Abstract
To investigate the alleviating effects of zinc (Zn) against gradually increasing cadmium (Cd) stress in aquatic environment, dry weight, polyamines and proline contents as well as metabolic enzymes were studied in Lemna minor L. after 4 days exposure. Dry weight was significantly decreased as the concentration of Cd increased. Cd stress also increased the putrescine (Put) content, while decreasing spermidine (Spd) content, whereas no significant change was observed in spermine (Spm) content. Hence, the ratio of (Spd + Spm)/Put rapidly reduced. In addition, the activities of arginine decarboxylase (ADC), ornithine decarboxylase and polyamine oxidase (PAO) enhanced accordingly. Cd treatment also induced a continuous accumulation of proline. Meanwhile, pyroline-5-carboxylate synthetase (P5CS) activity increased initially only to decline later and ornithine δ-aminotransferase (OAT) activity was only significantly stimulated at 4 μM Cd, while the proline dehydrogenase (PDH) activity declined. However, Zn supplementation lowered accumulation of Put and proline contents and raised the Spd content, via decreasing the activities the ADC and PAO and keeping the activities of P5CS, OAT and PDH at the control levels, but failed to generate a statistically significant difference in content of dry weight. These results suggested that Zn application can maintain polyamines and proline homeostasis, thus conferring the tolerance of L. minor to Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a kind of toxic heavy metal to most plants which generates from industrial and agricultural activities and ubiquitously exists in water (Sun et al. 2009). It is readily taken up by aquatic plants and is toxic at cellular, physiological, biochemical, and molecular levels, including growth retardation, inhibition of photosynthesis, induction and inhibition of enzymes, disruptions in water relations, and ultrastructural changes (Ding et al. 2007; Piotrowska et al. 2010; Prasad 1995; Singh et al. 2006; Tripathi et al. 1996; Yang et al. 2010). The trace metal zinc (Zn) is essential to all organisms functioning as a co-factor in a variety of biomacromolecules, including metabolic enzymes, transcription factors and cellular signaling proteins (Mendoza-Cozatl et al. 2005; Morrissey and Guerinot 2009; Palmer and Guerinot 2009). Due to chemical similarity between Cd and Zn, they both can be taken by plants as divalent cations and therefore moderate Zn supply can alleviate the effect caused by Cd in plants. Early studies have demonstrated that supplemented with Zn could suppress Cd accumulation, increase plant biomass, affect energy transfer in photosystem II and photochemical quenching, lower level of reactive oxidative species (ROS), protein and DNA damages (Aravind et al. 2009; Balen et al. 2011; Bunluesin et al. 2007; Malec et al. 2008). However, less is known about polyamines and proline metabolism. In Arabidopsis, differential expression of ADC has been detected under stress conditions like drought, high salinity, mechanical injury, potassium deficiency (Alcázar et al. 2006; Hummel et al. 2004; Peréz-Amador et al. 2002; Urano et al. 2003). In addition, Yang et al. (2011) and Chen et al. (2001) have demonstrated that increased level of proline in response to lead and copper treatment is due to the elevations of OAT or GK activities in wheat and rice seedlings.
Polyamines are polybasic aliphatic amines that play a major role in various physiological and developmental processes in plants (Martin-Tanguy 2001). They may work as an antioxidant, a free radical scavenger and a membrane stabilizer (Larher et al. 2003; Velikova et al. 2000) and contribute in enhancing tolerability (Alcázar et al. 2006; Groppa and Benavides 2008). Moreover, it was found that aquatic plants may accumulate PAs as cellular reducing agents in response to the increase in ROS production triggered by Cd (Verbruggen et al. 2009). Proline has also been widely reported presenting a remarkable accumulation in response to heavy metal exposure among plants (Chen et al. 2001; Radic et al. 2010; Zengin and Munzuroglu 2005). Simultaneously, proline accumulation was demonstrated that it is positively correlated with Put, Spd and titers of total polyamines in Cd-treated pear shoots (Wen et al. 2011).
Lemna minor L., a member of the duckweed family, lives in many types of fresh water ecosystems (Bog et al. 2010). In particular, it has been commonly used as a test organism in ecotoxicological and environmental studies (Horvat et al. 2007; Khellaf and Zerdaoui 2010), owing to the physiological properties (small size, high multiplication rates and vegetative propagation). Therefore, we chose it as the experimental material and polyamines (Put, Spd, Spm and (Spd + Spm)/Put) and proline contents as well as critical metabolic enzymes (ADC, ODC, PAO, P5CS, OAT and PDH) were investigated in detail, aiming to further explore mitigative strategies of Zn on Cd-induced stress responses.
Materials and methods
Plant material and treatments
Lemna minor were collected from unpolluted freshwater bodies in Nanjing, China, washed with distilled water, and acclimated in 1/10 Hoagland solution. They were cultured in a totally enclosed incubator (Forma 3744, UK) at a day/night temperature of 24 ± 2 °C for more than 2 weeks. The illumination procedure consisted of a 16/8 light/dark cycle and aphoton flux density of 50 μmol m−2 s−1 (Horvat et al. 2007). Plant materials were treated as follows: (1) control: 1/10 Hoagland solution; (2) Cd treatment: 1/10 Hoagland solution containing 1, 2, 3, 4 μM Cd; (3) Zn treatment: 1/10 Hoagland solution containing 20 μM Zn; (4) Cd + Zn treatment: 1/10 Hoagland solution containing 1 μM Cd + 20 μM Zn, 2 μM Cd + 20 μM Zn, 3 μM Cd + 20 μM Zn, 4 μM Cd + 20 μM Zn. After 4 days treatment, the whole plants were sampled. All experiments were performed in triplicate.
Determination of dry weight
The plant samples were thoroughly washed with tap water, rinsed with distilled water and oven-dried to constant weight at 85 °C as described by Chatterjee et al. (1998). The dry weight was expressed as mg g−1 fresh weight.
Determination of polyamine
1.5 g fresh weight (FW) of L. minor was homogenized in 4 mL of 6 % (v/v) cold perchloric acid (PCA), kept on ice for 1 h, and then centrifuged (4 °C) at 21,000×g for 30 min. The pellet was extracted twice with 2 mL 5 % (v/v) PCA and centrifuged again. The three supernatants were pooled and used to determine the contents of free and PS-conjugated polyamines, whereas the pellet was used to determine the contents of PIS-bound polyamines. The pellet was re-suspended in 5 % (v/v) PCA and hydrolyzed for 24 h at 110 °C in flame-sealed glass ampoules after being mixed with 12 N HCl (1:1, v/v). The hydrolyzates were filtered, dried at 70 °C, and then re-suspended in 1 mL of 5 % (v/v) PCA for analysis of PIS-bound polyamines. For PS-conjugated polyamines, 2 mL of the supernatant were mixed with 2 mL of 12 N HCl and hydrolyzed for 24 h at 110 °C in flame-sealed glass ampoules. The supernatant, hydrolyzed supernatant and the pellet were then benzoylated (Aziz and Larher 1995).
The benzoyl derivatives were separated and analyzed using a HPLC system (Agilent 1100, USA) equipped with an UV detector under the following conditions: 200 mm × 4.6 mm C18 reverse-phase column (Kromasil, Sweden); particle size, 5 μm; column temperature, 30 °C; mobile phase, 64 % (v/v) methanol; a flow rate of 0.8 ml min−1, a detected wavelength of 254 nm. The internal standard was 1,6-hexanediamine.
Analysis of ADC, ODC and PAO activities
The ADC and ODC activities were determined according to Zhao et al. (2003) with some modifications. 1.5 g fresh plant material was homogenized in 50 mM phosphate buffer (pH 6.3) containing 0.1 mM phenylmethylsulfonylfluoride (PMSF), 40 μM pyridoxal phosphate (PLP), 5 mM dithiothreitol (DTT), 5 mM ethylene diamine tetra acetic acid (EDTA), 20 mM ascorbic acid (Vc) and 40 μM polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 12,000×g for 40 min and the supernatants were used for the enzyme assay. For ADC and ODC measurement, Reaction mixture (1.5 mL) consisted of 1 mL of the assay buffer with 100 mM Tris–HCl (pH 8.5), 5 mM EDTA, 40 μM pyridoxal phosphate and 5 mM DTT, 0.3 mL of either the ADC or ODC enzyme extract and 0.2 mL of 25 mM l-arginine (l-ornithine). The reaction mixture was incubated at 37 °C for 60 min, and centrifuged (4 °C) at 3,000×g for 10 min after which 0.5 mL of the supernatant was mixed with 1 mL of 2 mM NaOH, and then 10 μL benzoyl chloride was added to the mixture and stirred continuously for 20 s. After the reaction proceeded at 25 °C for 60 min, 2 mL of saturated NaCl and 2 mL of ether were added to the reaction mixture and stirred thoroughly, then centrifuged (4 °C) at 1,500×g for 5 min, 1 mL of ether phase was collected and evaporated at 50 °C. The remainder was dissolved in 0.5 mL of methanol (HPLC grade), and its absorption value at 254 nm was measured by a spectrophotometer (Thermo GENESYS 10, USA) for ADC (the solution was diluted into 20 mL fords before measuring) and an HPLC system (Agilent 1100, USA) for ODC respectively. A standard curve with Agmatine (Agm) or Put was used to calculate the activity of ADC (ODC). ADC and ODC activities were expressed as μmol Agm g−1 FW h−1 (U) and μmol Put g−1 FW h−1 (U) respectively.
PAO activity was determined by the improved method of Smith (1972) described by Wang et al. (2004) with some modifications. 0.5 g fresh plant material was ground on ice, in 1.6 mL phosphate buffer (0.1 mM, pH 6.5); then separated centrifugally at 10,000×g for 20 min at 4 °C. The supernatants were used to assay enzyme activity. For PAO measurement, 3 mL of reaction mixture consisted of 2.5 mL phosphate buffer (pH 6.5), 0.2 mL chromogenic reagent [25 mL N,N-dimethylaniline and 10 mg 4-aminoantiprine in 100 mL phosphate buffer (0.1 mM, pH 6.5)], 0.1 mL horseradish peroxidase (250 U mL−1) and 0.2 mL enzyme extract. Then 0.1 mL PAs (10 mM Spd + 10 mM Spm) was added. The reaction was conducted at 25 °C for 30 min, and measured spectrophoto-metrically at 550 nm where 0.001 ΔA550 g−1 FW min−1 was equal to one enzyme activity unit (U).
Determination of proline
Proline content was estimated using ninhydrine acid reagent according to Bates et al. (1973). Plant samples (0.5 g) were homogenized with 5 mL sulfosalicylic acid (3 %, w/v) in a cold mortar and pestle. The homogenate was centrifuged (4 °C) at 10,000×g for 15 min, and 2 mL of the supernatant was mixed with 2 mL of glacial acetic acid and 2 mL of acid ninhydrine. After agitation, the reaction mixture was incubated at 100 °C for 30 min. After cooling, 4 ml of toluene was added to each tube and vortexed for 30 s. The chromophore containing toluene was separated and absorbance was taken at 520 nm in spectrophotometer against toluene blank. The concentration of proline was quantified using the standard curve of l-proline and expressed as μg g−1 FW.
Analysis of P5CS, OAT and PDH activities
P5CS activity was assayed by the method of Smith et al. (1984) with some modifications. 2.0 g fresh plant material was grounded in 2 mL TD buffer containing 50 mM Tris–HCl buffer (pH 7.0), 1 mM dithiothreitol and 10 % glycerol. After centrifugation (4 °C) at 14,000×g for 20 min, the supernatant was collected and precipitated by adding solid ammonium sulfate (40 % saturation). Then, the soluble fraction obtained by centrifugation (14,000×g for 20 min at 4 °C) was saturated with dry ammonium sulfate to a concentration of 80 %. After centrifugation at 14,000×g for 15 min at 4 °C, the pellet was collected and completely dissolved with 1 mL TD buffer. The crude enzyme solution was obtained after a 24 h dialysis against TD buffer at 4 °C. A total volume of 1 mL assay mixture containing 50 mM glutamate, 10 mM ATP, 20 mM MgCl2, 100 mM oxammonium hydrochloride, 50 mM Tris–HCl buffer (pH 7.0) and an appropriate amount of enzyme was incubated at 37 °C for 30 min and then the reaction was stopped by adding 1 mL stop solution (5.5 % FeCl3, 2.0 % HClO4, 2 M HCl). The precipitate was removed by centrifugation, and the absorbance of the supernatant at 535 nm was recorded against a blank identical to the one mentioned above but lacking ATP. 0.01 ΔA535 h−1 at 535 nm was defined as one unit (U) of P5CS activity.
OAT activity was assayed with ninhydrin according to Kim et al. (1994). 1.0 g fresh plant material was immediately homogenized in 100 mM potassium phosphate buffer (pH 7.9) containing 1 mM EDTA, 15 % glycerol and 10 mM β-mercaptoethanol. The homogenate was centrifuged at 14,000×g for 15 min at 4 °C and the supernatant was collected for OAT activity measurement. 1 mL of the reaction mixture consisted of 50 mM Tris–HCl (pH 8.0), 50 mM l-ornithine, 5 mM β-ketoglutarate, 0.05 mM pyridoxal phosphate and the appropriate amount of crude enzyme extract was incubated at 37 °C for 20 min. After the addition of 0.3 mL of 3 M perchloric acid and 0.2 mL of 2 % ninhydrin, the reaction was stopped by boiling for 5 min. The precipitate was collected by centrifugation (14,000×g, 30 min, 4 °C) and completely dissolved with 1.5 mL of ethanol, and the absorbance of 0.01 at 510 nm was defined as one unit (U) of OAT activity.
PDH activity was measured as described by Rena and Splittstoesser (1975) with a slight modification. 0.5 g fresh plant material was homogenized in the ice-cold extraction buffer (100 mM sodium phosphate, 1 mM cysteine, 0.1 mM EDTA, pH 8.0). After centrifugation at 14,000×g for 10 min at 4 °C, the supernatant was used as crude enzyme preparation for measurement of PDH activity. The crude extraction was incubated in the reaction buffer [100 mM Na2CO3–NaHCO3, 10 mM nicotinamide adenine dinucleotide (NAD), 20 mM l-proline, pH 10.3] at 32 °C for 5 min, and then PDH dependent NAD reduction was monitored at 340 nm for 4 min. 0.001 ΔA340 min−1 was defined as one unit (U).
Statistical analysis
All values are expressed as mean ± standard deviation from three individual experiments. The data were subjected to an analysis of variance in SPSS Statistics 17.0. The correlation coefficients were expressed using r values.
Results
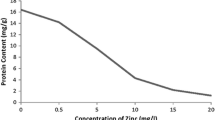
Effects of Zn on contents of dry weight under Cd stress
As shown in Fig. 1, The dry weight of L. minor decreased markedly in response to single Cd concentration compared with the control (r = 0.9889, P < 0.01). In contrast, the application of Zn weakened the decline to a certain extent, but no significant change was observed.
Effects of Zn on levels of polyamines under Cd stress
In comparison to the control plants, single Cd stress induced a massive accumulation of Put (Fig. 2a, b) (r put = 0.8857, P < 0.05; r spd = − 0.9884, P < 0.01). When plants were treated with 4 μM Cd, the content of Put reached the peak at 849.74 nmol g−1 FW, and it was 57.06 % higher than control plants. The content of Spd reduced dramatically under single Cd stress (r spd = −0.9746, P < 0.01), and it was only 293.80 nmol g−1 FW, 79.34 % of the control, when the fronds were grown in 4 μM Cd. However, as the concentration of Cd continued to rise, no outstanding changes were observed in Spm content (Fig. 2c). Therefore, due to the combined action of Put, Spd and Spm under induced Cd stress, the ratio of total (Spd + Spm)/Put decreased fiercely with the increase of the Cd concentrations (Fig. 2d). Zn addition significantly decreased the content of Put and increased the content of Spd (Fig. 1a), except for the 1 μM Cd treatment, but failed to generate a statistically significant difference in the content of Spm. So, application of Zn restored the Cd-induced decline in the (Spd + Spm)/Put ratio (Fig. 2).
Effects of Zn on the activities of ADC, ODC and PAO under Cd stress
It can be seen from Fig. 2a that ADC activity increased markedly under Cd treatment (r = 0.8818, P < 0.05) (Fig. 3a). When plants were treated with 3 μM Cd, ADC activity reached the peak at 22.22 U g−1 FW, and it was 1.61-fold higher than control plants. ODC and PAO activity changed in a similar pattern as ADC activity under single Cd stress (Fig. 3b, c). The maximum inductions were being 270.55 and 150.01 %, respectively, following the application of 4 and 3 μM Cd. However, Zn application distinctly weakened the enhancement of ADC and PAO activities, while there was no statistically significant difference in ODC activity (Fig. 3).
Effects of Zn on level of proline under Cd stress
As shown in Fig. 4, the proline content of L. minor increased conspicuously with the increase of the Cd concentrations, reaching its peak value at 3 μM Cd. However, it decreased when fronds were treated with 4 μM Cd. Compared to single Hg-treated fronds, the application of Zn dramatically reduced the proline content, but there is no remarkable difference among Zn added groups (Fig. 4).
Effects of Zn on the activities of P5CS, OAT and PDH under Cd stress
P5CS activity increased initially, reaching peak values at 2 μM Cd. However, as the concentration of Cd continued to rise, it declined afterwards (Fig. 5a). However, OAT activity was only significantly stimulated at 4 μM Cd, which was 1.18-fold higher than that of control plants (Fig. 5b). In contrast, PDH activity was inhibited with increasing Cd concentration (Fig. 5c). Application of Zn displayed a significant decrease in P5CS activity, except for the 4 μM Cd treatment. When the fronds were treated with 4 μM Cd, P5CS activity was slightly increased whereas OAT activity was decreased progressively combined with Zn addition. Additionally, Zn supplementation failed to generate a statistically significant difference in PDH activity compared to the Cd treatments only (Fig. 5).
Discussion
Reduction in biomass production is general response of higher plants to heavy metal toxicity (Ouariti et al. 1997). In our experiments, dry weight of L. minor was significantly inhibited. Similar results have been shown by plant dry weight of rice, ryegrass and Vetiveria zizanioides (Hassan et al. 2005; Xu et al. 2006, 2009). However, a decline in dry weight was weakened by Zn addition in the present study (Fig. 1), suggesting that Zn addition contributes to an increased tolerance capacity to Cd poisoning.
The homeostasis of inner polyamines metabolism in plants is essential for cell division, growth and death (Alcázar et al. 2010). In plants, polyamines levels depend not only on their synthesis but also on their degradation. The initial step in polyamines biosynthesis is the decarboxylation of arginine or ornithine to produce Put by ADC or ODC activities. Then, Spd and Spm synthesize on base of Put (Martin-Tanguy 2001). In addition, PAO activity which oxidizes Spd and Spm at their secondary amino groups participates in the polyamines degradation (Alcázar et al. 2010). Early studies had pointed out polyamines homeostasis was disturbed by Cd stress in H. dubia and P. crispus (Yang et al. 2010, 2013), and the results of our study are in accordance with the idea, which revealed that Cd stress resulted in a sharp increase in Put content and a significant decrease in Spd content and (Spd + Spm)/Put ratio (Fig. 2). However, Spm content remained constant with the increasing concentration of Cd. This could be a result of increases in ADC, ODC and PAO activities (Fig. 3), which accelerated the synthesis of Put and degradation of Spd, as suggested by a number of reports (Groppa et al. 2007; Moschou et al. 2008; Yang et al. 2010). Nevertheless, Zn addition effectively maintained the polyamines metabolism balance. It decreased Put content and increased Spd content, via reducing ADC and PAO activities. Unfortunately, no changes in Spm content and ODC activity were shown. As a result, the (Spd + Spm)/Put ratio was elevated compared to single Cd treatments. Endogenous Put content is generally considered that it may affect the redox state and a mass accumulation of Put is toxic in plants (Górecka et al. 2007; Groppa et al. 2001; Panicot et al. 2002). Spd acts as a protectant for the plasma membrane against stress damage by maintaining membrane integrity, preventing the activation of superoxide-generating NADPH oxidases and/or inhibiting protease and RNase activity (Roussos and Pontikis 2007; Roy et al. 2005). The elevation of the (Spd + Spm)/Put ratio is confirmed as a criterion of tolerance properties in plants regarding salt, osmotic, heat, chilling and heavy metals stresses (Bouchereau et al. 1999). So, it is safe to draw a conclusion that the decreasing of Put and the increasing of Spd and (Spd + Spm)/Put ratio were critical in improving Cd tolerance of L. minor, which also would be in agreement with the idea of a protective role for Zn against Cd stress.
Though consensus had not been achieved on the exact roles of proline, it was agreed that the proline accumulation is a common physiological response to various metal ions (Verbruggen and Hermans 2008). A similar result was observed in the present study in relation to proline content under single Cd stress (Fig. 4). The coordinate regulation of proline biosynthesis is achieved via two different pathways from either glutamate or orinithine/ariginine and proline degradation (Szabados and Savouré 2010). The rate-limiting step in glutamate pathway is P5CS enzyme activity, while in the ornithine pathway is OAT activity (Kishor et al. 2005). Meanwhile, PDH activity participates in the proline catabolism (Kishor et al. 2005). The present data in L. minor suggest that Cd induced effect on proline accumulation may be explained partially by the glutamate pathway involved in the elevation of P5CS activity (except for 4 μM Cd treatment) and the ornithine pathway involved in the sole stimulation of OAT activity under 4 μM Cd. A decline seen in PDH activity also participated in suppression of proline catabolism (Fig. 5). Nonetheless, Zn supplementation performed a suppressive effect on proline accumulation in L. minor exposed to Cd, by modulating P5CS and OAT activities and inhibiting the reduction in PDH activity. Consequently, application of Zn contributed to the proline level intrinsic balance, indicating that it is closely implicated in the protection of L. minor from Cd stress. In plants, proline accumulation was also closely linked with polyamines catabolism in plants exposed to abiotic stresses (Aziz et al. 1998; Bouchereau et al. 1999), owing to the reason that proline and Put share a common substrate, glutamate (Seki et al. 2007; Sharma and Dietz 2006; Simon-Sarkadi et al. 2006). In the present study, synchronous changes of proline and Put were observed in single Cd stress and Zn added groups, which were in good agreement with the viewpoint. Simultaneously, this effect supported this standpoint that proline and polyamines metabolism might exert mutual regulatory mechanism (Aziz et al. 1998).
In conclusion, Cd altered the dry weight, polyamines and proline contents as well as metabolic enzymes, inducing metabolic disturbances in L. minor. Application of Zn alleviated Put and proline accumulation, increased the dry weight and Spd contents and the (Spd + Spm)/Put ratio, which indicated that Zn were involved in the adaptation of L. minor to Cd-induced stress.
Abbreviations
- ADC:
-
Arginine decarboxylase
- Agm:
-
Agmatine
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylene diamine tetra acetic acid
- FW:
-
Fresh weight
- OAT:
-
Ornithine δ-aminotransferase
- ODC:
-
Ornithine decarboxylase
- P5CS:
-
Pyroline-5-carboxylate synthetase
- PAO:
-
Polyamine oxidase
- PAs:
-
Polyamines
- PDH:
-
Proline dehydrogenase
- PIS-bound:
-
Perchloric acid insoluble bound
- PLP:
-
Pyridoxal phosphate
- PMSF:
-
Phenylmethylsulfonylfluoride
- PS-conjugated:
-
Perchloric acid soluble conjugated
- Put:
-
Putrescine
- PVP:
-
Polyvinylpyrrolidone
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Alcázar R, Marco F, Cuevas JC et al (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Alcázar R, Altabella T, Marco F et al (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Aravind P, Prasad MNV, Malec P et al (2009) Zinc protects Ceratophyllum demersum L. (free-floating hydrophyte) against reactive oxygen species induced by cadmium. J Trace Elem Med Biol 23:50–60
Aziz A, Larher F (1995) Changes in polyamine titers associated with the proline response and osmotic adjustment of rape leaf discs submitted to osmotic stresses. Plant Sci 112:175–186
Aziz A, Martin-Tanguy J, Larher F (1998) Stress-induced changes in polyamine and tyramine levels can regulate proline accumulation in tomato leaf discs treated with sodium chloride. Plant Physiol 104:195–202
Balen B, Tkalec M, Šikić S et al (2011) Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology 20:815–826
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bog M, Baumbach H, Schween U et al (2010) Genetic structure of the genus Lemna L. (Lemnaceae) as revealed by amplified fragment length polymorphism (AFLP). Planta 232:609–619
Bouchereau A, Aziz A, Larher F et al (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bunluesin S, Pokethitiyook P, Lanza GR et al (2007) Influences of cadmium and zinc interaction and humic acid on metal accumulation in Ceratophyllum demersum. Water Air Soil Pollut 180:225–235
Chatterjee C, Jain R, Dube BK, Nautiyal N (1998) Use of carbonic anhydrase for determining zinc status of sugarcane. Trop Agric (Trinidad) 75:1–4
Chen CT, Chen LM, Lin CC et al (2001) Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci 160:283–290
Ding BZ, Shi GX, Xu Y et al (2007) Physiological responses of Alternanthera philoxeroides (Mart.) Griseb leaves to cadmium stress. Environ Pollut 147:800–803
Górecka TK, Cvikrová M, Kowalska U et al (2007) The impact of Cu treatment on phenolic and polyamine levels in plant material regenerated from embryos obtained in anther culture of carrot. Plant Physiol Biochem 45:54–61
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Groppa MD, Tomaro ML, Benavides MP (2007) Polyamines and heavy metal stress: the antioxidant behavior of spermine in cadmium- and copper-treated wheat leaves. Biometals 20:185–195
Hassan MJ, Zhang GP, Wu FB et al (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168:255–261
Horvat T, Vidaković-Cifrek Ž, Oreščanin V et al (2007) Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci Total Environ 384:229–238
Hummel I, Gouesbet G, El Amrani A et al (2004) Characterization of the two arginine decarboxylase (polyamine biosynthesis) paralogues of the endemic subantarctic cruciferous species Pringlea antiscorbutica and analysis of their differential expression during development and response to environmental stress. Gene 342:199–209
Khellaf N, Zerdaoui M (2010) Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicology 19:1363–1368
Kim HR, Rho HW, Park JW et al (1994) Assay of ornithine aminotransferase with ninhydrin. Anal Biochem 223:205–207
Kishor PBK, Sangam S, Amrutha RN et al (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Larher FR, Aziz A, Gibon Y et al (2003) An assessment of the physiological properties of the so-called compatible solutes using in vitro experiments with leaf discs. Plant Physiol Biochem 41:657–666
Malec P, Waloszek A, Prasad MNV et al (2008) Zinc reversal of cadmium-induced energy transfer changes in photosystem II of Ceratophyllum demersum L. as observed by whole-leaf 77 K fluorescence. Plant Stress 2:121–126
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Mendoza-Cozatl D, Loza-Tavera H, Hernandez-Navarro A et al (2005) Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev 29:653–671
Morrissey J, Guerinot ML (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev 109:4553–4567
Moschou PN, Paschalidis KA, Roubelakis-Angelakis KA (2008) Plant polyamine catabolism: the state of the art. Plant Signal Behav 3:1061–1066
Ouariti O, Gouia H, Ghorbal MH (1997) Responses of bean and tomato plants to cadmium: growth, mineral nutrition and nitrate reduction. Plant Physiol Biochem 35:347–354
Palmer CM, Guerinot ML (2009) Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol 5:333–340
Panicot M, Masgrau C, Borrell A et al (2002) Effects of putrescine accumulation in tobacco transgenic plants with different expression levels of oat arginine decarboxylase. Physiol Plant 114:281–287
Peréz-Amador MA, Leon J, Green PJ et al (2002) Induction of the Arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130:1454–1463
Piotrowska A, Bajguz A, Godlewska-Zylkiewicz B et al (2010) Changes in growth, biochemical components, and antioxidant activity in aquatic Plant Wolffia arrhiza (Lemnaceae) exposed to cadmium and lead. Arch Environ Contam Toxicol 58:594–604
Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35:525–545
Radic S, Babic M, Skobic D et al (2010) Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minor L. Ecotoxicol Environ Saf 73:336–342
Rena AB, Splittstoesser WE (1975) Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons. Phytochemistry 14:657–661
Roussos PA, Pontikis CA (2007) Changes of free, soluble conjugated and bound polyamine titers of jojoba explants under sodium chloride salinity in vitro. J Plant Physiol 164:895–903
Roy P, Niyogi K, SenGupta DN et al (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Sci 168:583–591
Seki M, Umezawa T, Urano K et al (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10:296–302
Sharma S, Dietz K (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Simon-Sarkadi L, Kocsy G, Várhegyi Á et al (2006) Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Plant Biol 50:793–796
Singh S, Eapen S, D’Souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Smith TA (1972) Purification and properties of the polyamine oxidase of barley plants. Phytochemistry 11:899–910
Smith CJ, Deutch AH, Rushlow KE (1984) Purification and characteristics of a γ-glutamyl kinase involved in Escherichia coli proline biosynthesis. J Bacteriol 157:545–551
Sun Y, Zhou Q, Wang L et al (2009) Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J Hazard Mater 161:808–814
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tripathi RD, Rai UN, Gupta M (1996) Induction of phytochelatins in Hydrilla verticillata (l.f.) Royle under cadmium stress. Bull Environ Contam Toxicol 56:505–512
Urano K, Yoshiba Y, Nanjo T et al (2003) Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ 26:1917–1926
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wang T, Guo SR, Liu J et al (2004) An improved method for measuring polyamine oxidase and its application to the study of cucumber root under hypoxic stress. Plant Physiol Commun 40:358–360
Wen XP, Ban Y, Inoue H et al (2011) Antisense inhibition of a spermidine synthase gene highlights the role of polyamines for stress alleviation in pear shoots subjected to salinity and cadmium. Environ Exp Bot 72:157–166
Xu WH, Wang HX, Liu H et al (2006) Effects of zinc, cadmium and their combined pollution on nutrient uptake and Zn, Cd accumulation in ryegrass (Lofium perenne L.). Asian J Ecotoxicol 1:70–74
Xu WH, Li WY, He JP et al (2009) Effects of insoluble Zn, Cd, and EDTA on the growth, activities of antioxidant enzymes and uptake of Zn and Cd in Vetiveria zizanioides. J Environ Sci 21:186–192
Yang HY, Shi GX, Wang HX et al (2010) Involvement of polyamines in adaptation of Potamogeton crispus L. to cadmium stress. Aquat Toxicol 100:282–288
Yang YL, Zhang YY, Wei XL et al (2011) Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol Environ Saf 74:733–740
Yang HY, Shi GX, Li WL et al (2013) Exogenous spermidine enhances Hydrocharis dubia cadmium tolerance. Russ J Plant Physiol 60:770–775
Zengin FK, Munzuroglu O (2005) Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Crac Bot 47:157–164
Zhao FG, Sun C, Liu YL et al (2003) Relationship between polyamine metabolism in roots and salt tolerance of barley seedlings. Acta Bot Sin 45:295–300
Acknowledgments
This study was supported by Project 30870139 of the National Natural Science Foundation and Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xuqiang Qiao and Penghe Wang are co-first authors.
Rights and permissions
About this article
Cite this article
Qiao, X., Wang, P., Shi, G. et al. Zinc conferred cadmium tolerance in Lemna minor L. via modulating polyamines and proline metabolism. Plant Growth Regul 77, 1–9 (2015). https://doi.org/10.1007/s10725-015-0027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0027-0