not have any stress and bacteria and one was a positive control that was uninoculated and underwent salt stress. After 80 days all plants were carefully uprooted and various vegetative parameters like root length, shoot length, number of leaves, chlorophyll content, fresh weight, flowering, and fruiting were studied (Pesqueira and others 2006).
Soil Analysis
Soil samples were drawn after 80 days from each respective treatment and soil analysis was carried out. The soils were analyzed for pH, total organic carbon (TOC) (Walkley and Black 1934), available phosphorus (Olsen and others 1954), and available potassium (Schollenberger and Simon 1945). Estimation of zinc, copper, manganese, and iron content of the soil samples was performed using an atomic absorption spectrophotometer (Chapman and Pratt 1978).
Statistical Analysis
To evaluate the efficiency of rhizobacteria in pot experiments under saline conditions, a completely randomized block design was used. To identify significant treatment, analysis of variance (ANOVA) was carried out. Mean values were compared at significance levels of 1 and 5%. The ANOVA indicated significances of treatment and effects.
Genomic DNA Sequencing and Phylogenetic Analysis
Genomic DNA isolation was performed (Sambrook and others 1989) and a complete 1.6-kb 16S rDNA region was amplified using the universal primer 1FAGCGGCGGACGGGTGAGTAATG and 1509RAAGGAGGGGATCCAGCCGCA. The DNA sequencing was performed using an ABI Prism Sequence Detection System. The BLASTn search program (http://www.ncbi.nlm.nih.gov) was used to look for nucleotide sequence homology. The sequences obtained were then aligned by ClustalW using MEGA 4.0 software (Tamura and others 2007) and a neighbor-joining (NJ) and maximum parsimony tree was generated using the software.
Nucleotide Sequence Accession Number
The sequences obtained in this study were deposited in the NCBI GenBank nucleotide sequence database under the accession numbers GU553133 (Pseudomonas putida) and GU564407 (Pseudomonas pseudoalcaligens).
Results
Isolation of Bacterial Strains from Saline Soils
Soil sample 1, that is, Khambhat soil, is alkaline with pH 8.0, electrical conductivity of 1.42 mmhos/cm, and salinity of 0.9%. Whereas soil sample 2, that is, Bhavnagar soil, had pH 7.8 and electrical conductivity of 2.7 mmhos/cm, making the soil sodic saline in nature. A total of 176 bacterial isolates were obtained from both sites in Gujarat using different media. Eighty-six isolates were obtained from the Bhavnagar site and 90 isolates from the Khambhat site of Gujarat. Among the total of 176 isolates, 82 were screened out on the basis of their fast growth potential.
Tolerance to Salinity
To select those isolates that are tolerant to salinity in vitro, screening in culture media containing different NaCl concentrations was performed. Results show that from 86 bacterial isolates, 71 were able to tolerate nutrient agar medium containing 500 mM NaCl. Upon further increasing the concentration of NaCl, only 62 isolates were selected that were able to tolerate 1 M NaCl. These 62 isolates were then studied for their PGP characteristics.
Phosphate Solubilization
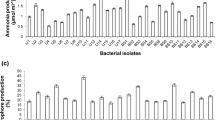
When the phosphate solubilization potential of the 62 isolates was studied, 28 isolates showed a zone of phosphate solubilization on Pikovskyaya’s agar medium using TCP. The zone of phosphate solubilization on agar plates ranged from 9 to 22 mm within 4 days of incubation. The MSC1 isolate yielded the best phosphate solubilization, as shown in Fig. 1. Of the 28 isolates that had good phosphate solubilization on agar plates, 15 of the best phosphate solubilizers were then further studied for phosphate solubilization in liquid medium. All 15 isolates showed phosphate solubilization in Pikovskyaya’s liquid medium in the range of 9–45 μg/ml, as shown in Fig. 2. Isolate MSC4 showed the maximum phosphate solubilization in liquid medium. A decrease in pH of the liquid medium was observed in all 15 isolates. The pH of the isolates declined from 7.2 to 4.5 (Fig. 2).
Siderophore Production
Siderophore production of the 15 selected isolates was determined by change in color of the CAS agar medium from dark blue to yellow or purplish pink around the colony. Of the 15 selected isolates, 13 showed siderophore production. The zone of siderophore production on the CAS agar plate ranged from 9 to 30 mm, as shown in Fig. 3. Isolate MSC1 showed the highest siderophore production among all the other isolates. Quantitative analysis was performed to check for the type of siderophore produced, that is, hydroxymate or catecholate. All the isolates were observed to produce the hydroxymate type of siderophore. It was observed that all 13 isolates produced siderophore in the range of 11–50 mM. Among all the isolates, the MSC1 isolate showed better siderophore production after 24 h of incubation (Fig. 3).
Indole Acetic Acid Production
Indole acetic acid production was estimated in all 13 selected isolates. Of these, ten isolates were able to produce IAA in tryptone yeast medium. The range of production was 10–26 μg/ml after 72 h of incubation (Fig. 4). Maximum IAA production was observed in isolate MSC4. The pattern of production of IAA was an increase up to 96 h and thereafter a continuous decrease after 120 h of incubation was observed.
Growth Promotion of Chickpea Under Salinity Conditions
Plant growth in the presence of 300 mM NaCl resulted in the conclusion that adding NaCl to soil reduced growth of chickpea. Plants grown in saline soil showed a reduction in root length to about 48% of that of chickpea plants grown in normal soil. A decrease of 16% was observed in the shoot length of plants in soil treated with NaCl compared to plants in untreated soil. Plants grown in the presence of NaCl without treatment with PGPR showed an almost 71% reduction in root length and about a 34% reduction in shoot length in comparison to chickpea plants grown in the presence of isolate MSC1. A reduction was also observed in the number of leaves and lateral roots. Comparisons between all the vegetative parameters were made between normal soil (control −ve), saline soil (control +ve), and saline soil inoculated with different PGPR.
Enhancement of root length was the greatest in MSC4-treated plants, followed by MSC1-treated plants, compared to untreated controls. Maximum shoot length was observed in MSC1-treated plants, which was about 34% higher than untreated plants.
Plants treated with isolates MSC1, MSC4, and B7 showed an increased number of leaves compared to untreated plants, where the maximum increase in the number of leaves (31%) was observed in plants treated with MSC1. The MSC1-treated plants also showed an increase in the number of branches (8.6%) compared to all other treatments. In MSC1-treated plants, fresh weight and dry weight were higher than uninoculated NaCl added plants. Plants treated with isolate B7 showed a minimum increase in fresh weight and dry weight, which was less than that in untreated plants. The number of lateral roots was reduced compared with that of plants grown without salinity stress, whereas it was the greatest in plants treated with isolate B15 in comparison with other treatments and control plants with salinity stress.
In MSC4-treated plants, there was an increase in chlorophyll content compared to both NaCl-added and NaCl-free plants (Table 1). Plants treated with the isolate MSC4 had a maximum increase in the number of fruits (112%) compared to uninoculated controls. Plants treated with isolate MSC1 also showed an increase in the number of fruits (105%) in comparison to controls. MSC4-treated plants had a maximum increase in the number of flowers (63%) under saline stress compared to uninoculated plants. Plants inoculated with MSC1 also showed an increase in the number of flowers (54%) in comparison to uninoculated controls. Plants treated with isolates B7, B8, and B15 showed a negligible increase in the number of fruits and flowers compared to controls.
The study of various vegetative parameters, that is, leaf size, number of leaves, number of branches, number of lateral roots, and number of flowers and fruits showed that the best growth promotion was observed in plants treated with MSC1. MSC4 was the next most effective treatment in terms of growth promotion. B7, B8, and B15 showed negligible increases in root length and shoot length compared to NaCl-added plants.
Soil Analysis
Soil analysis showed that there was an increase in TOC in the soil inoculated with isolate MSC4. Phosphorus content also increased in the soil treated with MSC4 in comparison to uninoculated controls. Soil treated with isolate MSC1 showed an increase in potassium content which was beneficial to plant growth. The increase in phosphorus and potassium content in the soil treated with isolates MSC1 and MSC4 is due to their potential to solubilize insoluble P and release K from silicate in soil and thus enhance mineral uptake by plants. Plants treated with isolate MSC4 showed an increase in iron, zinc, and manganese content in the soil in comparison with controls. Isolates MSC1 and MSC4 have the potential to produce chelators like siderophores which can chelate minerals like iron, copper, zinc, and manganese and thus increase the nutrient content in the soil. Treatment with isolate MSC1 also yielded an increase in copper content in the soil. Isolates B7, B8, and B15 also yielded negligible increases in TOC, phosphorus, potassium, iron, copper, zinc, and manganese as compared to untreated uninoculated plants (Table 2).
Phylogenetic Analysis
To identify the potential rhizobacteria, phylogenetic analysis was carried out. Using MEGA 4.0, through the NJ method a consensus tree was constructed. The selected strains were run through the BLAST search program, presenting the highest sequence homology proportion and query coverage and the lowest E values. The sequence of another genus was also used to mediate the actual relationship in the participating sequencing group. The results of the BLAST search program revealed that isolate MSC1 had a sequence homology with Pseudomonas putida (Fig. 5) and isolate MSC4 had a sequence homology with Pseudomonas pseudoalcaligens (Fig. 6).
Phylogenetic analysis of MSC1 based on 16S rRNA gene sequences available from the European Molecular Biology Laboratory data library constructed after multiple alignments of data by ClustalX. Distances and clustering with the NJ method were calculated by using MEGA 4.0. Bootstrap values based on 500 replications are listed as percentages at the branching points
Phylogenetic analysis of MSC4 based on 16S rRNA gene sequences available from the European Molecular Biology Laboratory data library constructed after multiple alignments of data by ClustalX. Distances and clustering with the NJ method were calculated by using MEGA 4.0. Bootstrap values based on 500 replications are listed as percentages at the branching points
Discussion
Preliminary studies revealed the salt tolerance of different cultures. Research work that tested the PGP potential of the isolates and at the same time eliminated isolates that did not show desired characteristics was systematically carried out. Solubilization of phosphates by rhizobacteria was observed by 15 isolates. A decrease in the pH of the liquid medium was observed in all 15 isolates. However, no correlation between P solubilization and pH reduction was observed by Tank and Saraf (2003). Similarly, El-Azeem and others (2007) reported that all 81 isolates studied for the solubilization of TCP were able to solubilize TCP in broth cultures (quantitative method), 53 isolates solubilized phosphate in solid medium (qualitative method), and the pH values of the cultures were reduced from the initial value of 7.1 to values that varied between 4.16 and 6.45. On the other hand, Rajankar and others (2007) reported that bacteria like Bacillus subtilis and Bacillus megatherium isolated from the saline belt of the Purna River Basin were able to solubilize phosphate and release a minute quantity of acid that reduced the salinity of the soil by neutralization.
Only 13 of the 15 isolates were able to produce iron-chelating substances, that is, siderophores, which were found to be the hydroxamate type. However, only 10 were found to produce phytohormones (IAA) and maximal levels were produced by MSC4. Similarly, Chandra and others (2007) reported that Mesorhizobium loti MP6, isolated from root nodules of Mimosa pudica, showed production of the hydroxamate type of siderophore. Pseudomonas putida produced siderophore and induced systemic resistance in watermelon against gummy stem rot, whereas the siderophore-negative mutants failed to induce resistance (Lee and others 2005). Sarode and others (2009) reported that during a screening study of PGPR, of 32 strains, 8 were able to grow in iron-deficient medium and produce siderophore. Maximum siderophore production was observed by Acinetobacter calcoaceticus, which was shown to produce the catechol type of siderophore. Datta and Basu (2000) reported that a decrease in IAA production in Rhizobium sp. from Cajanus cajan after 72 h might be due to the release of IAA-degrading enzymes such as IAA oxidase and peroxidase.
The effect of the five isolates in our study was then observed in pot trials using chickpea. The effect of salinity could be relieved in plants inoculated with selected PGPR compared to plants not treated with PGPR. Primary root length enhancement was maximal in MSC4 followed by MSC1. Similarly, Vivas and others (2003) reported enhanced root and shoot growth of lettuce inoculated with Bacillus spp. under dry salt stress conditions. Munns (2003) mentioned that suppression of plant growth under saline stress may be due to the decreasing availability of water or toxicity of high salt concentrations.
A substantial increase in root length may be responsible for increased nutrient uptake by plants. The seeds treated with MSC4 and MSC1 showed maximum growth of roots and shoots, resulting in growth promotion and higher yields. Zahir and others (2008) reported increases in the shoot length, fresh weight, and number of leaves per peaplant. This result is in accordance with our results where we observed an increase in root length, shoot length, number of leaves, and lateral root count of chickpea when inoculated with MSC1 and MSC4 under salt stress. An increase in chlorophyll content in all plants treated with PGPR under saline stress has also been reported by Nadeem and others (2006), whereas Lee and others (2001) reported an increase in chlorophyll a and b in lettuce plants grown in the presence of NaCl stress. Increased chlorophyll content may be the result of an increase in the photosynthetic area of plant leaves at high salt stress by PGPR inoculation compared to control untreated plants where leaf area was reduced due to stress. An increase in chlorophyll content may also be attributed to the increase in the photosynthesis load on plants to fulfill plant nutrient requirements under stress conditions. According to Cheeseman (1988), salinity stress diverts metabolic carbon to storage pools, so as a result less carbon is available for growth, leading to reduced growth of plants.
Mishra and others (2010) reported that PGPR under salinity stress are able to enhance the production of IAA and solubilization of phosphorus, thereby improving root length, shoot length, and dry weight of roots and shoots of C. arietinum L. plants. Similarly, Pandya and Saraf (2010) reported that application to chickpea of bioinoculants with phosphate solubilization and siderophore production potential yielded increases in all vegetative growth parameters under saline conditions.
In light of the present results, it may be concluded that saline soils inhibit growth, metabolic activity, and yield of chickpea. However, due to the plant growth-promoting properties of the isolates MSC1 (Pseudomonas putida) and MSC4 (Pseudomonas pseudoalcaligens), the stress of salinity could be reduced and the growth of plants is almost at par with normal soils.
Conclusion
The use of soil rhizosphere bacteria possessing the traits of plant growth promotion under saline stress is becoming prevalent worldwide to achieve sustainable agriculture along with soil reclamation through phytoremediation as well as bioremediation. The present study demonstrated the ability of native strains in saline areas to tolerate salinity and show plant growth-promoting potential. From a total of 176 bacterial isolates, 62 have the potential to tolerate salinity up to 1 M NaCl concentration. Five bacterial isolates were screened out that not only can tolerate high salinity but play an essential role in helping plants to establish and grow in salinity conditions. Isolate MSC1 increased the root length of treated plants by 71% and shoot length by 34% compared to untreated plants. MSC4 was another effective growth promoter and increased root length by 76%. Treatments with isolates MSC4 and MSC1 also yielded the maximum increase in the number of flowers by 63 and 54%, respectively. Plants inoculated with MSC4 and MSC1 showed an increase in the number of fruits by 112 and 105% in comparison with uninoculated controls. Thus, using strains with all the PGPR attributes can enhance plant growth under saline conditions.
References
Abd El-Azeem SAM, Mehana TA, Shabayek AA (2007) Some plant growth promoting traits of rhizobacteria isolated from Suez Canal region, Egypt. Afr Crop Sci Conf Proc 8:1517–1525
Arnow LE (1937) Colorimetric estimation of the components of 3,4-dihyroxyphenylalanine tyrosine mixtures. J Biol Chem 118:531–535
Ashraf M, Waheed A (1993) Responses of some genetically diverse lines of chickpea (Cicer arietinum) to salt. Plant Soil 154:257–266
Autoun H, Kleopper JW (2001) Plant growth promoting rhizobacteria (PGPR). In: Brenner S, Miller JF (eds) Encyclopedia of genetics. Academic Press, New York, pp 1477–1480
Brick JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Chandra S, Choure K, Dubey RC, Maheshwari DK (2007) Rhizosphere competent Mesorhizobium loti MP 6 induces root hair curling, inhibits Sclerotium sclerotiorum, and enhances growth of Indian mustard (Brassica campestris). Braz J Microbiol 38:124–130
Chapman HD, Pratt PF (1978) Methods of analysis for soils, plants and waters. Univ Calif Div Agric Sci 3034, Berkeley
Cheeseman JM (1988) Mechanisms of salinity tolerance in plants. J Plant Physiol 87:547–550
Cicek N, Cakirlar H (2002) The effect of salinity on some physiological parameters in two maize cultivars. Bulg J Plant Physiol 281:66–74
Datta C, Basu PS (2000) Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol Res 155:123–127
Garg N, Singla R (2004) Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz J Plant Physiol 16(3):137–146
Gaur AC (1990) Phosphate solubilizing micro-organisms as biofertilizer. Omega Scientific Publishers, New Delhi
Gibson F, Magrath DI (1969) The isolation and characterization of hydroxamic-acid (Aerobactin) formed by Aerobacter aerogenes 62-1. Biochem Biophys Acta 192:175–184
Lee YH, Lee H, Lee DK, Shim HK (2001) Factors relating to induced systemic resistance in watermelon by plant growth promoting Pseudomonas spp. Plant Pathol 17:174–179
Lee KD, Bai Y, Smith D, Han HS, Supanjani (2005) Isolation of plant-growth-promoting endophytic bacteria from bean nodules. Res J Agric Biol Sci 1:232–236
Mishra M, Kumar U, Mishra PK, Prakash V (2010) Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Adv Biol Res 4(2):92–96
Munns R (2003) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nadeem SM, Hussain I, Naveed M, Asghar HN, Zahir ZA, Arshad M (2006) Performance of plant growth promoting rhizobacteria containing ACC-deaminase activity for improving growth of maize under salt stressed conditions. Pak J Agric Sci 43:114–121
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture Circular No. 939. US Government Printing Office, Washington, DC
Pandya U, Saraf M (2010) Role of single fungal isolates and consortia as plant growth promoters under saline conditions. Res J Biotechnol 5(3):5–9
Pesqeira J, Garcia MD, Staltari S, Molina M, Del C (2006) NaCl effects in Zea mays L. x Tripsacum dactyloides (L.) L. hybrid calli and plants. Elect J Biotechnol 9:286–290
Rajankar PN, Tambekhar DH, Wate SR (2007) Study of phosphate solubilization efficiencies of fungi and bacteria isolated from saline belt of Purna river basin. Res J Agric Biol Sci 3:701–703
Sambrook J, Fritsch FE, Maniatis TA (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sarode PD, Rane MR, Chaudhari BL, Chincholkar SB (2009) Siderophoregenic Acinetobacter calcoaceticus isolated from wheat rhizosphere with strong PGPR activity. Malays J Microbiol 5(1):6–12
Schollenberger CJ, Simon RH (1945) Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci 59:13–24
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Subba Rao NS (1982) Biofertilizers. In: Subba Rao NS (ed) Advances in agricultural microbiology. Butterworth Scientific, London, pp 219–303
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Bio Evol 24:1596–1599
Tank ND, Saraf MS (2003) Phosphate solubilization, exopolysaccharide production and indole-3-acetic acid secretion by rhizobacteria isolated form Trigonella foenum-graenum. Ind J Microbiol 43:37–40
Tank ND, Saraf MS (2010) Salinity resistant PGPR ameliorates NaCl stress on tomato plants. J Plant Interact 5:51–58
Vivas A, Marialanda JM, Ruiz-Lozano, Barea JM (2003) Influence of Bacillus spp. on physiological activities of two arbuscular mycorrhizal fungi and plant responses to PEG-induced drought stress. Mycorrhiza 13:249–256
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Xu G, Magen H, Kafkaf U (2000) Advances in chloride nutrition in plants. Adv Agron 68:97–150
Yildirim E, Taylor AG, Spittler TD (2006) Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sci Hortic 111:1–6
Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M (2008) Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J Microbiol Biotechnol 18(5):958–963
Acknowledgment
Funding for this study was obtained by the lead author, Prof. Meenu Saraf, from British Petroleum International Ltd. (BP). We are thankful to BP international Ltd., for their fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, D., Jha, C.K., Tank, N. et al. Growth Enhancement of Chickpea in Saline Soils Using Plant Growth-Promoting Rhizobacteria. J Plant Growth Regul 31, 53–62 (2012). https://doi.org/10.1007/s00344-011-9219-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9219-7