Abstract
Underground excavations are naturally under deteriorating environments due to corrosive ground water, type of minerals, and rock mass structures. The abundant of water (e.g., humidity and the inflow of groundwater), and the presence of chemical materials are among the main reasons creating such environments. Rock formations also contain minerals, which may be dissolved in the stream of groundwater and react with support measures (e.g., rock bolts, shotcrete and concrete linings). Deterioration is the main factor leading to the decay of the support systems and consequently affecting their functioning. The deterioration of support measures may result in significant consequences such as the failure of supports and the collapse of underground excavations, in long-term. It is, therefore, vitally required to investigate the long-term deterioration and behaviour of supports, and to find suitable approaches for mitigating the undesirable consequences of this natural phenomenon. Hence, the main scope of this study is to investigate the hydrogeological factors resulting in the deterioration of the segmental concrete linings at Zagros water transfer tunnel, located in the West of Iran. The key factors governing the deterioration mechanisms are identified and discussed based on a combination of field observation, laboratory testing and the literature studying of similar cases. The outcomes of these studies indicate that sulfate attack has been the crucial mechanism leading to the deterioration of the concrete linings in the Zagros tunnel. The hydrogeological condition of the tunnel well satisfies the four risk factors previously found by the UK Thaumasite Expert Group. The tunnel segmental concrete linings are, therefore, highly prone to sulfate attack. This study also shows that tunnelling through H2S and water bearing grounds has been the main reason providing such a deteriorating environment. The existence of organic materials (e.g., oil and coal) in the surrounding sedimentary rocks is expected to be the main source of H2S gases. The study is eventually complemented by proposing a few practical techniques to prevent further damage of the segmental linings, due to chemo-physical deteriorations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
To estimate the remaining service life of tunnel excavations, it is required to decipher the parameters affecting the decaying process of support measures. The present study, therefore, aims at investigating the main mechanisms of deterioration of the segmental tunnel linings at the Zagros water transfer tunnel. It is noted that the Zagros tunnel has been constructed in an extremely complex hydrogeological condition, containing fault zones, and water and gas bearing grounds. The rush of groundwater rich of Hydrogen Sulfide (H2S) and Methane (CH4) gases resulted in significant technical issues during tunneling. A few of the main hydrogeological issues encountered during the construction of the tunnel and the proposed mitigating strategies were reported in the relevant references (Bayati and Hamidi 2017; Eftekhari and Hamidi 2009; Khave 2013, 2014; Morsali and Rezaei 2017).
In this study, the prominent factors leading to the corrosion of the concrete linings, at the Zagros tunnel, are identified and briefly discussed. The method of analyses is based on a combination of field observations, laboratory testing and comparing the results with similar case studies available in the literature. The comparison is mainly performed to verify the results achieved from the interpretation of filed inspections and laboratory tests. Additionally, a number of practical techniques are suggested to prevent further deterioration of the concrete linings. The outcomes of this study might be useful to prevent the aggressive effects of deterioration (e.g. corrosion, weathering, and erosion) on tunnel structures as well as to ensure the long-term durability and the service life of segmental concrete linings, in similar conditions.
2 Project Information
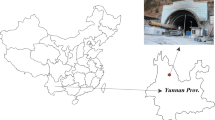
The Zagros water transfer tunnel with total length of 49 km and 6.73 m diameter has been designed to convey around 70 m3/s water from the Sirvan River to Dashtezahab area in Kermanshah province, Iran (see Fig. 1). This project is one of the longest water transfer project in Iran. The tunnel was divided into three main parts consisting of Lot 1A (14 km) as northeast section, Lot 1B (9 km) as middle section, and Lot 2 (26 km) as southwest section. The Zagros tunnel was also supported with pre-cast concrete segments with hexagonal arrangement and thickness of 250 mm (Hassanpor et al. 2016; Shahriar et al. 2008).

Adopted and modified from Ghafarian et al. (2016)
A schematic view of the location of Zagros tunnel.
The construction of Lot 2 has recently been completed with a double shield TBM in extremely harsh hydrogeological conditions. Tunnel was excavated from two portals at south and north ends. Two other access tunnel were also constructed from portals located in two deep valleys crossing the tunnel route to facilitate the excavation and to supply the required materials (e.g., the Kordighaseman adit) (Bayati and Hamidi 2017; Hassanpor et al. 2009, 2016; Shahriar et al. 2008).
Groundwater inflow was one of the dominant issues affecting the construction of the Zagros tunnel. Substantial ingress of water and toxic gases, like H2S and CH4, resulted in noticeable unpredictable delays in tunnelling. Water inflow also brought about challenging technical problems and highly reduced the performance of excavation. The problems associated with tunnelling in gas and water bearing grounds, in the area of the study, have well been studied and documented in the relevant literature (Bayati and Hamidi 2017; Eftekhari and Hamidi 2009; Hassanpor et al. 2016; Shahriar et al. 2008).
Tunnelling at the Zagros project has derived in different geological formations, which include but are not limited to soft and swelling shales; squeezing grounds; highly faulted and folded sedimentary rocks; and gas and water bearing rocks. Based on relevant studies, over 25% of the Lot 2 of the tunnel was excavated in heavily water and gas bearing grounds (Bayati and Hamidi 2017; Eftekhari and Hamidi 2009). A proper understanding of the geological and hydrogeological conditions of the tunnel may help to decipher the mechanisms leading to the deterioration of the segmental linings. The key geological and hydrogeological features of the tunnel are, therefore, briefly explained here.
2.1 Geological Condition
In terms of structural geology, the Zagros tunnel is located in the core of the Zagros Mountain range. This zone is a wide Tertiary thrust and fold belt stretching across the entire western region of Iran with a roughly aligned NW–SE strike. The fold-thrust belt of Zagros contains very complex structural units which include, but are not limited to, flexural folds, ramp and flat, detachment folds, fault propagation folds, fault-bend folds, klippen, imbricate fans, duplexes, late stage strike-slip and normal faulting (Khave 2013). It is also noted that the major principal stress of these structures is normally aligned in north-east to south-west direction (Khave 2013).
The Zagros tunnel was excavated in variety of formations, with varying ages from Mesozoic to Cenozoic. The youngest geological units encountered in the tunnel route are alternative thick greenish-gray shale and thin-bedded siltstone, sandstone and argillaceous limestone of Amiran formation (chainage 11 + 800 to 12 + 100). These units have been located in the core of Baneh-dar syncline. Gurpi formation underlies Amiran formation and spreads throughout almost the entire tunnel path. Limy shale and argillaceous limestone are the dominant type of rocks in this specific formation. Relevant studies in the area have revealed that a few rock strata in the Gurpi formation contain pyrites rich minerals in nodules form (Khave 2013; Mirmehrabi et al. 2011).
Garou formation also underlies the Gurpi formation and comprises of alternating thin to thick-bedded shale and argillaceous limestone. Moreover, according to the geological studies, Gotnia formation is the oldest unit along the tunnel path (chainage 19 + 500 to 21 + 500). The prevailing rocks in this formation are anhydrite, gypsum and evaporate cast. The Gotnia formation intersects the tunnel at several locations in Zimkan structure (Khave 2013).
The geological and geotechnical characteristics of the ground have well been studied and reported in the relevant literature (Hassanpor et al. 2016; Shahriar et al. 2008). Based on these studies the main lithological units consist of shale, limestone and marl layers. The tunnel passes through several formations with rock mass conditions varies from weak to good, with RMR ranging from 17 to 75. The overburden strata frequently changed from hard rock to soft, dry to wet, stable to unstable, sticky to non-sticky ground, and vice versa. The maximum depth of the tunnel is also around 1000 m with the average depth equal 400 m (Khave 2013; Shahriar et al. 2008). A schematic longitudinal profile of the geological formation along the Lot 2 of the tunnel (chainage 3 + 600 to 5 + 100) is shown in Fig. 2. Extensive H2S and water flow were observed in this part of the tunnel.
2.2 Hydrogeological Condition
The complex and unique hydrogeological condition of the Zagros tunnel has been the topic of several technical papers. In this study, we briefly explain the main hydrogeological features of the tunnel. Further details related to the hydrogeological condition of this tunnel can be found in the relevant literature (Bayati and Hamidi 2017; Eftekhari and Hamidi 2009; Tali et al. 2014). In the Zagros tunnel, the groundwater level varies from 30 to 340 m above the tunnel crown. During the construction of the tunnel, flow rates in excess of 1200 (L/s) were encountered. Since the tunnel passed through gas bearing grounds, the inflow of water accompanied with a considerable amount of gases into the tunnel environment (see Figs. 3, 4).
The rush of groundwater into the tunnel (Nazemi 2017)

(Courtesy of Mirmehrabi et al. 2010)
Excessive water flow and the harsh environment at the Zagros tunnel.
Generally speaking, the permeability of a rock mass is a function of its porosity as well as the existence of discontinuities. The intersections between fractures or faults are normally critical areas where high water flow is expected. This is because discontinuities produce open network to transfer water into the excavations. The in situ rock mass permeability, which measured from Lugeon tests at the Zagros tunnel, ranges from 1.0 to 5.0 (1.15 × 10−7 to 5.57 × 10−7 m/s) in the Gurpi and 30.0 to 60.0 Lugeon (3.45 × 10−6 to 6.9 × 10−6 m/s) in the Garu formations.
Site inspections indicated that water inrushes mainly seeped up through the filling washed discontinuities and conduits. Figure 5 shows the inflow of water and gas into the tunnel. The peaks show that water flows along filling washed discontinuities and conduits of folding hinge parts. Field investigation revealed that the inflow of water increases when dip of discontinuities (including bedding) increases. Additionally, the discharge of water decreases where joints with orientation orthogonal to the tunnel axis are encountered.
It is also noteworthy that the rock masses surrounding the tunnel are saturated and H2S is mainly dissolved in water, whereas CH4 is not dissolved in the ground water and flow into the tunnel as a gas. The value of the H2S gas emission is, therefore, proportional to the amount of water ingress into the tunnel (Eftekhari and Hamidi 2009).
Field inspections and groundwater sampling at the tunnel, revealed that, in normal situations, almost 1 L of H2S is released per 100 L of water inflow into the tunnel. The volume of the released gas varies based on the groundwater discharge rate (Morsali and Rezaei 2017). A precise estimation of groundwater inflow is, therefore, required to predict the volume of H2S emission.
3 The State of the Art on the Mechanisms of Concrete Deterioration
A proper understanding of the concrete deterioration mechanisms is needed to find factors resulted in the decaying of the linings at the Zagros tunnel. In this section, the main mechanisms of concrete deterioration, including sulfate attack, are investigated. The study especially focused on the deterioration of concrete and shotcrete supports used in underground excavations.
Concrete is a composite material consisting of fine and coarse aggregate which are bounded together with a cement paste and this cement past is hardened over time. Additive materials like pozzolans or superplasticizers are sometimes added to the mixture in order to improve the mechanical characteristics of the wet mix or the final products. Reinforced materials, like rebar, are also embedded to improve the tensile and shear strength of the concrete and is known as reinforced concrete (Mostoufinejad 2003). Concrete durability assessment and concrete petrography are special aspects of concrete design that involve the geo-mechanical engineering. Numerous studies have been devoted to investigating the long-term durability of concrete based on the interactions between aggregate and cements as well as the physical and chemical processes acting on the concrete elements (Alsinawi and Al-Bazzaz 1975; Fookes 1980; Larsen 1966; Talesnick and Baker 1999).
Concrete is widely used in the construction of buildings and infrastructure, due to its high durability in severe environments. However, concrete in structures may be damaged because of excessive loading or chemical reactions. These reactions may occur due to the ingress of certain reactive species from the surrounding environment into the microstructure of concrete or even because of the alteration of concrete components (Bellmann et al. 2012; Sims and Huntley (née Hartshorn) 2004).
Segmental linings and shotcrete are typical examples of concrete structures which are widely used to stabilise underground excavations for long-term (Hoff 2009). These supports are subjected to internal and external loads (e.g., rock loads, water head) as well as environmental effects (e.g., weathering and deterioration), during the construction and the service life of excavations (Attiogbe and Rizkalla 1988; Usman and Galler 2013; Usman et al. 2011; Zhiqiang and Mansoor 2013). Such loadings and chemical reactions degrade the mechanical properties of supports and subsequently affect the remaining service life of excavations (Usman and Galler 2013). Generally speaking, as a tunnel ages, its lining’s deterioration frequency tends to increase (Lei et al. 2013). Since underground excavations are humid, the rate of concrete lining deterioration is substantial. The impact of deterioration could be significant, and it is normally expensive and time-consuming to repair such damage (Inokuma and Inano 1996; Romer 2003a, b). Deterioration may also result in the failure of support systems and the catastrophic collapse of excavations. For example, the gradual decaying of support measures resulted in the sudden collapse of Oyamano tunnel on the national highway route 127, in Chiba prefecture in 1990, in Japan (Inokuma and Inano 1996). Sims and Huntley (née Hartshorn) (2004) also reported several cases of concrete structure deterioration around the world, containing an interesting case of tunnel lining damage in the Formazzo valley, in Italy. They mentioned that deterioration was extremely severe and in some places, the entire 300 mm lining of the tunnel was detached.
John (1982) investigated the unusual mechanisms of concrete deterioration in the linings of New Zealand railway tunnels. Two main reasons of concrete corrosion were identified in this study. The decaying of the concrete at the tunnel arches which was accompanied by gypsum formation. This mechanism was attributed to the reaction of sulfate ions in coal smoke accumulated at the tunnel crown with the concrete materials (John 1982). Severe exfoliation was also observed along the base of the tunnel lining, around 0.25 to 1.5 m above the ballast level. In this part of the tunnels, concrete surfaces were substantially removed in sheets and severe exfoliation extended to the depth of 60 to 100 mm. Laboratory investigation confirmed the formation of Mirabilite (Na2SO4·10H2O) on the surface of concrete. However, Mirabilite was not observed in the interior parts of the cored concrete samples. The reactions between the chemically rich groundwater and concrete materials was identified as the main suspect of concrete corrosion in the linings of tunnels in this area (John 1982).
Romer and co-workers investigated the deterioration of concrete linings in a few road tunnels in Swithserland (Romer 2003a, b; Romer et al. 2003). The interaction of concrete and groundwater and its effect on the mechanisms of deterioration were well explained in their studies.
3.1 Mechanisms of Sulfate Attack
Generally due to the abundant of water, reactive chemical minerals, and temperature, tunnel environments are severely corrosive (Lei et al. 2013). The deterioration of concrete and shotcrete lining has been the subject of several studies (Hagelia 2011; Hagelia et al. 2003; John 1982; Usman and Galler 2013; Zhiqiang and Mansoor 2013). Physical damage by crystal erosion, chemical dissolution or a combination of both mechanisms is normally observed in underground excavations (Lei et al. 2013). The governing mechanisms of concrete deterioration are complex and may involve different reactions (Attiogbe and Rizkalla 1988; Bellmann et al. 2012; Romer et al. 2003). However, a few common features are normally observed in the majority of deteriorating environments. For example, sulfate attack is among the most serious deteriorating processes affecting the integrity of concrete structures. The main mechanisms of sulfate attack are briefly explained in this study.
3.1.1 Formation of Ettringite and Thaumasite
Sulfate attack is a common mechanisms of concrete deterioration. Significant efforts have been devoted to investigating the mechanisms of concrete deterioration and the effects of sulfate attack on concrete structures (Crammond 2003; Irassar 2009; Rahman and Bassuoni 2014; Suleiman et al. 2014). Literature review indicated that the most common products of sulfate attack are gypsum (CaSO4·2H2O), ettringite (3CaO·Al2O3·3CaSO4·31H2O), and thaumasite (CaSiO3·CaCO3·CaSO4·15H2O) (Crammond 2003; Hobbs and Taylor 2000; Sims and Huntley (née Hartshorn) 2004). The chemical reactions and the formation of deterioration products depends on the composition of the cement (e.g., the mineralogy of mortar) and the environmental conditions (e.g., temperature, the chemistry of groundwater and surrounding rocks) (Bellmann et al. 2012; Nielsen et al. 2014; Romer et al. 2003). The conventional form of sulfate attack results in the formation of ettringite (± gypsum). This form of chemical reaction usually occurs early on in the deterioration process. Field investigations by Crammond (2003), however, showed that the procedure of sulfate attack may not always start with the formation of ettringite. Although Sulfate Resisting Portland Cements (SRPC) with low level of Aluminate hydrate have been used to prevent the formation of ettringite, such cements may not necessarily protect the concrete structures against thaumasite reactions. In Thaumasite Sulfate Attack (TSA) the Calcium Silicate Hydrates (CSH) and not the aluminate phases are attacked by sulfate ions. The replacement of CHS by thaumasite gradually leads to the softening of the cement matrix and transfers it into a white mushy non-cohesive mass. The formation of chemical products results in the expansion and spalling of the concrete surface.
Crammond (2003) stated that there are two main mechanisms of thaumasite generation in concrete structures, especially in underground and buried cases. The first mechanisms is thaumasite development due to sulfate attack which is known as TSA. The second mechanism, which is known as Thaumasite Formation (TF) is not as aggressive as the first mechanism. In other words, thaumasite may, like ettringite, precipitate in cracks and voids without necessarily affecting the structure of the host concrete or mortar (Crammond 2003). No obvious sign of damage due to sulfate attack is seen in such cases. Although the presence of TF is normally harmless for concrete structures, it may be a precursor of TSA. TF may, therefore, be considered as an indication of a potential failure problem (Crammond 2003; Sims and Huntley (née Hartshorn) 2004). Crammond (2003) reported the outcomes of 15 years research on the mechanisms of sulfate attack in the UK. Over 80 cases of significant sulfate attack were identified and carefully investigated. The majority of the observed deterioration occurred due to TSA. Field inspections showed that TSA almost entirely disrupts the cementitious binding- ability of the concrete materials by transforming it to mush.
3.2 Factors Controlling TSA
The mechanisms of thaumasite formation in the lining of underground tunnels were well explained by Romer et al. (2003). One of the main mechanism is the direct formation of C–S–H phase, which is related to the transformation of hard concrete (cement) paste into thaumasite. This stage depends on the qualitative chemical and mineralogical characteristics of concrete as well as the composition of groundwater (Romer et al. 2003). The process of deterioration is affected by chemical parameters such as the composition of paste, the pH value of groundwater and its chemistry as well as the physical characteristics of concrete (e.g., porosity, permeability and homogeneity). These parameters may significantly vary over time, highlighting the complexity of the process of long-term concrete deterioration (Hobbs and Taylor 2000; Romer et al. 2003).
Groundwater may contain substantial amount of sulfate ions because of the dissolution of some minerals (e.g., pyrite). The effect of such minerals on the corrosion of concrete structures was investigated by Bellmann et al. (2012). They also observed severe deteriorations where concrete structures were in contact with pyrite or gypsum because of proximity to old abandoned mine workings. Bellmann’s study highlighted the impact of minerals on the mechanisms of sulfate attack and the corrosion of concrete structures. In particular, the combination of pyrite oxidation, which initiates the sulfate attack, together with carbonation transfer the cement phase into calcite, gypsum, and thaumasite. This has resulted in the loss of adhesion of shotcrete layers in several underground excavations in Germany (Bellmann et al. 2012).
Romer (2003a, b) indicated that the process of concrete deterioration involves several stages including leaching, dissolution, transportation, (re)precipitation and the formation of new products. Romer (2003a, b) also stated that the dilute concentration of water-soluble reactive components (like magnesium or sulfate) may deeply penetrate into the concrete with pore fluid and leach the cement paste. Potassium, sodium and chloride may also be transported in higher concentration, but accompanied by lower degree of leaching (Romer et al. 2003). The chemical composition of groundwater may also significantly vary from a representative pore solution. These studies revealed that significant mass transfer interaction may occur between groundwater and the cement paste in the concrete (Romer 2003a, b). The following outcomes have also been extracted:
-
1.
Component of cement paste is dissolved and leached away by groundwater
-
2.
Components of groundwater (e.g. magnesium or sulfate) can create stable compounds with constituent of the pore solution or cement pastes.
Sulfate attack involves the movement of sulfate ions through the pores of concrete (cement) based on different transportation mechanisms. The classical models of sulfate attack are based on the assumption that sulfate ions permeate from an aggressive environment to adjacent concrete structures. This attack results in a phase transformation and subsequently damage the microstructure of concrete (Bellmann et al. 2012). The extent of damage is mainly controlled by the concentration of the sulfate ions and other accompanying aggressive substances, as well as the chemo-mechanical parameters of cement (Bellmann et al. 2012). Salt materials may also penetrate through the matrix of concrete based on capillary absorption, permeation, and diffusion. Such reactions, when are combined with the influence of humidity, may severely damage concrete structures and lead to spalling and deterioration (Lei et al. 2013).
Crammond (2003) also investigated the factors that are required for triggering TSA. Based on this study, SO −24 ions, SiO3, CO −23 ions, water and cold temperature (below 15 °C) are the main requirements for the TSA in concrete structures. Sims and Huntley (née Hartshorn) (2004) also identified the primary and secondary risks factors leading to the formation of thaumasite. The main primary factors contain (1) presence of the source of sulfate, including sulfide that may decay to sulfate, (2) presence of mobile water or groundwater in the case of buried concrete, (3) presence of carbonate that may be provided by the aggregates, and (4) low temperature (generally below 15 °C). The secondary risk factors also contain (1) type and quality of the cement used in concrete, (2) the quality of concrete, (3) change in ground chemistry or water regime due to construction, and (4) type, depth and geometry of concrete structure (Sims and Huntley (née Hartshorn) 2004).
The dominant glue that is found in the matrix of Portland cement pastes, including sulfate resistant Portland cements (SRPC), consists of CSH. This component is the main source for producing the required silicate to trigger TSA. The remnant unhydrated clinker grains of tri-calcium silicate (C3Si) and di-calcium silicate (C2Si) conforms secondary sources for the TSA reactions. The accessibility of these materials to aggressive SO −24 ions depends on the quality of the cement/mortar and cement and aggregate types. Crammond (2003) stated that the required CO −23 ions are mainly produced from particles of limestone used as an aggregate or cement filler in concrete. These ions may even be supplied as carbonate or bi-carbonate ions dissolved in the sulfate bearing external water sources. Generally speaking, smaller limestone particles have higher tendency to enter reactions. Apparently, dolomite limestone (CaMg(CO3)2) particles are more reactive and vulnerable to TSA rather than CaCO3 based limestone particles. Nielsen et al. (2014) also studied the effects of cement type and aggregate on sulfate attack and thaumasite development in concrete. Outcomes of this study indicated that the mortar prisms that contain limestone filler are highly vulnerable to sulfate attack. A mobile water source is also needed for the formation of all types of sulfate attack.
4 Data Collection and Methodology
Field inspections at different tunnel locations were performed to assess the deterioration of the concrete linings. These inspections revealed that damage on the surface of linings is associated with swelling, spalling and the severe degradation of mortar. Deterioration was also significant where the segmental linings prone to cyclic wetting and drying. Filed observations also indicated that sulfate attack could be a reason of the segmental linings deterioration. Figure 6 well depicts the decaying of the concrete linings at the Zagros tunnel.
Field investigations also showed that the process of concrete lining deterioration produced a new white powdery material, mush, which is well consistent with the signs of sulfate attack described in the relevant literature (Crammond 2003; Hobbs and Taylor 2000). Figure 7 shows typical examples of mush materials on the surface of concrete linings. To decipher the mechanisms of concrete corrosion, a few samples of this product were collected and tested. X-ray Diffraction, XRD, analysis was performed on the collected samples of deteriorated concretes and on the new products, mush, to identify the phases existed in the materials.
Samples of concrete from the segmental tunnel linings were collected by coring in proper locations, where severe damage was observed. These samples were later analysed to assess the effects of deterioration on the mechanical characteristics of concrete. Several specimens of exfoliated concrete and the deterioration product were also collected for further laboratory investigations. Photographic analysis based on Microscopy Analysis, MA, and XRD technique were also performed to investigate the mechanisms of deterioration in micro-scale level. Additionally, to assess the effects of water chemistry, samples of groundwater were collected from different locations in the tunnel. Care was taken to fill the entire bottles of water to exclude air and to preserve the chemistry of the water samples. Outcomes of all the performed tests are briefly explained here.
5 Results and Discussion
5.1 Field Inspections to Identify the Sources of Sulfate Ions in the Tunnel
As mentioned earlier, the presence of toxic gases (e.g., H2S, CH4) brought about several problems during the construction of the Zagros tunnel. Not only the emission of gases, during tunnelling, led to loss of lives and resulted in a very harsh environment for the crews to work, but resulted in a very deteriorating atmosphere that damaged the tunnelling equipment (e.g., TBM, rails, haulage system), and more importantly the tunnel linings (Khave 2013).
To mitigate the adverse effects of deterioration, it is needed to clearly identify the sources of H2S emission and corrosive sulfate ions. Considerable studies have been dedicated to study the main sources of H2S, in terms of geological and petrological points of views (Khave 2013, 2014; Mirmehrabi et al. 2010, 2011). The associated hazards and the remedial measures for reducing the relevant risks were also investigated and well documented in the literature (Bayati and Hamidi 2017; Eftekhari and Hamidi 2009; Khave 2013, 2014; Morsali and Rezaei 2017). Several field investigations were, therefore, carefully performed to assess the hydrogeological condition of the tunnel and to identify the sources of H2S gas and sulfate ions in the tunnel.
Studies indicated that gas emission may be encountered during tunnelling in numerous sedimentary formations. H2S and SO2 are, especially, formed in fractured rock masses containing Pyrite (Mittermayr et al. 2012). The oxidation of iron sulfate minerals, mainly pyrites, within the rock mass adjacent to the concrete structures results in the formation of sulfuric acid and provides the required sulfate ions to trigger sulfate attack (Crammond 2003; Hobbs and Taylor 2000).
Outcomes of field inspections indicated that in addition to rocks that contain Pyrite (FeS2), around the Zagros tunnel, a number of the sedimentary formation (e.g., Pabde, Gurpi, and Garo formations) have been identified as host rocks for hydrocarbon reservoirs. It is expected that such hydrocarbon reservoirs may exist in the tunnel underlying sedimentary formations, even though in small scale. Apparently, these organic materials have been the main sources of H2S and CH4 gases in the tunnel. This is interestingly consistent with the other relevant studies on sulfate attack. In particular, Crammond (2003) showed that the spoils and bituminous materials left from abandoned coal collieries and oil shales have been among the main sources of SO −24 ions attacking concrete structures in several locations, in the UK.
Crude oil is formed in sedimentary rocks and seeps through the permeable formations. If the rock masses and the geological structures are proper to trap the migrating crude oil, the accumulation of such organic materials may create a hydrocarbon reservoir. The existence of soft and plastic strata, such as shale, mudstone and other clay-reach rocks with very low permeability, can trap the hydrocarbon materials in the rock masses (Ghazban and Motiei 2010; Rezaee 2001). Figure 8 shows the seepage of organic materials from the rock mass into the Zagros tunnel, proving the above-mentioned theory.

(Courtesy of Mirmehrabi et al. 2010)
Seepage of organic bitumen into the Zagros tunnel from segments’ joints.
It is noteworthy that, in the Zagros tunnel area, although there are rock formations suitable for the creation of crude oil, the generated oil and gases migrate upward and spread out through the jointed rocks. This is due to the lack of proper geological structures to trap these organic materials. Therefore, merely the trace of such materials has remained in the original host formations and in the overlaying strata. The organic materials are mainly black liquid bitumens as dense as tar (see Fig. 8).
The existence of sulphur springs, organic traces, organic argillaceous rocks, exposure of H2S odour from the fresh surface of rocks and the smell of H2S during boreholes drilling, all prove the accuracy of the above-mentioned theories on the formation of Hydrogen Sulfide gas in the Zagros tunnel (Eftekhari and Hamidi 2009; Mirmehrabi et al. 2011).
The presence of sulfuric ions may, therefore, be attributed to the chemical reaction of water with H2S, which produced Sulfuric Acid (H2SO4) as:
5.2 Groundwater Chemistry Analysis
Previous studies have shown that groundwater chemistry may play a significant role in the deterioration of construction materials used in tunnelling (Mittermayr et al. 2012; Mossmark 2014; Saglamoglu 2012). To examine the potential of sulfate attack on the concrete linings, the existence of sulfate ions in the groundwater ran into the tunnel was examined. Water samples were collected from different chainages, where severer concrete deterioration were observed. Test were performed according to the procedure explained in the relevant references (Mossmark 2014; Rajesh et al. 2015). Typical examples of the geochemical tests performed on the water samples collected from the tunnel are shown in Tables 1 and 2. Note that in Table 1, TDS, TH, and EC show the total dissolved solid, electrical conductivity, and total hardness of water, respectively. Further information related to these parameters can be found in the relevant references (Mossmark 2014; Rajesh et al. 2015).
The analyses revealed that water contains considerable amount of SO −24 , especially in the form of CaSO4 and MgSO4, and Na2 SO4. Outcomes of the groundwater chemistry also showed that the major anions are HCO3−and SO −24 . As mentioned, H2S may naturally be produced in the tunnel because of the decaying of organic materials. The formations of sulfuric acid has, therefore, been very probable at the Zagros tunnel. Test results showed that the ground water, in the tunnel, is rich of Sulfuric Acid and can react with the concrete linings and leads to severe degradation of their mechanical properties.
5.3 XRD Analysis
A few XRD analyses were also performed to investigate the mechanisms of concrete corrosion. The outcomes of XRD tests on three deteriorated concrete samples and one sample of the corrosion product, which have been collected from the Zagros tunnel, are shown in Fig. 9. The results of XRD analyses revealed that the white deposition on the surface of concrete linings mainly consists of Calcium Sulfate (CaSO4), and is expected to be the final product of Calcium Hydroxide dissolution in the presence of Sulfuric acid. This reaction can be described by Eq. (4).
It is noted that the formation of mush on the surface of segmental tunnel linings, in severely deteriorated areas, supports the proposed corrosion theory and reactions (see Fig. 10). The results of the XRD tests indicated that the concrete samples mainly contain calcite (CaCO3) and negligible amount of Quartz. Quartz is a durable mineral that is not dissolved in acidic solution.
Figure 9 also shows that the sample of white corroded products contains gypsum. Gypsum is a soft Calcium Sulfate mineral with the chemical formula of CaSO4·2H2O, and is the most common mineral produced due to sulfate attack. Based on the results of the XRD tests on the collected samples, the most likely chemical reactions in the tunnel lining can be described as Eqs. (5) and (6).
The formation of gypsum is accompanied by volume expansion and swelling in the concrete materials. Swelling leads to spalling and the disintegration of concrete surface, which subsequently degrade the mechanical properties (e.g., strength) of concrete.
5.4 Microscopy Analysis (MA)
Petrographic examination is widely used to investigate the effects of deterioration on the structural integrity of concretes (Hobbs and Taylor 2000; Larsen 1966). Microscopy Analysis (MA) was, therefore, used for further investigation of the deterioration mechanisms occured in the segmental concrete linings at the Zagros tunnel. A number of thin sections were produced from the collected cored samples of deteriorated concrete linings and well prepared based on the existing standards (Mays 1992). Thin sections from the top, middle and bottom of the concrete cores were made in order to check whether deterioration has developed into the segmental concrete linings. Figures 11, 12 and 13 show typical results of the MA performed on the thin sections prepared form the top, middle and bottom sections of three different concrete cores, A, B, and C, respectively. Particles of limestone micrite and dolomite, which contain crystals of calcite and some clay minerals, can be seen in the thin sections. A limited amount of micro-fossil particles were also detected in a few of the prepared thin sections. Some calcite crystals also contain iron oxides (magnetite, Fe3O4, and hematite, Fe2O3), which do not seem to be reactive in the concrete matrix. The MA also disclosed the existence of some calcite crystals around the small blocks of cherts in the concrete. There is also a limited amount of clinkers in the paste that have not reacted and may never be hydrated during the lifetime of the structure.
A number of calcite crystals were also observed in the concrete matrix. These crystals can be formed due to the precipitation of calcite or have likely been introduced because of the acidic attack on the concrete. Such crystals were even observed in the thin sections prepared from the middle part of the concrete cores. The role of carbonation in the formation of these crystals may simply be ruled out, as it is unlikely for the carbon monoxide, CO2, to penetrate that deep into the core of concrete lining whose porosity is relatively very low. Apparently, acidic attack has been the main source for the formation of calcite crystals.
Interestingly the outcomes of petrographic examination shown in Figs. 11, 12 and 13 are well consistent with the results of tests presented in the relevant references on the sulfate attack in concrete materials. For example, Hobbs and Taylor (2000) reported the outcomes of a few petrographic investigations performed on thin sections of samples collected from a number of TSA affected bridge foundation in the UK. This investigation confirmed the existence of three different zones in the affected concretes. The first zone, which was in direct contact with the backfill materials, had 5 to 10 mm thickness and showed significant transition of cement paste into thaumasite. The second zone with 10 mm to 15 mm thickness contained cracks that were parallel to the concrete surface. These cracks were normally filed with thaumasite. Additionally, white thaumasite haloes were observed around the coarse and fine aggregates including the siliceous sand particle. This zone, normally does not contain other sulfate minerals. However, little amount of calcium hydroxide and calcium carbonate occasionally were precipitated into the cracks and voids. The third and deeper zone did not contain any significant visual sign of deterioration. However, very thin layers of ettringite (around 5 mm) were frequently observed along the cracks. Additionally, field observation of concrete strip foundation in a personal property subjected to sulfate groundwater revealed three different zones of deterioration effects. The first zone at the concrete/clay interface showed complete transformation of cement paste to thaumasite. The second zone contains intact concrete, but thaumasite and ettringite were observed within the voids and cracks. No evidence of visual damage was observed in the third zone. However, ettringite was accumulated within the voids in this zone (Hobbs and Taylor 2000). In cases that concrete are in contact with aggressive groundwater, the thaumasite type of sulfate attack is mainly formed due to natural sulfate ion ingress into the matrix or sulfate acid attack on concrete (Hobbs and Taylor 2000). Petrographic observations also showed that sulfate attack occurs within surface regions of concrete largely deplete them of calcium.
Figure 14 also shows typical examples of the performed Microscopy Analysis (MA) of a deteriorated concrete sample. This figure well shows that sulfate ions gradually react with the limestone/dolomite particles and cement paste, and alter the concrete materials. These chemical reactions gradually led to the deterioration of the concrete linings.
5.5 Effects of Sulfate Attack on the Mechanical Properties of Concrete
To design a suitable segmental tunnel linings, it is needed to ensure that the concrete has the required strength and mechanical characteristics. The concrete components should, therefore, carefully be selected to meet these requirements. It is also necessary to compute the proportion of each component to achieve a robust concrete. The concrete mixture not only must satisfy the technical requirements, but should be cost effective for mass production. In other words, the component should easily be available. The construction process of the segmental tunnel linings, for the Zagros tunnel, were performed according to the relevant references and standards (Mostoufinejad 2003; Ramezanianpour 2011). The 28-day strength of the concrete samples was around 45 MPa which is well above the concrete strength considered in the design of concrete linings (35 MPa).
Several laboratory tests were performed on concrete samples collected from the Zagros tunnel to analyse the effects of sulfate attack on the physico-mechanical characteristics of the concrete linings. Outcomes of the tests have well been documented in the local and international literature (Ahmadkhani 2013; Moodi et al. 2015). These tests included compressive strength, water absorption, electric resistivity, and sulfate ion on 69 cored samples collected from the deteriorated segmental linings from different locations of the tunnel. Outcomes of the study indicated that sulfate attack has affected the strength and the stiffness of the concrete linings. In particular, the outcomes of the study by Moodi et al. (2015) shown that concrete deterioration has reduced the strength (cohesion) of the concrete lining. Water absorption test were also conducted on 64 specimens. Further 84 sulfate ion tests were also performed to assess the effects of sulfate attack on the mechanical characteristics of concrete.
The compressive strength tests were performed on cores with 75 mm diameter. To saturate the specimens, cores were placed in water, with 20 °C temperature, for at least 48 h. Outcomes indicated that the compressive strength of 3%, 53.3% and 43.7% of specimens varied between 5 and 20 MPa, 20 and 35 MPa, and 35 and 50 MPa, respectively. Since the design value of the 28-days compressive strength of concrete was 35 MPa, almost 56.3% of the specimen represented lower compressive strength than the design value. This strength degradation could be because of sulfate attack or the poor construction quality of concrete (Ahmadkhani 2013; Moodi et al. 2015). Moodi et al. (2015) also reported the outcomes of 24 tests on samples of concrete collected from 8 different non-deteriorated segments. The strength achieved from all tests were above 35 MPa. This shows that the strength loss in the deteriorated areas was mainly due to the physical and chemical impacts of sulfate attack.
Concrete electrical resistance depends on its permeability and environmental condition (Biczok 1967; Ramezanianpour 2011). Higher permeability allows aggressive ions to penetrate concrete easier. This increases the electrical conductivity of the concrete and proportionally decrease its electrical resistance. This shows that a higher electric resistivity represents a better performance against aggressive ions (Ramezanianpour et al. 2018). The outcomes of the electrical resistance tests showed that the electric resistance of concrete specimens varies between 16 and 55 kΩ-cm. Studies also showed that if the electric resistance of concrete specimen is less than 55 kΩ-cm the potential of aggressive ion ingress in the concrete may not be significant (Moodi et al. 2015).
Concrete porosity is also an important parameter. It allows water containing chemical species to leave off aggressive components. Remaining chemical species can react with concrete materials and cause chemical attack. This highlights the importance of assessing the porosity and permeability of concrete specimens. The water absorption test is an indicative of the porosity and permeability of concrete specimens. To assess the porosity and the permeability, the collected samples were dried in an oven at 105 °C for 72 h. They then were carefully weighted and exposed to ambient temperature. These specimens then were submerged in water for 30 min and again were weighted. The water absorption was then calculated as the difference between the weights of specimens for dried and fully saturated conditions (Ahmadkhani 2013; Moodi et al. 2015). Outcomes of these studies indicated that the water absorption value for concrete specimens varies between 3.2 and 7% with a mean value around 5%. Almost 92% of specimens represented water absorption values exceeding 4% (Moodi et al. 2015). These indicate the potential of sulfate attack and deterioration is significant at the Zagros tunnel.
A few sulfate ion tests were also performed on the collected concrete samples (Ahmadkhani 2013; Moodi et al. 2015). Based on the local concrete standard the water soluble sulfate content (in the form of SO3) shall not exceed 4% of the cement weight and the total sulfate content should not exceed 5% of the cement weight (Moodi et al. 2015; Ramezanianpour 2011). Studies indicated that the percentage of water-soluble sulfate content and total sulfate content should be less than 0.72% and 0.9%, respectively. To assess the impact of deterioration, Moodi et al. (2015) performed sulfate ion tests on powders collected from every 5 mm of the first 25 mm depth of the cored concrete specimens. The outcomes of the analyses indicated that for the majority of collected samples form the inner side of the tunnel segments, the value of water soluble sulfate content is less than 0.72%. Acid extraction test also showed that the majority of specimens had total sulfate content above 0.9%. However, this value decreased with increasing the depth into the concrete segment and reached an almost allowable value at 20 mm depth, except for a few samples. Tests on samples extracted from the outer side of the concrete segmental linings also showed a smaller value of sulfate content in compare with the ones extracted from the inner sides. For all outer side samples, the sulfate content was less than 0.72%. Except for three samples the total sulfate content was less than 0.9% at all depth. However, three samples that their total level of sulfate content exceed 0.9% at 5.0 mm depth reached an allowable level of total sulfate content at almost 10 mm depth. Further details related to the effects of sulfate attack on the mechanical properties of concrete, especially segmental lining, can be also be found elsewhere (Ahmadkhani 2013; Moodi et al. 2015; Ramezanianpour et al. 2018).
6 Discussion and Proposals
The mechanisms of concrete deterioration at the Zagros tunnel was carefully discussed and the sources of sulfate attack were identified. The role of deterioration on the mechanical behavior of concrete lining was also investigated. The question then would be to find a practical approach to prevent further sulfate attack and the consequent damage of the segmental linings. This questions is briefly explained and addressed in this section.
It is unlikely that the change of cement can highly improve the resistance of segmental linings, at the Zagros tunnel, against the deteriorating factors and completely eliminate further damage. Adding pozzolan may reduce the porosity of the concrete to some extent (Ramezanianpour et al. 2018), but it cannot totally prevent the acidic reactions (TSA), particularly on the surface of the concrete linings. Reducing the water/cement ratio may also help to decrease the permeability of the concrete, to some extent. It, however, may not completely preclude the acidic attack at the exposed surfaces of the concrete linings (Biczok 1967).
To reduce any deterioration damage, the contact of concrete with aggressive environment should be prevented. Alternatively, materials (cements) that represent superior resistance to aggressive spices may be used (Biczok 1967; Ramezanianpour 2011). Bellmann et al. (2012) investigated the mechanisms of deterioration of twenty real cases in Germany. In particular, they represented the outcomes of corrosion assessment in a concrete bridge, which was exposed to water with high sulfate concentration (2500 mg/L) for 35 years. However, due to the bituminous coating the concrete showed minimum deterioration and remained in very good condition (Bellmann et al. 2012). Inokuma and Inano (1996) also suggested the following countermeasure to protect the linings of underground structures against corrosion:
-
Applying a layer of chemical grout or shotcrete over the inner part of tunnel linings
-
Using rock bolts to reduce the ground pressure on linings
-
Backfilling of the tunnel linings in order to suppress the loosening of the ground as well as to reduce tensile forces that may develop because of bending of rocks strata.
There are variety of surface treatments that can be used for concrete structures protection. Such techniques have well been explained in the relevant references (Bellmann et al. 2012; Biczok 1967). For example, carbon textile sheet may be applied to reduce the potential of deterioration. These materials can be bonded into the inner surfaces of linings, by agents, to prevent cracks from opening and widening. Although textile sheets are expensive, they can effectively restrain the deformation of the tunnel lining and strengthen the load-bearing capacity of the linings (Inokuma and Inano 1996).
Further investigation might be needed to find proper coating polyester layers to protect the inner surface of segmental tunnel linings where severe deterioration damage was observed. Liu and Vipulanandan (2001) conducted a combination of full-scale and laboratory tests in order to assess the performance of a polyester-based polymer concrete coating material for the protection of dry and wet concretes and clay brick substrates against deterioration. Acidic environments was considered to represent the worst sewer and accelerated test conditions. The performance of the polymer concrete coating material was studied for over 3 years. Such polymer coating can easily be applied on both dry and wet surfaces of concretes. Outcomes of the study indicated that the polymer coating achieves a considerable bond strength with clay bricks and wet concrete surfaces. The bounding in between the coating and concrete was sufficient to ensure the stability of the layers in long-term. Indeed, no bond failure was recorded during the 3 years of the tests. This study also showed that the coating extended the lifetime of dry and wet concrete by a factor of 29 and 71, respectively. This indicates that the polymer concrete coating was even more effective for wet rather than dry concrete surfaces (Liu and Vipulanandan 2001).
Pre-excavation grouting (e.g., face pre-grouting and peripheral pre-grouting) are also widely used in tunnelling in weak and water bearing grounds. Pre-excavation grouting not only reduces the ingress of water because of sealing discontinuities, but also strengthens the rock mass a head of the tunnel and helps to improve the tunnel long-term stability (Fouladgar 2004; Tseng et al. 2001). A cone (fan) shape pattern is normally suggested for pre-excavation grouting (Fouladgar 2004; Tseng et al. 2001). Schematic views of such grouting pattern are shown in Fig. 15. Pre-excavation grouting technique has proven to be an effective technique for tunnelling in harsh hydrogeological conditions. This technique has previously been used in numerous tunnels with complex hydrogeological conditions (Fouladgar 2004; Tseng et al. 2001). For example, pre-grouting was used at Koohrang-III water transfer tunnel in order to reduce the water rush as well as to reinforce the encountered weak fault zones to facilitate TBM tunnelling (Fouladgar 2004). Pre-grouting may, however, be very time-consuming and can, therefore, highly affect the rate of tunnelling.
Since the Zagros tunnel excavated by a Double Shield TBM and lined by segmental linings, the exact locations of the discharging conduits and their morphology have not well been identified. An elaborate post-grouting plan may, therefore, be useful to reduce the inflow of water and to reclaim the aquifer. The cost of such a systematic grouting might be considerable and at best this scenario may not be conclusive (Khave 2014).
Chemical grout may also be used to fill the gap behind the segmental linings and to protect the outer surfaces of the linings. Grout may also be injected into the rock mass behind the installed segmental linings. This can help to reduce the permeability of the rock mass around the tunnel and to decrease the ingress of water. Reinforcing the rock mass can also reduce the rock loads on the tunnel support and increase the long-term stability of the tunnels. Filling the gap behind the segmental linings may also protect the outer surfaces of the concrete linings against deterioration (Annett 1994). The use of chemical grouting agents may, however, bring about a new emission problem which should be considered in the design. Chemical grouting may pollute the groundwater and the tunnel environment (Vik et al. 2000). The use of grouting products in tunnel construction may lead to relatively large local releases of chemical materials into the environment. This can result in considerable environmental issues. Excessive care must be taken and continuous monitoring should be considered to ensure that the selected chemical grout does not affect the environment. Hence, it is suggested to avoid unnecessary use of the chemical grouts in tunnelling, especially where the tunnel is used for the transmission of water. These are also important where the amount of chemical grout is large and/or the dilution of discharged water to the recipient is low or the sensitivity of the receiving water is considerable (Vik et al. 2000).
It is also noted that non-destructing testing (NDT) method can be used to identify the gaps between the segmental linings and the tunnel walls. Such areas are normally prone to water rush and vulnerable to the deterioration of the outer surfaces of lining. NDT tests may also be used to check the quality of the installed segmental concrete linings, in-place, and to ensure that deterioration has not affected the functioning of the supports. Additionally, NDT may be used for investigating the role of grouting in strengthening of affected linings (Aggelis et al. 2008; Davis et al. 2005).
Further analyses is also needed to identify the potential of the corrosion of concrete reinforcement in the tunnel environment. For example, Hobbs and Taylor (2000) investigated the nature of thaumasite sulfate attack in concrete in several real cases in the UK. Several bridges and houses were considered in the study. Field inspections revealed that in some cases the overall integrity of the concrete structures were not severely impacted by the procedure of sulfate attack. However, significant thaumasite formation was accompanied by concrete deterioration which subsequently reduced the effective cover to the reinforcement. This indeed has increased the potential of reinforcement corrosion due to chloride ion ingress. Similar phenomenon was observed in a few locations at the Zagros tunnel (see Fig. 16). The corrosion of reinforced pre-cast concrete segmental linings has been investigated by researchers and well documented in the references (Mohammed and Hamada 2006; Mohammed et al. 2004). In particular, Abbas and co-workers (2012, 2014) performed large-scale tests to assess the performance of conventional Reinforced Concrete, RC, and Steel-Fiber Reinforced Concrete, SFRC, segmental linings against chloride ions. Outcomes of these studies indicated that SFRC segments showing a higher cracking load and more stable post-peak behavior in comparison with the conventional RC segments. The SFRC segments might, therefore, be a more durable alternative for precast segmental tunnel linings in terms of resistance against the corrosive factors (Abbas et al. 2012, 2014). The analytical approach proposed by Wang and Liu (2003) can also be used to investigate the softening of steel and concrete bonds due to corrosion effects.
Further investigation should also be done to assess the effects of deterioration on the long-term stability of the tunnel. Perhaps the model proposed by Wells and Melchers (Wells and Melchers 2014, 2015) for predicting the rate of concrete corrosion due to H2S effects, in sewers pipes, can be adopted to compute the reduction in the thickness of the concrete linings, in long-term. The role of excessive rock load because of creep and the deterioration of rock mass in long-term must also be included. Highly damaged segmental linings may also be replaced with galvanised steel linear to ensure the service life of the tunnel. However, this technique could be challenging and costly, in practice.
7 Conclusion
The Zagros tunnel was excavated in a very harsh hydrogeological condition. In particular, the existence of gas and water bearing grounds was not identified in the feasibility studies and in the primary geological investigations, prior to the tunnel construction. The presence of such gassy ground conditions was a challenging problem during the tunnel excavation. Not only it resulted in a very harsh environment for the tunnel crews to operate, but also it led to the decaying of tunnelling equipment and support measures.
It is postulated that the organic materials buried in the ground have been the main source of H2S at the Zagros tunnel. Relevant studies indicate that a great proportion of hydrocarbon reservoirs, in Iran, located in limestone formations (Ghazban and Motiei 2010; Rezaee 2001). The geological formations pertaining to hydrocarbon resources have been crucial in the formation and entrapment of H2S gas in this area. High porosity and permeability are also the dominant features of limestone rocks in the tunnel area. The existence of beddings and joints also provide a suitable path for the organic materials to permeate through the rock strata and enter the tunnel.
In this study, the effects of ground water and H2S on the deterioration of the tunnel pre-cast segmental concrete linings were investigated. Outcomes of the study indicated that the emission of H2S and its reaction with groundwater produce H2SO4 and formed a very corrosive environment. Field observations also revealed that the main suspect of concrete deterioration is sulfate attack. Sulfate ions attack the cement paste structures and partially or entirely replace this hard part of the concrete. This gradually disrupt the bonding between components and transfer the concrete to a non-cohesive mass loosely holding the aggregate particles together. In sulfate attack, sulfate ions react with calcium aluminate phases and calcium hydroxide, in the hardened cement paste to form ettringite (3CaO·Al2O3·3CaSO4·31H2O) or gypsum (CaSO4·2H2O) or may significantly react with carbonates/bicarbonates and results in thaumasite (CaSiO3·CaCO3·CaSO4·15H2O). The new products of reactions in sulfate attack normally have a larger volume than the original cement materials. This results in noticeable swelling and spalling of concrete linings. Sulfate attack also results in the gradual loss of the strength of the concrete linings. Results of XRD and microscopy analyses verified the proposed theory of concrete deterioration at the Zagros tunnel.
Interestingly enough, all the substances and conditions required for sulfate attack (Crammond 2003; Hobbs and Taylor 2000; Sims and Huntley (née Hartshorn) 2004) have been available at the Zagros tunnel to easily cause TSA on the segmental tunnel linings. The abundant of water and bituminous materials could simply generate H2SO4 and produce the required SO −24 ions. Chemical analyses have shown that the required carbonate ions for the formation of thaumasite can be provided from the limestone and dolomite aggregate, in the concrete. The cold and wet environments of the tunnel has also produced a proper atmosphere for TSA. Further investigation is, however, needed to ensure if the acidic attack has penetrated deep into the middle section of the concrete lining and affected the performance of the reinforcements.
This paper also highlighted the importance of geological studies prior to the excavating of tunnels. A proper geological study can help to identify the majority of hazards encountered during tunnelling even in challenging conditions, like the Zagros tunnel. For example, a deep geological study and a proper estimation of water inflow and gas emission based on the hydrogeological conditions at the area of the Zagros tunnel could reveal some of the associated geological and geotechnical hazards, before starting tunnelling. Such information could indeed help to find the best location for the construction of the tunnel.
Apparently protecting the concrete linings against the deteriorating elements can be the best approach to prevent further deterioration. Hence, it is suggested to use a coating layer, like polyester, in order to reduce the interactions between the concrete linings and the groundwater containing aggressive SO −24 , at the Zagros tunnel. It is noted that such chemical coating materials should be safe in terms of environmental issues. The Zagros tunnel will be used to transfer water. Utilizing any type of materials in the construction of the tunnel must, therefore, satisfy the safety requirements. The penetration of H2S may also be prevented by the injection of appropriate grout behind the tunnel segments. It is also noteworthy that the complete sealing of the segmental concrete linings can increase the water pressure on them and may not be technically practical. Grouting the gaps in between the segmental linings and the tunnel walls may, however, protect the concrete lining and prevent severe deterioration, in long-term.
References
Abbas S, Soliman AM, Nehdi M (2012) Mechanical and durability performance of precast tunnel linings. Paper presented at the Montreal TAC 2012, tunnels and underground spaces: sustainability and innovations, Montreal, Canada
Abbas S, Soliman AM, Nehdi ML (2014) Chloride ion penetration in reinforced concrete and steel fiber-reinforced concrete precast tunnel lining segments. ACI Mater J 111:613–621
Aggelis D, Shiotani T, Kasai K (2008) Evaluation of grouting in tunnel lining using impact-echo. Tunn Undergr Space Technol 23:629–637
Ahmadkhani M (2013) Assessing the factors leading to the acidic attack and decaying of concrete and the consequences (in Persian). Paper presented at the 5th Iranian annual concrete conference
Alsinawi SA, Al-Bazzaz AH (1975) A brief review of the alkali–silica in concrete and their applications to Iraqi aggregates. Eng Geol 9:123–139
Annett MF (1994) Grouting of tunnel linings, Structural Grouts In: Domone PLJJ, Jefferis SA (eds) Blackie academic & professional - an imprint of Chapman & Hall, Chap 9, pp 176–193
Attiogbe EK, Rizkalla SH (1988) Response of concrete to sulfuric acid attack. ACI Mater J 85:481–488
Bayati M, Hamidi JK (2017) A case study on TBM tunnelling in fault zones and lessons learned from ground improvement. Tunn Undergr Space Technol 63:162–170
Bellmann F, Erfurt W, Ludwig HM (2012) Field performance of concrete exposed to sulphate and low pH conditions from natural and industrial sources. Cem Concr Compos 34:86–93
Biczok I (1967) Concrete corrosion and concrete protection (second impression). Chemical Publishing Company Inc, Palm Springs
Crammond NJ (2003) The thaumasite form of sulfate attack in the UK. Cem Concr Compos 25:809–818
Davis AG, Lim MK, Petersen CG (2005) Rapid and economical evaluation of concrete tunnel linings with impulse response and impulse radar non-destructive methods. NDT E Int 38:181–186
Eftekhari A, Hamidi JK (2009) Geological hazards in TBM tunnelling—a case study from Zagros long tunnel. Paper presented at the 8th Iranian tunnelling conference, Tarbiat Modares University
Fookes PG (1980) An introduction to the influence of natural aggregates on the performance and durability of concrete. Q J Eng GeolHydrogeol 13:207–229
Fouladgar A (2004) An overview of Koohrang-III water conveyance project and a brief review of the methods of excavation in different tunnel faces and adits (in Persian). Paper presented at the 6th Iranian tunnelling conference, Iran University of Science and Technology, Tehran, Iran
Ghafarian P, Gholami S, Owlad E, Gerivani H (2016) Rainfall–runoff temporal variability in Kermanshah province, Iran and distinguishing anthropogenic effects from climatic effects. J Earth Syst Sci 125:1299–1311. https://doi.org/10.1007/s12040-016-0731-9
Ghazban F, Motiei H (2010) Petroleum geology of the Persian Gulf. Tehran University Press, Tehran
Hagelia P (2011) Deterioration mechanisms and durability of sprayed concrete for rock support in tunnels. The University of Oslo, Oslo
Hagelia P, Sibbick RG, Crammond NJ, Larsen CK (2003) Thaumasite and secondary calcite in some Norwegian concretes. Cem Concr Compos 25:1131–1140
Hassanpor J, Rostami J, Khamehchiyan M, Bruland A (2009) Developing new equations for TBM performance prediction in carbonate-argillaceous rocks: a case history of Nowsood water conveyance tunnel. Geomech Geoeng Int J 4:287–297
Hassanpor J, Ghaedi Vanani AA, Rostami J, Cheshomi A (2016) Evaluation of common TBM performance prediction models based on field data from the second lot of Zagros water conveyance tunnel (ZWCT2). Tunn Undergr Space Technol 52:147–156
Hobbs DW, Taylor MG (2000) Nature of the thaumasite sulfate attack mechanism in field concrete. Cem Concr Res 30:529–533
Hoff CHWD (2009) Water transport in brick, stone and concrete. CRC Press, Boca Raton
Inokuma A, Inano S (1996) Road tunnels in Japan: deterioration and countermeasures. Tunn Undergr Space Technol 11:305–309
Irassar EF (2009) Sulfate attack on cementitious materials containing limestone filler—a review. Cem Concr Res 39:241–254
John DAS (1982) An unusal case of ground water sulphate attack on concrete. Cem Concr Res 12:633–639
Khave GJ (2013) TBM tunnelling in hydrogen sulfide gas bearing ground and its solutions. Geotech Geol Eng 31:1621–1638. https://doi.org/10.1007/s10706-013-9669-8
Khave GJ (2014) Delineating subterranean water conduits using hydraulic testing and machine performance parameters in TBM tunnel post-grouting. Int J Rock Mech Min Sci 70:308–317
Larsen G (1966) Petrographic method used in the study of leaching of cement paste in concrete. Eng Geol 1:189–199
Lei M, Peng L, Shi C, Wang S (2013) Experimental study on the damage mechanism of tunnel structure suffering from sulfate attack. Tunn Undergr Space Technol 36:5–13
Liu J, Vipulanandan C (2001) Evaluating a polymer concrete coating for protecting non-metallic underground facilities from sulfuric acid attack. Tunn Undergr Space Technol 16:311–321
Mays GC (1992) Durability of concrete structures: investigation, repair, protection. E & FN Spon, London
Mirmehrabi H, Ghafoori M, Lashkaripour G, Hassanpor J (2010) Hazards associated with tunnelling in gas bearing grounds—a case study of Aspar (lot 2 of Zagros) water transmission tunnel (in Persian). Sci Technol Environ Eng 93:51–66
Mirmehrabi H, Ghafoori M, Lashkaripour G, Azali ST, Hassanpour J (2011) Hazards of mechanized tunnel excavation in H2S bearing ground in Aspar tunnel, Iran. Environ Earth Sci 66:529–535
Mittermayr F, Bauer C, Klammer D, Böttcher ME, Leis A, Escher P, Dietzel M (2012) Concrete under sulphate attack: an isotope study on sulphur sources. Isot Environ Health Stud 48:105–117
Mohammed TU, Hamada H (2006) Corrosion of steel bars in concrete with various steel surface conditions. ACI Mater J 103:233–242
Mohammed TU, Hamada H, Yamaji T (2004) Concrete after 30 years of exposure—part II: chloride ingress and corrosion of steel bars. ACI Mater J 101:13–18
Moodi F, Ramezanianpour AA, Chenar QB, Esteghamati MZ (2015) Evaluation of sulfate damages in a tunnel concrete segments. In: Concrete repair, rehabilitation and retrofitting IV: proceedings of the 4th international conference on concrete repair, rehabilitation and retrofitting (ICCRRR-4), 5–7 October 2015, Leipzig, Germany, 2015. CRC Press, p 251
Morsali M, Rezaei M (2017) Assessment of H2S emission hazards into tunnels: the Nosoud tunnel case study from Iran. Environ Earth Sci 76:227
Mossmark F (2014) Prediction of groundwater chemistry in conjunction with underground construction—field studies and hydrochemical modelling. Chalmers University of Technology, Göteborg
Mostoufinejad D (2003) Concrete technology. Arkan Publication, Isfahan
Nazemi M (2017) Personal photos gallery of geotechnical problems at the Zagros tunnel
Nielsen P, Nicolai S, Darimont A, Kestemont X (2014) Influence of cement and aggregate type on thaumasite formation in concrete. Cem Concr Compos 53:115–126
Rahman MM, Bassuoni MT (2014) Thaumasite sulfate attack on concrete: mechanisms, influential factors and mitigation. Constr Build Mater 73:652–662
Rajesh R, Brindha K, Elango L (2015) Groundwater quality and its hydrochemical characteristics in a shallow weathered rock aquifer of Southern India Water Quality. Expo Health 7:515–524
Ramezanianpour AA (2011) Concrete design (in Persian). Sanat-Gostar Publication, Tehran
Ramezanianpour AA, Zolfagharnasab A, Bahmanzadeh F, Ramezanianpour AM (2018) Assessment of high performance concrete containing mineral admixtures under sulfuric acid attack (in Persian). Amirkabir J Civil Eng 50:121–138
Rezaee MR (2001) Petroleum geology (in Persian). Alavi Publication, Tehran
Romer M (2003a) Detachment of shotcrete due to long term interaction with ground water. Paper presented at the international seminar on the thaumasite form of sulfate attack of concrete
Romer M (2003b) Steam locomotive soot and the formation of thaumasite in shotcrete. Cem Concr Compos 25:1173–1176
Romer M, Holzer L, Pfiffner M (2003) Swiss tunnel structures: concrete damage by formation of thaumasite. Cem Concr Compos 25:1111–1117
Saglamoglu S (2012) Groundwater chemistry and its influence on the selection of construction materials—a review of four traffic tunnels in Sweden and evaluation of technical requirements. Chalmers University of Technology, Göteborg
Shahriar K, Sharifzadeh M, Khademi Hamidi J (2008) Geotechnical risk assessment based approach for rock TBM selection in difficult ground conditions. Tunn Undergr Space Technol 23:318–325
Sims I, Huntley (née Hartshorn) SA (2004) The thaumasite form of sulfate attack-breaking the rules. Cem Concr Compos 26:837–844
Suleiman AR, Soliman AM, Nehdi ML (2014) Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr Build Mater 73:674–681
Talesnick M, Baker R (1999) Investigation of the failure of a concrete-lined steel pipe. Geotech Geol Eng 17:99–121
Tali N, Lashkaripour GR, Ghafoori M, Hafezi Moghadas N (2014) Problems of mechanized tunnelling in gassy ground conditions—a case study of Nosoud water transmission tunnel. Int J Geogr Geol 3:101–113
Tseng D-J, Tsai B-R, Chang L-C (2001) A case study on ground treatment for a rock tunnel with high groundwater ingression in Taiwan. Tunn Undergr Space Technol 16:175–183
Usman M, Galler R (2013) Long-term deterioration of lining in tunnels. Int J Rock Mech Min Sci 64:84–89
Usman M, Volderauer C, Gschwandtner G, Galler R (2011) Three dimensional load analysis of tunnel linings including weathering processes of the shotcrete. Berg Hüttenmännische Monatshefte (BHM) 156:487–491
Vik E et al (2000) Experiences from environmental risk management of chemical grouting agents used during construction of the Romeriksporten tunnel. Tunn Undergr Space Technol 15:369–378
Wang X, Liu X (2003) A strain-softening model for steel–concrete bond. Cem Concr Res 33:1669–1673
Wells T, Melchers RE (2014) An observation-based model for corrosion of concrete sewers under aggressive conditions. Cem Concr Res 61–62:1–10. https://doi.org/10.1016/j.cemconres.2014.03.013
Wells T, Melchers RE (2015) Modelling concrete deterioration in sewers using theory and field observations. Cem Concr Res 77:82–96. https://doi.org/10.1016/j.cemconres.2015.07.003
Zhiqiang Z, Mansoor YA (2013) Evaluating the strength of corroded tunnel lining under limiting corrosion conditions. Tunn Undergr Space Technol 38:464–475
Acknowledgements
The technical supports provided by Dr. B. Rafiei, Dr. M. Tadayon, Dr. M. Heidari, Mr. M. Zaker Esteghamati and Mr. M. Nazemi, during the course of this study, are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fathi Salmi, E., Soltani Asadi, Z., Bayati, M. et al. Assessing the Hydrogeological Conditions Leading to the Corrosion and Deterioration of Pre-cast Segmental Concrete Linings (Case of Zagros Tunnel). Geotech Geol Eng 37, 3961–3983 (2019). https://doi.org/10.1007/s10706-019-00886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-019-00886-1